Abstract
Inflammatory Bowel Disease (IBD) is a chronic inflammatory condition that usually affects younger adults but has a second incidence peak in the older population. Although diagnosis of IBD is driven by symptoms, some patients are asymptomatic and incidentally discovered while participating in colon screening program (CSP). We aimed to identify the incidence and outcome of IBD in fecal immunochemical test (FIT) positive patients in the British Columbia CSP. We conducted a retrospective chart review of patients who had colonoscopies for positive FIT and were found to have colitis based on endoscopic and histological assessment. Of 93,994 patients who underwent screening colonoscopy for positive FIT between 2009 and 2017, 608 (0.6%) were found to have colitis. From 11 CSP sites, 191 patients met the inclusion criteria. 58 patients (30.4%) were diagnosed with ulcerative colitis, 109 (57.1%) with Crohn’s disease (CD), and 24 (12.6%) with IBD unclassified. 124 patients (64.9%) received treatment, of which 34 (17.8%) received biologics and 4 (2.1%) required surgery. Our study demonstrated a clinically significant incidence of IBD, with novel finding of CD predominance, within a Canadian provincial CSP. Further research is needed to guide management of older patients with varying rates of IBD progression after incidental diagnosis.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Inflammatory bowel disease in elderly, Immunologic factors
Subject terms: Autoimmunity, Inflammatory bowel disease
Introduction
The exact pathophysiology of IBD remains unknown but is believed to arise from dysregulation of the complex interplay between genetic factors, environmental factors, gut microbiome, as well as innate and adaptive immunity that leads to the onset and progression of disease1. The highest prevalence of IBD is reported in Europe and North America (UC 505 per 100,000 persons in southeast Norway; CD 318.5 per 100,000 persons in Nova Scotia, Canada) with a lower incidence in East Asian and African countries2. Recent studies have shown a rising incidence of IBD in newly industrialized countries in Asia, Africa, and South America, thus, implicating a greater role of environmental factors, such as diet as well as stress in the pathogenesis of IBD2.
The peak incidence of IBD occurs between ages of 20–40 years, with a second smaller peak between ages 50–70. It is estimated that 10–15% of cases of IBD are diagnosed in individuals over the age of 603. The diagnosis is made with colonoscopy after patients commonly present with abdominal pain and bloody diarrhea. However, a small subset of older patients is diagnosed during screening colonoscopy. Studies performed in Japan, Nottingham, and Spain reported an incidence of 0.056–0.35% in asymptomatic participants in their respective colon screening programs4–7. The disease course, treatment efficacy and treatment side effects differ in older patients with IBD as compared to their younger counterparts. The health status of older patients with new IBD diagnoses is also more heterogenous, which affects management strategies especially in patients who remain asymptomatic. A systematic review demonstrated that although the treatment strategies were similar in older patients diagnosed with IBD, use of biologic agents was associated with lower response and higher adverse event rates3. Hence a strategy of “start low-go slow” was advocated. There are still limited guidelines on the management of asymptomatic patients with IBD. The diagnosis of subclinical IBD in older patients is a less studied phenomenon that could provide the opportunity to study the pathophysiology of the IBD, disease progression, and impact of various treatment modalities. It also poses further diagnostic and clinical challenges in older patients who may be more frail or comorbid.
Materials and methods
Study population
This is a retrospective chart review of patients who received a colonoscopy as part of the British Columbia Colon Screening Program (BCCSP) (British Columbia, Canada) between January 1, 2009, and December 31, 2017. In alignment with the Canadian Task Force on Preventative Health Care, the BCCSP screens asymptomatic individuals 50–74 years old with biennial FIT and follow-up colonoscopy for abnormal results. The BCCSP uses NS-Plus (April 2013 to May 2019) and OC-Sensor (January 2009 to April 2013) with a positivity cut-off of 10ng globin/gram feces. Individuals with a personal history of colorectal cancer or inflammatory bowel disease are not eligible for the screening program. Individuals with a personal history of neoplastic colorectal lesions or a high-risk family history of colorectal cancer undergo colonoscopy within the program.
