Abstract
Background
Questions persist around whether and how to use race or geographic ancestry in biomedical research and medicine, but these forms of self-identification serve as a critical tool to inform matching algorithms for human leukocyte antigen (HLA) of varying levels of resolution for unrelated hematopoietic stem cell transplant in large donor registries.
Methods
Here, we examined multiple self-reported measures of race and ancestry from a survey of a cohort of over 100,000 U.S. volunteer bone marrow donors alongside their high-resolution HLA genotype data.
Results
We find that these self-report measures are often non-overlapping, and that no single self-reported measure alone provides a better fit to HLA genetic ancestry than a combination including both race and geographic ancestry. We also found that patterns of reporting for race and ancestry appear to be influenced by participation in direct-to-consumer genetic ancestry testing.
Conclusions
While these data are not used directly in matching for transplant, our results demonstrate that there is a place for the language of both race and geographic ancestry in the critical process of facilitating accurate prediction of HLA in the donor registry context.
Subject terms: Genetic testing, Transplant immunology
Plain language summary
Self-identification with respect to race and ancestry is an important component in the process of finding a matching unrelated bone marrow donor for a patient in large donor registries. Here, we considered whether terms specific to either race or the geographic ancestry of donors would be more useful in the matching process. We found that rather than using either of these terms alone, collecting responses for both race and geographic ancestry from potential donors is most likely to provide the information necessary to find a genetic match among millions of donors for a patient in need of a transplant.
Damotte et al. examine the utility of multiple measures of race and ancestry self-identification in the context of matching HLA for potential unrelated bone marrow donors with patients. They show that combining both race and geographic ancestry provides a better fit to HLA than either measure alone.
Introduction
While not used directly for matching donors and recipients, self-report data regarding race and ancestry are a critical part of the bioinformatic algorithm matching for human leukocyte antigen (HLA) of prospective donors with patients in need of hematopoietic stem cell transplant. Modern genomic methods may provide granular detail regarding ancestry, but genome-wide data is not collected routinely in bone marrow donor registries. Given the ongoing reliance on self-identification, how do we ensure that the methods that we employ for this critical task are best-suited toward inclusion of diverse populations? Beyond the transplant setting, these questions apply as well to the next generation of genomic, biomarker, behavioral research, clinical trials, and biobanks. Likewise, consideration of race continues to play a part in medical practice1,2. Historically, self-identification using race categories as defined by the United States Office of Management and Budget (OMB)3 has been standard; indeed, federally funded researchers are mandated to collect and report this information. Further complexity is added by inconsistent use of the term “ethnicity,” which is often used to describe a group sharing culture, language, or other features. However, many in the biomedical community have sought to focus rather on identification according to geographic ancestry4–7 It is argued that these measures better reflect human history and are more likely to represent biological differences compared to race, which is understood to be a social construct8. Meanwhile, there remains a need to incorporate some form of this information to expedite the matching process for patients in search of an unrelated bone marrow donor, where it is used to narrow the search space of possible donors by facilitating identification of the most likely high-resolution HLA haplotypes in the donor pool.
HLA data for potential volunteer unrelated donors stored in registries is of varying resolution; while HLA genotyping for donors recruited in the last several years is typically very high resolution (generally from sequence-based typing (SBT) methods) and complete with respect to loci genotyped, this varies for a substantial number of donors whose data were collected up to decades ago. Some data is incomplete with respect to the HLA loci typed (for example, often missing data for HLA-C or HLA-DQB1) and/or is low resolution (for example, was typed with serological methods or much lower resolution molecular methods). In order to perform efficient searches for a donor match for a given patient, these lower resolution HLA genotypes need to undergo algorithmic imputation to predict the most likely high-resolution HLA genotypes for a given donor. Among the inputs for this algorithm are known haplotypic associations in combination with known patterns of variation based on ancestry9—this is the primary use for self-identification data collected at the time of donor recruitment. Thus, while donors and patients are not matched according to race and ancestry, they are matched according to known or predicted high-resolution HLA genotype; the predicted genotypes having been informed, critically, by self-identified race and ancestry.
