Abstract
Early peritoneal dialysis (PD)-related infection is a severe complication. This study investigated the relationship between patient–doctor contact (PDC) duration and early PD-related infection. In the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) Korea, incident dialysis patients receiving PD were divided into two groups based on PDC duration (< 15 min versus ≥ 15 min), which was defined as the duration a nephrologist typically spends with a patient receiving PD during each visit according to the facility practice pattern. Early risks of PD-related infections, such as peritonitis and catheter-related infection (onset within 3 and 12 months of PD), were compared to the PDC duration using Cox regression. The study included 276 patients (184 [66.7%] in the shorter PDC group [< 15 min] and 92 [33.3%] in the longer PDC group [≥ 15 min]). The average age did not differ between the groups. The incidences of 3- and 12-month PD-related infections were significantly lower in the longer PDC group than in the shorter PDC group (3 months: 1.1% versus 9.8%, P = 0.007; 12 months: 9.8% versus 23.4%, P = 0.007). Longer PDC was independently associated with a lower risk of PD-related infections at 3 and 12 months (3 months: adjusted hazard ratio [aHR], 0.11; 95% confidence interval [CI], 0.02–0.85, P = 0.034; 12 months: aHR, 0.43; 95% CI 0.19–0.99, P = 0.048). Overall, a longer PDC duration was associated with a significantly lower risk of early PD-related infection.
Keywords: Catheter-related infection, Patient–doctor contact hour, PD-related infection, Peritoneal dialysis, Peritonitis
Subject terms: Renal replacement therapy, Peritoneal dialysis
Introduction
Peritoneal dialysis (PD)-related infection is a serious complication that can result in PD discontinuation1–3. PD-related infection has been linked to 20% of PD discontinuation and 2–6% of patient deaths4–6. In particular, PD-related infection in the early stages of dialysis is associated with an increased risk of early hemodialysis (HD) transfer, which limits PD use in individuals with kidney failure7.
Several studies have evaluated the factors that contribute to the occurrence of PD-related infections, and they have identified several risk factors, including male sex, obesity, and hypoalbuminemia5. However, most studies have focused on non-modifiable risk factors, with few reporting on potentially modifiable factors. Recent results from the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) found no link between several factors related to PD training practice and peritonitis risks8.
Increasing the frequency and duration of face-to-face patient–doctor contact (PDC) may improve patient outcomes, particularly in those suffering from chronic diseases9–11. Increased PDC enhances patient engagement and builds trust between patients and clinicians12,13. However, the relationship between PDC duration and the occurrence of PD-related infections in incident dialysis patients receiving PD has yet to be investigated. This study evaluated the relationship between PDC duration and the risk of early PD-related infections, such as peritonitis and catheter-related infections, in patients with PD participating in the PDOPPS in South Korea.
Results
Baseline characteristics
Table 1 shows the patients’ baseline characteristics. The average age was 53.6 ± 13.2 years and 69.2% of the patients were male. The leading cause of kidney failure was diabetes (47.5%), followed by hypertension (24.6%) and glomerulonephritis (14.9%). Differences in age, gender, body mass index, and comorbidities were small to moderate, without any statistically significant differences. The PD connection system did not differ between the two groups (Supplementary Table S1).
Table 1.
Baseline patient characteristics.
| Total (n = 276) | Shorter PDC group (n = 184) | Longer PDC group (n = 92) | P | |
|---|---|---|---|---|
| Age, years | 53.6 ± 13.2 | 53.6 ± 13.4 | 53.6 ± 13.0 | 0.982 |
| Sex, male, n (%) | 191 (69.2) | 121 (65.8) | 70 (76.1) | 0.080 |
| Body mass index, kg/m2 | 24.1 ± 3.9 | 24.1 ± 4.0 | 24.1 ± 3.6 | 0.994 |
| Primary kidney disease, n (%) | ||||
| Diabetes mellitus | 131 (47.5) | 90 (48.9) | 41 (44.6) | 0.495 |
| Hypertension | 68 (24.6) | 47 (25.5) | 21 (22.8) | 0.621 |
| Glomerulonephritis | 41 (14.9) | 28 (15.2) | 13 (14.1) | 0.811 |
| Polycystic kidney disease | 6 (2.2) | 2 (1.1) | 4 (4.3) | 0.098 |
| Others | 30 (10.9) | 17 (9.2) | 13 (14.1) | 0.218 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 154 (55.8) | 102 (55.4) | 52 (56.5) | 0.864 |