The BCCSP database maintains a comprehensive prospective database for all program participants including colonoscopy findings and pathology. All program participants who had a positive FIT and underwent colonoscopy between January 1, 2009 and December 31, 2017 and were diagnosed with colitis on endoscopy and/or histology were identified. Of the 41 endoscopy sites participating in the BCCSP, eleven major sites were included in this study. Charts were reviewed for the patients who had a screening colonoscopy for a positive FIT result, with endoscopic and/or histologic evidence of colitis from the initial assessment were screened. Those who had a previous diagnosis of inflammatory bowel disease or any treatment for colitis were excluded. Additionally, patients who were diagnosed with microscopic colitis, lymphocytic colitis, collagenous colitis, ischemic colitis, segmental colitis associated with diverticulosis (SCAD), or did not have charts available were excluded from the study.
Data collection
Charts were reviewed to collect demographic information, including sex, race, age at the time of diagnosis, and cigarette smoking history. Patients’ medical history, such as, family history of IBD or colorectal cancer, were recorded if available. Disease characteristics, including presence of symptoms prior to screening colonoscopy, such as, abdominal pain or diarrhea, clinical, endoscopic, and histological findings, and treatment modalities used, were collected. The extent of colonoscopy, to the cecum or terminal ileum, was also recorded.
The stratification of colitis between IBD subtypes, e.g., Ulcerative colitis (UC), Crohn’s disease (CD), and IBD unclassified (IBDU) was performed based on endoscopic and histological assessments from the screening colonoscopy. The Copenhagen Diagnostic Criteria for CD and UC were used to classify the colitis presentation as UC, CD, or IBDU8–10. Patients were classified as having UC when there were endoscopic findings of continuous ulceration or granulated mucosa from the rectum proximally, and histopathological findings of cryptitis, crypt distortion, crypt abscess, neutrophils in epithelial structures, or chronic active colitis. Patients were classified with CD when there were endoscopic findings of patchy colitis, aphthous ulcerations, or cobblestoning, with histopathological findings of transmural discontinuous focal or patchy ulceration or presence of granulomas. All cases which demonstrated endoscopic and histopathological features of IBD colitis but did not fit the criteria for either UC or CD were classified as IBDU. The Copenhagen criteria also characterizes based on clinical symptoms. However, since most patients in the study were asymptomatic, this was not applicable. Three of the researchers (HB, BS, and JT) reviewed all endoscopy and histology reports individually. Fleiss kappa was calculated to determine the interrater reliability between the three raters. Any disagreements in IBD subtype classification were sorted during discussions amongst the above-mentioned researchers.
The total follow-up period was documented from the initial colonoscopy to the last gastroenterologist visit for each patient. Further information was obtained on disease characteristics for all patients, including severity, location of disease, and whether stricturing or penetrating disease was present, as per the Montreal classification of IBD11. Treatment modalities used, if any, from the initial visit to the last follow-up were recorded.
Statistical analysis
Continuous variables were analyzed as medians with range. Categorical variables were assessed using frequencies and percentages for each variable. Mann-Whitney U test was used to compare continuous dependent variables. Chi-square test and Fisher test were used to compare categorical dependent variables and determine statistical differences based on IBD subtype. All categories, such as, patient demographics, presence of symptoms prior to colonoscopy, severity of disease, and treatment modalities used were compared for statistically significant difference based on IBD subtypes, with the null hypothesis that there is no difference between the groups. All statistical analyses were performed using Rstudio version 2022.02.3. Statistical significance was set at p < 0.05. The study was approved by the University of British Columbia Research Ethics Board and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants in the study.
