Abstract
Although the ultimate target of infection is the central nervous system (CNS), there is evidence that the enteric nervous system (ENS) and the peripheral nervous system (PNS) are involved in the pathogenesis of orally communicated transmissible spongiform encephalopathies. In several peripherally challenged rodent models of scrapie, spread of infectious agent to the brain and spinal cord shows a pattern consistent with propagation along nerves supplying the viscera. We used immunocytochemistry (ICC) and paraffin-embedded tissue (PET) blotting to identify the location and temporal sequence of pathological accumulation of a host protein, PrP, in the CNS, PNS, and ENS of hamsters orally infected with the 263K scrapie strain. Enteric ganglia and components of splanchnic and vagus nerve circuitry were examined along with the brain and spinal cord. Bioassays were carried out with selected PNS constituents. Deposition of pathological PrP detected by ICC was consistent with immunostaining of a partially protease-resistant form of PrP (PrPSc) in PET blots. PrPSc could be observed from approximately one-third of the way through the incubation period in enteric ganglia and autonomic ganglia of splanchnic or vagus circuitry prior to sensory ganglia. PrPSc accumulated, in a defined temporal sequence, in sites that accurately reflected known autonomic and sensory relays. Scrapie agent infectivity was present in the PNS at low or moderate levels. The data suggest that, in this scrapie model, the infectious agent primarily uses synaptically linked autonomic ganglia and efferent fibers of the vagus and splanchnic nerves to invade initial target sites in the brain and spinal cord.
Although the clinical signs and pathological damage are indicative of central nervous system (CNS) disease, the most likely natural portal of entry of nonexperimental transmissible spongiform encephalopathy (TSE) infection is via the gastrointestinal (GI) tract. Bovine spongiform encephalopathy (BSE) and a new variant of Creutzfeldt-Jacob disease (vCJD) are linked (13, 25) and almost certainly entered their hosts via the food chain. However, little is known about how the pathogenic agent enters the GI tract and subsequently spreads to target sites in the CNS.
Evidence from natural (23, 46, 47) and experimental (2, 4, 5, 24, 30, 39) scrapie and BSE (48) implicates the peripheral nervous system (PNS) and the enteric nervous system (ENS) in the spread of the infectious agent to the CNS. In several peripherally infected rodent models of scrapie, including hamster 263K scrapie, the model of oral challenge used in this study, neural targeting of infection shows a pattern consistent with spread along visceral nerves.
A membrane-bound glycoprotein, PrP, is fundamental to the development of scrapie and related diseases (7, 11, 36). PrP mRNA and the normal cellular form of the protein (PrPC) are widely expressed in the CNS (33, 35) and, less abundantly, in a number of adult and embryonic peripheral tissues (6, 15, 35, 41). A partially protease-resistant form of PrP (PrPSc) extracted from infected brains copurifies with infectivity (2, 3, 8, 16, 45). Disease-associated forms of PrP (variously termed abnormal or pathological PrP or PrPSc) accumulate in the CNS and a variety of extraneural tissues during the incubation periods of experimental and naturally occurring TSEs (5, 12, 18, 19, 37, 38, 39). When such disease-associated forms are shown to be associated with infectivity, as is the case in this study (2, 3), they may also be considered surrogate markers for an infectious agent.
Regardless of the initial mechanisms of uptake from extraneural sites, infectivity (29, 31) and pathological PrP (2, 3, 4) are first seen in the hindbrain and spinal cord of the CNS of rodents peripherally infected with scrapie. After oral challenge of hamsters with 263K scrapie, the earliest CNS target sites for pathological PrP deposition are the dorsal motor nucleus of the vagus nerve (DMNV) and the solitary tract nucleus (SN) of the brain and the gray matter of thoracic vertebrae 4 to 9 of the spinal cord (4). Subsequent spread of infection in the spinal cord occurs in both cranial and caudal directions (2, 3, 4, 29, 32), and at the end point of disease, most brain areas and all cervical and thoracic segments of the spinal cord and the corresponding dorsal root ganglion (DRG) contain substantial accumulations of pathological PrP (39). The presence of BSE agent in sensory ganglia of preclinical, orally challenged cows (48) or pathological PrP in sympathetic, sensory (39, 47) and enteric (1, 5, 46) ganglia of scrapie-fed hamsters and sheep with natural scrapie suggests that the PNS and the ENS harbor the infectious agent early in the disease process.
It was previously proposed (4, 39) that the 263K scrapie agent reaches its initial target sites in the CNS by spreading, in a retrograde direction, along autonomic PNS pathways and ganglia supplying the viscera, i.e., along sympathetic and parasympathetic efferents of the splanchnic and vagus nerves (Fig. 1). To investigate this further, we carried out a time course study using our hamster model of 263K oral challenge and immunocytochemistry (ICC) and paraffin-embedded tissue (PET) blotting to identify and compare PrP deposition in the ENS, PNS, and CNS. Specific components of the vagus and splanchnic nerve circuitry were examined in addition to the brain and spinal cord, with which they form neuronal relay circuits. Jejunal and ileal ENS was included, as it forms synaptic links with both nerves. Selective bioassays were also carried out to assess the levels of infectivity in two key PNS components, the cervical vagus nerve and the celiac and mesenteric ganglion complex (CMGC).