| Hypertension | 257 (93.1) | 173 (94.0) | 84 (91.3) | 0.401 |
| Congestive heart failure | 19 (6.9) | 16 (8.7) | 3 (3.3) | 0.093 |
| Cerebrovascular accident | 14 (5.1) | 8 (4.3) | 6 (6.5) | 0.561 |
| Peripheral vascular disease | 7 (2.5) | 5 (2.7) | 2 (2.2) | 0.807 |
| Liver cirrhosis | 9 (3.3) | 8 (4.3) | 1 (1.1) | 0.280 |
| Cancer | 13 (4.7) | 8 (4.3) | 5 (5.4) | 0.765 |
| Smoking, n (%) | 0.944 | |||
| None | 143 (51.8) | 95 (51.6) | 48 (52.2) | |
| Ex-smoker | 82 (29.7) | 54 (29.3) | 28 (30.4) | |
| Current smoker | 51 (18.5) | 35 (19.0) | 16 (17.4) | |
| Starting PD mode, n (%) | 0.361 | |||
| CAPD | 230 (83.3) | 156 (84.8) | 74 (80.4) | |
| APD | 46 (16.7) | 28 (15.2) | 18 (19.6) | |
| Education (n = 196), n (%) | 0.300 | |||
| ≤ 12 years | 114 (58.2)) | 77 (55.8) | 37 (63.8) | |
| > 12 years | 82 (41.8) | 61 (44.2) | 21 (36.2) | |
| Marital status, n (%) | 0.414 | |||
| Single/divorced/separate/widowed | 90 (32.6) | 63 (34.2) | 27 (29.3) | |
| Married | 186 (67.4) | 121 (65.8) | 65 (70.7) | |
| Living alone, n (%) | 42 (15.2) | 26 (14.1) | 16 (17.4) | 0.477 |
| Employment, n (%) | 0.670 | |||
| Jobless including students | 142 (51.4) | 93 (50.5) | 49 (53.3) | |
| Employed | 134 (48.6) | 91 (49.5) | 43 (46.7) | |
| Self-ambulatory, n (%) | 0.305 | |||
| No | 4 (1.4) | 4 (2.2) | 0 | |
| Yes | 272 (98.6) | 180 (97.8) | 92 (100.0) | |
| Caregiver-assisted PD, n (%) | 0.530 | |||
| No | 254 (92.0) | 168 (91.3) | 86 (93.5) | |
| Yes | 22 (8.0) | 16 (8.7) | 6 (6.5) | |
| PD nurse advice after working hours, n (%) | 93 (33.7) | 41 (22.3) | 52 (56.5) | < 0.001 |
| Average duration during one training session < 2 h, n (%) | 205 (74.3) | 154 (83.7) | 51 (55.4) | < 0.001 |
| Total training time > 5 h, n (%) | 134 (48.6) | 112 (60.9) | 22 (23.9) | < 0.001 |
PDC patient–doctor contract, PD peritoneal dialysis, CAPD continuous ambulatory peritoneal dialysis, APD automated peritoneal dialysis.
Most incident dialysis patients who underwent PD (83.3%) initially received continuous ambulatory PD (CAPD). The patients’ education level, marital status, and employment status were not statistically different between the groups. There was also no difference in the proportions of patients who were self-ambulatory or dependent on caregivers for PD. However, more patients in the longer PDC group than in the shorter PDC group had access to a PD nurse via an after-hours hotline (56.5% versus 22.3%, P < 0.001).
Patients in both groups started training on the day of PD catheter insertion. More patients in the shorter PDC group than in the longer PDC group had shorter training sessions (< 2 h/session) (83.7% versus 55.4%, P < 0.001), and the proportion of patients who had longer total training time (> 5 h) was higher in the shorter PDC groups than in the longer PDC group (60.9% versus 23.9%, P < 0.001). These findings indicate that patients in the shorter PDC group had a higher frequency of training sessions and a greater cumulative training time than those in the longer PDC group.
In the comparison of PDC duration by facility, facilities with more than 50 patients receiving PD tended toward shorter PDC (P = 0.062), but there was no significant difference by whether the facility was a university-affiliated hospital or a tertiary hospital.
Incidence of early PD-related infections
Table 2 provides details relating to PD-related infections. Overall, 52 (18.8%) patients developed PD-related infections during the 12 months following PD initiation. The incidence of PD-related infections was significantly higher in the shorter PDC group compared to the longer PDC group (23.4% versus 9.8%, P = 0.007). When the incidence of PD peritonitis and catheter-related infections were separately compared, the shorter PDC group tended to experience a higher rate of both PD peritonitis and catheter-related infections than the longer PDC group (PD peritonitis: 17.4% versus 8.7%, respectively, P = 0.053; catheter-related infection: 6.5% versus 1.1%, respectively, P = 0.067). Overall, there were 73 PD-related infection events at 1 year. The incidence rate in the shorter PDC group was 0.34 episodes/patient-year, while the longer PDC group had 0.12 episodes/patient-year, which was significantly lower than that of the shorter PDC group (P = 0.010). The longer PDC group had a significantly lower incidence of PD peritonitis than the shorter PDC group (0.11 episode/patient-year vs. 0.27 episode/patient-year, P = 0.002).
Table 2.