Results
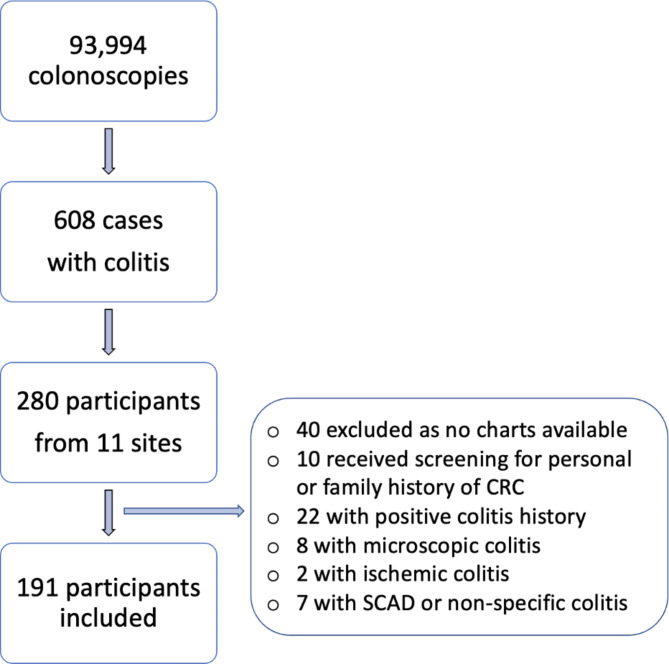
Between January 1, 2009, and November 31, 2017, 93,994 patients underwent screening colonoscopies in the British Columbia Colon Screening Program for a positive fecal immunochemical test at 41 different healthcare facilities. Of those, 608 patients (0.6%) were identified to have colitis due to various etiologies on endoscopic or histological assessments on screening colonoscopies. Chart review was undertaken for all patients who received care at one of the 11 study sites, a total of 280 patients screened and 191 included in the study (Fig. 1).
Fig. 1.
Flow chart of patient inclusion and exclusion for the study. CRC colorectal cancer, SCAD segmental colitis associated with diverticulosis.
Patient characteristics
191 patients were included in the study. The median age at the time of screening colonoscopy was 58 (range 46–75 years). Majority of patients were male and Caucasian; baseline characteristics are shown in Table 1.
Table 1.
Patient characteristics of all patients and stratified by IBD subtype.
| All patients n = 191 |
UC n = 58 |
CD n = 109 |
IBDU n = 24 |
p-value* | |
|---|---|---|---|---|---|
| Age, median (range) | 58 (46–75) | 57.5 (46–74) | 58 (50–75) | 60 (50–70) | 0.82 |
| Male sex, n (%) | 112 (58.6) | 34 (58.6) | 63 (57.8) | 15 (62.5) | 0.91 |
| Active smoking, n (%) | 23 (12) | 4 (6.9) | 17 (15.6) | 2 (8.3) | 0.13 |
| Family CRC history, n (%) | 6 (3.1) | 2 (3.4) | 4 (3.7) | 0 (0) | 0.64 |
| Family IBD history, n (%) | 24 (12.6) | 8 (13.8) | 13 (11.9) | 3 (12.5) | 0.73 |
| Symptoms prior to colonoscopy, n (%) | 42 (22) | 19 (32.8) | 20 (18.3) | 3 (12.5) | 0.04 |
| Abdominal pain prior to colonoscopy, n (%) | 14 (7.3) | 5 (8.6) | 7 (6.4) | 2 (8.3) | 0.61 |
| Diarrhea prior to colonoscopy, n (%) | 34 (17.8) | 18 (31.0) | 14 (12.8) | 2 (8.3) | 0.006 |
| Extent of UC | |||||
| Ulcerative proctitis, n (%) | 15 (25.8) | ||||
| Left-sided colitis, n (%) | 22 (37.9) | ||||
| Pancolitis, n (%) | 21 (36.2) | ||||
| Extent of CD | |||||
| Ileal, n (%) | 16 (14.7) | ||||
| Colonic, n (%) | 49 (45) | ||||
| Ileocolonic, n (%) | 44 (40.3) | ||||
| TI intubation, n (%) | 136 (71.2) | 34 (58.6) | 85 (78) | 17 (70.8) | 0.07 |
| Follow-up period, median (range) (months) | 25 (0–85.9) | 30.9 (0–84.5) | 27.9 (0–85.9) | 1.7 (0–64.4) | 0.57 |
*p-values demonstrate comparison between UC and CD.
UC ulcerative colitis, CD Crohn’s disease, IBDU Inflammatory Bowel Disease unclassified, IBD Inflammatory Bowel Disease, CRC colorectal cancer, TI terminal ileum.
