Abstract
Background
Cardiovascular disease and low bone mineral density are major health problems in the elderly. These two conditions are considered independent of each other and age-related diseases. The aim of this study is to investigate the association between low bone mineral density (BMD) and cardiovascular disease (CVD) incidents, and the effect of vitamin D and calcium supplement on the incidence of CVD in patients with low BMD.
Methods
A total of 1047 patients (597 females/450 males) with the age of 65 years and more were diagnosed with osteopenia for 13 years or more. The study also included 220 patients (107 females/113 males) with osteopenia who already took calcium and vitamin D continually since their diagnosis. BMD was measured by dual-energy X-ray absorptiometry. The incidence of any cardiovascular diseases in the study patients and the presence of corresponding risk factors were collected and analyzed.
Results
In both elderly Arab females and males, there was an association between total hip and femoral neck BMD and the possibility to have CVD. On the other hand, the results showed that patients who use calcium and vitamin D supplements showed a significant reduction in the incidence of CVD comparing to the non-treated patients.
Conclusion
Low total hip and femoral neck BMD were associated with a higher chance to have CVD incidents in both elderly Arab males and females; moreover, calcium and vitamin D supplements have a possible protective role in reducing cardiovascular disease in elderly patients with osteopenia.
Keywords: Bone mineral density, Calcium, Cardiovascular disease, Dax scan, Vitamin D
Introduction
Cardiovascular diseases (CVD) and low bone mineral density (BMD) are serious health problems that hit many countries and cause significant morbidity and mortality. Both CVD and low BMD often occur concurrently and are associated with shared risk factors such as age, smoking, low physical activity, and hypertension [1]. Due to the life-threatening consequences of both diseases, recurrent prolonged hospitalizations may be needed, which impose heavy burden on the healthcare system of many countries.
Although CVD and low BMD are thought initially as unrelated diseases, several studies investigated the possible relationship between the two conditions. Many elderly patients who were diagnosed to have heart disease found to have low BMD as one of the contributing risk factors [2–4]. Some studies suggested a noticeable increase in acute myocardial infarction incidents in elderly people who have experienced fractures [5, 6].
Epidemiological studies have found that low BMD predisposes postmenopausal female patients to coronary heart disease and stroke [7]. Moreover, a study conducted by the American Heart Association revealed that 38% of people with osteopenia also have atrial fibrillation [8].
Research suggests a noteworthy link between BMD and an elevated risk of CVD; several studies indicated that low BMD can cause CVD by increasing the tendency to have vascular calcification that leads to narrowing and clogging the lumen of coronary vessels [9, 10]. The increase in the risk of vascular calcification in patients with low BMD contributes to the stiffening of coronary vessels and the development of atherosclerosis [10, 11]. Moreover, fractures resulting from reduced bone density can serve as a warning sign for potential cardiovascular issues mediated by enkindling massive inflammation that can affect the normal heart function [12].
The available literature regarding the relation between low BMD and CVD still has some contradictions regarding the possible relation between the two conditions. Relatively, few studies have addressed the relation between low BMD and the CVD. Diverse ethnicities may influence the relation between low BMD and CVD. Some studies investigated the impact of race on the relationship between BMD and CVD by comparing blacks and whites [13, 14]. The studies found that lower total hip BMD was associated with higher heart failure (HF) risk in white men but lower risk in black men. According to our knowledge, there is no study that described the relationship between low BMD and CVD in the Arab population.
Calcium and vitamin D play an important role in mineral homeostasis and the maintenance of skeletal health. Calcium and vitamin D supplements have been widely used for fracture prevention in elderly populations. Many trials have studied the effectiveness and cardiovascular safety of calcium and vitamin D supplementation, with disparate results [15, 16]. In this study, we evaluated the effect of calcium and vitamin D supplements on decreasing the risk of CVD in elderly Arabian patients with low bone mineral density using dual-energy X-ray absorptiometry (DXA). The findings of this study can be used for future comparative studies by providing an evidence that can confirm or disprove the association between low BMD and CVD.
Materials and methods
Study sample
This retrospective study was approved by the institutional research board at Jordan University of Science and Technology (IRB # 5/135/2020).
The study participants were included from King Abdullah University Hospital. The subjects were 65 years and more who were diagnosed with osteopenia for 13 years or more (from 2011 until 2020) and have complete filed datasets.