Early PD-related infection rates and episodes per patient-year.
| Total (n = 276) | Shorter PDC group (n = 184) | Longer PDC group (n = 92) | P | |
|---|---|---|---|---|
| PD-related infection within 12 months, n (%) | 52 (18.8) | 43 (23.4) | 9 (9.8) | 0.007 |
| PD peritonitis within 12 months, n (%) | 40 (14.5) | 32 (17.4) | 8 (8.7) | 0.053 |
| Catheter-related infection within 12 months, n (%) | 13 (4.7) | 12 (6.5) | 1 (1.1) | 0.067 |
| PD-related infection episode/patient-year | 0.27 | 0.34 | 0.12 | 0.010 |
| PD peritonitis episode/patient-year | 0.22 | 0.27 | 0.11 | 0.002 |
| Catheter-related infection episode/patient-year | 0.05 | 0.07 | 0.01 | 0.071 |
| Recurrent, relapsing, or repeat peritonitis within 12 months, n (%) | 10 (3.6) | 9 (4.9) | 1 (1.1) | 0.173 |
| PD-related infection within 3 months, n (%) | 19 (6.9) | 18 (9.8) | 1 (1.1) | 0.007 |
| PD peritonitis within 3 months, n (%) | 15 (5.4) | 14 (7.6) | 1 (1.1) | 0.024 |
| Catheter-related infection within 3 months, n (%) | 4 (1.4) | 4 (2.2) | 0 | 0.305 |
| Repeat catheter-related infection within 12 months, n (%) | 1 (0.4) | 1 (0.5) | 0 | 1.000 |
| Change modality because of infection within 12 months, n (%) | 3 (1.1) | 2 (1.1) | 1 (1.1) | 1.000 |
PD peritoneal dialysis, PDC patient–doctor contact.
Regarding the timing of the infections, 19 (6.9%) patients developed a PD-related infection within 3 months. The incidence of infections within 3 months was significantly higher in the shorter PDC group than in the longer PDC group (9.8% versus 1.1%, P = 0.007). The shorter PDC group also had a significantly higher incidence of PD peritonitis within 3 months compared to the longer PDC group (7.6% versus 1.1%, P = 0.024).
The incidences of recurrent, relapsing, or repeat peritonitis, as well as repeat catheter-related infection, were similar between the groups. Three patients had their treatment modality changed to HD within 12 months due to PD-related infections. There were no differences between groups in terms of treatment modality change.
The peritonitis organisms listed in Supplementary Table S2 show a significantly higher rate of Gram-positive peritonitis in the shorter PDC group than in the longer PDC group (47.9% vs. 10.0%, P = 0.027). There was no difference in the incidence of Gram-negative peritonitis. Among the catheter-related infections, Gram-positive bacteria were more frequent in the shorter PDC group, but the difference was not statistically significant (53.8% vs. 0%, P = 0.299; Supplementary Table S3).
Association between patient–doctor contact time and early PD-related infections
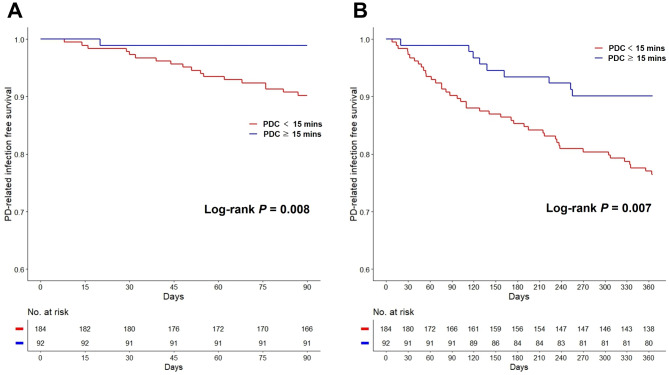
Figure 1 depicts Kaplan–Meier curves for early PD-related infections based on PDC durations. The shorter PDC group had significantly more PD-related infections than the longer PDC group, both within 3 (log-rank P = 0.008; Fig. 1a) and 12 months (log-rank P = 0.007; Fig. 1b). Of note, only one case of PD-related infection was reported in the longer PDC group within 3 months. Interestingly, one out of 20 facilities had a PDC duration of ≥ 30 min. Supplementary Fig. S1 displays Kaplan–Meier curves for three groups based on PDC durations (shorter [< 15 min], longer [15 to 30 min], and longest [≥ 30 min] groups). The group with the longest PDC duration had no PD-related infections within 12 months (log-rank P = 0.019).
Fig. 1.
Kaplan–Meier curves for early PD-related infections. (A) PD-related infection-free survival within 3 months. (B) PD-related infection-free survival within 12 months. PD peritoneal dialysis, PDC patient–doctor contact.
Tables 3 shows the results of the Cox proportional hazard regression analyses of PD-related infections within 3 and 12 months, respectively. The longer PDC group had a significantly lower risk of PD-related infections within 3 months in all models (model 3: adjusted hazard ratio [aHR], 0.11; 95% confidence interval [CI], 0.02–0.85; P = 0.034) (Table 3). When analyzing the risk of PD-related infections within 12 months, all models confirmed that those in the longer PDC group had a consistently lower risk than those in the shorter PDC group (model 4: aHR, 0.43, 95% CI, 0.19–0.99, P = 0.048) (Table 3).