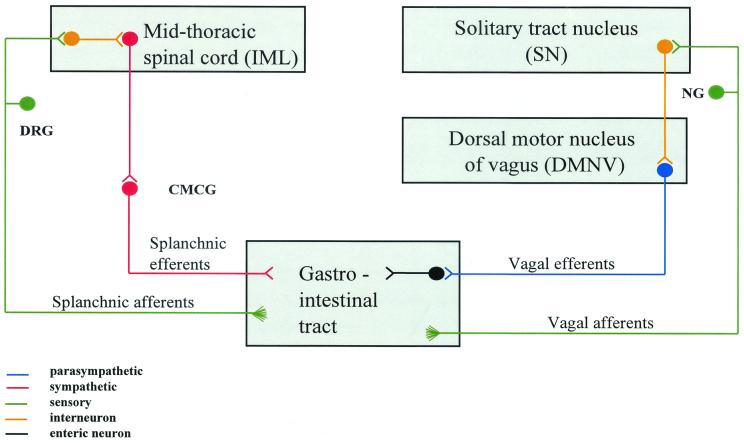
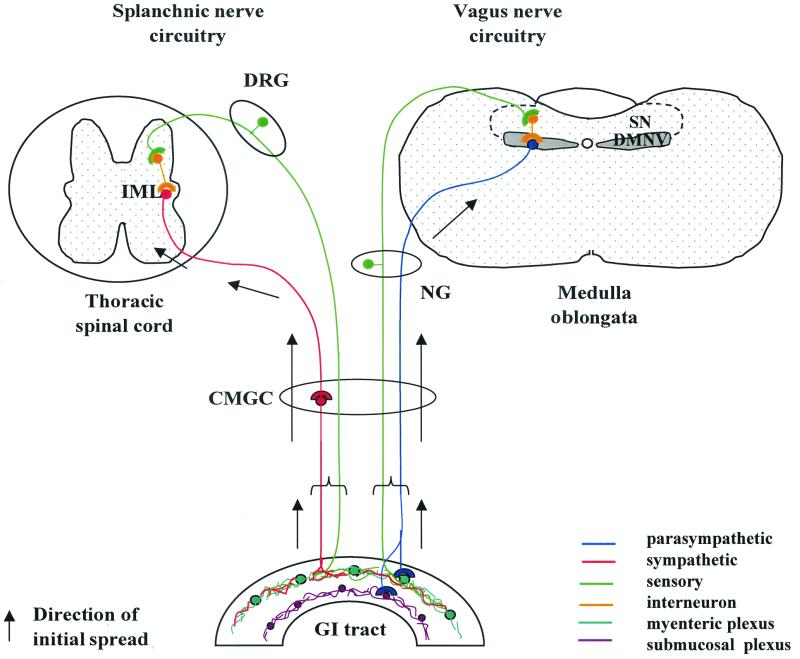
FIG. 1.
Diagrammatic representation of the neural pathways that link the GI tract with the brain and spinal cord. Circuitry has been simplified to show major routes only. The infectious agent may reach the CNS by spreading along either efferent (motor) or afferent (sensory) fibers of vagus or splanchnic nerves. Efferent fibers of the vagus nerve have their nerve cell bodies in the DMNV and synapse with neurons of the ENS in ganglia of the submucosal and myenteric plexuses in the wall of the alimentary canal. The nerve cells of vagus nerve afferent fibers are located in the NG and directly innervate the alimentary canal. These fibers run to the SN, where they synapse with interneurons projecting to the DMNV. The cell bodies of splanchnic nerve efferent fibers are located in the IML and synapse with CMGC neurons that, in turn, innervate the GI tract. Afferent fibers of the splanchnic nerve originate in the DRG, run through the CMGC, and directly innervate target organs such as the alimentary canal. Adapted from reference 39, with permission from Elsevier Science.
Using these approaches, it was possible to identify the temporal sequence and location of PrPSc deposition within the CNS, PNS, and ENS and thereby to define the neuroanatomical pathways involved in early pathogenesis. The findings strongly support and expand our hypothesis that the infectious agent primarily uses autonomic ganglia and efferent fibers of the vagus and splanchnic nerves to reach and invade initial CNS target sites.
MATERIALS AND METHODS
Experimental design.
Outbred Syrian hamsters were fed individual food pellets doused with 100 μl of a 10% hamster brain homogenate from 263K scrapie-infected donors or uninfected controls as previously described (2). For PrP studies, four or five hamsters were humanely sacrificed at specific time points throughout the incubation period: at 56, 62, 69, 76, 83, 90, 97, 104, 111, 118, 126, and 132 days postinfection (dpi) or at the clinical end point of disease (159 ± 4 [mean and standard error] dpi). Two mock-challenged hamsters, similarly fed with normal brain homogenate, were sacrificed at 161 dpi. For bioassay experiments, specimens were taken from five terminally ill and two mock-challenged hamsters.
Histological procedures.
Hamsters were transcardially perfused with periodate-lysine-paraformaldehyde (PLP) (39). The brain and CMGC were removed from all hamsters. Spinal cord with attached DRG, jejunum, ileum (adjacent to the ileocecal sphincter), and left and right nodose ganglion (NG) with attached cervical vagus nerve were included from 69 dpi to the end point of disease. After perfusion, tissues were immersed for 5 h in PLP, transferred to and kept in 70% alcohol for a further 48 h, processed for 6 h in an enclosed tissue processor, and embedded in paraffin wax. Prior to processing and embedding, brains were trimmed coronally into 2-mm-thick slices using a brain slicing mold (SEMAT, St. Albans, United Kingdom). Spinal cords were sliced coronally between nerve roots to provide individual cervical, thoracic, and sometimes lumbar segments with corresponding left and right DRG. Pieces were marked on their cranial surface with India ink, numbered, and processed separately but embedded, in sequence, in one block (49). Sections (6 μm) of brain and spinal cord with attached DRG were taken at 100-μm intervals for PrP ICC, PET blot analysis, and cresyl violet-Luxol fast blue histochemistry. For other tissues these procedures were carried out either with serial sections or with sections taken at 50-μm intervals.
