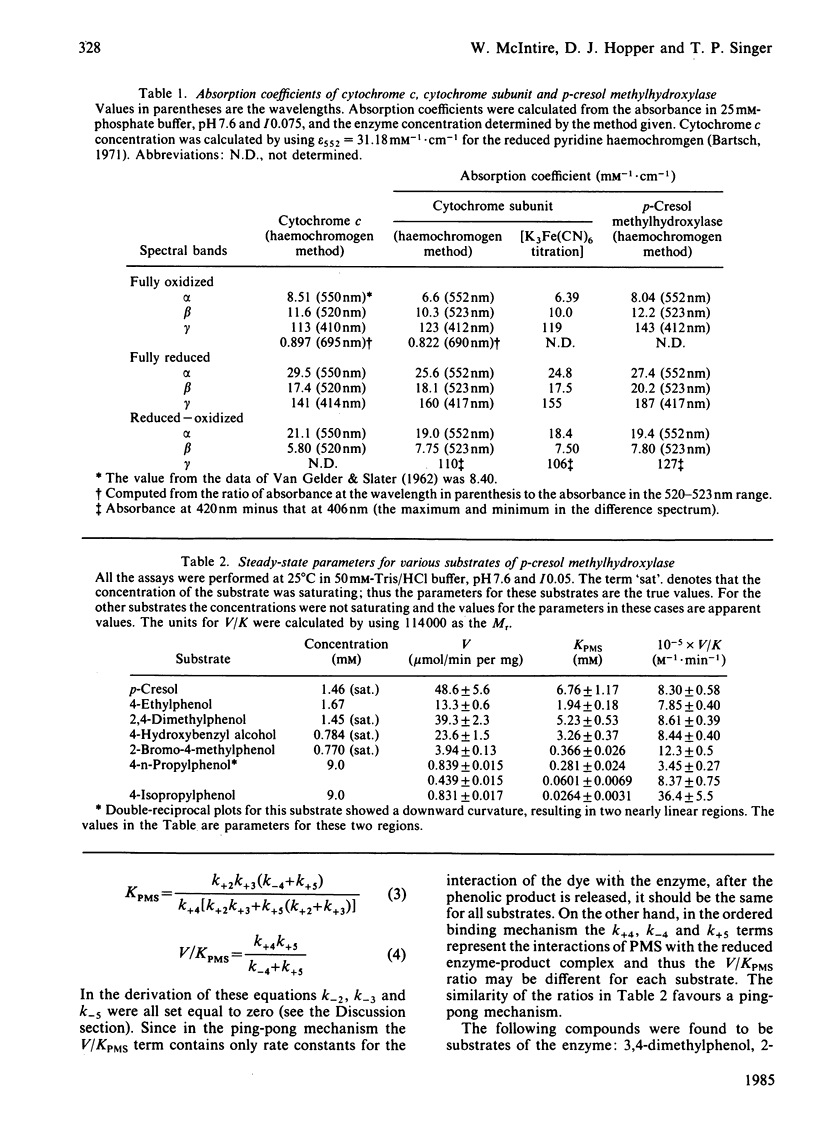
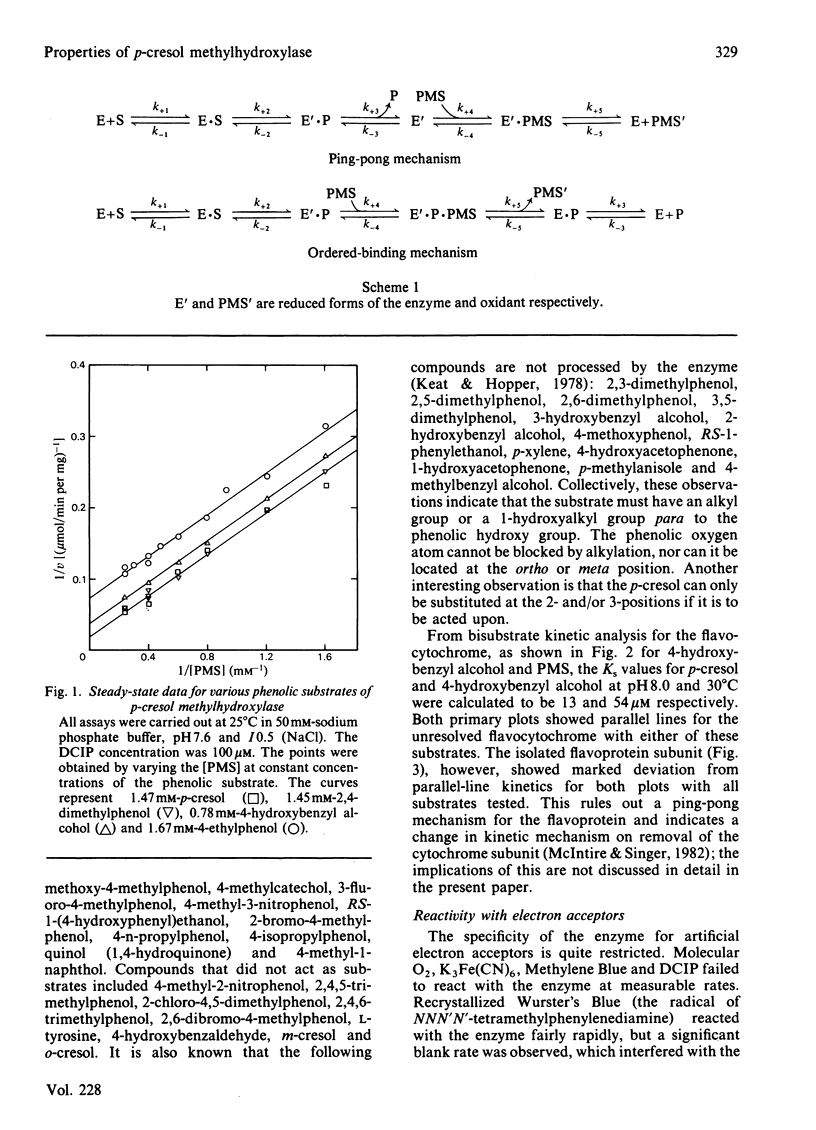
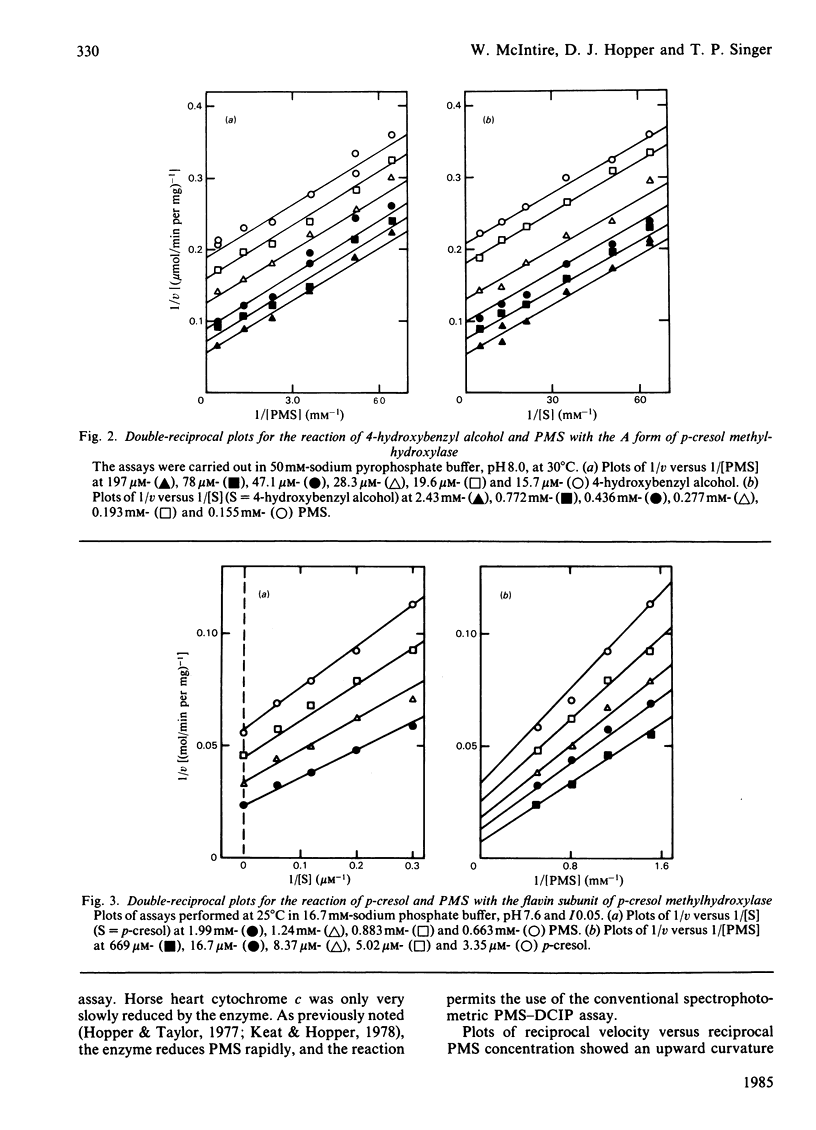
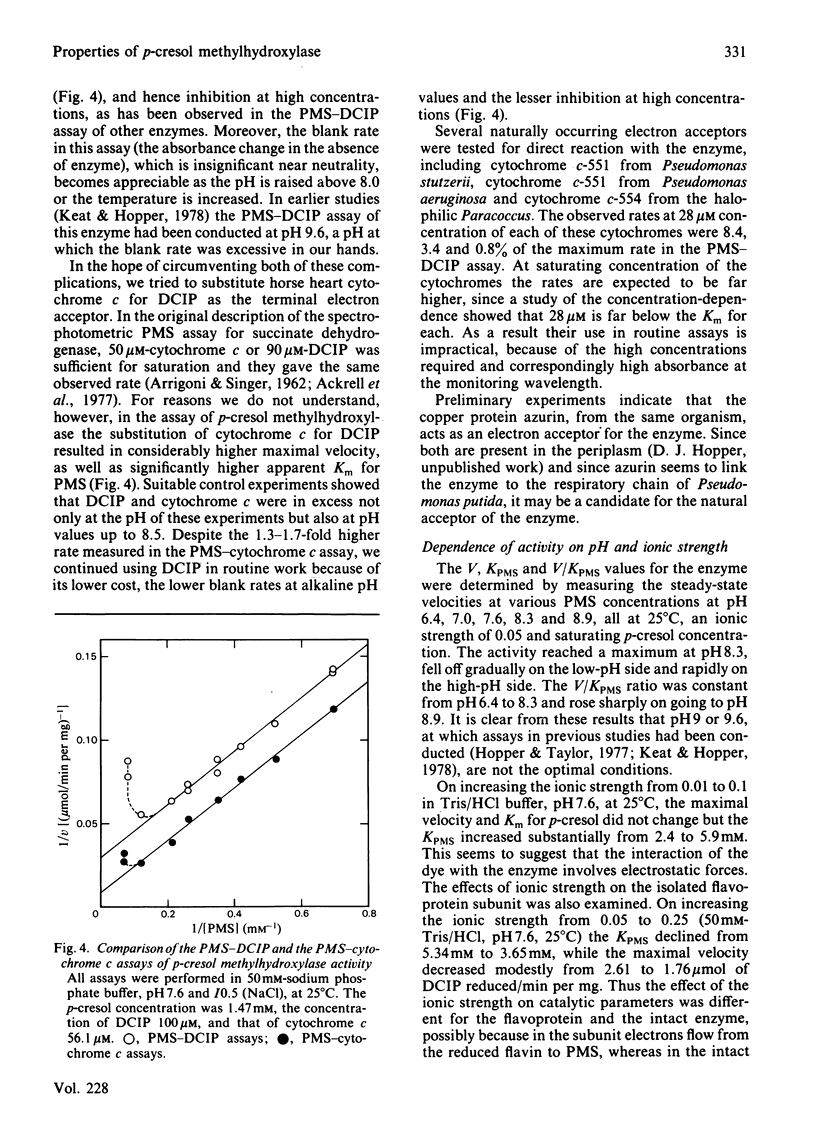
Abstract
p-Cresol methylhydroxylase from Pseudomonas putida, an anaerobic dehydrogenase that catalyses the oxidation of p-cresol to p-hydroxybenzyl alcohol and then to p-hydroxybenzaldehyde, is an enzyme of great interest in several respects. One of these is the fact that its flavoprotein and cytochrome c subunits may be reversibly dissociated with ease, with full regeneration of the activity and its native properties on recombining the components. Bisubstrate kinetic analysis of the unresolved enzyme gives parallel-line kinetics in double-reciprocal plots, whereas the reaction of the separated flavoprotein subunit with substrates is described by converging lines. The mechanistic implications of these behaviours are discussed. Reductive titration with dithionite results in the uptake of 3 electrons by the enzyme, with the intermediate formation of the anionic flavin radical [McIntire, Edmondson, Hopper & Singer (1981) Biochemistry 20, 3068-3075]. Reductive titration with substrates resulted initially only in reduction of the cytochrome subunit, followed by formation of the anionic radical and finally the fully reduced enzyme. These observations suggest rapid intermolecular electron transfer between p-cresol methylhydroxylase molecules. This paper also