Table 3.
Cox regression analysis of early PD-related infections within 3 months and 12 months.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P | Model 4 | P |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |||||
| PD-related infections within 3 months | ||||||||
| PDC ≥ 15 min | 0.11 (0.01–0.80) | 0.030 | 0.11 (0.01–0.80) | 0.029 | 0.11 (0.02–0.85) | 0.034 | 0.10 (0.01–0.78) | 0.029 |
| PD-related infections within 12 months | ||||||||
| PDC ≥ 15 min | 0.39 (0.19–0.79) | 0.010 | 0.39 (0.19–0.81) | 0.011 | 0.41 (0.20–0.84) | 0.015 | 0.43 (0.19–0.99) | 0.048 |
Model 1: Unadjusted.
Model 2: Adjusted for age and sex.
Model 3: Adjusted for age, sex, underlying diabetes mellitus, and congestive heart failure.
Model 4: Adjusted for age, sex, underlying diabetes mellitus and congestive heart failure, PD nurse advice after working hours, average duration during a training session, and total training time.
PD peritoneal dialysis, HR hazard ratio, CI confidence interval, aHR adjusted hazard ratio, PDC patient–doctor contact.
Subgroup analysis of early PD-related infection according to the patient–doctor contact time
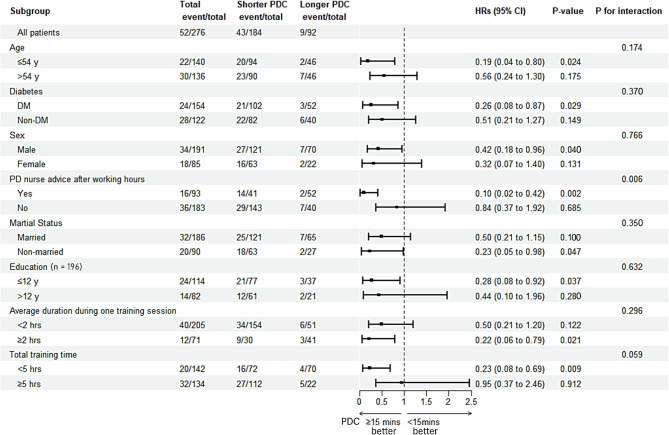
Figure 2 depicts the findings from a subgroup analysis of PD-related infections within 12 months. Patients with longer PDC had better results in early PD-related infections in various subgroups, particularly among younger patients, diabetics, males, nonmarried patients, and those with a low education level. Furthermore, in the longer PDC group, the risk of early PD-related infections was lowered among patients with a total training time of < 5 h, but not among those with a total training time of ≥ 5 h.
Fig. 2.
Subgroup Cox regression analysis of PD-related infections within 12 months. PD peritoneal dialysis, PDC patient–doctor contact, HR hazard ratio, CI confidence interval, DM diabetes mellitus.
Discussion
This study found that longer PDC duration was associated with a lower risk of early PD-related infections among incident dialysis patients with PD. To minimize bias, we analyzed prospectively collected nationwide cohort data of patients who had recently started PD. Although a previous study evaluated the association between PDC intervals and prognosis in patients on PD14, this is the first study to confirm an association between PDC duration and PD-related infections in incident dialysis patients with PD. The data indicate that increasing PDC may improve PD-related infection outcomes in patients undergoing PD.
PDC is important, particularly for the management of patients with chronic diseases. Regular PDC may improve patient adherence, provide opportunities for disease-related education, and aid in the prevention and detection of new medical issues12,13. Providing adequate PDC improves not only disease control but also patient satisfaction9. Our research confirmed that by spending quality time with patients, nephrologists may contribute to reducing the risk of Gram-positive infections by lowering the risk of touch contamination in the early stages of PD. In addition to the importance of proper PDC duration, research has shown that proper PDC intervals are also important. Yi et al. found that an optimal PDC interval is associated with better outcomes in patients with CAPD14. They retrospectively examined the relationship between PDC frequency and clinical outcomes, such as peritonitis, hospitalization, and survival rates, in 433 CAPD patients at a single center. The results showed that the high PDC frequency group (PDC interval of < 2 months) had a higher patient-survival rate, lower peritonitis rate, and lower hospitalization rate than the low PDC frequency group. However, the same study found no relationship between PDC duration and patient outcomes. To the best of our understanding, no previous study has confirmed the link between PDC duration and clinical outcomes in patients requiring PD. The results of this present study demonstrated that the duration of PDC during outpatient treatment may play a role in preventing PD-related infections, emphasizing the significance of appropriate PDC duration.