ICC.
Immunostaining was carried out according to the ABC method using mouse anti-hamster PrP monoclonal antibody 3F4 (27) or normal serum and diaminobenzidine to visualize the reaction product. Sections were pretreated with formic acid for 10 min to enhance PrP visualization. Similarly treated sections from mock-challenged hamsters served as control tissue.
PET blotting.
Sections were mounted on nitrocellulose membranes (0.45 μm pore size; Bio-Rad Laboratories, Hemel Hempstead, Herts, United Kingdom), dried flat at room temperature (approximately 2 h), and incubated overnight at 37°C. PET blotting was carried out as described by Schulz-Schaeffer et al. (42). After being washed in Tris-buffered saline (TBS; 10 mM Tris-HCl, 100 mM NaCl [pH 7.8]) with 0.05% Tween 20, sections were digested with 250 μg of proteinase K/ml in a buffer containing 10 mM Tris-HCl (pH 7.8), 100 mM NaCl, and 0.1% (wt/vol) Brij 35 for 8 h at 55°C. Sections were then denatured with guanidine isothiocyanate. Immunostaining was carried out with mouse anti-hamster PrP antibody 3F4 and nitroblue tetrazolium (NBT)–5-bromo-4-chloro-3-indolylphosphate (BCIP) to visualize the reaction product. PET blots were assessed using a dissecting microscope.
Infectivity bioassays.
Vagus nerve and CMGC samples were removed from two terminally ill animals (S1 and S2) and two mock-infected controls (N1 and N2). From another three terminally ill hamsters (S3 to S5) only vagus nerve samples were taken. Left and right portions (approximately 1 cm) of cervical vagus nerves were removed remote from the NG and carefully separated from surrounding tissue. The CMGC was removed attached to the abdominal aorta between the celiac, superior mesenteric, left renal, and right renal arteries. Two 0.5-cm pieces from the abdominal artery cranially and caudally adjacent to the CMGC were also taken. Samples were washed three times in TBS (10 mM Tris-HCl, 133 mM NaCl, pH 7.4), incubated at 37°C for 1.5 h in 200 μl of TBS containing 0.25% (wt/vol) collagenase (Boehringer Mannheim) and 0.025% (wt/vol) CaCl2, and heated to 80°C for 10 min. The volume was adjusted to 500 μl with TBS. Aliquots (50 μl) were inoculated intracerebrally (i.c.) into groups of five recipient hamsters. Infectivity titers were estimated from the incubation periods using dose-response curves as previously described (3). The experiment was terminated at 370 dpi.
RESULTS
Early temporal and spatial deposition of PrPSc in enteric, splanchnic, and vagus nerve relays.
Although the amounts of PrPSc varied between individuals culled at a given time point, the site and sequence of deposition were consistent. PrPSc was found earlier and with more frequency in autonomic (i.e., efferent) components of the circuitry than in sensory components. In the splanchnic nerve circuitry, PrPSc was seen in the CMGC and intermediolateral cell column (IML) of the spinal cord before DRG; in the vagus nerve circuitry, it was seen in the DMNV before NG. The ENS contains autonomic and sensory NS networks. Table 1 summarizes the temporal and spatial deposition of PrPSc in enteric, splanchnic, and vagus nerve relays.
TABLE 1.
Presence of PrPSc in enteric, splanchnic, and vagus nerve relays after ingestion of 263K scrapie
| No. of hamsters affected/no. challenged
| ||||||||
|---|---|---|---|---|---|---|---|---|
| dpia | Enteric ganglia | CMGC | Mid-T IMLb | Mid-T DRGb | DMNV | SN | Right NG | Left NG |
| 56 | ND | 4/4 | ND | ND | 0/4 | 0/4 | ND | ND |
| 62 or 63 | ND | 4/4 | ND | ND | 2/4 | 1/4 | ND | ND |
| 69 | 4/4 | 4/4 | 4/4 | 0/4 | 4/4 | 2/4 | 1/4 | 0/4 |
| 76 | 4/4 | 4/4 | 4/4 | 2/4 | 4/4 | 1/4 | 3/4 | 1/2c |
| 83 | 4/4 | 4/4 | 4/4 | 3/4 | 3/3c | 3/3c | 3/4 | 2/4 |
| 90 | 4/4 | 4/4 | 4/4 | 3/4 | 4/4 | 3/4 | 3/4 | 4/4 |
Early time points are shown (the incubation period was ∼160 dpi).
Mid-T IML and mid-T DRG, = IML from thoracic cord segments 3 to 8 with corresponding DRG; ND, not done.
There were four animals in the group, but the region was present only in the specified number of animals.
(i) ENS.
PrPSc was found from the earliest available time point (69 dpi) to the end point of disease in and around neurons of the myenteric and submucosal plexuses of the ileum and jejunum of all four hamsters (Fig. 2A). As the incubation period progressed, accumulations increased in individual ganglia, and the number of affected ganglia increased.
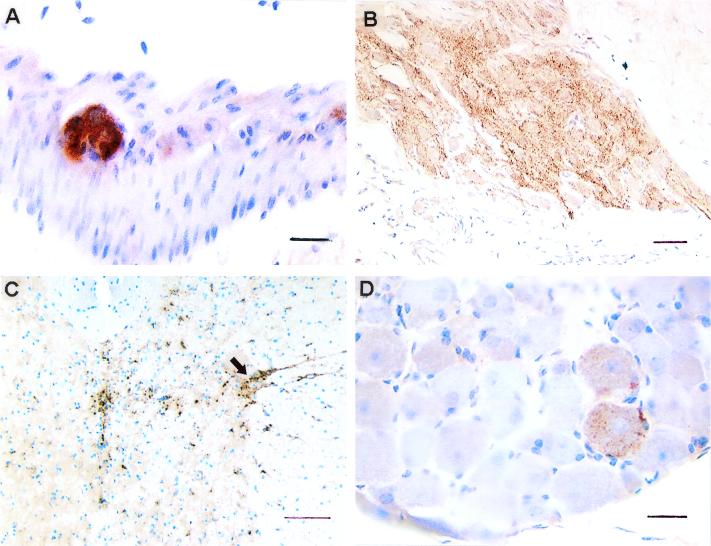
FIG. 2.
