Abstract
A sufficiently high current output of nano energy harvesting devices is highly desired in practical applications, while still a challenge. Theoretical evidence has demonstrated that Coulomb drag based on the ion-electron coupling interaction, can amplify current in nanofluidic energy generation systems, resulting in enhanced energy harvesting. However, experimental validation of this concept is still lacking. Here we develop a nanofluidic chemoelectrical generator (NCEG) consisting of a carbon nanotube membrane (CNTM) sandwiched between metal electrodes, in which spontaneous redox reactions between the metal and oxygen in electrolyte solution enable the movement of ions within the carbon nanotubes. Through Coulomb drag effect between moving ions in these nanotubes and electrons within the CNTM, an amplificated current of 1.2 mA cm−2 is generated, which is 16 times higher than that collected without a CNTM. Meanwhile, one single NCEG unit can produce a high voltage of ~0.8 V and exhibit a linear scalable performance up to tens of volts. Different from the other Coulomb drag systems that need additional energy input, the NCEG with enhanced energy harvesting realizes the ion-electron coupling by its own redox reactions potential, which provides a possibility to drive multiple electronic devices for practical applications.
Subject terms: Nanofluidics, Electron transfer
A nanofluidic chemoelectrical generator utilizing the Coulomb drag effect is developed to achieve enhanced current density, generating an amplified current of 1.2 mA cm−2 and voltage of approximately 0.8 V, scalable linearly to tens of Volts.
Introduction
The phenomenon of Coulomb drag was initially documented as electron-electron interactions between two closely spaced, yet electrically isolated conductors, referred to as “layers”. Due to long-range interactions between these two distinct layers, an electrical current flowing through one of the layers, known as the “active layer”, can induce an electrical current in the other layer, the “passive layer”. During this process, both the energy and momentum of the carriers in the “active layer” transfer to the carriers in the “passive layer”, resulting in the generation of an open circuit voltage and short circuit current through efficiently “dragging” them along1–3.
A similar dragging effect has also been observed in nanofluidic-based devices, extending the concept of Coulomb drag to interactions between a moving ionic fluid and electrons in conductors4–9. It has been discovered that a nanoampere electrical current in graphene can be induced by ionic flow which is driven by applying voltage to the fluidic chamber10, as the flow of fluids induces electrical polarization, driving electrical currents. Conversely, electrical excitations also contribute to ion movement. By applying an externally biased voltage to the single-walled carbon nanotube (SWCNT) shell layer, an electrical current is generated which drags ions through the SWCNT nanochannels, resulting in an ionic drag current with opposite direction11. Recently, Xiong et al. demonstrated that this generated current originates from the confined ion-electron interactions via a nanofluidic Coulomb drag mechanism through calculations and simulations12. The model predicts the amplification of ionic current by the electronic membrane due to the significant difference between ion mass and hole mass on the semiconductive membrane, deepening the understanding of the ion-electron interactions in nanochannels and demonstrating the potential for enhancing the current output in energy harvesting. With the amplified current resulting from the Coulomb drag effect, the bottleneck of low current in the practical application of nanoenergy generators can be addressed13–15. However, further experimental work is still needed to explore the amplification of ionic current. In addition, all previously reported studies on the Coulomb drag effect needed an external electrical field to drive the electrons or ionic flow10,11. These additional energy input components increase the complexity of the energy-harvesting devices, hindering their practical application. Therefore, it is important to develop nanofluidic-based energy generators that can not only exploit the current amplification effect by Coulomb drag but also power the ionic flow itself.
In this work, we present a high-performance nanofluidic chemoelectrical generator (NCEG) based on a carbon nanotube membrane (CNTM), which is fabricated by sandwiching a CNTM with an area of around 25 mm2 between two metal electrodes. To drive the ionic flow by the NCEG itself, we utilize metal-air redox reactions that can convert chemical energy into electrical energy without external stimulation16–18. Through the spontaneous redox reactions, a chemical potential is established across the membrane, which provides a driving force for the migration of ions in the nanochannels of CNTM. As a result, the current coupling of the ion-electron is significantly amplified, which is 16 times higher than that collected without a CNTM. Furthermore, this device can generate a stable and sustained direct voltage of ~0.8 V and a short-circuit current density of ~1.2 mA cm−2, with a power density of 74 μW cm−2, under ambient conditions for up to 80 hours. This work is the first to provide experimental evidence that ion-electron Coulomb drag interactions can amplify currents for energy harvesting, offering a scalable strategy for energy harvesting devices based on nanofluidic systems.