examines the effect of pH and ionic strength on the activity and specificity of the enzyme with respect to substrates and natural, as well as artificial, electron acceptors. The absorption coefficients of the enzyme and of its subunits in various oxidation states are also presented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Ackrell B. A., Coles C. J., Singer T. P. On the reaction of succinate dehydrogenase with Wurster's blue and its implications on the effect of the membrane environment on catalytic activity. FEBS Lett. 1977 Mar 15;75(1):249–253. doi: 10.1016/0014-5793(77)80097-5. [DOI] [PubMed] [Google Scholar]
- Bar-Tana J., Cleland W. W. Rabbit muscle phosphofructokinase. II. Product and dead end inhibition. J Biol Chem. 1974 Feb 25;249(4):1271–1276. [PubMed] [Google Scholar]
- Bhattacharyya A., Tollin G., McIntire W., Singer T. P. Laser-flash-photolysis studies of p-cresol methylhydroxylase. Electron-transfer properties of the flavin and haem components. Biochem J. 1985 Jun 1;228(2):337–345. doi: 10.1042/bj2280337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Adolescent ambivalence: a therapeutic issue. J Psychiatr Nurs Ment Health Serv. 1980 Sep;18(9):29–33. [PubMed] [Google Scholar]
- Ghisla S., Massey V., Lhoste J. M., Mayhew S. G. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry. 1974 Jan 29;13(3):589–597. doi: 10.1021/bi00700a029. [DOI] [PubMed] [Google Scholar]