PrPSc in the ENS and splanchnic nerve circuitry of the same hamster 90 days after oral challenge with 263K scrapie. (A) Myenteric ganglion. (B) CMGC. (C) Spinal cord segment T5 (arrow indicates IML). (D) DRG from segment T5. PrPSc deposition is already extensive in the ENS and CMGC but minimal in DRG. Scale bars: A and D, 20 μm; B, 50 μm; C, 100 μm.
(ii) Splanchnic nerve circuitry (CMGC-IML-DRG).
PrPSc was observed in the CMGC of all four hamsters at the first time point of 56 dpi (Table 1). Initial deposits were few, but PrPSc accumulated rapidly. By 76 dpi, deposition was substantial, and by 90 dpi, strongly labeled PrPSc was apparent throughout most of the complex (Fig. 2B). In the spinal cord, PrPSc was invariably seen in the IML at 69 dpi, the earliest time point at which the cord was available. Deposition was present in thoracic cord segments 3 to 8, with the largest amounts in the IML of segments 5, 6, and 7 (Fig. 2C). PrPSc was not detected in DRG until 76 dpi (two out of four hamsters). At later time points, deposition remained scant and the amount was always less than that in the corresponding IML (Fig. 2D). In all instances, PrPSc was found in DRG only after accumulations were fairly widespread within the corresponding cord segments.
(iii) Vagus nerve circuitry (DMNV-SN-NG).
In the brain, the first PrPSc deposits were seen in and around neurons of the DMNV at 62 dpi (two out of four brains) and the commissural portion of the SN (one out of four brains). Deposition was minimal in both areas but was greater in the DMNV. At later time points, accumulations were typically greater in the DMNV than in the SN (Fig. 3A to C). PrPSc was either present at low levels or undetected in NG until 90 dpi (Fig. 3D), and even at this time the numbers of positively labeled cells were, at best, only moderate. As with DRG (and in contrast to the CMGC), accumulation was slow, and at the end point of disease, several neurons remained unlabeled (Fig. 3E).
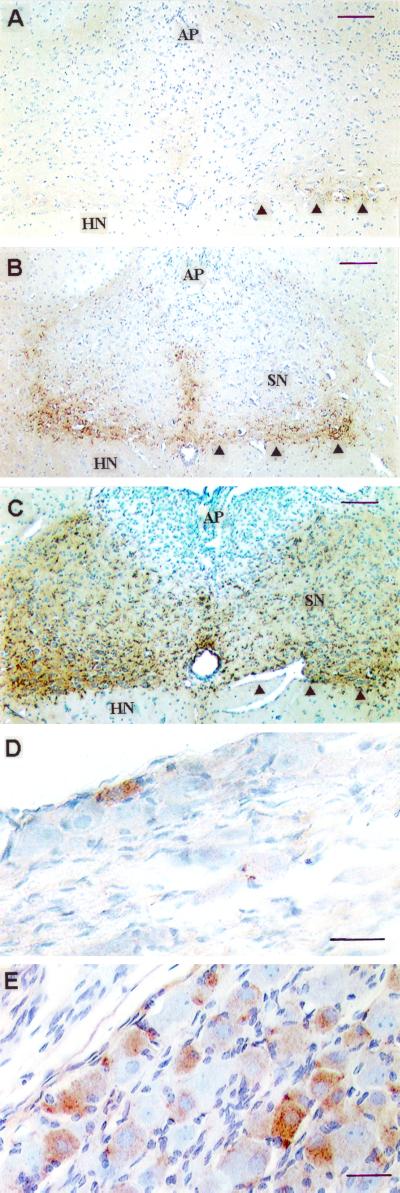
FIG. 3.
PrPSc immunolabeling in the vagus nerve circuitry of hamsters orally challenged with 263K scrapie agent. Sequential accumulation in DMNV-SN (A to C) and NG (D and E) is shown. (A) 76 dpi. (B and C) 90 dpi. The amounts of PrPSc vary between individuals culled at any time point, but the sequence of deposition is consistent. Deposition is more abundant in the DMNV (arrowheads) than in the adjacent SN. The area postrema (AP) and hypoglossal nucleus (HN) are largely unlabeled. (D) Right NG at 90 dpi. (E) Right NG at end stage of disease; several sensory NG neurons remain unlabeled. Scale bars: A to C, 100 μm; D and E, 30 μm.
Subsequent pattern of PrPSc accumulation in the PNS and the CNS.
Subsequent to the IML, PrPSc was observed in the intermediate zone at 69 dpi and then extending from the IML into the adjacent white matter at 76 dpi. Accumulations became progressively greater in the initial sites and generally more widespread within ventral and dorsal gray matter. Concurrently, PrPSc appeared in the IML of thoracic segments located cranial and caudal to those first affected. This pattern of spread continued with increasing incubation, and at late stages of disease, all infected hamsters showed marked granular deposition of PrPSc throughout the brain and entire gray matter “butterfly” of each cervical, thoracic and, where present, lumbar spinal cord segment.