Results
Schematic and electrical output characteristics of NCEG
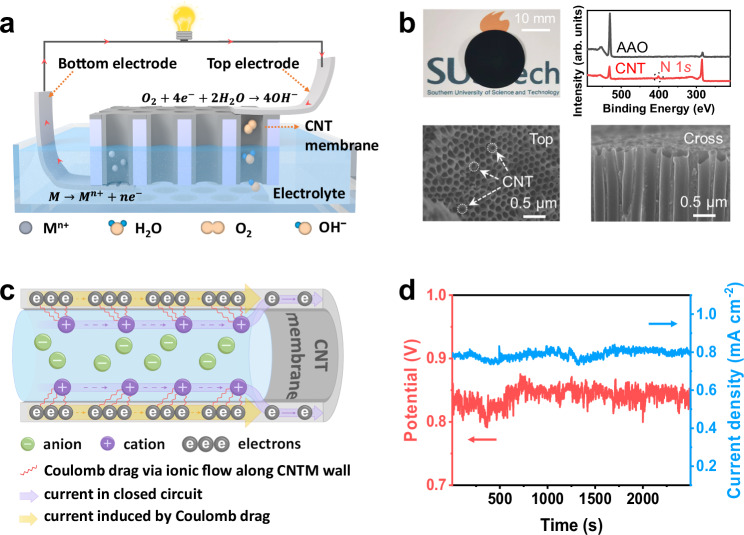
In general, an applied voltage is needed to drive ion movement by the Coulomb drag phenomenon in the nanofluidic system10,11. However, for a nanofluidic-based energy generator through Coulomb drag, it is essential that the device itself can facilitate ion transport within the nanochannels without requiring additional voltage components, thereby simplifying its structure. Here, we report a compact nanofluidic energy generator device (Fig. 1a) with an active electrode, in which the redox reactions on the electrode were employed to provide chemical potential, driving the transport of ions in nanochannels and enhancing the collected current by Coulomb drag. As shown in Fig. 1a, a highly arrayed porous CNTM is sandwiched between a pair of metal electrodes, which are a gold foil and a conductive carbon tape (Nissin SEM double-sided conductive carbon tape) containing an aluminum substrate, respectively. The nitrogen-doped CNTM was fabricated using a template of porous anodic aluminum oxide (AAO), through the conventional chemical vapor deposition (CVD) approach using humidified acetonitrile bubbler as the single source of carbon and nitrogen. The black color of the membrane from the optical photograph and the peak of nitrogen from X-ray photoelectron spectroscopy (XPS) indicates the successful fabrication of CNTM on the AAO template (Fig. 1b). From the morphology characterization in Fig. 1b, the hollow thin CNTs exhibit a density of 1011−1012 cm−2, a length of about 25 μm, and diameter gradient from 100 to 250 nm. Different from the ion transport in bulk water, the CNTM provides a nano-confined environment for the interactions between ions in the aqueous and electrons in the CNTM wall. As shown in Fig. 1c, with nanochannels of CNTM half-immersed by electrolyte solution, a closed circuit is built between the electrolyte and CNTM while the current is significantly restricted due to the high resistance of the CNTM. However, the movement of the cations along the surface of the CNTM gives rise to the Coulomb drag phenomenon, in which a large number of free electrons are dragged by the ionic flow based on the principle of momentum conservation between ions and holes. As a result, the current from the closed circuit of electrolyte and CNTM (purple arrow) and the coupled electrons (yellow arrow) in the CNTM dragged by ionic flow are collected. Therefore, high energy outputs of an approximately 0.8 V open-circuit voltage (VOC) and a 1.2 mA cm−2 persistent current density can be realized by the device designed in this work in 0.1 M NaCl electrolyte (Fig. 1d).
Fig. 1. Schematic and electrical output characteristics of the nanofluidic chemoelectrical generator (NCEG).
a The structure diagram of the NCEG: A highly arrayed porous a carbon nanotube membrane (CNTM) is sandwiched between a pair of metal electrodes, which are a gold foil and a conductive carbon tape containing an aluminum substrate, respectively. Once the electrolyte solution is added on only one side of the CNTM and half-filled in the nanochannels of the CNTM, redox potential forms and then powers the ionic movement in the nanochannels, which amplifies the current in the whole circuit due to the ion-electron Coulomb drag effect. b Morphologies of the CNTM and the X-ray photoelectron spectroscopy (XPS) characterization of the pristine anodic aluminum oxide (AAO) membrane and the CNTM. c Schematic diagram of the ionic Coulomb drag effect between the ionic movement along the CNTM and the free electrons dragged in the CNTM. d Open-circuit voltage and short-circuit current density generated by the NCEG in 0.1 M NaCl electrolyte.
