Abstract
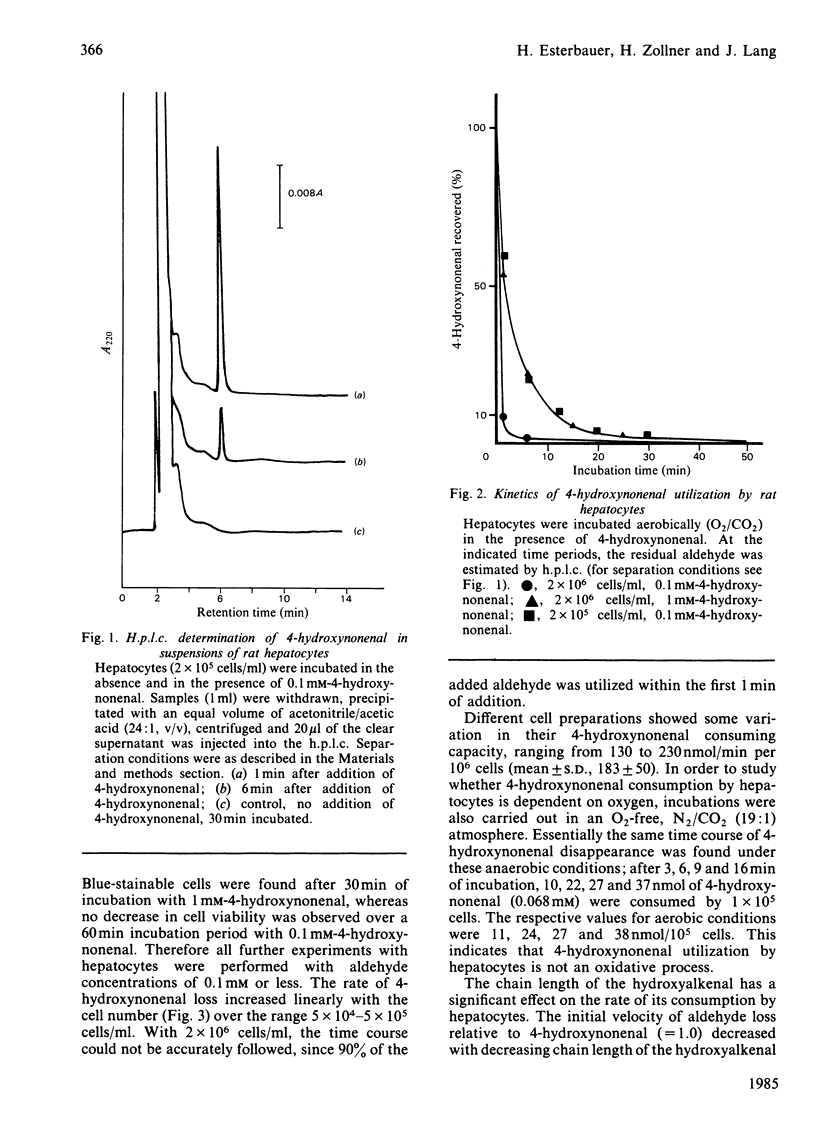
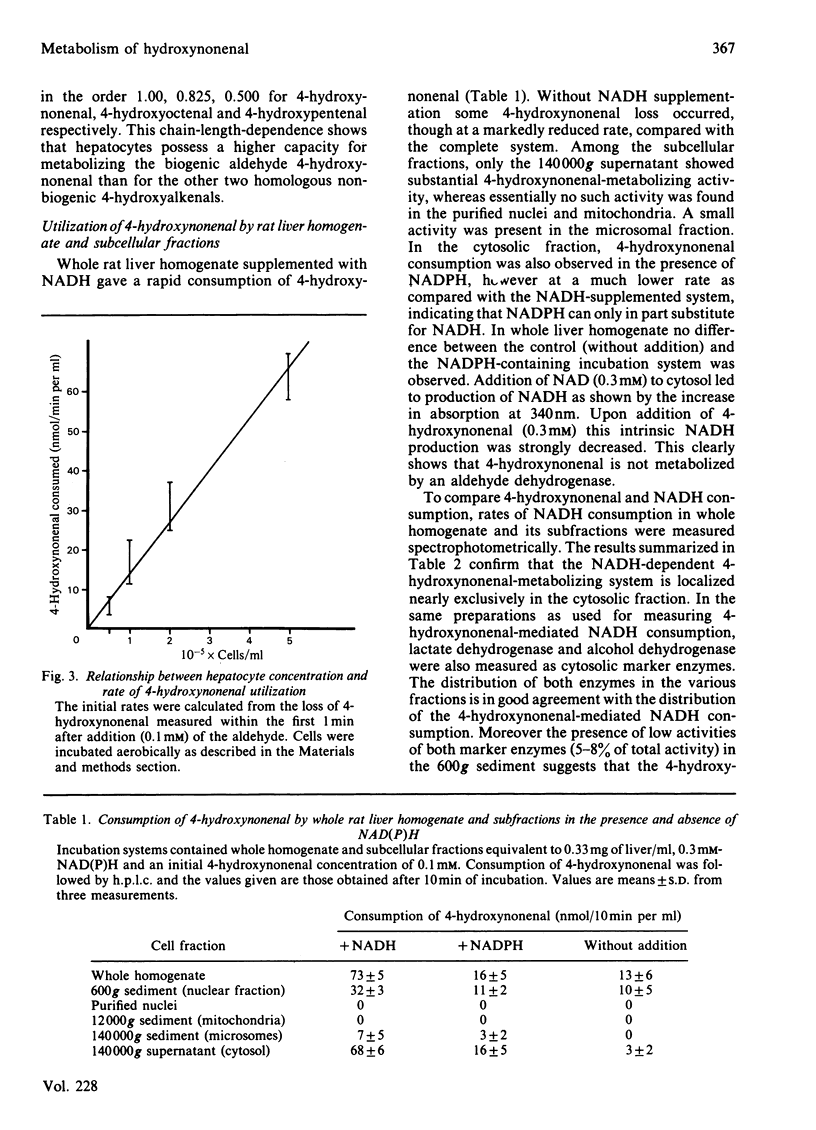
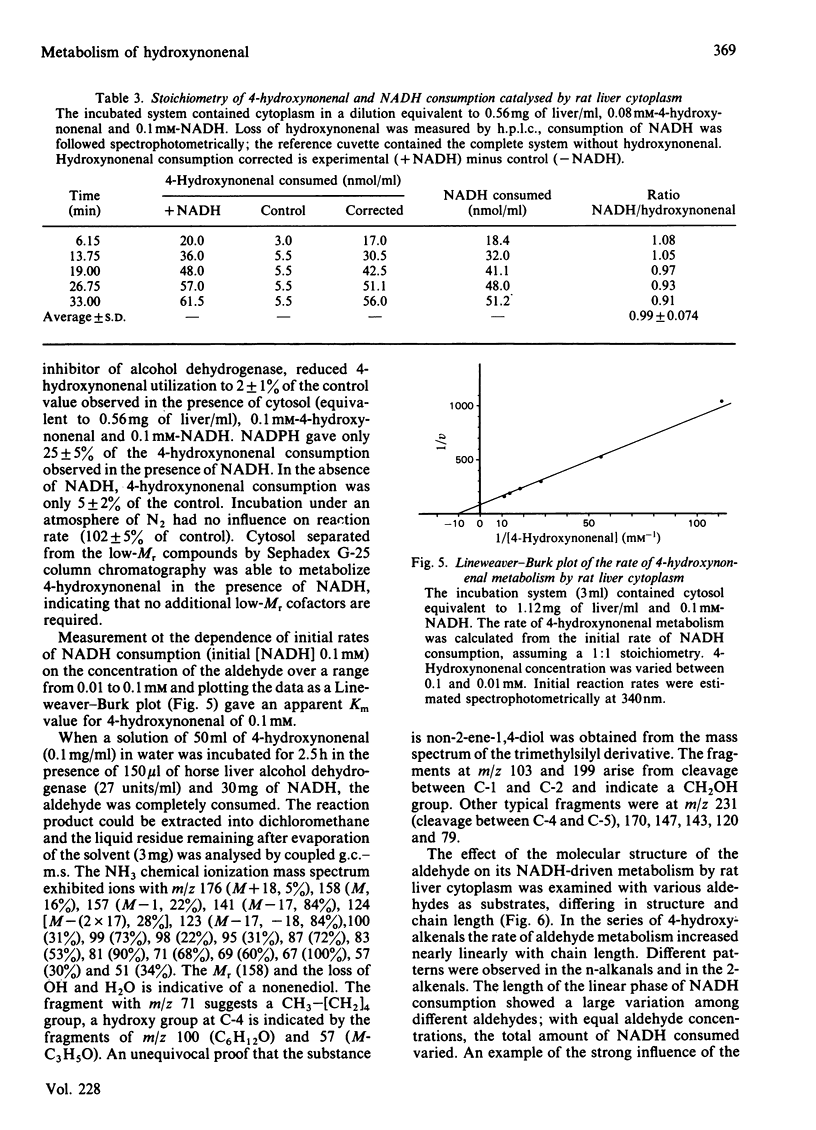
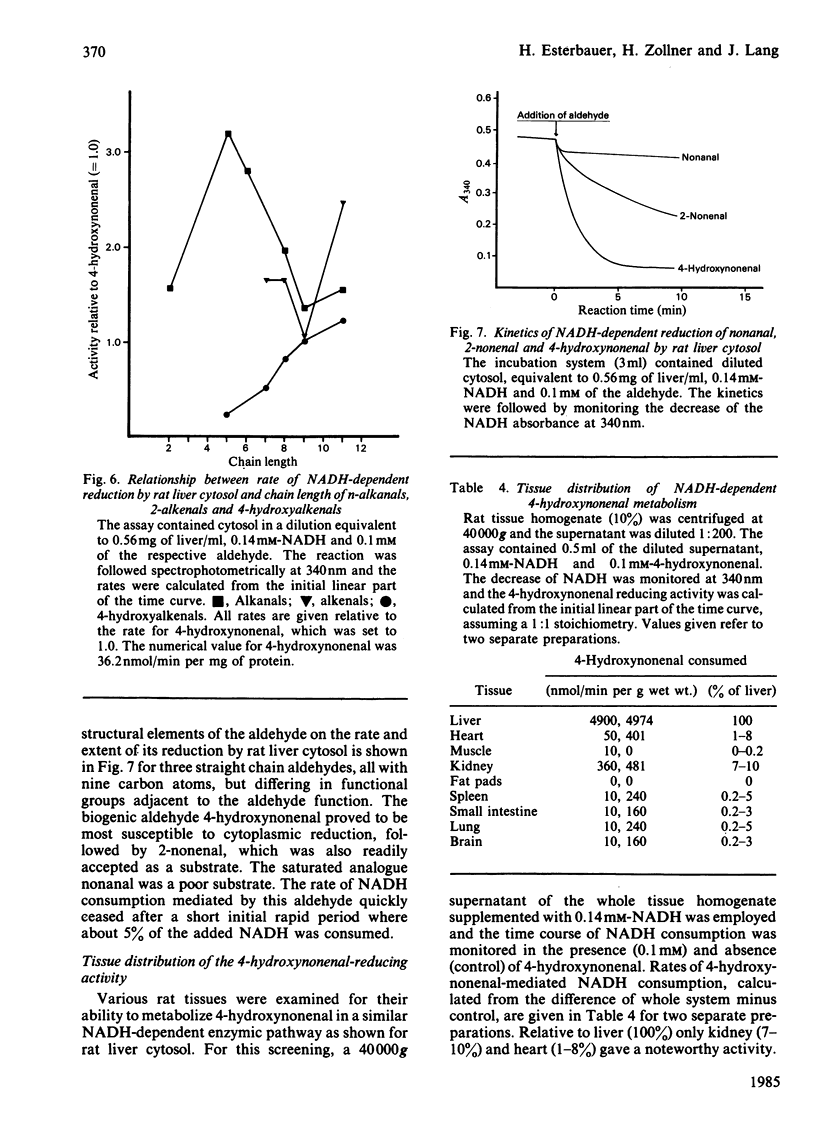
The metabolism of the lipid peroxidation product 4-hydroxynonenal and of several other related aldehydes by isolated hepatocytes and rat liver subcellular fractions has been investigated. Hepatocytes rapidly metabolize 4-hydroxynonenal in an oxygen-independent process with a maximum rate (depending on cell preparation) ranging from 130 to 230 nmol/min per 10(6) cells (average 193 +/- 50). The aldehyde is also rapidly utilized by whole rat liver homogenate and the cytosolic fraction (140 000 g supernatant) supplemented with NADH, whereas purified nuclei, mitochondria and microsomes supplemented with NADH show no noteworthy consumption of the aldehyde. In cytosol, the NADH-mediated metabolism of the aldehyde exhibits a 1:1 stoichiometry, i.e. 1 mol of NADH oxidized/mol of hydroxynonenal consumed, and the apparent Km value for the aldehyde is 0.1 mM. Addition of pyrazole (10 mM) or heat inactivation of the cytosol completely abolishes aldehyde metabolism. The various findings strongly suggest that hepatocytes and rat liver cytosol respectively convert 4-hydroxynonenal enzymically is the corresponding alcohol, non-2-ene-1,4-diol, according to the equation: CH3-[CH2]4-CH(OH)-CH = CH-CHO + NADH + H+----CH3-[CH2]4-CH(OH)-CH = CH-CH2OH + NAD+. The alcohol non-2-ene-1,4-diol has not yet been isolated from incubations with hepatocytes and liver cytosolic fractions, but was isolated in pure form from an incubation mixture containing 4-hydroxynonenal, isolated liver alcohol dehydrogenase and NADH and its chemical structure was confirmed by mass spectroscopy. Compared with liver, all other tissues possess only little ability to metabolize 4-hydroxynonenal, ranging from 0% (fat pads) to a maximal 10% (kidney) of the activity present in liver. The structure of the aldehyde has a strong influence on the rate and extent of its enzymic NADH-dependent reduction to the alcohol. The saturated analogue nonanal is a poor substrate and only a small proportion of it is converted to the alcohol. Similarly, nonenal is much less readily utilized as compared with 4-hydroxynonenal. The effective conversion of the cytotoxic 4-hydroxynonenal and other reactive aldehydes to alcohols, which are probably less toxic, could play a role in the general defence system of the liver against toxic products arising from radical-induced lipid peroxidation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985 Jan 7;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Casini A. F., Ferrali M., Comporti M. Extraction and partial characterization of dialysable products originating from the peroxidation of liver microsomal lipids and inhibiting microsomal glucose 6-phosphatase activity. Biochem Pharmacol. 1979 Oct 1;28(19):2909–2918. doi: 10.1016/0006-2952(79)90585-9. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980 Nov 7;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Fulceri R., Esterbauer H. Cytotoxic aldehydes originating from the peroxidation of liver microsomal lipids. Identification of 4,5-dihydroxydecenal. Biochim Biophys Acta. 1984 Feb 9;792(2):172–181. doi: 10.1016/0005-2760(84)90219-4. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Ferrali M., Ciccoli L., Esterbauer H., Comporti M. Detection of carbonyl functions in phospholipids of liver microsomes in CCl4- and BrCCl3-poisoned rats. Biochim Biophys Acta. 1982 Sep 14;712(3):628–638. doi: 10.1016/0005-2760(82)90292-2. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. P., Draper H. H. Uptake and oxidation of malonaldehyde by cultured mammalian cells. Lipids. 1982 Aug;17(8):519–523. doi: 10.1007/BF02535378. [DOI] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. Enzymes catalysing conjugations of glutathione with alpha-beta-unsaturated carbonyl compounds. Biochem J. 1968 Oct;109(4):651–661. doi: 10.1042/bj1090651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Müller A., Brigelius R., Esterbauer H., Sies H. Effects of 4-hydroxynonenal on isolated hepatocytes. Studies on chemiluminescence response, alkane production and glutathione