Once accumulation was established in the DMNV and commissural portion of the SN, PrPSc was observed in medial and intermediate regions of the SN and then in other specific brain stem nuclei, notably the medullary reticular formation (76 dpi) and vestibular complex, pons, and red nucleus (83 dpi). Labeling was then rapidly disseminated to several other sites throughout the brain.
Throughout the incubation period, the sequence and spatial precision of targeting were consistent. The final PrPSc distribution pattern was reproduced in all brains, spinal cords, and ganglia and was identical to that described previously for hamsters orally infected with 263K (4, 39). PrPSc was not found in vagus, splanchnic, or spinal nerve roots, apart from a tiny amount in the vagus nerve of one terminally ill hamster. In mock-infected control hamsters, PrPC, which differs in appearance from the characteristic granular forms of PrPSc (4, 5, 38, 39), was seen only in some neuronal cell bodies of the brain and the spinal cord. Staining was absent from tissues when normal serum replaced PrP antibody.
Although cellular detail cannot be resolved with PET blots, aggregations of protease-resistant PrP (PrPSc) were seen at the same sites in all tissues and at all time points that immunostaining was detected in adjacent ICC sections. However, in PET blots, deposits were stained more intensely by the blue-black chromogen and were more easily detected at low power than corresponding ICC-labeled deposits (Fig. 4).
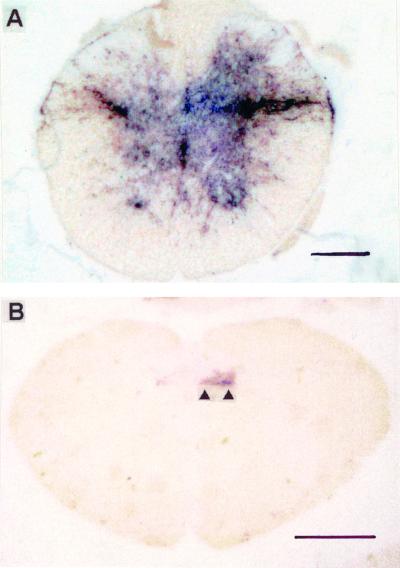
FIG. 4.
Early PrPSc deposition (blue-black) in the IML and DMNV of hamsters orally challenged with 263K scrapie, as demonstrated by PET blotting. (A) IML at 90 dpi. (B) DMNV (arrowheads) at 76 dpi. Specimens represent sections adjacent or nearly adjacent to those in Fig. 2C (A) and 3A (B). Scale bars: A, 200 μm; B, 1 mm.
Scrapie infectivity in the vagus and splanchnic nerve circuitry.
Two PNS components, the cervical vagus nerve and the CMGC, were analyzed by bioassays for the presence of infectivity. Samples of the vagus nerve were excised from a position remote from NG to avoid the inclusion of ganglion cell bodies.
The results for the vagus nerve samples are summarized in Table 2. Mortality and incubation time for the recipients in the bioassays demonstrated a low but consistent presence of infectivity in the cervical vagus nerve trunks. The estimated amount of infectivity was approximately 102 50% i.c. infective doses (ID50i.c.), corresponding to about 105 ID50i.c. per g of tissue.
TABLE 2.
Detection of infectivity in cervical trunks of the vagus nerve by bioassaysa
| Donor | Vagus nerve sample | Incubation time (days)
|
Estimated titer (ID50i.c./sample)b | |
|---|---|---|---|---|
| Range | Mean ± SE | |||
| S1 | Left | 119–292 | 162 ± 30 | 101.3 |
| Right | 117–155 | 126 ± 7 | 102.7 | |
| S2 | Left | 117–239 | 144 ± 22 | 101.8 |
| Right | 117–180 | 134 ± 11 | 102.3 | |
| S3 | Left | 120–260 | 151 ± 25 | 101.6 |
| Right | 131–222 | 167 ± 18 | 101.3 | |
| S4 | Left | 131–183 | 159 ± 10 | 101.5 |
| Right | 127–152 | 139 ± 4 | 102.0 | |
| S5 | Left | 124–242 | 153 ± 20 | 101.6 |
| Right | 145–274 | 190 ± 21 | <101.2 | |
Aliquots (50 μl) of homogenized left and right vagus nerves from terminally ill hamsters orally challenged with 263K were inoculated i.c. into groups of five recipients. Mortality was 100%. Recipients similarly inoculated with vagus nerves from donors previously orally mock infected with normal brain homogenate showed no clinical signs of scrapie. The experiment was terminated at 370 dpi.
Total amount of infectivity in one equivalent of excised tissue, i.e., the entire donor sample, calculated by applying mean incubation time to a dose-response curve.
Infectivity levels in the two CMGC samples (Table 3) were higher (approximately 103 and 104 ID50i.c.), even though the CMGC represented only a minor constituent of the homogenized tissue samples. Artery samples were located cranially or caudally adjacent to the CMGC. The trace levels of infectivity detected for these control specimens probably originated from residual CMGC nervous tissue.
TABLE 3.
Detection of infectivity in the CMGC and adjacent parts of the abdominal aortaa
| Donor | Sample | Mortality in bioassay | Incubation (days)
|
Estimated titer (ID50i.c./sample)b | |
|---|---|---|---|---|---|
| Range | Mean ± SE | ||||
| S1 | CMGC | 5/5 | 100–107 | 104 ± 1 | 104.1 |
| Cran. A. | 1/5 | 318 | |||
| Caud. A. | 1/5 | 156 | |||
| S2 | CMGC | 5/5 | 107–128 | 118 ± 4 | 103.1 |
| Cran. A. | 1/5 | 230 | |||
| Caud. A. | 5/5 | 120–240 | 171 ± 24 | 101.2 | |
Aliquots (50 μl) of homogenized CMGC and cranially (Cran.) or caudally (Caud.) adjacent artery (A.) from terminally ill donors orally challenged with 263K were inoculated i.c. into groups of five recipients. Mortality is given as number of hamsters that succumbed to infection/number challenged. Recipients similarly inoculated with CMGC from donors previously orally mock infected with normal brain homogenate showed no clinical signs of scrapie. The experiment was terminated at 370 dpi.