Potential induced by redox reactions
The voltage output signal is from the redox reactions between the metal electrode and oxygen. In the presence of the electrolyte solution, the oxidation of the bottom metal electrode occurs, generating an electrical potential, thus realizing the conversion of chemical energy into electrical energy19–21. The significant role of the redox reaction site is demonstrated by manipulating the position of the added electrolyte, as illustrated in Supplementary Fig. 1, facilitating the achievement of both positive and negative output voltages. To further explore the reactions occurring after the addition of electrolyte, symmetric (Fig. 2a) and asymmetric (Fig. 2d) electrode configurations are employed and their voltage and current outputs in three different electrolyte-contacting positions are compared. As shown in Fig. 2b, in the symmetrical electrode configuration with two carbon conductive tape electrodes, a significant voltage output of 0.8 V can be observed only when one electrode was placed in contact with the electrolyte (Fig. 2bii). However, voltage output sharply decreased and fluctuated around 0 V after submerging the electrodes on both sides (Fig. 2biii). In this case, the oxidation-reduction reactions take place on both sides of the carbon conductive electrode, resulting in the offset of the generated voltages. However, due to the standard electrokinetic effect of ion transport, an immediate drop to zero in current output was not observed (Fig. 2ciii). In contrast, in the asymmetric electrode configuration with the carbon conductive tape electrode and inert gold electrode (Fig. 2d), the voltage output remained unchanged when both sides were immersed in the electrolyte (Fig. 2eiii) since there was no reaction in the inert electrode. Moreover, the current collected in the asymmetric electrode configuration (Fig. 2fiii) is significantly greater than that in the symmetric electrode configuration (Fig. 2ciii), due to the much lower resistance of the gold electrode compared to the carbon conductive electrode. To exclude the effect from CNTM, two different connection statuses between the electrode and CNTM were explored (Supplementary Fig. 2). In both cases, the output voltages have no obvious difference, which can further confirm that the output voltage in the nanofluidic chemoelectrical generator is only related to the redox potential between the metal electrode and O2 in the electrolyte. The redox reactions that occurred between the metal electrode and oxygen are summarized as follows:
| 1 |
| 2 |
where M represents the metal electrode.
Fig. 2. Potential induced by redox reactions.
a The schematics of the nanofluidic chemoelectrical generator (NCEG) with carbon conductive tape containing an aluminum substrate as symmetrical electrodes on both sides of the carbon nanotube membrane (CNTM). b, c The electrical outputs of NCEG with symmetric electrode configuration in intermittent contact with solution. d The schematics of NCEG with carbon conductive tape containing an aluminum substrate and gold as asymmetric bottom/top electrodes on both sides of CNTM. e, f The electrical outputs of NCEG with asymmetric electrode configuration in intermittent contact with solution. The electrolyte solution used here was 0.1 M NaCl.
In these reactions, the pH of the electrolyte22–24 and electrode activity25–28 have significant effects on the output voltage. The output voltage shows a higher value up to 1.2 V but decays gradually when the neutral NaCl electrolyte solution is substituted by a strong acid or alkaline (Supplementary Fig. 3a), indicating the accelerated redox reactions caused by the more reactive electrolyte solutions29. The presence of bubbles at the reaction interface (Supplementary Fig. 3b) also confirmed the chemical reactions, as evidenced by chemical Eqs. (3) and (4) below:
| 3 |
| 4 |
The decayed output voltage can be attributed to the corrosive depletion of the bottom electrode30–32. The electrode activities of various metals determine the potentials in the redox reactions33. As shown in Supplementary Fig. 4, the output voltages are highly correlated with the electrode activities, in which more active electrodes result in higher output voltages. After the redox reactions, the metal ions (Zn2+ as an example) will be released from Zn electrode and can be driven to move from the bottom side to the top side (Supplementary Fig. 5), which can further confirm the redox reactions and the generation of transmembrane potential.
Current enhanced by ionic Coulomb drag effect and its working mechanism
Benefiting from the ionic selectivity in a surface-charged nanochannel8–12, cations moving along the solid channel surface in a nanofluidic device can induce electron migration via ionic Coulomb drag. Theoretical study has demonstrated that Coulomb drag between ions within nanochannels and electrons/holes on semiconductors holds the potential for amplifying ionic current due to a significant disparity between the ion mass and the effective mass of electrons/holes12. To validate the hypothesis that the enhanced current in the NCEG arises from the Coulomb drag effect, the role of CNTM and the ion-electron interactions within the nanochannels of CNTM are discussed.