Although previous investigations have examined the relationship between single measures of self-identification and genetic ancestry10–13, here we expand on our earlier work considering self-identification for donors registered with the National Marrow Donor Program (NMDP)14 with an approach that differs from other studies in several important ways. We directly incorporate findings from the social sciences15 to perform a large-scale study comparing multiple measures of self-identification simultaneously with HLA genetic ancestry in the same cohort. Here, we specifically leverage genetic information for HLA to facilitate comparison between measures and understand whether some are more closely related to genetic ancestry in this region than others. We do so in a larger and more diverse sample of the U.S. adult population than previously examined, considering how both self-identified race and ancestry can be used to best describe human diversity, with a focus on the relevance for donor-patient matching algorithms16. In the National Marrow Donor Program Registry there are nearly 7 million volunteer bone marrow donors, the majority of which have missing or ambiguous typing at loci that are critical for matching. Population specific haplotype frequency data is used to make predictions but these predictions are only as good as the accuracy of the assignment of an individual to a population17,18. Finally, we consider the role that direct-to-consumer genetic testing may play in shifting patterns of self-identity, and the extent to which this may provide potential advantage in the registry context.
Methods
We collected multiple self-reported measures of race and ancestry from a cohort of more than 100,000 U.S. adults who also provided genetic data for HLA as potential donors registered with the National Marrow Donor Program (NMDP). To ascertain genetic ancestry, we used the registry’s data for the human leukocyte antigen (HLA) complex on the short arm of chromosome 6, which is critical to matching in tissue transplant. The HLA loci exhibit extreme levels of variability and differentiation among human populations and the region is relatively well-maintained during gametogenesis, and thus can be used as ancestry informative markers19–22. Our survey of potential NMDP donors, conducted for this study in spring 2015, included questions about racial self-identification and multiple (geographic) ancestry items. All participants provided informed consent (available in Supplementary files) and this study was approved by the Institutional Review Board at the University of California San Francisco (study #14-13977).
Survey questions
For self-reported ancestry, we included three measurement approaches: (1) personal ancestry (PA), a check-all-that-apply option using a series of geographic categories; (2) personal ancestry salience (PAS), a measure that asked people to “weight” their ancestry self-reports on a 100-point scale; and 3) family ancestry (FA), check-all-that-apply ancestry questions about specific biological relatives, such as grandparents. In order to fully exploit the FA responses, we also computed a summary family fractional ancestry (FFA) value from the family responses based on the number of ancestry selections per parent or grandparent (Supplementary Methods). In addition to asking respondents to describe themselves using official racial categories (RC), we also asked that they tell us how they think other Americans would classify them using the same categories, which we term “reflected race” (RR)23; we were interested in this measure as a proxy for race coding that might be contributed by a third party, such as a clinical provider. The complete survey is provided in the Supplementary Material.
Assignment of HLA haplotype ancestry
To understand how these measures of self-reported race and ancestry relate to genetic ancestry, we employed a Bayesian classifier to assign the most probable geographic origin for subjects’ HLA haplotypes (Supplementary Table S1). Our previous work had shown that population-level HLA haplotype ancestry assignments using this method are equivalent to ancestry proportions derived from a well-characterized panel of ancestry informative markers14. To further validate the classifier, we examined prediction of the HLA-based ancestry classifications from ancestry proportions derived from over 700,000 single nucleotide polymorphism (SNP) markers for an independent dataset of 1983 individuals, with cross-validation revealing accuracy approaching 85% (Supplementary Fig. S1).
Data analysis
We tested the fit of all self-reported race and ancestry responses alone and in specific combinations as predictors of genetic (HLA haplotype) ancestry in a multinomial logistic regression model, including covariates for age, sex, and educational attainment (Detailed in Supplementary Methods). Our survey methodology included randomly switching the order in which the race vs. ancestry sections were presented, which yielded some variation in the number of responses for each section, and thus we adjusted for this feature. Likewise, we adjusted for the email outreach recruiting participants, the specific language of which varied (Supplementary Fig. S2). To test for genetic differences between groups of respondents, we calculated Edward’s genetic distance and tested for significance using a permutation procedure (Detailed in Supplementary Methods).
Statistics and reproducibility
All data analysis was performed in the R environment for statistical computing. All analysis is reproducible using the code linked below in “Code availability24.”