status. Biochem J. 1983 Aug 15;214(2):479–487. doi: 10.1042/bj2140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzio M., Torrielli M. V., Giroud J. P., Esterbauer H., Dianzani M. U. Neutrophil chemotactic responses to aldehydes. Res Commun Chem Pathol Pharmacol. 1982 Jun;36(3):463–476. [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Volatile hydrocarbon and carbonyl products of lipid peroxidation: a comparison of pentane, ethane, hexanal, and acetone as in vivo indices. Lipids. 1979 Dec;14(12):989–995. doi: 10.1007/BF02533435. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Scholz N. Reaction of glutathione with conjugated carbonyls. Z Naturforsch C. 1975 Jul-Aug;30(4):466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- Ferrali M., Fulceri R., Benedetti A., Comporti M. Effects of carbonyl compounds (4-hydroxyalkenals) originating from the peroxidation of liver microsomal lipids on various microsomal enzyme activities of the liver. Res Commun Chem Pathol Pharmacol. 1980 Oct;30(1):99–112. [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelle J. J., Petersen D. R. Metabolism of malondialdehyde by rat liver aldehyde dehydrogenase. Toxicol Appl Pharmacol. 1983 Aug;70(1):57–66. doi: 10.1016/0041-008x(83)90179-5. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Some aspects of the regulation of fuel supply in omnivorous animals. Adv Enzyme Regul. 1972;10:397–420. doi: 10.1016/0065-2571(72)90025-8. [DOI] [PubMed] [Google Scholar]