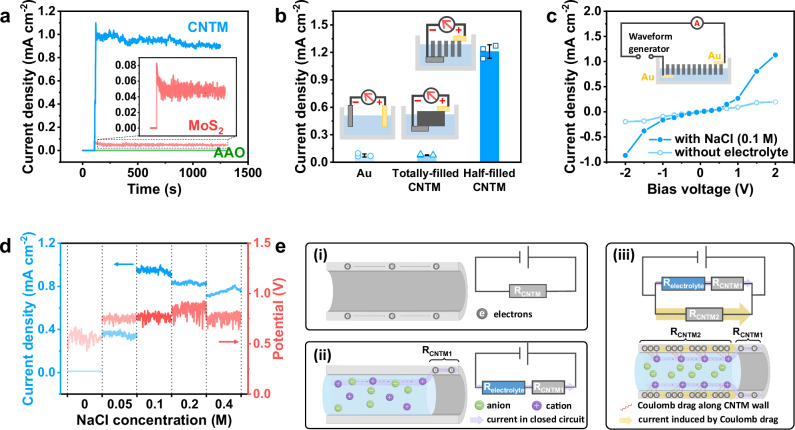
Three different nanochannel membranes with different conductivity, including CNTM, semiconducting molybdenum disulfide (MoS2), and insulated anodized aluminum oxide film (AAO), are used to confirm the ion-electron Coulomb drag effect. Despite similar structures observed in Supplementary Fig. 6 for all three membranes, their current and voltage outputs are so different, elucidating the effect of their electronic properties on the energy outputs. As shown in Supplementary Fig. 7a and Fig. 3a, the AAO membrane does not exhibit any induced voltage or current due to its insulator characteristics. In contrast, as a semi-conductor, the MoS2 membrane shows an induced current of 48.9 μA cm−2 and an open-circuit voltage of 600 mV. The CNTM with the lowest resistance exhibits the highest induced current of 942 μA cm−2 and an open-circuit voltage of 768 mV. Given that the previously reported materials capable of utilizing the Coulomb drag effect all possess a semiconductor nature10–12, these results underscore that the materials that are suitable for Coulomb drag are essential for achieving an enhanced current in our NCEG. To further validate the effect of CNTMs for Coulomb drag applications, we employed a classical configuration widely used in the literature10,11. The membrane was encapsulated in PDMS (Supplementary Fig. 8a, b) and the setup was shown in Supplementary Fig. 8c, f. When a voltage (±1 V) was applied to the electrolyte, an electronic current was collected on the CNTM (Supplementary Fig. 8d, e). Conversely, an induced ionic current through the nanochannel of CNTM can also be observed when voltage (±1 V) was directly applied to the CNTM (Supplementary Fig. 8g, h). The electron current on the CNTM, as well as the ion current in the electrolyte, show a variation with the on/off state of the voltage stimulus (±1 V). The results exhibit a typical behavior of Coulomb drag reported in the literature, suggesting the mutual coupling between the ions in the electrolyte solution and the electrons on the CNTM10–12.
Fig. 3. Mechanism validation of the ionic Coulomb drag effect.
a Different short-circuit current densities from membranes with different electronic properties: bare anodic aluminum oxide (AAO) membrane (insulator), MoS2 membrane (low conductance), and the carbon nanotube membrane (CNTM) (high conductance). b Different short-circuit current densities in 0.1 M NaCl electrolyte solution from three configurations: without CNTM, with half-filled CNTM, and with totally-filled CNTM. For every data point, three individual experiments were performed, which are represented by three data dots. The error bars were given as the standard deviation. c Short-circuit current density of the nanofluidic chemoelectrical generator (NCEG) with and without the contact of electrolyte solution (0.1 M NaCl) as a function of external voltages. Both the bottom and top electrodes in the NCEG are replaced with gold electrodes to avoid the potential difference. d The influence of NaCl concentration on current density and potential. e Schematic illustrations of the ion-electron coupling interactions via ionic Coulomb drag in CNTM nanochannels. Without the electrolyte, the electrical current that flows through the CNTM nanochannels is limited (i). Once the electrolyte is added to the nanochannels of CNTM, a closed circuit is built between the electrolyte and partial of the CNTM (ii). Driven by the potential difference, the movement of ions induces the free electrons in the CNTM (Coulomb drag effect), effectively amplifying the current density in the circuit (iii).
To investigate the ion-electron interactions within the nanochannels of CNTM further, the role of these nanochannels was examined. Figure 3b presents a comparison among three configurations of the NCEG: one without CNTM, one with half-filled CNTM, and one with totally-filled CNTM. Their surface morphologies are provided in Supplementary Fig. 9a,b. The same Al-Au electrode pair was applied in all configurations to maintain a consistent potential difference. As shown in Fig. 3b, the current density collected by the half-filled CNTM is 1.2 mA cm−2, whereas the ionic current in bulk water and electronic current in the totally-filled CNMT are only 0.073 mA cm−2 and 0.076 mA cm−2, respectively. This substantial disparity in current density emphasizes the significance of the ion transport within the nanochannels of CNTMs for enhancing current outputs. To validate both Coulomb drag occurrence and understand how ionic flow interacts with the electrons in the CNTM, Fig. 3c exhibits the NCEG currents with and without the presence of an electrolyte solution (0.1 M NaCl). As shown in Fig. 3c, consistently higher current densities were detected when using NaCl electrolytes compared to those obtained without them, indicating the reduction of electrical resistance with electrolytes. Importantly, the current density without NaCl electrolytes displays a linear response with varying voltages from −2 to 2 V, while an exponential increase in current density was observed in the condition of NaCl electrolytes. Similar results were also found in the work of Feng et al.10, where they observed the Coulomb drag current in graphene induced by ionic flow. This exponential increase in the current density suggests that the behavior of the ionic flow along CNTMs governs the movement of dragged electrons. Meanwhile, as the bias voltage applied in the circuit increases, there is a sharp rise in the number of ions moving along the nanochannels, consequently leading to a significant enhancement in current density.