Data extraction from the database was performed independently by two researchers. Any disagreement between the two recruited researchers was solved by a third researcher, who also was anonymous to the other two researchers. A total of 1047 (597 females/450 males) subjects were included in the study. Patients who had thyroid gland, parathyroid gland, and adrenal gland disorders were excluded. Patients who had a history of significant bone injury, liver failure, chronic diseases of gastrointestinal tract, cancer or life-threatening illness, diabetes mellitus, and smoking were also excluded from the study. The baseline characteristics of the study group, stratified by gender, are summarized in Table 1.
Table 1.
Clinical characteristics of the study cohort stratified by gender
| Characteristics | Females (n = 597) | Males (n = 450) | P-value |
|---|---|---|---|
| Age (years) | 76.2 ± 6.1 | 77.8 ± 5.9 | 0.2 |
| Body mass index (kg/m2) | 26.6 ± 4.6 | 25.3 ± 3.4 | < 0.05 |
| Systolic blood pressure (mm Hg) | 134 ± 22 | 131 ± 18 | < 0.05 |
| Low-density lipoprotein (mg/dL) | 132 ± 35 | 125 ± 28 | < 0.05 |
| High-density lipoprotein (mg/dL) | 55 ± 12 | 46 ± 9 | < 0.05 |
| Triglycerides (mg/dL) | 125 (80–171) | 115 (73–142) | < 0.05 |
| Lipid-lowering medication, number (%) | 134 (22.4%) | 121 (26.8%) | 0.493 |
| Aspirin, No. (%) | 228 (38.1%) | 184 (40.8%) | 0.329 |
| Prevalent coronary heart disease, No. (%) | 91 (15.2%) | 127 (28.2%) | < 0.05 |
| Prevalent stroke/transient ischemic attack, No. (%) | 51 (8.5%) | 41 (9.1%) | 0.493 |
| Prevalent peripheral arterial disease, No. (%) | 62 (10.3%) | 46 (10.2%) | 0.641 |
| Prevalent atrial fibrillation, No. (%) | 31 (5.19%) | 29 (6.4%) | 0.519 |
| Serum creatinine (mg/dL) | 0.9 ± 0.3 | 1.1 ± 0.2 | 0.172 |
| Estimated glomerular filtration rate (cystatin-based) (mL/min per 1.73 m2) | 84 ± 18 | 81 ± 15 | 0.227 |
| C-reactive protein (mg/L) | 2.4 (1.2–5.6) | 1.9 (1–4.2) | |
| Total hip BMD (g/cm2) | 0.7 ± 0.1 | 0.9 ± 0.1 | < 0.001 |
| Femoral neck BMD (g/cm2) | 0.6 ± 0.1 | 0.8 ± 0.1 | < 0.001 |
| Total hip, WHO categories, No. (%) | < 0.001 | ||
| Normal | 223 (37.3%) | 200 (44.4%) | |
| Osteoporosis | 374 (62.6%) | 250 (55.6%) | |
| Femoral neck, WHO categories, No. (%) | < 0.001 | ||
| Normal | 268(44.8%) | 207 (46%) | |
| Osteoporosis | 329 (55.1%) | 243 (54%) |
Data for continuous variables are presented as mean ± SD
BMD bone mineral density, WHO World Health Organization
Patients with osteopenia used a bisphosphonate or denosumab as a treatment course. The number of osteopenia patients who were on calcium and vitamin D3 medications in addition to bisphosphonate or denosumab was 220 patients, and the subjects were 65 years and more and diagnosed with osteopenia for 10 years or more. The records of those patients showed that they were on calcium (1000–1200 mg daily) and vitamin D3 (600–800 IU daily) since their diagnosis. Those patients, who underwent treatment, were followed up by performing regular dual-energy X-ray absorptiometry (DXA) scans. Among the 220 patients, there were 107 females (48 females were diagnosed with femoral neck osteopenia, 59 females with total hip osteopenia) and 113 males (52 males were diagnosed with femoral neck osteopenia, 61 males with total hip osteopenia).
Measurement of bone mineral density
Dual-energy X-ray absorptiometry (DXA) scans were performed (Hologic 4500A, version 9.03; Hologic, Inc., Waltham, MA, USA) to measure bone mineral density (BMD), using the array beam mode. Scans were read blindly at the King Abdullah University Hospital using Hologic software version 9.03. The primary measures of interest were BMD of the total hip and BMD of the femoral neck. DXA scan protocols that were used for all subjects were identical.
For the DXA scan results, the World Health Organization classification chart was used for osteopenia using a T-score. The T-scores were categorized as follows: (i) normal, when the T-score is − 0.99 or above; (ii) osteopenia, the T-score between − 1 and − 2.5; (iii) osteoporosis, the T-score below − 2.5.