Total amount of infectivity in one equivalent of excised tissue, i.e., in the entire donor sample, calculated by applying mean incubation time to a dose-response curve.
DISCUSSION
We used ICC, PET blotting, and selective infectivity assays in a time course study to determine the temporal sequence and location of scrapie infection in the ENS, splanchnic and vagal PNS, and CNS of hamsters after oral challenge with scrapie strain 263K. While bioassays provide the gold standard for detection of the scrapie agent per se, ICC and PET blot analyses facilitate studies on the spread of infection by using disease-associated forms of PrP (pathological PrP or PrPSc) as surrogate markers for infectivity. A close correlation between infectivity and PrPSc has been previously established in our animal model (2, 3).
Available antibodies are unable to discriminate between host- and disease-associated forms of PrP in histologically processed tissues. We can distinguish pathological PrP from PrPC by differences in morphological appearance and distribution (4, 38, 39), but until now there was no formal proof that the disease-associated PrP detected by ICC corresponded to PrPSc. PET blot pretreatments destroy PrPC, leaving only the proteinase K-resistant fraction (42). Here, the deposits visualized by ICC were consistent with the PrPSc immunostaining in adjacent PET blots, even at early stages of incubation. These results provide strong evidence that pathological PrP detected by our ICC method is PrPSc.
Based on a series of studies examining the pathogenesis of 263K scrapie after oral challenge in hamsters, we proposed that the infectious agent reaches its initial CNS target sites by spreading in a retrograde direction along autonomic PNS pathways and ganglia supplying the viscera, i.e., along sympathetic and parasympathetic efferents of the splanchnic and vagus nerves (4, 39). Large parts of the alimentary canal, in particular, the esophagus, stomach, small intestine, and ascending colon, are innervated by these two nerves (17, 21), which contain fibers of autonomic (efferent) and sensory (afferent) neurons. With the neuroanatomy of the splanchnic and vagus nerve circuitry in mind (Fig. 1), the location, timing, and progression of PrPSc deposition revealed by this study strongly support and expand our hypothesis. PrPSc appeared and accumulated in a predictable temporal sequence in specific sites that accurately reflect the described autonomic and sensory relays. Deposition was always present in the CMGC and IML before the corresponding DRG. The same holds true with respect to the DMNV and NG. The results also show that, at least in this animal model, the ENS may be a key portal of entry for the infectious agent into the splanchnic and vagus nerve circuitry. As efferent and afferent fibers of both vagus and splanchnic nerves contact myenteric ganglia (17, 21), these would be the most likely sites for ENS-mediated neuroinvasion. However, infection may occur via intestinal nerve terminals not linked to ENS ganglia or other visceral tissues.
Our findings suggest that after uptake from the GI tract, the infectious agent primarily spreads by two neuroanatomical pathways: (i) along the vagus nerve to the DMNV in the brain and (ii) along the splanchnic nerve to the IML of the midthoracic spinal cord. Intramural ganglia of the gut and the CMGC are respective intervening relay points (Fig. 5). Within the CNS, the infectious agent probably travels along interneurons and sensory afferents to the SN-NG and the DRG, respectively. The reproducibility and spatial precision of PrPSc deposition indicate that spread is not random but occurs in a stepwise fashion along the synaptically linked neuronal populations. The observations indicate that initial spread occurs in a retrograde direction along efferent motor pathways, but dual efferent and sensory spread to the brain is also a possibility. The observed pattern of spread shows striking similarities to that of conventional neurotropic viruses, such as herpes simplex virus type 1 (22, 34), reovirus serotype 3 isolate T3C9 (40), and pseudorabies virus (14).
FIG. 5.
Pictorial representation of the neuronal pathways used in the oral routing of 263K scrapie. Initial spread (arrows) occurs in a retrograde direction along sympathetic and parasympathetic fibers of the splanchnic and vagus nerves. Enteric and abdominal ganglia (CMCG) have an early involvement in pathogenesis.
The most logical way for PrPSc to spread along peripheral nerves is by established axonal transport mechanisms. Several studies have reported that scrapie spreads within the nervous system by means of axonal pathways (20, 28), and the suggested rate of spread (0.5 to 2 mm/day) is consistent with that of slow axonal transport (10, 29, 44). It has been claimed that PrPC can be transported in an anterograde direction along peripheral nerve axons (9), but it is not known whether PrPSc is so transported. Transportation per se was not formally established in this study, but the evidence presented here would be compatible with this. While an abundance of PrPSc was detected in association with cell bodies of CNS neurons or peripheral ganglia, deposition in nerves (cell processes) was minimal or undetected, even at the terminal stage of disease. The low levels of infectivity found at the end stage of disease in vagus nerves compared to those found in the brain or CMGC are a further indication that in nerve fibers, the agent is in transit rather than being actively replicated.
The study did not reveal any evidence for hematogenous spread of infection to the brain. PrPSc was not detected early in infection at sites with an impaired blood-brain barrier, such as the area postrema (Fig. 3) or the choroid plexus. In addition, routing via the blood would not be consistent with the observed selectivity of targeting.