Among patients requiring HD, Kawaguchi et al. found an association between PDC frequency, duration, and mortality using data from the Dialysis Outcomes and Practice Patterns Study (DOPPS)9. A correlation between mortality- and facility-based PDC duration in HD patients was investigated. The study found that each 5-min reduction in PDC duration was associated with a 5% increased risk of death in patients undergoing HD. Moreover, a shorter PDC duration was linked to higher all-cause hospitalization. The study concluded that a longer PDC duration with patients requiring HD is beneficial. They emphasized that, despite advancements in medical technology, significant changes in patient behaviors are required to improve health9. Extending PDC may encourage behavioral changes and improve prognosis. Similarly, in patients undergoing PD, a longer PDC duration was associated with fewer early PD-related infections, probably as a result of improved patient adherence and education.
Preventing early PD-related infections is critical for preserving long-term peritoneal function and avoiding HD transfer among patients on PD15–17. Although there is disagreement about the best cut-off time for early PD peritonitis, it has traditionally been defined as an infection that occurs within 3 or 12 months5,18. Furthermore, numerous studies have identified the risk factors for early PD-related infections5,19–22. However, most of these studies had several limitations, including a small number of patients, a retrospective study design, and the possibility of unmeasured confounding factors. In this study, we found that PDC duration, which can be moderated by healthcare providers but has not been identified in previous studies, may be linked to early PD-related infections. Furthermore, the overall incidence of PD-related infection is lower in patients who have recently begun PD than in those who have previously initiated PD23,24. Our study reduced potential bias based on treatment within different eras by using data from patients who had recently developed PD, illuminating current trends.
Unified treatment and training guidelines for PD are limited. Furthermore, guidelines for PD and its application differ by country25,26. A recent study compared PD training practices and their association with peritonitis using PDOPPS global data8. Contrary to expectations, the results findings suggested that the risk of peritonitis was not related to when, where, how, or for how long patients with PD were trained. In particular, the total training time was found to be associated with the risk of peritonitis in a previous PDOPPS study that included both incident and prevalent patient data27. Results from the recent PDOPPS study8 and our present study using incident PD cohort data found no significant relationship between total training time and peritonitis risk. This may be because patients with more complex medical histories may require more training time. Our findings showed that the PDC duration at the outpatient clinic was strongly associated with the risk of early PD-related infections, even after adjusting for PD training practices. In particular, the group with shorter PDC duration had a higher risk of infections despite longer training time, highlighting the potential significance of PDC duration. To verify our findings, further studies on total training time in patients of varying complexities are warranted.
Physician reimbursement, cultural differences, and individual tendencies all have an impact on PDC duration9. Facility-related factors, such as university-affiliated hospitals, tertiary hospitals, and the large number of in-center patients receiving PD, did not affect the PDC duration in our study. All citizens in South Korea are covered by national health insurance. As such, treatment fees are uniformly applied based on the number of times the patient visits the clinic, and not according to PDC duration. This is a significant factor that reduces the PDC duration in the outpatient clinic. If nephrologists extend the PDC duration, they will reduce early PD-related infections and hospitalization rates, while also contributing to lower long-term medical costs. In particular, patient groups with younger ages, diabetes, and lower education levels, benefited the most from longer PDC durations. Based on this information, nephrologists should strive to provide adequate PDC duration, particularly for these patients. Furthermore, a government policy to extend the PDC duration would be highly beneficial.
This study has a few limitations. The PDC duration was calculated using the PDOPPS Unit Practices Survey (UPS) data from each facility. The UPS was completed by a nurse at each facility, therefore reflected the facility’s overall PDC duration rather than the individual experience of each patient. Therefore, the data could not provide specific PDC durations for each patient in the same facility. Hence, a PD facility effect could influence the results24. Furthermore, while the training-related items in each institution’s UPS data, including duration of training, did not exhibit a significant difference between the short and long PDC groups, it is possible that center’s experience or PD program of each center may impact on the incidence of peritonitis. Moreover, we did not review the specific activities included during PDC. Face-to-face appointments in the clinic can cover various topics, including treatment-related discussions, patient condition assessment, medication information, and preventive education. This study could not determine which activities during PDC are closely related to the prevention of early PD-related infection. However, a longer PDC duration may have other beneficial effects on reducing PD-related infection risk, such as investing more time in education or reinforcing patient motivation. Furthermore, the link between PDC duration and long-term prognosis could not be confirmed by investigating patients who had newly started PD. Additionally, as an observational study, causal inferences cannot be made from our findings. There may also be unmeasured confounding factors. Further research is required to address these limitations in the future.
In conclusion, a longer PDC duration was linked to a lower risk of early PD-related infections within 3 and 12 months of PD onset. A longer PDC duration may help to reduce the likelihood of touch contamination. Nephrologists should devote enough time to outpatient care for patients with PD, and policies supporting the extension of PDC duration may contribute to reducing PD-related infections and maintaining long-term PD.