The incidence of cardiovascular diseases in osteopenia patients
The recruited expert panel reviewed all relevant CVD data in the files of the patients included in the study. The history, physical examination, report of chest radiography, and medication usage were assessed. In this study, the incidence of cardiovascular disease was defined as the initiation of one or more of the heart conditions after being diagnosed with osteopenia. The CVDs included in this study are coronary heart disease, stroke/transient ischemic, peripheral arterial disease, and atrial fibrillation. For atrial fibrillation confirmation, the experts combined the annual ECGs performed for the recruited patients upon hospitalization. Hospital records for the recruited patients were collected, abstracted, and reviewed by the attending cardiologist.
Assessment and definition of baseline variables
We obtained the following baseline variables: body weight, height, systolic blood pressure, smoking, glucose level, C-reactive protein, and treatment with antihypertensive medication from the patient’s medical records.
Smoking increases mortality from all causes and has a crucial role in atherosclerotic cardiovascular disease [17], so we excluded the smoker patients from this study. Moreover, patients with diabetes mellitus who were prescribed hypoglycemic medications were excluded as well.
Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Patients with a BMI above 30 are excluded from the study to minimize the influence of obesity-related confounding variables, which is known as a risk factor for CVD and bone disorders. Moreover, patients using antihypertensive medications were excluded from the study populations. Cystatin C was used to calculate the estimated glomerular filtration rate (eGFR) using standard methods.
Statistical analysis
Baseline characteristics and BMD measures of groups with or without incident cardiovascular disease in each sex group were performed using t-tests for unequal variances and the chi-square test for categorical and continuous variables. Total hip and neck BMD were evaluated both as continuous and categorical variables.
Post hoc pair-wise tests were planned to ascertain any group differences. Statistical significance was tested at the level of P = 0.05. The data are presented as mean ± standard error of the mean (SEM).
Results
The association between the baseline variables and BMD
This study included 1047 subjects with a mean age of 77 ± 6 years. Of these, 57% were women and 43% were men. Females in this study were younger in comparison to males and had more prevalent use of lipid-lowering drugs and aspirin and had higher eGFR levels and triglycerides (Table 1).
Results describing the association between baseline variables and total hip BMD and femoral neck BMD are summarized in Tables 2 and 3.
Table 2.
The association of continuous total hip BMD with baseline characteristics of the study cohort
| Characteristics | Total hip BMD | |||||||
|---|---|---|---|---|---|---|---|---|
| Females (n = 597) | Males (n = 450) | |||||||
| No. | Correlation coefficient | Mean ± SD | P-value | No. | Correlation coefficient | Mean ± SD | P-value | |
| Age (year) | 597 | − 0.32 | < 0.05 | 450 | − 0.21 | < 0.05 | ||
| Body mass index (kg/m2) | 597 | 0.52 | < 0.05 | 450 | 0.36 | < 0.05 | ||
| Systolic blood pressure (mm Hg) | 597 | 0.03 | 0.505 | 450 | 0.03 | 0.510 | ||
| Low-density lipoprotein (mg/dL) | 523 | 0.001 | 0.982 | 425 | 0.03 | 0.524 | ||
| High-density lipoprotein (mg/dL) | 596 | ˗0.05 | 0.149 | 450 | 0.01 | 0.892 | ||
| Triglycerides (mg/dL) | 597 | 0.12 | 0.004 | 450 | 0.09 | 0.04 | ||
| Lipid-lowering medication | ||||||||
| Yes | 134 | 0.75 ± 0.14 | 0.313 | 121 | 0.95 ± 0.15 | 0.332 | ||
| No | 463 | 0.77 ± 0.15 | 329 | 0.92 ± 0.18 | ||||
| Aspirin | ||||||||
| Yes | 228 | 0.75 ± 0.15 | 0.457 | 184 | 0.95 ± 0.16 | 0.742 | ||
| No | 369 | 0.75 ± 0.13 | 266 | 0.95 ± 0.16 | ||||
| Prevalent coronary heart disease | ||||||||
| Yes | 91 | 0.75 ± 0.14 | 0.771 | 127 | 0.95 ± 0.16 | 0.492 | ||
| No | 506 | 0.75 ± 0.15 | 323 | 0.95 ± 0.18 | ||||
| Prevalent stroke/transient ischemic attack | ||||||||
| Yes | 51 | 0.75 ± 0.15 | 0.928 | 41 | 0.95 ± 0.18 | 0.255 | ||
| No | 546 | 0.75 ± 0.14 | 409 | 0.95 ± 0.16 | ||||
| Prevalent peripheral arterial disease | ||||||||
| Yes | 508 | 0.75 ± 0.14 | 0.937 | 367 | 0.96 ± 0.19 | 0.167 | ||
| No | 89 | 0.74 ± 0.15 | 83 | 0.91 ± 0.16 | ||||
| Prevalent atrial fibrillation | ||||||||
| Yes | 31 | 0.69 ± 14 | 0.011 | 29 | 0.95 ± 0.18 | 0.968 | ||
| No | 566 | 0.75 ± 15 | 421 | 0.95 ± 0.16 | ||||
| Creatinine (mg/dL) | 595 | 0.07 | 0.062 | 426 | − 0.01 | 0.914 | ||
| Estimated glomerular filtration rate (cystatin-based) (mL/min per 1.73 m) | 586 | 0.06 | 0.086 | 439 | 0.09 | 0.033 | ||
| C-reactive protein (mg/L) | 595 | 0.11 | 0.004 | 450 | 0.02 | 0.734 | ||
Table 3.