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
The relationship between measures of self-reported race and ancestry is complex and non-redundant
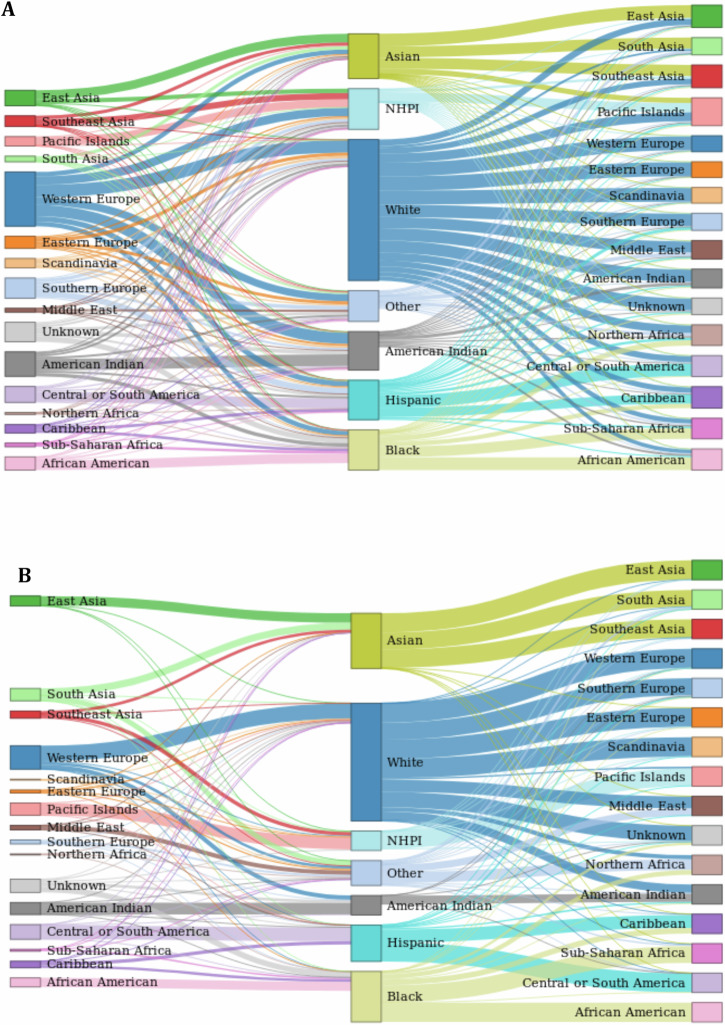
Respondent demographics detailed by sex and age with respect to place of birth and response to RC are shown in Tables 1 and 2, respectively. Despite often being treated interchangeably, we found that measures of self-reported race and ancestry are often non-overlapping, even when administered simultaneously in the same cohort. On the surface, responses for RC and PA might seem to provide redundant information, with many respondents identifying as White and also reporting PA from Western Europe, for example. However, cross-tabulating the measures with one another showed they are not as interchangeable as they might appear at first glance. When comparing racial self-identification and PA, every possible PA was connected to every possible RC in our sample (Fig. 1A), yielding a total of 3,582 different RC/PA combinations (Supplementary Table S2). Nearly 60% of the sample self-reported two or more PA responses, and close to 12% provided two or more RC responses. Even when we restrict to individuals who selected a single PA and single RC response to describe themselves (39% of our sample), much of the complexity between ancestry and race reporting remains (Fig. 1B).
Table 1.
Survey respondent demographics (gender and age groups) separated by place of birth
| Total | Female | Male | [18–24] | [25–34] | [35–44] | [45–54] | [55–64] | [65+] | |
|---|---|---|---|---|---|---|---|---|---|
| All respondents | 103348 | 82226 | 21121 | 13576 | 35487 | 27804 | 18105 | 8313 | 9 |
| Respondent US born | |||||||||
| Yes | 95770 | 80.1% | 19.9% | 13.3% | 34.5% | 26.6% | 17.4% | 8.2% | 0% |
| No | 7487 | 72.7% | 27.3% | 11.4% | 32.8% | 31.1% | 18.5% | 6.1% | 0% |
| Parents US born | |||||||||
| Neither | 11107 | 73.8% | 26.2% | 16.7% | 35.6% | 27.4% | 15.4% | 4.9% | 0% |
| One | 8190 | 80.7% | 19.3% | 17.2% | 35.9% | 26.2% | 14.5% | 6.2% | 0% |
| Both | 84012 | 80.2% | 19.8% | 12.3% | 34.0% | 26.9% | 18.1% | 8.6% | 0% |
| Grandparents US born | |||||||||
| None | 15319 | 74.7% | 25.3% | 14.8% | 31.7% | 25.6% | 18.7% | 9.1% | 0% |
| One | 3031 | 79.8% | 20.2% | 12.9% | 27.6% | 25.1% | 22.3% | 12.1% | 0% |
| Two | 13639 | 80.6% | 19.4% | 13.6% | 31.7% | 25.1% | 19.5% | 10.1% | 0% |
| Three | 9939 | 81.8% | 18.2% | 12.9% | 34.8% | 26.1% | 17.7% | 8.6% | 0% |
| Four | 61365 | 80.2% | 19.8% | 12.7% | 35.9% | 27.9% | 16.5% | 7.0% | 0% |
Gender and age group were missing for 1 and 63 individuals, respectively. Place of birth was missing for 91 individuals, parents place of birth was missing for 39 individuals. Grand-parents place of birth was missing for 235 individuals.