Infection via the oral route is strongly indicated (but not formally proven) in vCJD, BSE, and natural scrapie. Disease-specific PrP is found in the DMNV as a characteristic feature of both vCJD (26) and early BSE (43) infections, and spreading pathways similar to those described here have been described recently for sheep with natural scrapie (47). As the pattern of pathological PrP deposition in these nonexperimental infections closely resembles that observed in orally transmitted hamster scrapie, our findings provided new indirect evidence that vCJD in humans, BSE in cattle, and natural scrapie in sheep were caused by ingestion of TSE agent. The findings reported here for experimental 263K hamster scrapie strongly indicate that after oral challenge, infection of the CNS occurs via the splanchnic and vagus nerves. As similar pathogenic mechanisms are likely to operate in other orally acquired TSEs, this work provides baseline information about the peripheral routing of infection and a rodent model with which to study it.
ACKNOWLEDGMENTS
The skillful technical assistance of Marion Joncic and Stephanie Collishaw is gratefully acknowledged.
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and in part by grants from the German Bundesministerium für Bildung und Forschung and the German Bundesministerium für Gesundheit.
REFERENCES
- 1.Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen J-M, Lantier F. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 2.Baldauf E, Beekes M, Diringer H. Evidence for an alternative direct route of access for the scrapie agent to the brain bypassing the spinal cord. J Gen Virol. 1997;78:1187–1197. doi: 10.1099/0022-1317-78-5-1187. [DOI] [PubMed] [Google Scholar]
- 3.Beekes M, Baldauf E, Diringer H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J Gen Virol. 1996;77:1925–1934. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- 4.Beekes M, McBride P A, Baldauf E. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J Gen Virol. 1998;79:601–607. doi: 10.1099/0022-1317-79-3-601. [DOI] [PubMed] [Google Scholar]
- 5.Beekes M, McBride P A. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci Lett. 2000;278:181–184. doi: 10.1016/s0304-3940(99)00934-9. [DOI] [PubMed] [Google Scholar]
- 6.Bendheim P E, Brown H R, Rudelli R D, Scala L J, Goller N L, Wen G Y, Kascsak R J, Cashman N R, Bolton D C. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992;42:149–156. doi: 10.1212/wnl.42.1.149. [DOI] [PubMed] [Google Scholar]
- 7.Beuler H, Aguzzi A, Sailer A, Greiner R-A, Autenreid P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 8.Bolton D C, McKinley M P, Prusiner S B. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 9.Borchelt D R, Koliatsos V E, Guarnieri M, Pardo C A, Sisodia S S, Price D L. Rapid anterograde axonal transport of the cellular prion glycoprotein in the peripheral and central nervous systems. J Biol Chem. 1994;269:14711–14714. [PubMed] [Google Scholar]
- 10.Brady S T. Molecular motors in the nervous system. Neuron. 1991;7:521–533. doi: 10.1016/0896-6273(91)90365-7. [DOI] [PubMed] [Google Scholar]
- 11.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 12.Bruce M E, McBride P A, Farquhar C F. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett. 1989;102:1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- 13.Bruce M E, Will R G, Ironside J W, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock C J. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 14.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbin A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caughey B, Race R, Chesebro B. Detection of prion protein mRNA in normal and scrapie-infected tissues and cell lines. J Gen Virol. 1988;69:711–716. doi: 10.1099/0022-1317-69-3-711. [DOI] [PubMed] [Google Scholar]
- 16.Diringer H, Gelderblom H, Himert H, Ozel M, Edelbluth C. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983;306:476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- 17.Dockray G J. The brain-gut axis. In: Yamada T, editor. Textbook of gastroenterology. 3rd ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 1999. pp. 67–81. [Google Scholar]
- 18.Farquhar C F, Dornan J, Somerville R A, Tunstall A M, Hope J. Effect on Sinc genotype, agent isolate and route of infection on the accumulation of protease-resistant PrP in non-central nervous system tissues during the development of murine scrapie. J Gen Virol. 1994;75:495–504. doi: 10.1099/0022-1317-75-3-495. [DOI] [PubMed] [Google Scholar]
- 19.Foster J D, Wilson M, Hunter N. Immunolocalisation of the prion protein (PrP) in the brains of sheep with scrapie. Vet Rec. 1996;139:512–515. doi: 10.1136/vr.139.21.512. [DOI] [PubMed] [Google Scholar]
- 20.Fraser H, Dickinson A G. Targeting of scrapie lesions and spread of agent via the retino-tectal projection. Brain Res. 1985;346:32–41. doi: 10.1016/0006-8993(85)91091-1. [DOI] [PubMed] [Google Scholar]
- 21.Furness J B, Bornstein J C, Kunze W A A, Clerc N. The enteric nervous system and its extrinsic connections. In: Yamada T, editor. Textbook of gastroenterology. 3rd ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 1999. pp. 11–35. [Google Scholar]
- 22.Gesser R M, Koo S C. Oral inoculation with herpes simplex virus type 1 infects enteric neurons and mucosal nerve fibers within the gastrointestinal tract in mice. J Virol. 1996;70:4097–4102. doi: 10.1128/jvi.70.6.4097-4102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groschup M H, Weiland F, Straub O C, Pfaff E. Detection of scrapie agent in the peripheral nervous system of a diseased sheep. Neurobiol Dis. 1996;3:191–195. doi: 10.1006/nbdi.1996.0019. [DOI] [PubMed] [Google Scholar]