- Hopper D. J. Incorporation of [18O]water in the formation of p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from Pseudomonas putida. Biochem J. 1978 Oct 1;175(1):345–347. doi: 10.1042/bj1750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. Redox potential of the cytochrome c in the flavocytochrome p-cresol methylhydroxylase. FEBS Lett. 1983 Sep 5;161(1):100–102. doi: 10.1016/0014-5793(83)80738-8. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. The purification and properties of p-cresol-(acceptor) oxidoreductase (hydroxylating), a flavocytochrome from Pseudomonas putida. Biochem J. 1977 Oct 1;167(1):155–162. doi: 10.1042/bj1670155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. The hydroxylation of P-cresol and its conversion to P-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976 Mar 22;69(2):462–468. doi: 10.1016/0006-291x(76)90544-1. [DOI] [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. P-cresol and 3,5-xylenol methylhydroxylases in Pseudomonas putida N.C.I.B. 9896. Biochem J. 1978 Nov 1;175(2):649–658. doi: 10.1042/bj1750649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambeth D. O., Palmer G. The kinetics and mechanism of reduction of electron transfer proteins and other compounds of biological interest by dithionite. J Biol Chem. 1973 Sep 10;248(17):6095–6103. [PubMed] [Google Scholar]
- Massey V., Hemmerich P. Photoreduction of flavoproteins and other biological compounds catalyzed by deazaflavins. Biochemistry. 1978 Jan 10;17(1):9–16. doi: 10.1021/bi00594a002. [DOI] [PubMed] [Google Scholar]