Table 2.
Survey respondents demographics (gender and age groups) separated by race
| Total | Female | Male | [18–24] | [25–34] | [35–44] | [45–54] | [55–64] | [65+] | |
|---|---|---|---|---|---|---|---|---|---|
| All respondents | 103348 | 82226 | 21121 | 13576 | 35487 | 27804 | 18105 | 8313 | 9 |
| American Indian | 279 | 80.6% | 19.4% | 10.4% | 22.9% | 35.8% | 24.0% | 6.8% | 0% |
| Asian | 3461 | 67.6% | 32.4% | 18.2% | 42.1% | 25.1% | 11.2% | 3.4% | 0% |
| Black | 3044 | 84.3% | 15.7% | 14.1% | 30.7% | 29.1% | 18.4% | 7.7% | 0% |
| Hispanic | 4889 | 80.6% | 19.4% | 21.5% | 34.9% | 27.5% | 12.4% | 3.7% | 0% |
| Native Hawaiian or Pacific Islander | 129 | 76.7% | 23.3% | 10.1% | 34.9% | 36.4% | 12.4% | 6.2% | 0% |
| White | 78489 | 79.8% | 20.2% | 11.5% | 33.4% | 27.0% | 18.9% | 9.1% | 0% |
| Other | 1146 | 66.8% | 33.2% | 11.2% | 34.9% | 28.4% | 17.0% | 8.5% | 0% |
| Multi-race | 11903 | 81.4% | 18.6% | 18.9% | 39.2% | 25.4% | 11.9% | 4.5% | 0% |
Gender and age group were missing for 1 and 63 individuals, respectively.
Fig. 1. Sankey diagram of connection between racial categories and geographic ancestries selected by respondents.
A Considering all respondents. B Only respondents who selected a single race category and a single geographic ancestry were considered.
Combining self-identification responses for race and ancestry provides the best fit to HLA variation
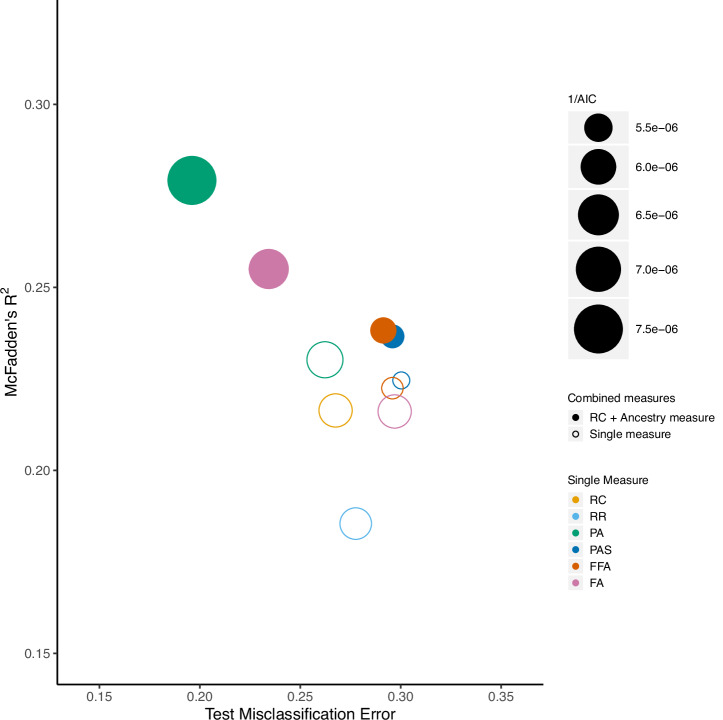
We found that no single self-reported measure of race or ancestry alone provides a better fit to HLA genetic ancestry classification than combined measures (Fig. 2). When examining single measures, PA provided the best model fit, lending support to the notion that geographic ancestry serves as a better proxy for genetic ancestry than self-identified race. However, RC provided a better fit than any of our other single measures, including FA, while RR fit very poorly, with the lowest R2 of any measure. Our quantitative measures, PAS and FFA, were highly correlated (Supplementary Table S3), but had the highest misclassification rates of any single measure we examined, diminishing the overall model fit. Although PA provided better fit than the RC response alone, fit to HLA genetic ancestry was significantly improved by incorporating the RC response with any of the ancestry measures, with the most significant improvements noted for combinations including PA and FA. Strikingly, the best-fitting model predicting genetic ancestry classification included a combination of RC self-identification and PA. This combined measure showed marked improvement in model fit compared to the PA single measure (p < 0.001).