The association of continuous femoral neck BMD with baseline characteristics of the study cohort
| Characteristics | Total hip BMD | |||||||
|---|---|---|---|---|---|---|---|---|
| Females (n = 597) | Males (n = 450) | |||||||
| No. | Correlation coefficient | Mean ± SD | P-value | No. | Correlation coefficient | Mean ± SD | P-value | |
| Age (year) | 195 | − 0.30 | < 0.05 | 152 | − 0.21 | < 0.05 | ||
| Body mass index (kg/m2) | 195 | 0.49 | < 0.05 | 152 | 0.32 | < 0.05 | ||
| Systolic blood pressure (mm Hg) | 195 | 0.02 | 0.505 | 152 | 0.02 | 0.510 | ||
| Low-density lipoprotein (mg/dL) | 523 | ˗0.01 | 0.8 | 425 | 0.02 | 0.62 | ||
| High-density lipoprotein (mg/dL) | 596 | ˗0.03 | 0.42 | 450 | 0.01 | 0.812 | ||
| Triglycerides (mg/dL) | 597 | 0.06 | 0.06 | 450 | 0.04 | 0.831 | ||
| Lipid-lowering medication | ||||||||
| Yes | 134 | 0.65 ± 0.12 | 0.77 | 121 | 0.78 ± 0.17 | 0.458 | ||
| No | 463 | 0.65 ± 0.15 | 329 | 0.77 ± 0.15 | ||||
| Aspirin | ||||||||
| Yes | 65 | 0.64 ± 0.15 | 0.457 | 184 | 0.78 ± 0.16 | 0.322 | ||
| No | 130 | 0.65 ± 0.12 | 266 | 0.79 ± 0.16 | ||||
| Prevalent coronary heart disease | ||||||||
| Yes | 91 | 0.64 ± 0.12 | 0.921 | 126 | 0.78 ± 0.16 | 0.672 | ||
| No | 506 | 0.64 ± 0.15 | 324 | 0.78 ± 0.17 | ||||
| Prevalent stroke/transient ischemic attack | ||||||||
| Yes | 22 | 0.64 ± 0.12 | 0.827 | 41 | 0.78 ± 0.18 | 0.255 | ||
| No | 575 | 0.64 ± 0.14 | 409 | 0.76 ± 0.16 | ||||
| Prevalent peripheral arterial disease | ||||||||
| Yes | 508 | 0.65 ± 0.14 | 0.826 | 367 | 0.76 ± 0.18 | 0.651 | ||
| No | 89 | 0.65 ± 0.12 | 83 | 0.77 ± 0.15 | ||||
| Prevalent atrial fibrillation | ||||||||
| Yes | 31 | 0.62 ± 12 | 0.013 | 34 | 0.77 ± 0.17 | 0.446 | ||
| No | 566 | 0.65 ± 13 | 416 | 0.74 ± 0.16 | ||||
| Creatinine (mg/dL) | 595 | 0.07 | 0.062 | 426 | 0.002 | 0.914 | ||
| Estimated glomerular filtration rate (cystatin-based) (mL/min per 1.73 m) | 586 | 0.06 | 0.115 | 439 | 0.08 | 0.123 | ||
| C-reactive protein (mg/L) | 595 | 0.14 | 0.002 | 450 | 0.04 | 0.371 | ||
In both sexes, all BMD measurements were negatively correlated with age. However, the BMD measurements showed a statistically significant association with a high triglyceride level. The incidence of atrial fibrillation was more frequent in patients with lower total hip BMD. Males with high total hip BMD were found to have a higher eGFR level.