The effect of the electrolyte concentration on the voltage and current output was also explored. Figure 3d shows that the voltage output has a small dependence on the electrolyte concentration since NaCl does not participate in the redox reactions. In contrast, the NaCl concentration exhibits significant influences on the current density. As shown in Fig. 3d, no current output can be observed with water due to its high resistance. With the increase of NaCl concentration from 0 to 0.1 M, the current output reached its maximum value and then slightly dropped with NaCl concentrations from 0.2 to 0.4 M. This can be attributed to the limited thickness of the electrical double layer (EDL) at high NaCl concentration, which corresponds to the trend observed in the literature10. These results indicate again that the amplified current density results from the movement of ions within the nanochannels of CNTM. Moreover, the ionic flow along the nanochannels of CNTM was further confirmed by EDS on the top surface of the CNTM (Supplementary Fig. 10).
The above-discussed mechanism can be schematically exhibited in Fig. 3e. In the absence of an ionic electrolyte within the nanochannels (Fig. 3e i), the current is limited because of the high resistance of CNTM. The introduction of ionic electrolytes will effectively reduce the resistance of the entire system and result in the ionic flow along the wall of CNTM (Fig. 3e ii)34. Consequently, ion-electron Coulomb drag occurs between this ionic flow and electrons within CNTM, leading to another parallel electron flow (Fig. 3e iii). Based on the principle of momentum conservation and the fact that the typical mass ratio between the ions and the holes is of the order of 105 to 106, the ion transport in the nanochannels promotes a great number of electrons moving in the CNTM, which is also experimentally validated by this exponential increase of current density with a linear increase of voltage in Fig. 3c. Therefore, a remarkably amplified current density can be achieved through this nanofluidic Coulomb drag. In contrast to the approach employed by Xiong et al.12, where ion-coupled hole Coulomb drag was achieved by adjusting both the diameter and the surface hole accumulation concentration of the Si/SiO2 channel to induce migration of counter-ions, our work utilizes the redox reaction potential difference between the two electrodes to drive ionic flow, thereby enabling Coulomb drag of ion-coupled electrons. Furthermore, while in Xiong et al.’s work, ions and holes are separated by the SiO2 between the electrolyte and the semiconductor, our work involves closer contact between CNTM and ions, potentially enhancing interactions between electrolyte ions and electrons in CNTM. As a result, an amplified current density can be observed in the NCEG device, which is 16 times higher than the current collected by the primary cell without CNTM (Fig. 3b).
The enhanced electrical performance outputs and application of NCEG
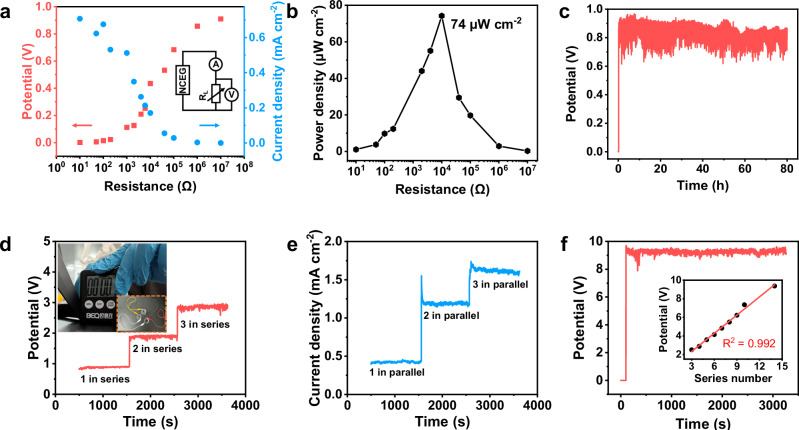
With the ability to amplify current and voltage, NCEG shows practical application value to power different resistive loads. With a 25 mm2 working area of the CNTM, the maximum volumetric power density can reach 74 μW cm–2 when an optimal resistance of 10 KΩ is connected with the NCEG (Fig. 4a, b). A long-term stability measurement shows that the NCEG can work steadily for 80 hours with a negligible output VOC decay (Fig. 4c). Meanwhile, the scalable output voltages of ~1.0 V, ~1.8 V, and ~2.9 V, can be obtained when one, two, and three NCEG units are in series connection, respectively (Fig. 4d). With three NCEG units connected in series, an electronic timer can be powered as shown in the inset of Fig. 4d. Similarly, the short-circuit current can be scaled to ~1.6 mA cm-2 with three units connected in parallel (Fig. 4e). A linear relationship between a series number and output voltage was obtained (the inset of Fig. 4f) and the output voltage reaches 10 V for 3000 s without degradation when 14 NCEGs are connected in series (Fig. 4f). Different from the evaporation potential, airflow disturbances and varied light environment have limited influence on the voltage outputs of the NCEG (Supplementary Fig. 11). Compared with the reported hydroelectric devices, the NCEG presents a huge advantage on the current density resulting from the Coulomb drag effect (Supplementary Table 1).