Fig. 2. Assessment of different races and/or ancestries models.
These models represent the observed fits of different models as predictors of genetic (HLA haplotype) ancestry (see Materials and Methods). Shown on the x-axis is the test misclassification error (rate of incorrect model prediction) and values for McFadden’s R2 are shown on the y-axis, which corresponds to goodness of fit. The predictors shown are as follows: RC race category; PR personal race; RR reflected race; PA personal ancestry; PAS personal ancestry salience; FFA fractional family ancestry; FA family ancestry.
Specific examples from our data illustrate why combining race and ancestry responses serves to better represent genetic variation than single measures of self-identification. For instance, complexity in reporting American Indian race and ancestry is well documented in demographic studies25,26. American Indian PA is reported frequently in our sample (15% of individuals), and is most often seen in combination with Western European PA (N = 5709). Despite the fact that “American Indian” is also provided as an option for the RC response, many individuals reporting this PA combination report only the White RC. We computed the HLA genetic distance between individuals reporting the specific combination of Western Europe and American Indian PA with only White RC (80%) and those who reported the same PA (Western Europe and American Indian) with White RC plus American Indian RC (17%) or only American Indian RC (1.6%); using a permutation procedure, we found that the White-only RC and White RC plus American Indian RC groups are not significantly divergent (p = 0.15). However, the American Indian-only RC group is significantly divergent from the White-only RC group (p < 0.001) and from the White RC plus American Indian RC group (p = 0.03), showing the added value of combining race and ancestry responses in more accurately imputing high-resolution HLA for transplant matching.
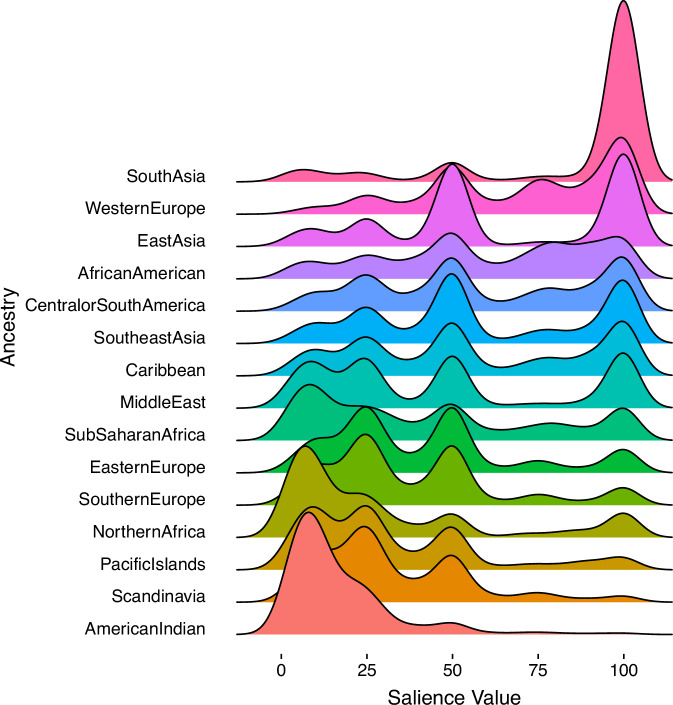
Whereas incorporation of salience values (PAS) did not improve the overall fit of our models, they do provide important insights into the underlying dynamics in ancestry identification. Although frequently reported, American Indian PA yields the lowest mean PAS value (16.8) of any PA response (Fig. 3). Even among individuals who report American Indian FA for all four of their biological grandparents, their mean American Indian PAS value is only 49; in comparison, individuals who report four South Asian grandparents FA report South Asian mean PAS of 99 (p < 0.001). These results may also explain why PA provided better overall model fit to HLA variation than FA. Notably, individuals who identify with American Indian RC report significantly higher American Indian PAS than those who did not (mean 26 vs. 14; p < 0.001).