The incidence of cardiovascular disease
The filed database of the patients recruited in the current study incorporated all the medical information, follow-up visits, regular medical examinations, images, and regular DXA scans after being diagnosed with osteopenia.
The records revealed 478 incidents of cardiovascular disease (CVD), with 235 (49.2%) occurring in females and 243 (50.8%) occurring in males. The number and incidence rates of CVD in relation to BMD categories were summarized in Table 4. The highest event rates occurred in females with osteopenia of the total hip. The analysis showed that the incidence of CVD significantly increased in patients with total hip osteopenia comparing to individuals with normal BMD. Moreover, the incidence of CVD significantly increased in patients with femoral neck osteopenia comparing to individuals with normal BMD. The results also showed that patient gender did not affect this CVD incidence related to BMD abnormalities.
Table 4.
CVD events with normal BMD and osteopenia stratified by sex
| CVD event | Female | Male |
|---|---|---|
| Total hip: No. (incidence rate) | ||
| Normal BMD | 55 (24.6%) | 31 (15.5%) |
| Osteopenia | 180 (48.1%)* | 55 (22%)* |
| Femoral neck (incidence rate) | ||
| Normal BMD | 33 (12.3%) | 31 (14.9%) |
| Osteopenia | 98 (29.7%)* | 51 (20.9%)* |
BMD bone mineral density, CVD cardiovascular disease
*P < 0.05 (t-test). The significant increase was between normal and osteopenia
Based on the above findings, there was an obvious association between BMD and CVD incidents in both males and females.
Correlation of low level of daily vitamin D and calcium with the risk of cardiovascular diseases
There is no cure for osteopenia. The goal of osteopenia treatment is to prevent its progression to osteoporosis which increases the fracture risk. The main treatment for osteopenia is bisphosphonate or denosumab with or without calcium and vitamin D3 supplements.
In this study, 220 patients (107 females, 113 males) were diagnosed with osteopenia and were on daily calcium and vitamin D3 supplements since their diagnosis. The data showed that the treated patients that used calcium and vitamin D3 supplements had a significant reduction in the incidence of CVD comparing to the treated patients that did not use calcium and vitamin D3 supplements. Moreover, the incidence of CVD in the treated patients was similar to the healthy individuals (Fig. 1).
Fig. 1.
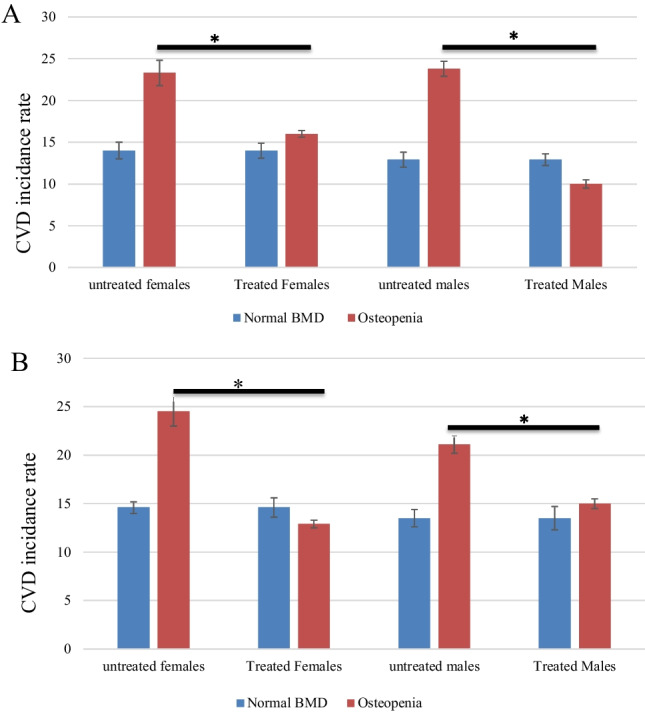
Correlation of low level of daily vitamin D and calcium with the risk of CVD events. A The comparison of the incidence of CVD between patients treated with calcium and vitamin D and the non-treated patients with normal BMD. Total hip osteopenia, a significant increase in the incidence of CVD, was in the non-treated group compared to the treated group. B The comparison of the incidence of CVD between patients treated with calcium and vitamin D and the non-treated patients with normal BMD. Femoral neck osteopenia, again, a significant increase in the incidence of CVD, was in the non-treated group compared to the treated group. Each column represents the percentage of incidence of CVD ± standard error of the mean (SEM). *P < 0.05 (t-test)
Discussion
Recent studies have shed light on a potential relationship between low BMD and CVD, suggesting that the two conditions may be interconnected [1, 3, 18]. Low BMD and heart diseases may share common risk factors such as aging, sedentary lifestyle, poor diet, and certain medical conditions. Moreover, evidence has suggested that individuals with low BMD are susceptible to CVD [19, 20]. In this study, we exclude the major risk factors that are commonly associated with CVD which made this study to focus more readily on the association between BMD and CVD.