Methods
Study participants and data collection
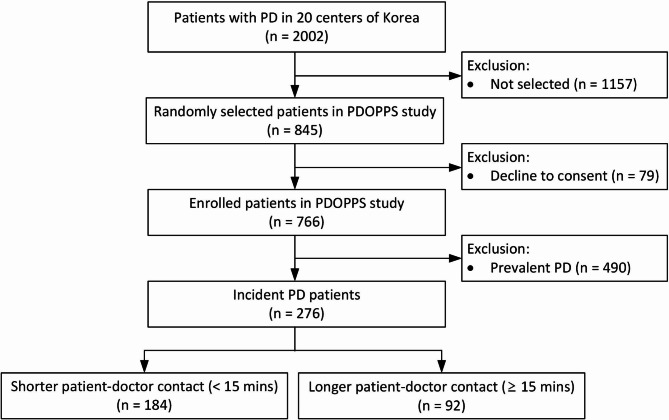
This prospective, nationwide, multicenter study included adult patients (age ≥ 18 years) who initiated PD between 2020 and 2021 in South Korea. They were included in the PDOPPS Korea cohort data. In 2019, South Korea joined the PDOPPS, which is an international prospective cohort study of PD treatment and patient outcomes28. PDOPPS Korea includes patient data from 20 randomly selected PD institutions among 81 in South Korea, each treating ≥ 20 patients with PD. The methods used in PDOPPS were previously described in detail28. The study enrolled 20–30 patients on PD from each institution at the outset. Incident dialysis patients on PD were defined as those starting PD for less than 60 days at the time of enrollment in PDOPPS Korea. All incident dialysis patients on PD (n = 276) during the study period were included. (Fig. 3). All PD patients enrolled in this study used the same PD connectology, twin-bag system. PDOPPS Korea was approved by the medical ethics committees of all participating centers, and all patients provided written informed consent before inclusion.
Fig. 3.
Study flowchart. PD peritoneal dialysis, PDOPPS Peritoneal dialysis outcomes and practice patterns study.
Patient demographics and comorbidities were recorded at the time of study enrollment. Data on the occurrence of PD-related infections was collected during the observation period. Data were extracted manually from electronic medical charts and entered into a secure web-based data collection tool. All data from each site were collected using consistent and standardized data collection methods.
Outcome and definitions
The primary outcome was early PD-related infection (defined as a combination of PD peritonitis and catheter-related infections within 12 months of PD commencement). The rate of earlier infections within 3 months (90 days) was also studied. Additionally, we determined the prevalence of PD peritonitis and catheter-related infections, respectively.
PD peritonitis was defined using the International Society for Peritoneal Dialysis (ISPD) Guidelines29,30. PD peritonitis was diagnosed when two of the following three criteria were satisfied: (1) abdominal pain, tenderness, and/or cloudy fluid; (2) peritoneal effluent containing a white blood cell count of > 100 cells/mL, including > 50% polymorphonuclear leukocytes; and (3) a positive effluent culture result. There were two types of catheter-related infections: exit site infection (ESI) and tunnel infection. ESI was defined as the presence of purulent drainage, regardless of erythema at the catheter exit site31. Catheter tunnel infection was defined as clinical inflammation or ultrasonographic evidence of collection within the catheter tunnel31. Recurrent, relapsing, and repeat peritonitis were defined according to the ISPD guideline8, while repeat ESI was defined as 2 or more ESIs with the same organism within 12 months and an initial positive response to antibiotic therapy32.
PDC duration was defined as the average amount of time a nephrologist spent with a patient on PD per visit. PDC duration was reported by each study facility, which was typically done by a head nurse or unit PD nurse, in the UPS. The PDC duration options ranged from < 15 min, 15–30 min, 30–60 min, and > 60 min. Patients were divided into two groups: those receiving < 15 min versus ≥ 15 min based on the PDC duration reported by each study site and the number of patients included in those sites. Of the 20 facilities that reported their PDC durations, 11 (55.0%) reported less than 15 min, 8 (40.0%) reported 15–30 min, and 1 (5.0%) reported 30–60 min. The proportion of university-affiliated hospitals did not differ significantly by PDC duration (PDC duration < 15 min: 10/11 [90.9%]; PDC duration ≥ 15 min: 5/9 [55.6%]; P = 0.347) and tertiary hospitals (PDC duration < 15 min: 7/11 [58.3%]; PDC duration ≥ 15 min: 3/9 [37.5%]; P = 0.178). Of the 276 incident dialysis patients receiving PD, 92 had a PDC duration of ≥ 15 min or more (the longer PDC group) and 184 had a PDC duration of < 15 min (the shorter PDC group).