Fig. 3. Density plots of personal ancestry salience (PAS) values given by individuals who selected specific geographic ancestry.
The x-axis represents salience values (range 0-100) provided by participants for specific ancestries.
Likewise, we observed complexity comparing racial self-identification as Black with sub-Saharan African PA, furthering support for combining measures of self-identified race and ancestry in the matching algorithms designed to improve resolution for HLA variation. Although tracing ancestry to the original peoples of sub-Saharan Africa is the official definition of the “Black or African American” racial category in the U.S.3, we offered both “Sub-Saharan Africa” and “African American” categories among our ancestry responses. Among respondents who identified RC as Black alone (N = 3038), 67% reported African American PA, compared to 17% who reported Sub-Saharan African PA. We analyzed the HLA genetic distance between individuals who identified as Black RC alone and who reported African American ancestry only and those who reported Sub-Saharan African ancestry only and found significant divergence (p < 0.001). One explanation for these observations may be found in respondents’ nativity: among respondents who identified as Black RC alone, respondents who reported sub-Saharan African ancestry were significantly less likely to have been born in the U.S. than those who did not report this ancestry (84% and 93% respectively; p < 0.001). Foreign-born Black RC respondents who reported sub-Saharan African PA also reported a mean sub-Saharan African PAS value of 82, compared to 45 for their U.S.-born counterparts who selected the same RC and PA responses (p < 0.001).
Participation in direct-to-consumer ancestry testing changes patterns of self-identification
Finally, we found that self-identification reporting patterns may be transformed by participation in direct-to-consumer genetic ancestry testing (GAT). Approximately 5% of our respondents reported having taken a GAT27. Overall, these individuals gave more responses for ancestry (mean responses 2.3 vs 1.9; p < 0.001) as well as distinctive combinations of race and ancestry reporting compared to those who did not use GAT. Among respondents who identified as Black RC alone, 62% reported sub-Saharan African PA if they had taken a GAT compared to 14% who have never taken a GAT (p < 0.001). In contrast, these groups reported African American PA nearly equivalently at 70% and 66%, respectively. In contrast to the larger sample, genetic distance measures were non-significant between Black RC individuals who did or did not report sub-Saharan African PA. Likewise, among GAT participants, 96% of Black respondents reporting sub-Saharan ancestry also reported being U.S. born. In addition to sub-Saharan African PA, a number of other PA responses were also found to differ in frequency according to whether respondents had used GAT. For example, among GAT takers, American Indian PA was reported less often by individuals identifying as White RC (pcorr = 0.004), but more often by individuals identifying as Hispanic RC (pcorr < 0.001) compared to those who did not use GAT. Thus, in contrast to individuals who did not participate in GAT, here the race response did not improve model fit and racial identification appears not relevant with respect to HLA genetic ancestry.
Discussion
Taken together, our results demonstrate that there is a place for the language of both race and (geographic) ancestry in the specific context of matching for HLA in donor registries. Although not used for matching itself, given the high level of ambiguity in HLA genotyping in donor registries and the critical role that self-identified race and ancestry play in bioinformatic predicting high-resolution, unambiguous HLA genotypes, more accurate self-identification of race and ancestry translates directly to more accurate matching and improved patient outcomes. A limitation of this study is the lack of genome-wide data for comparison to results for the HLA data. Given the objective here to consider prediction of high-resolution HLA genotypes, we acknowledge that the results might not be applicable to questions considering the relationship between genetics and self-identification outside of the registry setting. Likewise, these results are specific to the context of a U.S. donor registry and may not be applicable to other populations, which may be significantly more homogenous or have very different histories of immigration.
