Abstract
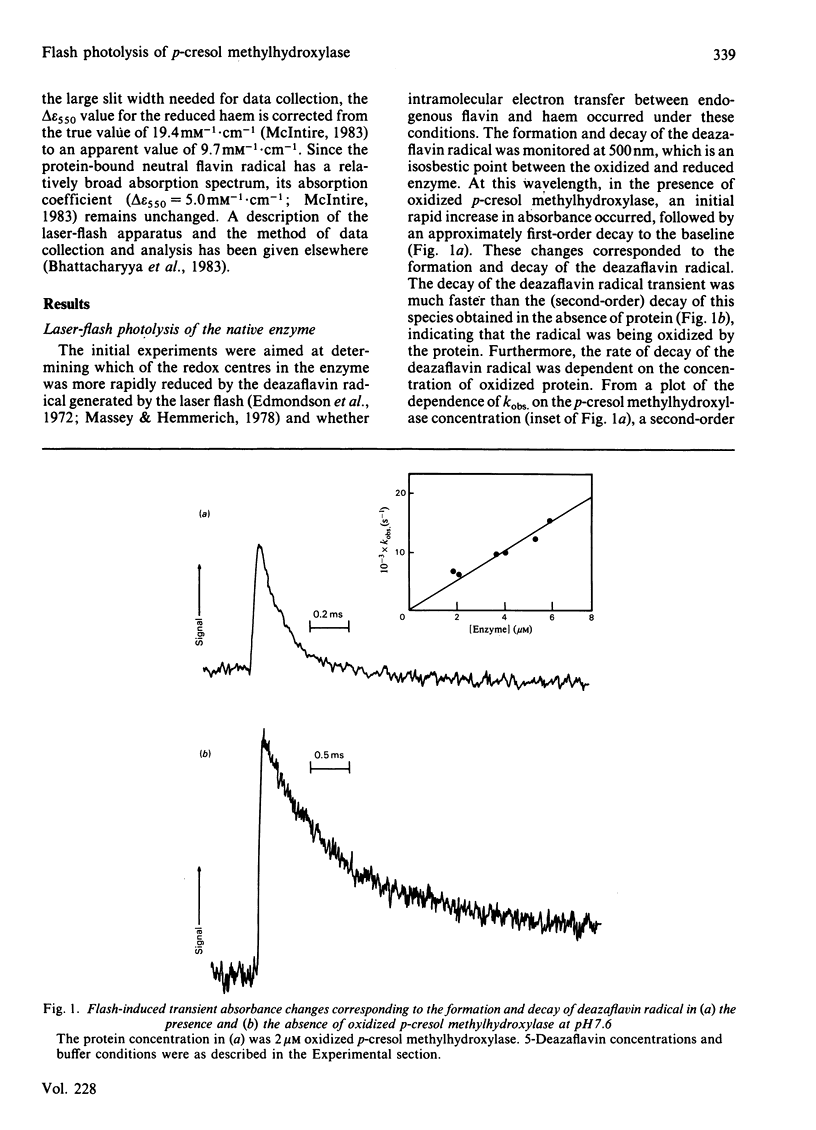
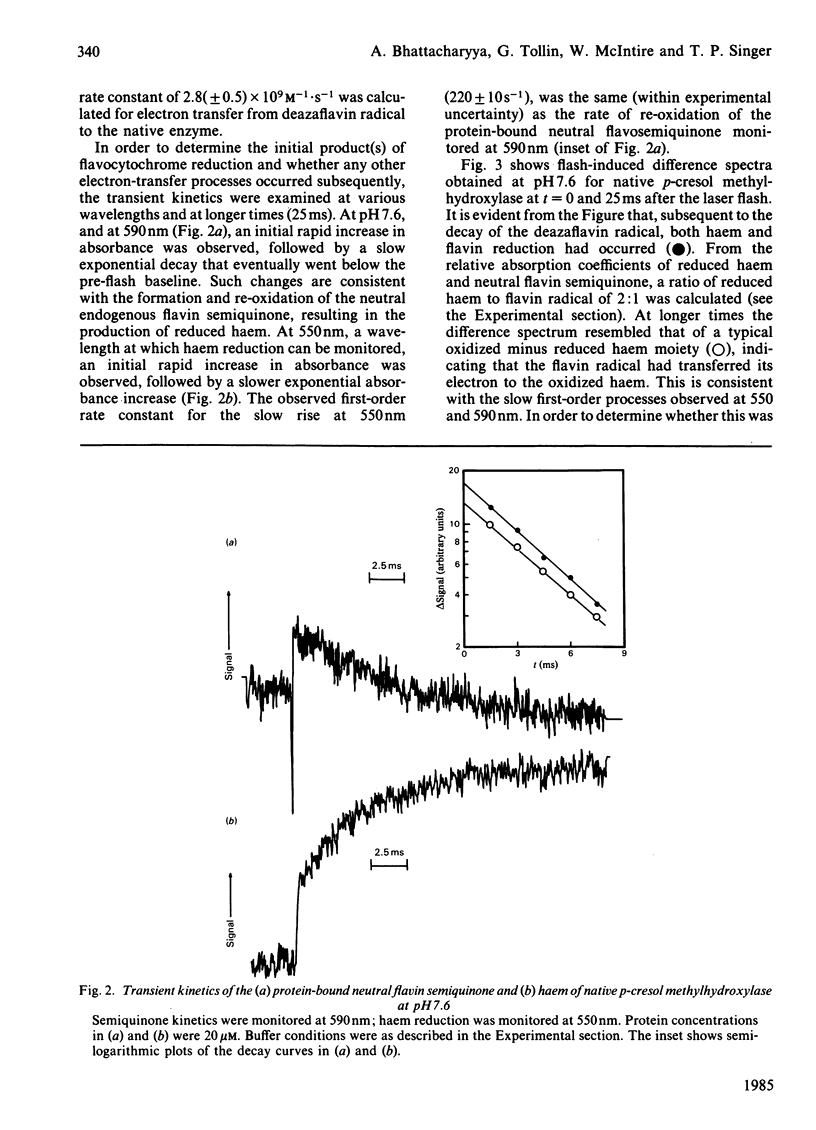
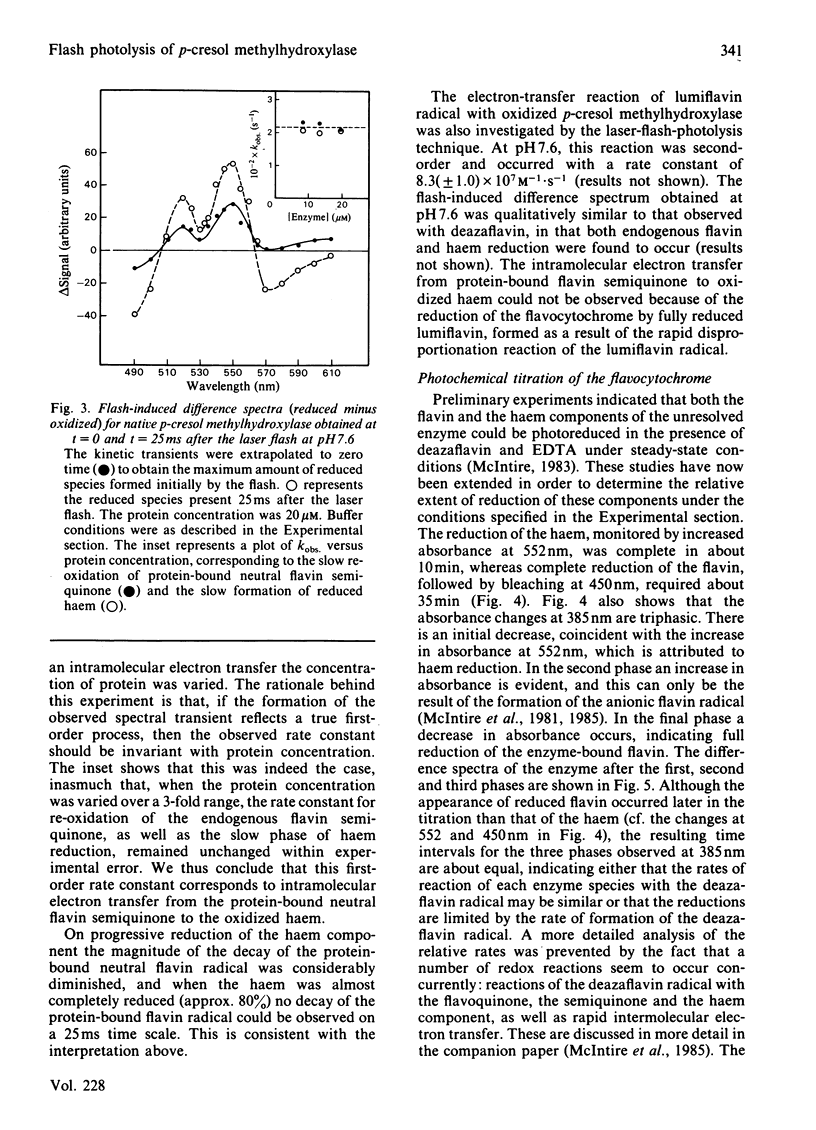
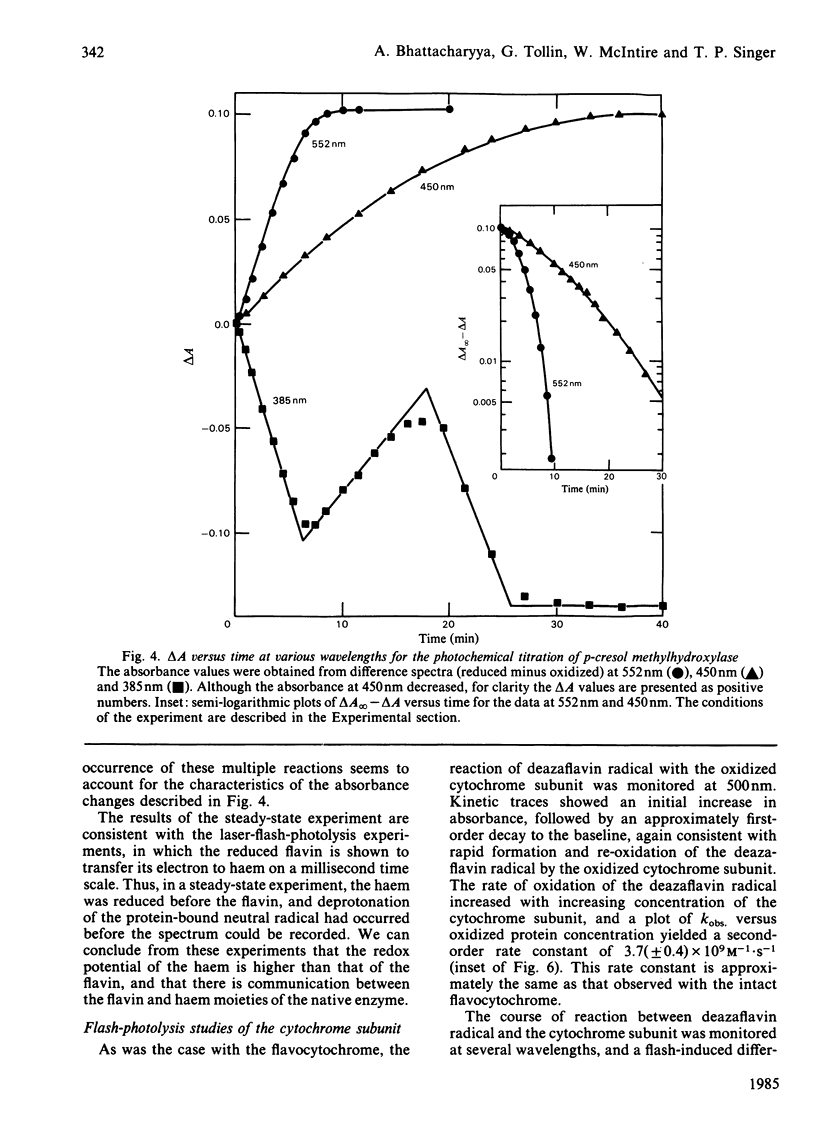
p-Cresol methylhydroxylase, a heterodimer consisting of one flavoprotein subunit and one cytochrome c subunit, may be resolved into its subunits, and the holoenzyme may then be fully reconstituted from the pure subunits. In the present study we have characterized the reduction kinetics of the intact enzyme and its subunits, by using exogenous 5-deazariboflavin semiquinone radical generated in the presence of EDTA by the laser-flash-photolysis technique. Under anaerobic conditions the 5-deazariboflavin semiquinone radical reacts rapidly with the native enzyme with a rate constant approaching that of a diffusion-controlled reaction (k = 2.8 X 10(9) M-1 X s-1). Time-resolved difference