Statistical analyses
All continuous variables are presented as means ± standard deviations, while categorical variables are presented as numbers with percentages. The student’s t-test was used to compare continuous variables, while Pearson’s chi-square or Fisher’s exact test was used to evaluate categorical variables. Kaplan–Meier curves with log-rank tests were used to compare the risk of early PD-related infection in shorter and longer PDC groups. A comparison of PD-related infection, PD peritonitis, and catheter-related infection event rates for the PDC group was analyzed using Poisson regression analysis. Multivariate Cox proportional hazard models were used to compare the risk of early PD-related infection in the longer PDC group versus the shorter PDC group. PDC was measured at the facility level, and adjustment variables were chosen based on clinical significance, differences in baseline characteristics, and association with PD-related infection to account for confounding factors at both the patient and facility levels. For early PD-related infection, subgroup analyses were performed using age, gender, diabetes, PD nurse advice after working hours, marital status, average training time during one session, and total training time. Of all the variables, only education level had missing data, and even with missing data, we did not exclude it from the overall analysis. For chi-square analysis and subgroup analysis for education, we deleted the missing data and compared the two groups. Statistical analyses were conducted with SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) software. P values of < 0.05 were considered statistically significant.
Ethics statement
This study was approved by the central Institutional Review Board in the US, as well as Kyungpook National University Hospital (IRB No. 2018-06-012), Seoul National University Hospital, and all twenty Korean PD centers participating in the phase 2 PDOPPS study. This study was conducted in accordance with the guidelines of the 2013 Declaration of Helsinki.
Supplementary Information
Acknowledgements
We express our gratitude to all the investigators, study teams, and patients for their participation in the PDOPPS Korea. Participating Investigators are listed in the Supplementary Information.
Author contributions
Research idea and study design: JHL, SHP, and YLK. Data acquisition: HYJ, JYC, JHC, CDK, KHO, SHP, and YLK. Data analysis/interpretation: JHL, YJS, RPF, BB, JP, and DWJ. Writing of the paper: JHL, SHP, and YLK. Supervision or mentorship: JP, DWJ, SHP, and YLK. All authors contributed to and reviewed the manuscript.
Funding
Global support for the ongoing DOPPS programs is provided without restriction on publications by a variety of funders. For details, visit https://www.dopps.org/AboutUs/Support.aspx. The PDOPPS Korea study was funded by Baxter, Korea, Fresenius Medical Care, Korea, Kyowa Hako Kirin, Korea and Chongkeundang, Korea. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR22C1832). No funding entity or sponsor had a role in the study design, data collection, analysis, reporting, or the decision to submit this work for publication.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
David Johnson has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca, Bayer, and AWAK, speaker’s honoraria from ONO and Boehringer Ingelheim & Lilly, and travel sponsorships from Ono and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. Jeffrey Perl reports support from AHRQ and Arbor Research Collaborative for Health. He has received consulting fees from Astra Zeneca, Baxter Healthcare, GSK, Otsuka, Amgen, Pfizer, and Bayer; honoraria from Baxter Healthcare, FMC, DaVita Healthcare, Innovative Renal Care, AMGEN, and US Renal Care; and owns stock in LiberDi. The remaining authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sun-Hee Park and Yong-Lim Kim.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Sun-Hee Park, Email: sh-park@knu.ac.kr.
Yong-Lim Kim, Email: ylkim@knu.ac.kr.
the PDOPPS Korea group:
Yong-Lim Kim, Sun-Hee Park, Kook-Hwan Oh, Young-Ki Lee, Se-Hee Yoon, Young-Joo Kwon, Sang Heon Song, Cheol Whee Park, Seung Hyeok Han, Min-Jeong Lee, Byoung Geun Han, Jung-Hwa Ryu, Joon Ho Song, Nam-Ho Kim, Byung Chul Shin, Eun Young Lee, Chung Sik Lee, Yang Wook Kim, Su-Ah Sung, Joong Kyung Kim, Tae Ik Chang, Jong-Hak Lee, and Jong-Woo Yoon
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74205-x.