- MAGGIO R., SIEKEVITZ P., PALADE G. E. STUDIES ON ISOLATED NUCLEI. I. ISOLATION AND CHEMICAL CHARACTERIZATION OF A NUCLEAR FRACTION FROM GUINEA PIG LIVER. J Cell Biol. 1963 Aug;18:267–291. doi: 10.1083/jcb.18.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett L. J., Hurd H. K., Hollstein M. C., Levin D. E., Esterbauer H., Ames B. N. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res. 1985 Jan-Feb;148(1-2):25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- Poli G., Dianzani M. U., Cheeseman K. H., Slater T. F., Lang J., Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985 Apr 15;227(2):629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. HEPATIC ALDEHYDE OXIDASE. 3. THE SUBSTRATE-BINDING SITE. J Biol Chem. 1964 Jun;239:2027–2035. [PubMed] [Google Scholar]
- Siu G. M., Draper H. H. Metabolism of malonaldehyde in vivo and in vitro. Lipids. 1982 May;17(5):349–355. doi: 10.1007/BF02535193. [DOI] [PubMed] [Google Scholar]
- THEORELL H., YONETANI T. LIVER ALCOHOL DEHYDROGENASE-DPN-PYRAZOLE COMPLEX: A MODEL OF A TERNARY INTERMEDIATE IN THE ENZYME REACTION. Biochem Z. 1963;338:537–553. [PubMed] [Google Scholar]
- Tam B. K., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. 3. Transient formation of phospholipid peroxides. J Biol Chem. 1970 May 10;245(9):2295–2300. [PubMed] [Google Scholar]