spectra at pH 7.6 indicate that both flavin and haem are reduced initially by the deazariboflavin semiquinone radical, followed by an additional slower intramolecular electron transfer (k = 220 s-1) from the endogenous neutral flavin semiquinone radical to the oxidized haem moiety of the native enzyme. During the steady-state photochemical titration of the native enzyme at pH 7.6 with deazariboflavin semiquinone radical generated by light-irradiation the haem appeared to be reduced before the protein-bound flavin and was followed by the formation of the protein-bound anionic flavin radical. This result suggests that the redox potential of the haem is higher than that of the flavin, and that deprotonation of the flavin neutral radical occurred during the photochemical titration. Reduction kinetics of the flavoprotein and cytochrome subunits were also investigated by laser-flash photolysis. The protein-bound flavin of the isolated flavin subunit was reduced rapidly by the deazariboflavin semiquinone radical (k = 2.2 X 10(9) M-1 X s-1), as was the haem of the pure cytochrome c subunit (k = 3.7 X 10(9) M-1 X s-1). Flash-induced difference spectra obtained for the flavoprotein and cytochrome subunits at pH 7.6 were consistent with the formation of neutral flavin semiquinone radical and reduced haem, respectively. Investigation of the kinetic properties of the neutral flavin semiquinone radical of the flavoprotein subunit at pH 7.6 and at longer times (up to 5s) were consistent with a slow first-order deprotonation reaction (k = 1 s-1) of the neutral radical to its anionic form.
Full text
PDF

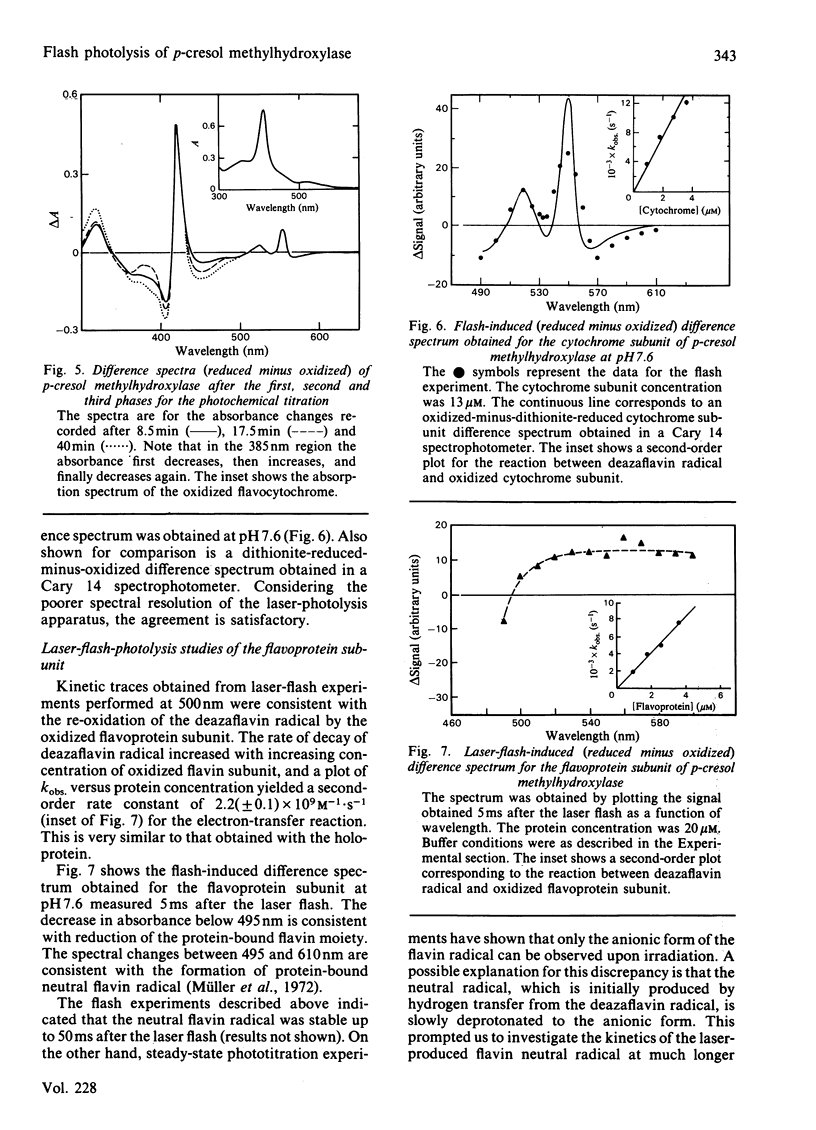
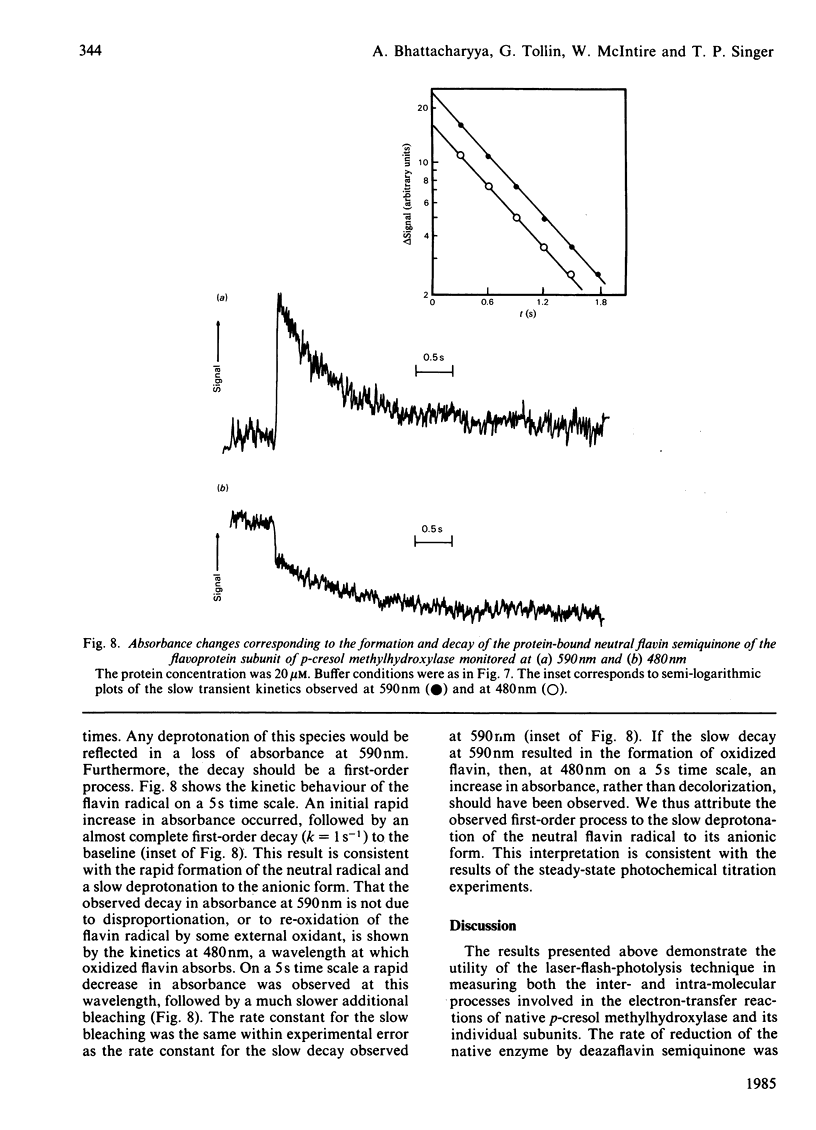