References
- 1.Wang, A. Y. Consequences of chronic inflammation in peritoneal dialysis. Semin. Nephrol. 31, 159–171. 10.1016/j.semnephrol.2011.01.005 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Cho, Y., Hawley, C. M. & Johnson, D. W. Clinical causes of inflammation in peritoneal dialysis patients. Int. J. Nephrol. 2014, 909373. 10.1155/2014/909373 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voinescu, C. G. & Khanna, R. Peritonitis in peritoneal dialysis. Int. J. Artif. Organs 25, 249–260. 10.1177/039139880202500402 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Boudville, N. et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J. Am. Soc. Nephrol. 23, 1398–1405. 10.1681/asn.2011121135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu, H. et al. Risk factors for early-onset peritonitis in Southern Chinese peritoneal dialysis patients. Perit. Dial. Int. 36, 640–646. 10.3747/pdi.2015.00203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, B. et al. Mortality in patients receiving renal replacement therapy in South Korea. Kidney Res. Clin. Pract. 10.23876/j.krcp.24.035 (2024). [DOI] [PubMed] [Google Scholar]
- 7.Béchade, C. et al. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol. Dial. Transplant. 29, 2127–2135. 10.1093/ndt/gft055 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Cheetham, M. S. et al. International peritoneal dialysis training practices and the risk of peritonitis. Nephrol. Dial. Transplant. 37, 937–949. 10.1093/ndt/gfab298 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi, T. et al. Associations of frequency and duration of patient-doctor contact in hemodialysis facilities with mortality. J. Am. Soc. Nephrol. 24, 1493–1502. 10.1681/asn.2012080831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plantinga, L. C. et al. Frequency of patient-physician contact in chronic kidney disease care and achievement of clinical performance targets. Int. J. Qual. Health Care 17, 115–121. 10.1093/intqhc/mzi010 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Goodkin, D. A., Mapes, D. L. & Held, P. J. The dialysis outcomes and practice patterns study (DOPPS): How can we improve the care of hemodialysis patients?. Semin. Dial. 14, 157–159. 10.1046/j.1525-139x.2001.00043.x (2001). [DOI] [PubMed] [Google Scholar]
- 12.McClellan, W. M., Soucie, J. M. & Flanders, W. D. Mortality in end-stage renal disease is associated with facility-to-facility differences in adequacy of hemodialysis. J. Am. Soc. Nephrol. 9, 1940–1947. 10.1681/asn.V9101940 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Plantinga, L. C. et al. Frequency of patient-physician contact and patient outcomes in hemodialysis care. J. Am. Soc. Nephrol. 15, 210–218. 10.1097/01.asn.0000106101.48237.9d (2004). [DOI] [PubMed] [Google Scholar]
- 14.Yi, C. et al. Patient-doctor contact interval and clinical outcomes in continuous ambulatory peritoneal dialysis patients. Am. J. Nephrol. 45, 346–352. 10.1159/000464258 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Hsieh, Y. P. et al. The negative impact of early peritonitis on continuous ambulatory peritoneal dialysis patients. Perit. Dial. Int. 34, 627–635. 10.3747/pdi.2013.00024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harel, Z., Wald, R., Bell, C. & Bargman, J. M. Outcome of patients who develop early-onset peritonitis. Adv. Perit. Dial. 22, 46–49 (2006). [PubMed] [Google Scholar]
- 17.Pérez Fontan, M. et al. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit. Dial. Int. 25, 274–284 (2005). [PubMed] [Google Scholar]
- 18.Vargas, E. et al. Early peritonitis in a large peritoneal dialysis provider system in Colombia. Perit. Dial. Int. 37, 30–34. 10.3747/pdi.2016.00030 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Oo, T. N., Roberts, T. L. & Collins, A. J. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am. J. Kidney Dis. 45, 372–380. 10.1053/j.ajkd.2004.10.008 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Chow, K. M. et al. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit. Dial. Int. 25, 374–379 (2005). [PubMed] [Google Scholar]
- 21.Han, S. H. et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol. Dial. Transplant. 22, 2653–2658. 10.1093/ndt/gfm242 (2007). [DOI] [PubMed] [Google Scholar]
- 22.McDonald, S. P., Collins, J. F., Rumpsfeld, M. & Johnson, D. W. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit. Dial. Int. 24, 340–346 (2004). [PubMed] [Google Scholar]
- 23.Marshall, M. R. A systematic review of peritoneal dialysis-related peritonitis rates over time from national or regional population-based registries and databases. Perit. Dial. Int. 42, 39–47. 10.1177/0896860821996096 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Bello, A. K. et al. Epidemiology of peritoneal dialysis outcomes. Nat. Rev. Nephrol. 18, 779–793. 10.1038/s41581-022-00623-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardini, J., Price, V., Figueiredo, A., Riemann, A. & Leung, D. International survey of peritoneal dialysis training programs. Perit. Dial. Int. 26, 658–663 (2006). [PubMed] [Google Scholar]
- 26.Zhang, L., Hawley, C. M. & Johnson, D. W. Focus on peritoneal dialysis training: working to decrease peritonitis rates. Nephrol. Dial. Transplant. 31, 214–222. 10.1093/ndt/gfu403 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Perl, J. et al. Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am. J. Kidney Dis. 76, 42–53. 10.1053/j.ajkd.2019.09.016 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Kim, Y. L. et al. Mon-077 exploring the status of peritoneal dialysis practices and outcomes in South Korea: Participation in the peritoneal dialysis outcomes and practice patterns study. Kidney Int. Rep. 4, S335–S336. 10.1016/j.ekir.2019.05.866 (2019). [Google Scholar]
- 29.Li, P. K. et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit. Dial. Int. 36, 481–508. 10.3747/pdi.2016.00078 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, P. K. et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int. 42, 110–153. 10.1177/08968608221080586 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Szeto, C. C. et al. ISPD Catheter-Related Infection Recommendations: 2017 Update. Perit. Dial. Int. 37, 141–154. 10.3747/pdi.2016.00120 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Beckwith, H. et al. Repeat peritoneal dialysis exit-site infection: Definition and outcomes. Perit. Dial. Int. 39, 344–349. 10.3747/pdi.2018.00216 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.