Fig. 4. The enhanced electrical performance outputs and application of the nanofluidic chemoelectrical generator (NCEG).
a Open-circuit voltage and short-circuit current density output of NCEG with different load resistances. The inset is an equivalent circuit schematic diagram of the test process. b Corresponding changes in output power density with different load resistances, and the best output performance can be achieved when the external resistance is 10 KΩ. c The electrical outputs of NCEG are sustained for 80 hours under ambient laboratory conditions. d Voltage output with one, two, and three NCEGs connected in series. The inset is an optical photograph of the electronic timer powered by three NCEGs connected in series. e Short-circuit current density with one, two, and three NCEGs connected in parallel. f Output-circuit voltage of 10 V from fourteen NCEGs that are connected in series. The inset is the open-circuit voltage when different numbers of NCEGs are connected in series. The red line is the best linear fit with a slope of 0.65.
Discussion
In this work, an NCEG that can achieve a milliampere-level current output was designed by sandwiching a CNTM between two metal electrodes. It has been demonstrated that the redox reactions between active metal and oxygen provide sufficient power to drive ionic flow within the nanochannels in the CNTM. Although the theoretical and experimental materials employed in our study differ from those utilized by Xiong et al.12, the validation of this theory in our work establishes Coulomb drag induced by ion movement in nanochannels as a universally applicable mechanism for amplifying current output. The developed NCEG device exhibits constant and stable electrical output with high environmental tolerance, achieving an output power density of 74 μW cm−2. We anticipate that this approach of amplifying the current output of NCEG not only enhances our fundamental understanding of the energy harvesting mechanism in nanofluidic systems but also facilitates the development of new-generation energy generators in practical applications. For its future exploration, the reasonable option of alternative materials (two-dimensional membrane with photoelectric or thermoelectric response such as graphene or molybdenum disulfide), and possible mechanism speculations (such as the interactions of ions, electrons under the light and thermal stimulation), will be potential for achieving composite harvesting of multiple energies. In addition, further work is still needed to optimize NCEG’s functionality for portable medical diagnostic tools or emergency communication systems.
Methods
Materials
The anodic aluminum oxide AAO membranes with a thickness of 60 μm, a top pore size of 200 nm, and a bottom pore size of 30 nm were purchased from Sterlitech Corporation, Germany. Different metal electrodes (Al, Zn, Cu, Fe, Ag, and Au) were purchased from Taizhou Sennuo Material Technology Co. Ltd, China. The aluminum base carbon conductive tape (Nissin SEM double-sided carbon conductive tape) and non-woven substrate carbon conductive tape (carbon conductive fiber cloth) were purchased from Guangzhou Li-ge Technology Co, Ltd, China. Acetonitrile was purchased from J&K Scientific China. (NH4)2MoS4 and dimethyl formamide (DMF) were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd, China.
Fabrication of nitrogen-doped CNTM and MoS2
An asymmetric porous AAO membrane was used as a template to fabricate the half-filled CNTM through the conventional chemical vapor deposition (CVD) process. In the CVD process, acetonitrile was employed as the carbon source precursor. Typically, the AAO substrate was first placed into the horizontal growth chamber of a standard atmospheric pressure CVD system. To conduct a reduction treatment on the AAO substrate, the temperature of the chamber was heated up to 1000 °C with a gas mixture (Ar, 100 sccm and H2, 50 sccm) for 0.5 h. Afterward, the carbon precursor was introduced to implement the growth step with a humidified acetonitrile bubbler. After 1.0 h of growth, the system was cooled down to room temperature under Ar protection. Furthermore, the procedure for totally-filled CNTM was identical to that of half-filled CNTM, except that the target temperature (1090 °C) is different. MoS2 membrane was obtained by completely infiltrating (NH4)2MoS4 solution into the AAO template and annealing. To be specific, by dissolving 0.06 g of (NH4)2MoS4 in 5 mL of DMF, the precursor solution was created. The AAO template was immersed in the solution and suctioned under a vacuum to allow full access to the channel. Then, the AAO template was placed on a 100 °C hotplate to dry the solution and annealed for 60 minutes at 300 °C in a 150 sccm flow of Ar at room pressure. Following the initial annealing, the AAO was reannealed for 30 minutes at 900 °C with a 150 sccm Ar flow.