- 24.Groschup M H, Beekes M, McBride P A, Hardt M, Hainfellner J A, Budka H. Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathol. 1999;98:453–457. doi: 10.1007/s004010051108. [DOI] [PubMed] [Google Scholar]
- 25.Hill A F, Desbruslais M, Joiner S, Sidle K C L, Gowland I, Collinge J, Doey L J, Lantos P. The same prion strain causes vCJD. Nature. 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 26.Ironside J W. Pathology of variant Creutzfeldt-Jacob disease. Arch Virol Suppl. 2000;16:143–151. doi: 10.1007/978-3-7091-6308-5_13. [DOI] [PubMed] [Google Scholar]
- 27.Kascsak R J, Rubenstein R, Merz P A, Tonna-Demasi M, Fersko R, Carp R I, Wisniewski H M, Diringer H. Mouse polyclonal and monoclonal antibodies to scrapie-associated fibril protein. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimberlin R H, Walker C A. Pathogenesis of mouse scrapie: evidence for neural spread of infection to the CNS. J Gen Virol. 1980;51:183–187. doi: 10.1099/0022-1317-51-1-183. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin R H, Walker C A. Pathogenesis of mouse scrapie: patterns of agent replication in different parts of the CNS following intraperitoneal infection. J Soc Med. 1982;75:618–624. doi: 10.1177/014107688207500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimberlin R H, Field H, Walker C A. Pathogenesis of mouse scrapie: evidence for spread of infection to central from peripheral nervous system. J Gen Virol. 1983;64:713–716. doi: 10.1099/0022-1317-64-3-713. [DOI] [PubMed] [Google Scholar]
- 31.Kimberlin R H, Walker C A. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol. 1986;67:255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- 32.Kimberlin R H, Walker C A. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 1989;12:213–220. doi: 10.1016/0168-1702(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar H A, Prusiner S B, Stowring L E, DeArmond S J. Scrapie prion proteins are synthesised in neurones. Am J Pathol. 1986;122:1–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Krinke G J, Dietrich F M. Transneuronal spread of intraperitoneally administered herpes simplex virus type 1 from the abdomen via the vagus nerve to the brains of mice. J Comp Pathol. 1990;103:301–306. doi: 10.1016/s0021-9975(08)80050-3. [DOI] [PubMed] [Google Scholar]
- 35.Manson J C, McBride P A, Hope J. Expression of the PrP gene in the brain of Sinc congenic mice and its relationship to the development of scrapie. Neurodegeneration. 1992;1:45–52. [Google Scholar]
- 36.Manson J C, Clarke A R, McBride P A, McConnell I, Hope J. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration. 1994;3:331–340. [PubMed] [Google Scholar]
- 37.McBride P A, Eikelenboom P, Kraal G, Fraser H, Bruce M E. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J Pathol. 1992;168:413–418. doi: 10.1002/path.1711680412. [DOI] [PubMed] [Google Scholar]
- 38.McBride P A, Wilson M I, Eikelenboom P, Tunstall A, Bruce M E. Heparan sulphate proteoglycan is associated with amyloid plaques and neuroanatomically targeted PrP pathology throughout the incubation period of scrapie-infected mice. Exp Neurol. 1998;149:447–454. doi: 10.1006/exnr.1997.6740. [DOI] [PubMed] [Google Scholar]
- 39.McBride P A, Beekes M. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neurosci Lett. 1999;265:135–138. doi: 10.1016/s0304-3940(99)00223-2. [DOI] [PubMed] [Google Scholar]
- 40.Morrison L A, Sidman R L, Fields B N. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci USA. 1991;88:3852–3856. doi: 10.1073/pnas.88.9.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oesch B, Westaway D, Walchi M, McKinley M P, Kent S B H, Aebersold R, Barry R A, Teplow D B, Tempst D B, Hood L E, Prusiner S B, Weissmann C. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 42.Schulz-Schaeffer W J, Tschöke S, Kranefuss N, Dröse W, Hause-Reitner D, Giese A, Groschup M H, Kretzschmar H A. The paraffin-embedded tissue blot detects PrPSc early in the incubation time in prion diseases. Am J Pathol. 2000;156:51–56. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz-Schaeffer W J, Fatzer R, Vandevelde M, Kretzschmar H A. Detection of PrPSc in subclinical BSE with the paraffin-embedded tissue (PET) blot. Arch Virol Suppl. 2000;16:173–180. doi: 10.1007/978-3-7091-6308-5_16. [DOI] [PubMed] [Google Scholar]
- 44.Scott J R, Davies D, Fraser H. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J Gen Virol. 1992;73:1637–1644. doi: 10.1099/0022-1317-73-7-1637. [DOI] [PubMed] [Google Scholar]
- 45.Somerville R A, Merz P A, Carp R I. Partial copurification of scrapie-associated fibrils and scrapie infectivity. Intervirology. 1986;25:48–55. doi: 10.1159/000149654. [DOI] [PubMed] [Google Scholar]
- 46.Van Keulen L J M, Schreuder B E C, Vromans M E W, Langeveld J P M, Smits M A. Scrapie-associated prion protein in the gastro-intestinal tract of sheep with natural scrapie. J Comp Pathol. 1999;121:55–63. doi: 10.1053/jcpa.1998.0300. [DOI] [PubMed] [Google Scholar]
- 47.Van Keulen L J M, Schreuder B E C, Vromans M E W, Langeveld J P M, Smits M A. Pathogenesis of natural scrapie in sheep. Arch Virol Suppl. 2000;16:57–71. doi: 10.1007/978-3-7091-6308-5_5. [DOI] [PubMed] [Google Scholar]
- 48.Wells G A H, Hawkins S A C, Green R B, Austin A R, Dexter I, Spencer Y I, Chaplin M J, Stack M J, Dawson M. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet Rec. 1998;142:103–106. doi: 10.1136/vr.142.5.103. [DOI] [PubMed] [Google Scholar]
- 49.Wilson M I, McBride P A. Technical aspects of tracking scrapie infection in orally dosed rodents. J Cell Pathol. 2000;5:17–22. [Google Scholar]