Most patients (77.8%) did not have any symptoms prior to their IBD diagnosis. 42 patients (22.2%) reported symptoms of abdominal pain or diarrhea that was either remote or mild enough to not require medical attention. Of those, 14 patients (7.4%) had non-specific abdominal pain, and 34 (18.0%) had at least one episode of diarrhea in the months preceding their screening colonoscopy.
Of the 191 charts reviewed, 58 patients (30.4%) were diagnosed with ulcerative colitis (UC), 109 patients (57.1%) with Crohn’s disease (CD), and 24 patients (12.6%) with IBD unclassified (IBDU). Three of the authors classified the disease phenotype based on endoscopic and histologic reports from initial colonoscopy, with an inter-rater reliability of 0.529, demonstrating moderate agreement amongst the three raters. Of the 191 charts reviewed, 136 (76%) of patients received terminal ileal intubation from 179 charts that reported the extent of colonoscopy.
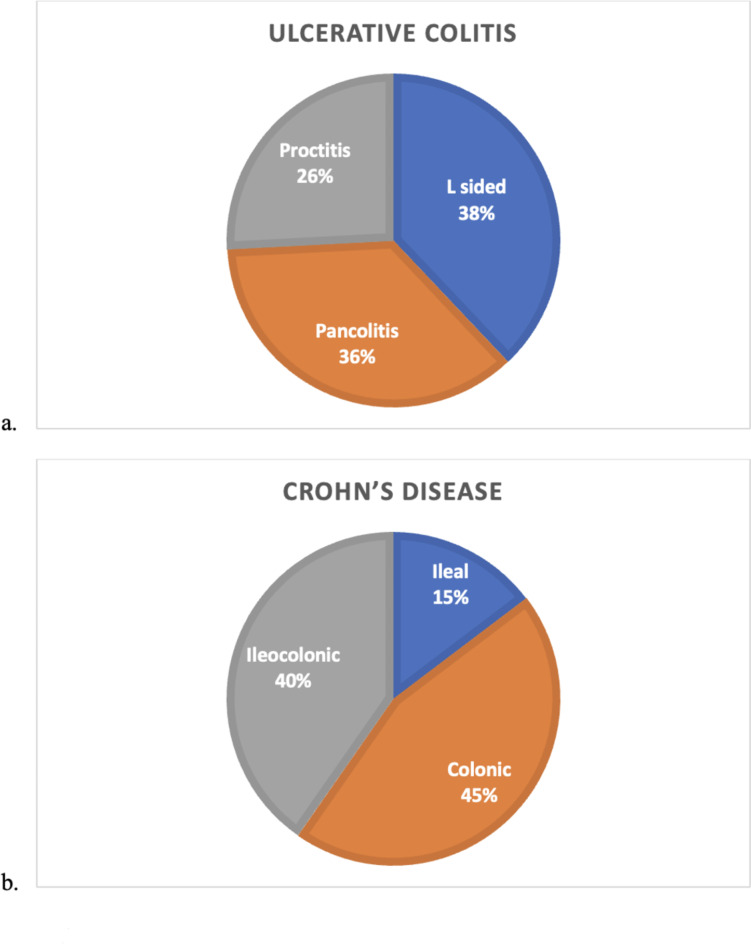
The distribution of inflammation is shown in Fig. 2. Of the 58 patients diagnosed with UC, the majority of patients (89.5%) had mild disease severity on endoscopy. Of the 109 patients diagnosed with CD, 97 patients (89%) had non-stricturing and non-penetrating disease, whereas 12 (11%) had stricturing disease.
Fig. 2.
Distribution of colitis in ulcerative colitis (a) and Crohn’s disease (b). Colonic inflammation was most prevalent in both Inflammatory Bowel Disease subtypes.