Design of NCEG
The NCEG device was simply built by attaching two electrodes on the upside and downside of the CNTM, respectively. Typically, aluminum-based conductive carbon tape (Nissin SEM double-sided carbon conductive tape) was used as the bottom electrode, and Au works as the top electrode. To investigate the effect of the potential difference in the redox reactions process, various materials, including Al, Zn, Cu, Fe, Au, and conductive carbon fiber cloth were used as the bottom electrode.
The polydimethylsiloxane (PDMS) was prepared with the mixture of the pre-polymer and curing agent with a 10:1 ratio. The mixture was placed in a vacuum-drying oven for 20 minutes to remove air bubbles. The encapsulated device based on CNTM was encapsulated by a resin mold with holes and PDMS. Both sides of the CNTM were attached to symmetrical silver (Ag) electrodes as a current collector. The surface of silver glue and Ag needed to be painted with PDMS which acts as an insulation from the ionic solution.
Characterizations
Scanning electron microscope (SEM) (Hitachi-SU8220) was used to characterize the morphologies of AAO substrate and CNTM grown of AAO template. X-ray photoelectron spectroscopy (XPS) (PHI 5000 VersaProbe, ULVAC-PHI, Inc) was used to analyze the surface element composition of CNTM and AAO. X-ray energy dispersion spectroscopy (EDS) (OXFORD X-Max 80) was used to characterize the elemental composition of the CNTM before and after electrical output measurement.
Electrical output measurement
The open-circuit voltage (VOC) and the short-circuit current (ISC) of the NCEG were measured using a digit multimeter (Keithley DMM7510). To validate the source of the voltage output signal, we manipulated the positions where the electrolyte solution made contact with the CNTM and recorded the corresponding voltage outputs. Meanwhile, the effects of both symmetric electrodes (carbon conductive tape containing aluminum) and asymmetric electrodes (carbon conductive tape containing aluminum and gold foil) on the potential outputs were also investigated by varying their positions in contact with the electrolyte solution. Moreover, to verify the electronic properties of solid membranes regarding the Coulomb drag effect, we explored electrical output performances using different membrane materials as well as membranes with and without nanochannels. To investigate the important role of the ionic flow within nanochannels in the current amplification, the current densities collected with and without the electrolyte (0.1 M NaCl) are compared. A waveform generator (SDG1000X, Shenzhen Dingyang Technology, China) was applied to provide varied external bias voltages. The top and bottom electrodes in the NCEG device were gold to eliminate any voltage generated due to chemical differences. Finally, electrical outputs of NCEG were measured in electrolytes with varying concentrations (0.05, 0.1, 0.2, 0.4 M NaCl).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We acknowledge the help from Xiaoliu Wen in drawing the schemes, including Figs. 1c and 3e. This work was financially supported by the National Key Technologies R&D Program of China (Grant No. 2023YFC2415900), the National Natural Science Foundation of China (No. 22275079), Shenzhen Science and Innovation Committee (20220815164834003), Shenzhen Science and Technology Program (KQTD20221101093559017), Guangdong Provincial Key Laboratory of Advanced Biomaterials (2022B1212010003) and Starting Grant from Southern University of Science and Technology (SUSTech). We also acknowledge the assistance of technical support from SUSTech Core Research Facilities.
Author contributions
K.X. conceived and supervised the project. Y.J., W.L., and T.W. designed the experiments, discussed the results, and wrote the paper. Y.J. and W.L. fabricated devices, carried out experiments, and performed data analysis. All the authors, including Y.W., T.M., L.W., G.X., Y.W., and N.L. analyzed the data and discussed the results.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data generated in this study are provided in the Source Data file including data in Figs. 1b, d, 2b, c, e, f, 3a–d, and 4a–f, and Supplementary Figs. 1, 2a, b, 3a, 4, 7a, b, 8d, e, g, h, 9c, d, and 11a, b. The other data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yisha Jiang, Wenchao Liu, Tao Wang.
Contributor Information
Tao Wang, Email: wangt329@163.com.
Yude Wang, Email: ydwang@ynu.edu.cn.
Nannan Liu, Email: liunannan@wzu.edu.cn.
Kai Xiao, Email: xiaok3@sustech.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-52892-4.