In this study, we evaluated the possible association between low BMD and CVD using a cohort of the Arab population who underwent a DAX scan. We found that BMD measurements can be used as an indicator for future CVD in both sexes, particularly HF. The subjects selected for this study did not have a history of CVD before being diagnosed with osteopenia, and they had CVD after a while of having osteopenia onset.
The outcomes of this study revealed a possible relation between low BMD and the incidence of CVD, and it is considered a non-gender-related correlation. An earlier observation by Farhat et al. studied the incidence of CVD and reported an inverse association between BMD and CVD in white males [21] but not in white females. The association between low BMD and CVD in males was also reported by Laroche et al. who recruited 18 males with asymmetrical symptomatic peripheral arterial disease. Laroche et al. showed that the BMD was significantly lower in the affected leg in comparison to the unaffected leg [22]. The possible explanation for the relationship between BMD and the incidence of HF in males can be due to the intense inflammation and high cytokine levels such as IL-6, TNF-α, and oxLDL that accompany the osteopenia in males [23]. As patient age increases, the decrease in the level of endogenous sex steroid hormones leads to an imbalance in the calciferol endocrine system [24, 25]. Patients with osteopenia are found to have sometimes a high level of PTH as a part of the pathological mechanism that causes bone thinning. Elevated PTH in osteopenia have been found to increase the incidence of HF particularly in males [26]. These suggested mechanisms are plausible, and future investigations are needed to elucidate the validity of these explanations.
In females, we observed an association between BMD and incident CVD, and this also was in consistent with Fohtung et al., who found an insignificant impact of low BMD on triggering HF incidents in females [13]. All women were over the age of 60 and suffering from menopause, which being suggested as a dominant risk factor for osteopenia.
Many patients with low BMD suffer from calcium and vitamin D deficiency. Calcium and vitamin D supplementations were effective in reducing osteoporotic fractures. Studies showed a reduction in the osteoporotic fractures between 25 and 70% with a daily intake of approximately 1000 mg/day of elemental calcium [27, 28]. Another study showed that calcium repletion with marginal vitamin D levels in study subjects resulted in bone sparing [27]. Therefore, optimal intake of vitamin D is influenced by calcium intake [27]. Vitamin D has been extensively studied regarding its impact on fracture risk reduction. In fact, vitamin D deficiency has been associated with a greater incidence of hip fracture in many populations [29]. Vitamin D is required for calcium absorption, and its deficiency increases the rates of bone loss which can increase the risk of fracture [28]. Studies also showed that concomitant supplementation of vitamin D and calcium may decrease fat deposition and reduce the risk of cardiovascular and metabolic abnormalities [30]. Moreover, hypovitaminosis D can relate to cardiac hypertrophy and heart failure [30]. Vitamin D deficiency leads to the development of arterial hypertension, cardiac hypertrophy, and atherosclerosis in animal model [31]. Vitamin D is a novel endocrine regulator of the renin-angiotensin system, as low vitamin D can overstimulate the renin-angiotensin system which causes cardiovascular injuries and HF [32]. Calcium associated with vitamin D also plays a role in reducing the risk of CVD [33]. Also, inadequate amount of calcium availability can negatively affect the intracellular signaling and smooth muscle contractions in the chambers of the heart which can lead to heart failure and cardiovascular diseases [15].
The intake of calcium supplements alone is still controversial as some studies showed that increasing calcium intake will cause an elevation in blood calcium level and the chance of calcium deposition in the lumen of the blood vessels causing their narrowing and stiffness [34]. Optimal bone and cardiovascular system healthiness requires intaking both dietaries, calcium and vitamin D, together.