Consideration of multiple measures here has revealed the underlying complexity in self-identification, with substantial variance between ancestries. For example, we show that while a substantial number of respondents claim American Indian ancestry, many acknowledge its relatively low salience; for those who do not simultaneously identify as American Indian in the context of race, we did not observe significant deviation in terms of HLA genetics from those who did not identify with this ancestry. In contrast, individuals who do select American Indian in the context of race typically gave higher salience values to that ancestry and were genetically distinct from those who did not. Thus, in this case racial self-identification appears to signal both personal and biological relevance. Our results also illustrate one of the pitfalls of using a check-all-that-apply format for reporting geographic origins as the sole self-identification measure in the registry setting.
Examination of HLA genetic differentiation among respondents who identified as Black in the context of race, but have variably selected between African American and Sub-Saharan African in the context of geographic ancestry, underscores conversely the pitfalls in using race as the only measure of self-identification. Here, although shared racial identification suggests a shared social experience of “blackness,” which likely has implications for health28,29, a registry that groups donors solely by racial self-identification might miss the HLA genetic variation among individuals and their differing immigration histories, which could be important for matching as well as understanding match disparities. For some other ancestries, racial self-identification has even more limitation. A high proportion of individuals claiming only Middle Eastern or North African ancestry do not identify with any of the standard OMB RC’s, and rather select Other. Likewise, South Asian ancestry is generally split between the Other category and Asian RC (Fig. 1).
These results underscore the notion that race and ancestry are describing distinct aspects of self-identification, which partially – but far from completely – overlap. Moreover, these patterns vary by population, emphasizing the need to embrace multiple measures in order to offer appropriate options to diverse cohorts. Accordingly, our results show that while providing important information, self-reported geographic ancestry alone is not as good a proxy for genetic variation in the context of HLA as when coupled with racial self-identification; there is also ample research that shows self-reported race has a role to play in studies of health disparities, and thus it might be important for the continued collection of this information by registries to continue to track and ameliorate longstanding inequalities in match rates across racial groups. Our results for individuals participating in GAT suggest that as genealogical tools and technologies increase in popularity and accessibility, individuals may move toward means of self-identification that are more geographically, and less racially, based; this may present an important opportunity for donor registries going forward as increasing numbers of engaged donors employ GAT.
In conclusion, this work demonstrates that we stand to improve current matching algorithms by recognizing the differences between measures of race and ancestry, and leveraging the instances of empirical convergence and divergence presented here to better reflect modes of identification that resonate with donors.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors wish to thank Aliya Saperstein for assistance in the conception and design of the survey, and Juliano Boquett with assistance in finalizing the manuscript. We also thank the study participants. This work was supported by grants from NIH National Human Genome Research Institute (R21HG00804) and the Department of the Navy, Office of Naval Research (N00014-17-1-2388).
Author contributions
Conceptualization: M.M., J.A.H., R.K., M.M., and E.W.; Data curation: V.D., C.Z., E.W., and M.M. Formal analysis: V.D., C.Z., C.L., Y.L., A.M., M.M., and J.A.H; Writing: V.D., P.J.N., M.M., and J.A.H.
Peer review
Peer review information
Communications Medicine thanks Claudio Anasetti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The full raw data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the University of California San Francisco. Processed data underlying all figures and tables (source data) are given in Supplementary Data Table 1.
Code availability
Analytical codes for this study are available at https://github.com/Hollenbach-lab/AQP_Paper1_PublicRelease, 10.5281/zenodo.1368551524.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Martin Maiers, Jill A. Hollenbach.
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-024-00620-w.
References
- 1.Stevens, L. A. et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol. Dial. Transpl.25, 449–457 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callier, S. L., Cunningham, B. A., Powell, J., McDonald, M. A. & Royal, C. D. M. Cardiologists’ perspectives on race-based drug labels and prescribing within the context of treating heart failure. Health Equity3, 246–253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office of Management and Budget. Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity (1997).