- McIntire W., Edmondson D. E., Hopper D. J., Singer T. P. 8 alpha-(O-Tyrosyl)flavin adenine dinucleotide, the prosthetic group of bacterial p-cresol methylhydroxylase. Biochemistry. 1981 May 26;20(11):3068–3075. doi: 10.1021/bi00514a013. [DOI] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Craig J. C., Everhart E. T., Webster R. V., Causer M. J., Singer T. P. Stereochemistry of 1-(4'-hydroxyphenyl)ethanol produced by hydroxylation of 4-ethylphenol by p-cresol methylhydroxylase. Biochem J. 1984 Dec 1;224(2):617–621. doi: 10.1042/bj2240617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W., Singer T. P. Resolution of p-cresol methylhydroxylase into catalytically active subunits and reconstitution of the flavocytochrome. FEBS Lett. 1982 Jul 5;143(2):316–318. doi: 10.1016/0014-5793(82)80124-5. [DOI] [PubMed] [Google Scholar]
- Morpeth F. F., Massey V. Steady-state kinetic studies on D-lactate dehydrogenase from Megasphera elsdenii. Biochemistry. 1982 Mar 16;21(6):1307–1312. doi: 10.1021/bi00535a031. [DOI] [PubMed] [Google Scholar]
- Oppenheimer L., Capizzi T. P., Miwa G. T. Application of jackknife procedures to inter-experiment comparisons of parameter estimates for the Michaelis-Menten equation. Biochem J. 1981 Sep 1;197(3):721–729. doi: 10.1042/bj1970721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