Certain limitations of this study should be noted. Information regarding the physical activity and the possibility that the recruited females were on systemic estrogen use was missing from patient files. Estrogen is playing an important role in keeping the bones strong and healthy; thus, estrogen deficiency can be related to the onset of osteopenia in both sexes and increases the CVD susceptibility.
Conclusion
Elderly Arab of both sexes suffering from osteopenia is at higher risk to develop CVD that can lead to HF. Moreover, a low level of daily vitamin D and calcium could protect from the incidence of future CVD in patients suffering from low BMD. The association between high calcium intake and CVD risk needs to be assessed further in additional studies.
Author contribution
Conceptualization: RRK. Methodology: EAE., AA., and RMA. Data collection: RRK and HSA. Statistical analysis: RRK, AA, EAE, and RMA. Data interpretation: RRK, A.A., and RMA. Manuscript preparation: RRK and HSA. Funding acquisition: RRK. Writing—review and editing: RRK, AA, and RMA. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding provided by the Qatar National Library. This study is funded by a grant from the Deanship of Scientific Research, Yarmouk University, Jordan (Grant # 29/2020). The publication of this article was funded by the Qatar National Library.
Availability of data and materials
The data that support the findings in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Research Board at Jordan University of Science and Technology (IRB No: 5/135/2020). All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from study participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khandkar C, Vaidya K, Karimi Galougahi K, Patel S (2021) Low bone mineral density and coronary artery disease: A systematic review and meta-analysis. Int J Cardiol Heart Vasc 23(37):100891. 10.1016/j.ijcha.2021.100891. PMID: 34746361; PMCID: PMC8554269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Truong TH, Nguyen M-NT, Kim NT et al (2022) Low bone mineral density and its related factors in adults with congenital heart disease in Vietnam: a cross‐sectional study. Health Sci Rep [Internet]. Available from: https://api.semanticscholar.org/CorpusID:251457858 [DOI] [PMC free article] [PubMed]
- 3.Fathala AL, Alkulaybi S, Khawaji A et al (2021) The association between low bone mineral density and coronary artery calcification in osteoporotic and non-osteoporotic patients in a tertiary center in Saudi Arabia. Ann Saudi Med 41:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Huang Y (2023) Association between bone mineral density and cardiovascular disease in older adults. Front Pub Health [Internet]. Available from: https://api.semanticscholar.org/CorpusID:259224131 [DOI] [PMC free article] [PubMed]
- 5.Fisher A, Srikusalanukul W, Davis M et al (2013) Cardiovascular diseases in older patients with osteoporotic hip fracture: prevalence, disturbances in mineral and bone metabolism, and bidirectional links. Clin Interv Aging 8:239–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Liu Y, Fu M et al (2023) Characteristics of preoperative acute myocardial infarction in elderly hip fracture patients and construction of a clinical prediction model: a retrospective cohort study. Clin Interv Aging 18:1985–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatta L, Cepelis A, Vikjord SAA et al (2021) Bone mineral density and risk of cardiovascular disease in men and women: the HUNT study. Eur J Epidemiol 36:1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H-K, Liu PP-S, Hsu J-Y et al (2020) Risk of osteoporosis in patients with atrial fibrillation using non–vitamin K antagonist oral anticoagulants or warfarin. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease [Internet]. Available from: https://api.semanticscholar.org/CorpusID:210132460 [DOI] [PMC free article] [PubMed]
- 9.Lello S, Capozzi A, Scambia G (2015) Osteoporosis and cardiovascular disease: an update. null 31:590–594 [DOI] [PubMed] [Google Scholar]
- 10.Lampropoulos CE, Papaioannou I, D’Cruz DP (2012) Osteoporosis—a risk factor for cardiovascular disease? Nat Rev Rheumatol 8:587–598 [DOI] [PubMed] [Google Scholar]
- 11.García-Gómez MC, Vilahur G (2020) Osteoporosis and vascular calcification: a shared scenario. Clinica e investigacion en arteriosclerosis: publicacion oficial de la Sociedad Espanola de Arteriosclerosis [Internet]. Available from: https://api.semanticscholar.org/CorpusID:195191111 [DOI] [PubMed]
- 12.West SL, O’Donnell E (2018) Cardiovascular disease and bone loss—new research in identifying common disease pathophysiologies and predictors. Available from: https://api.semanticscholar.org/CorpusID:212501056