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cusanovich M. A., Tollin G. Chromatium vinosum cytochrome c-552. Reduction by photoreduced flavins and intramolecular electron transfer. Biochemistry. 1980 Jul 8;19(14):3343–3347. doi: 10.1021/bi00555a037. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Barman B., Tollin G. On the importance of the N-5 position in flavin coenzymes. Properties of free and protein-bound 5-deaza analogs. Biochemistry. 1972 Mar 28;11(7):1133–1138. doi: 10.1021/bi00757a003. [DOI] [PubMed] [Google Scholar]
- Hopper D. J. Incorporation of [18O]water in the formation of p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from Pseudomonas putida. Biochem J. 1978 Oct 1;175(1):345–347. doi: 10.1042/bj1750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. The hydroxylation of P-cresol and its conversion to P-hydroxybenzaldehyde in Pseudomonas putida. Biochem Biophys Res Commun. 1976 Mar 22;69(2):462–468. doi: 10.1016/0006-291x(76)90544-1. [DOI] [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. P-cresol and 3,5-xylenol methylhydroxylases in Pseudomonas putida N.C.I.B. 9896. Biochem J. 1978 Nov 1;175(2):649–658. doi: 10.1042/bj1750649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Hemmerich P. Photoreduction of flavoproteins and other biological compounds catalyzed by deazaflavins. Biochemistry. 1978 Jan 10;17(1):9–16. doi: 10.1021/bi00594a002. [DOI] [PubMed] [Google Scholar]
- McIntire W., Edmondson D. E., Hopper D. J., Singer T. P. 8 alpha-(O-Tyrosyl)flavin adenine dinucleotide, the prosthetic group of bacterial p-cresol methylhydroxylase. Biochemistry. 1981 May 26;20(11):3068–3075. doi: 10.1021/bi00514a013. [DOI] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Singer T. P. p-Cresol methylhydroxylase. Assay and general properties. Biochem J. 1985 Jun 1;228(2):325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W., Singer T. P. Resolution of p-cresol methylhydroxylase into catalytically active subunits and reconstitution of the flavocytochrome. FEBS Lett. 1982 Jul 5;143(2):316–318. doi: 10.1016/0014-5793(82)80124-5. [DOI] [PubMed] [Google Scholar]
- Müller F., Brüstlein M., Hemmerich P., Massey V., Walker W. H. Light-absorption studies on neutral flavin radicals. Eur J Biochem. 1972 Feb;25(3):573–580. doi: 10.1111/j.1432-1033.1972.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Tollin G., Meyer T. E., Cusanovich M. A. Intramolecular electron transfer in Chlorobium thiosulfatophilum flavocytochrome c. Biochemistry. 1982 Aug 3;21(16):3849–3856. doi: 10.1021/bi00259a020. [DOI] [PubMed] [Google Scholar]