- 4.Rosenberg, N. A. et al. Genetic structure of human populations. Science298, 2381–2385 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Shriver, M. D. et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum. Genet.112, 387–399 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Tishkoff, S. A. & Kidd, K. K. Implications of biogeography of human populations for ‘race’ and medicine. Nat. Genet.36, S21–S27 (2004). [DOI] [PubMed] [Google Scholar]
- 7.New framework released on using population descriptors in genetics and genomics research. Am. J. Med. Genet. A191, 2462–2463 https://onlinelibrary.wiley.com/doi/10.1002/ajmg.a.62830 (2023). [DOI] [PubMed]
- 8.Ferrante-Wallace, J. & Brown, P. The Social Construction Of Race And Ethnicity In The United States (Prentice Hall, Upper Saddle River, 2001).
- 9.Madbouly, A. et al. Validation of statistical imputation of allele-level multilocus phased genotypes from ambiguous HLA assignments. Tissue Antigens84, 285–292 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Sucheston, L. E. et al. Genetic ancestry, self-reported race and ethnicity in African Americans and European Americans in the PCaP cohort. PLoS ONE7, e30950 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, Y. L., Teitelbaum, S., Wolff, M. S., Wetmur, J. G. & Chen, J. Comparing genetic ancestry and self-reported race/ethnicity in a multiethnic population in New York City. J. Genet.89, 417–423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banda, Y. et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics200, 1285–1295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang, H. et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am. J. Hum. Genet.76, 268–275 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollenbach, J. A. et al. Race, ethnicity and ancestry in unrelated transplant matching for the national marrow donor program: a comparison of multiple forms of self-identification with genetics. PLoS ONE10, e0135960 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saperstein, A. et al. Making the most of multiple measures: disentangling the effects of different dimensions of race in survey research. Am. Behav. Sci.60, 519–537 (2016). [Google Scholar]
- 16.Dehn, J. et al. HapLogic: a predictive human leukocyte antigen-matching algorithm to enhance rapid identification of the optimal unrelated hematopoietic stem cell sources for transplantation. Biol. Blood Marrow Transpl.22, 2038–2046 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Gragert, L. et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N. Engl. J. Med.371, 339–348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gragert, L., Madbouly, A., Freeman, J. & Maiers, M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum. Immunol.74, 1313–1320 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Fernandez Vina, M. A. et al. Tracking human migrations by the analysis of the distribution of HLA alleles, lineages and haplotypes in closed and open populations. Philos. Trans. R. Soc. Lond. B Biol. Sci.367, 820–829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Mazas, A. et al. Immunogenetics as a tool in anthropological studies. Immunology133, 143–164 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mountain, J. L., Lin, A. A., Bowcock, A. M. & Cavalli-Sforza, L. L. Evolution of modern humans: evidence from nuclear DNA polymorphisms. Philos. Trans. R. Soc. Lond. B Biol. Sci.337, 159–165 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Cavalli-Sforza, L. L., Menozzi, P. & Piazza, A. The History And Geography Of Human Genes (Princeton University Press, 1994).
- 23.Roth, W. D. The multiple dimensions of race. Ethn. Racial Stud.39, 1310–1338 (2016). [Google Scholar]
- 24.Damotte, V. Code for Multiple Measures For Self-Identification Improve Matching Donors with Patients in Unrelated Hematopoietic Stem Cell Transplant (2024). [DOI] [PMC free article] [PubMed]
- 25.Snipp, C. M. Who are American Indians? Some observations about the perils and pitfalls of data for race and ethnicity. Popul. Res. Policy Rev.5, 237–252 (1986). [Google Scholar]
- 26.Carolyn A. et al. “Dynamics of Race: Joining, Leaving, and Staying in the American Indian/Alaska Native Race Category between 2000 and 2010” Center for Administrative Records Research and Applications Working Paper #2014-10. (U.S. Census Bureau, 2014).
- 27.Horowitz AL, S. A., Little, J., Maiers, M. & Hollenbach, J. A. Consumer (dis-)interest in genetic ancestry testing: the roles of race, immigration, and ancestral certainty. N. Genet. Soc.38, 165–194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Health disparities experienced by black or African Americans--United States. MMWR Morb Mortal Wkly Rep.54, 1–3 (2005). [PubMed]
- 29.Gómez, L. E. & López, N. Mapping Race: Critical Approaches To Health Disparities Research. (Rutgers University Press, 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The full raw data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the University of California San Francisco. Processed data underlying all figures and tables (source data) are given in Supplementary Data Table 1.
Analytical codes for this study are available at https://github.com/Hollenbach-lab/AQP_Paper1_PublicRelease, 10.5281/zenodo.1368551524.