- 13.Fohtung RB, Brown DL, Koh WJH et al (2017) Bone mineral density and risk of heart failure in older adults: the Cardiovascular Health Study. J Am Heart Assoc 6:e004344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall LM, Zmuda JM, Chan BK et al (2008) Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res 23:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo M, Anderson ME (2013) Mechanisms of altered Ca2+ handling in heart failure. Circ Res 113:690–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scragg R, Stewart AW, Waayer D et al (2017) Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial. JAMA Cardiology 2:608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallucci G, Tartarone A, Lerose R et al (2020) Cardiovascular risk of smoking and benefits of smoking cessation.J Thorac Dis12(7). Available from: https://jtd.amegroups.com/article/view/37685. Cited 1 Jan 2020 [DOI] [PMC free article] [PubMed]
- 18.Park J, Yoon YE, Kim KM et al (2021) Prognostic value of lower bone mineral density in predicting adverse cardiovascular disease in Asian women. Heart 107:1040–1046 [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Ma S, Chen L et al (2023) Association between osteoporosis and cardiovascular disease in elderly people: evidence from a retrospective study. PeerJ [Internet]. Available from: https://api.semanticscholar.org/CorpusID:266131660 [DOI] [PMC free article] [PubMed]
- 20.Fohtung RB, Brown DL, Koh WJH et al (2017) Bone mineral density and risk of heart failure in older adults: the Cardiovascular Health Study. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease [Internet]. Available from: https://api.semanticscholar.org/CorpusID:3732837 [DOI] [PMC free article] [PubMed]
- 21.Farhat GN, Newman AB, Sutton-Tyrrell K et al (2007) The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int 18:999–1008 [DOI] [PubMed] [Google Scholar]
- 22.Laroche M, Moulinier L, Léger P et al (2003) Bone mineral decrease in the leg with unilateral chronic occlusive arterial disease. Clin Exp Rheumatol 21(1):103–106 [PubMed] [Google Scholar]
- 23.Huang Z, Xu Z, Wan R et al (2023) Associations between blood inflammatory markers and bone mineral density and strength in the femoral neck: findings from the MIDUS II study. Sci Rep [Internet]. Available from: https://api.semanticscholar.org/CorpusID:259308356 [DOI] [PMC free article] [PubMed]
- 24.Janjuha R, Bunn DK, Hayhoe RPG et al (2020) Effects of dietary or supplementary micronutrients on sex hormones and IGF-1 in middle and older age: a systematic review and meta-analysis. Nutrients [Internet]. Available from: https://api.semanticscholar.org/CorpusID:218856552 [DOI] [PMC free article] [PubMed]
- 25.van den Beld AW, Kaufman J-M, Zillikens C et al (2018) The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol 6(8):647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolaszko A, Nowalany-Kozielska E, Ceranowicz P et al (2018) The role of parathyroid hormone and vitamin D serum concentrations in patients with cardiovascular diseases, 2018. In Shi Z (ed.), Disease Markers, (pp 5287573) [DOI] [PMC free article] [PubMed]
- 27.Sunyecz JA (2008) The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag 4:827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voulgaridou G, Papadopoulou SK, Detopoulou P et al (2023) Vitamin D and calcium in osteoporosis, and the role of bone turnover markers: a narrative review of recent data from RCTs. Diseases [Internet]. Available from: https://api.semanticscholar.org/CorpusID:256757290 [DOI] [PMC free article] [PubMed]
- 29.Martinis MD, Allegra A, Sirufo MM et al (2021) Vitamin D Deficiency, osteoporosis and effect on autoimmune diseases and hematopoiesis: a review. Int J Mol Sci[Internet]. Available from: https://api.semanticscholar.org/CorpusID:237316032 [DOI] [PMC free article] [PubMed]
- 30.Condoleo V, Pelaia C, Armentaro G et al (2021) Role of vitamin D in cardiovascular diseases. Endocrines 2:417–426 [Google Scholar]
- 31.Wang TJ (2016) Vitamin D and cardiovascular disease. Annu Rev Med 67:261–272 [DOI] [PubMed] [Google Scholar]
- 32.Ferder M, Inserra F, Manucha W et al (2013) The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol 304(11):C1027–C1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong SH, Kim JH, Hong AR et al (2017) Dietary calcium intake and risk of cardiovascular disease, stroke, and fracture in a population with low calcium intake. Am J Clin Nutr 106:27–34 [DOI] [PubMed] [Google Scholar]
- 34.Tankeu AT, Ndip Agbor V, Noubiap JJ (2017) Calcium supplementation and cardiovascular risk: a rising concern. J Clin Hypertens 19:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings in this study are available from the corresponding author upon reasonable request.