References
- 1.Gorbachev, R. V. et al. Strong Coulomb drag and broken symmetry in double-layer graphene. Nat. Phys.8, 896–901 (2012). [Google Scholar]
- 2.Kim, S. & Tutuc, E. Coulomb drag and magnetotransport in graphene double layers. Solid State Commun.152, 1283–1288 (2012). [Google Scholar]
- 3.Song, J. C. W., Abanin, D. A. & LSJNl, Levitov. Coulomb drag mechanisms in graphene. Nano Lett.13, 3631–3637 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Xiao, K., Jiang, L. & Antonietti, M. Ion transport in nanofluidic devices for energy harvesting. Joule3, 2364–2380 (2019). [Google Scholar]
- 5.Zhang, Z., Wen, L. & Jiang, L. Nanofluidics for osmotic energy conversion. Nat. Rev. Mater.6, 622–639 (2021). [Google Scholar]
- 6.Feng, J. et al. Observation of ionic coulomb blockade in nanopores. Nat. Mater.15, 850–855 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Chen, F. et al. Ionic conductance oscillations in sub-nanometer pores probed by optoelectronic control. Matter4, 2378–2391 (2021). [Google Scholar]
- 8.Coquinot, B., Bocquet, L. & Kavokine, N. Quantum feedback at the solid-liquid interface: flow-induced electronic current and its negative contribution to friction. Phys. Rev. X13, 011019 (2023). [Google Scholar]
- 9.Yu, X., Principi, A., Tielrooij, K.-J., Bonn, M. & Kavokine, N. Electron cooling in graphene enhanced by plasmon–hydron resonance. Nat. Nanotechnol.18, 898–904 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, F. et al. Inducing electric current in graphene using ionic flow. Nano Lett.23, 4464–4470 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Rabinowitz, J., Cohen, C. & Shepard, K. L. An electrically actuated, carbon-nanotube-based biomimetic ion pump. Nano Lett.20, 1148–1153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong, M., Song, K. & Leburton, J.-P. Ionic coulomb drag in nanofluidic semiconductor channels for energy harvest. Nano Energy117, 108860 (2023). [Google Scholar]
- 13.Xue, G. et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol.12, 317–321 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Liu, X. et al. Power generation from ambient humidity using protein nanowires. Nature578, 550–554 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Wang, H. et al. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1,000 V output. Nat. Nanotechnol.16, 811–819 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Xu, S.-M. et al. Multistaged discharge constructing heterostructure with enhanced solid-solution behavior for long-life lithium-oxygen batteries. Nat. Commun.10, 5810 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Z. et al. Manipulating anion intercalation enables a high-voltage aqueous dual ion battery. Nat. Commun.12, 3106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun, W. et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science371, 46–51 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science334, 928–935 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Van Noorden, R. The rechargeable revolution: a better battery. Nature507, 26–28 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Lu, J. et al. The role of nanotechnology in the development of battery materials for electric vehicles. Nat. Nanotechnol.11, 1031–1038 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Wang, Q. et al. Interface chemistry of an amide electrolyte for highly reversible lithium metal batteries. Nat. Commun.11, 4188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, J. et al. Influence of electrolyte structural evolution on battery applications: Cationic aggregation from dilute to high concentration. Aggregate3, e153 (2022). [Google Scholar]
- 24.Xiao, P. et al. Insights into the solvation chemistry in liquid electrolytes for lithium-based rechargeable batteries. Chem. Soc. Rev.52, 5255–5316 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Li, L. et al. A novel, flexible dual-mode power generator adapted for wide dynamic range of the aqueous salinity. Nano Energy85, 105970 (2021). [Google Scholar]
- 26.Lu, X. et al. Hierarchically porous fiber-based nanofluidic diode as an efficient multimode hygroelectric generator. Adv. Energy Mater.12, 2202634 (2022). [Google Scholar]
- 27.Li, S. et al. Humidity-sensitive chemoelectric flexible sensors based on metal-air redox reaction for health management. Nat. Commun.13, 5416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang, Z. et al. Simultaneous electricity generation and steam production from a wide range of salinity by using unique nanofluidic diode. Nano Energy108, 108220 (2023). [Google Scholar]
- 29.Li, Y. et al. Quinone electrodes for alkali–acid hybrid batteries. J. Am. Chem. Soc.144, 8066–8072 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Hopkins, B. J., Shao-Horn, Y. & Hart, D. P. Suppressing corrosion in primary aluminum–air batteries via oil displacement. Science362, 658–661 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Li, Z. & Lu, Y.-C. Material design of aqueous redox flow batteries: fundamental challenges and mitigation strategies. Adv. Mater.32, 2002132 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Chen, Q. et al. Organic electrolytes for pH-neutral aqueous organic redox flow batteries. Adv. Funct. Mater.32, 2108777 (2022). [Google Scholar]
- 33.Zhang, Y. et al. Sustainable power generation for at least one month from ambient humidity using unique nanofluidic diode. Nat. Commun.13, 3484 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang, P., He, J., Park, J. H., Krstić, P. S. & Lindsay, S. Origin of giant ionic currents in carbon nanotube channels. ACS Nano5, 7277–7283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are provided in the Source Data file including data in Figs. 1b, d, 2b, c, e, f, 3a–d, and 4a–f, and Supplementary Figs. 1, 2a, b, 3a, 4, 7a, b, 8d, e, g, h, 9c, d, and 11a, b. The other data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.