Treatment
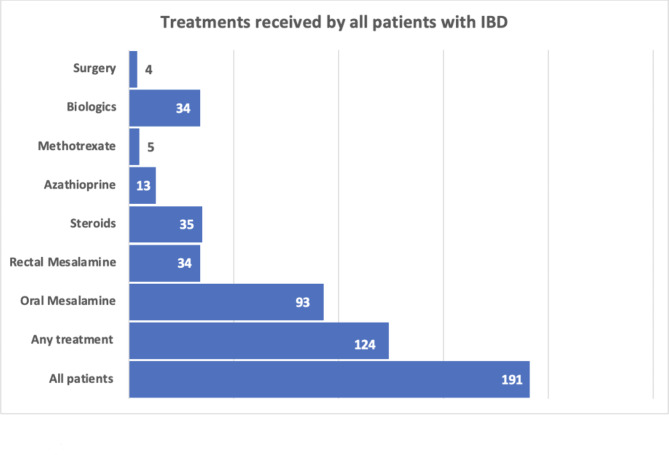
Of the 191 patients included in the study, 124 patients (64.9%) received treatment (Fig. 3). The patients were followed up by their gastroenterologists for a median of 25 months (range 0-85.9 months). 86.2% of patients with UC received treatment, compared to 60.6% of those diagnosed with CD, and 33.3% of those with IBDU (Table 2). Oral 5-ASA was the most frequently used therapy, being used in 70% in patients with UC, 77.3% with CD, and 87.5% in those with IBDu. Biologics were used in 20% and 34.8% of patients with UC and CD, respectively, and only in 12.5% diagnosed with IBDU. Tumour necrosis factor alpha inhibitor agents were used in 10 patients with UC, 17 patients with CD, and 1 patient with IBDU. Anti-integrin agent was used in 6 patients with UC, 7 patients with CD, and 1 with IBDU. Janus-Kinase inhibitor (JAKi) was used in 3 patients with UC and none in those with CD or IBDU, and Interleukin (IL)-12/IL-23 inhibitor was used in 2 patients with CD, and none in those with UC or IBDU. 2 patients required 4 different biologic therapies due to severity of disease and poor response to treatment, 1 patient required trial of 3 different biologics, whereas 5 patients required trial of 2 biologics. Surgical intervention was required in 1 patient with UC (2%), 3 patients (2.8%) with CD, and none with IBDU.
Fig. 3.
Distribution of patients who received treatment. Patients who received multiple treatments were included in each group. IBD, Inflammatory Bowel Disease.
Table 2.
Treatment characteristics of all patients and stratified based on IBD subtype.
| All patients n = 191 |
UC n = 58 |
CD n = 109 |
IBDU n = 24 |
p-values* | |
|---|---|---|---|---|---|
| Any treatment | 124 (64.9) | 50 (86.2) | 66 (60.6) | 8 (33.3) | 0.001 |
| Oral mesalamine, n (%) | 93 (48.7) | 35 (60.3) | 51 (46.8) | 7 (29.2) | 0.10 |
| Rectal mesalamine, n (%) | 34 (17.8) | 25 (43.1) | 5 (4.6) | 4 (16.7) | 1.87* 10 − 7 |
| Steroids, n (%) | 35 (18.3) | 10 (17.2) | 23 (21.1) | 2 (8.3) | 0.55 |
| Azathioprine, n (%) | 13 (6.8) | 4 (6.9) | 9 (8.3) | 0 (0) | 0.76 |
| Methotrexate, n (%) | 5 (2.6) | 2 (3.4) | 3 (2.8) | 0 (0) | 0.80 |
| Biologics, n (%) | 34 (17.8) | 10 (17.2) | 23 (21.1) | 1 (4.2) | 0.55 |
| Surgical intervention, n (%) | 4 (2.1) | 1 (1.7) | 3 (2.8) | 0 (0) | 0.68 |
*p-values demonstrate comparison between UC and CD.
UC, ulcerative colitis; CD, Crohn’s disease; IBDU, Inflammatory Bowel Disease unclassified.
Discussion
This large retrospective study identified subclinical IBD in FIT positive patients undergoing screening colonoscopy. The incidence rate in our study was comparable to previously reported studies with CD being the most common subtype of IBD diagnosed.
The incidence of IBD is highest in the third and fourth decades of life, but it can occur at any age12. Several studies have reported a bimodal distribution of IBD, with 10–30% new diagnosis of IBD occurring at an older age in the population13–15. The proportion of incidental endoscopic and histologic colitis in patients presenting for screening colonoscopies was 0.6% likely due to inclusion of microscopic colitis, ischemic colitis, and SCAD in the original cohort. The true proportion of patients with subclinical IBD in our study was comparable to 0.35% as reported in a Spanish cohort screened for FIT positivity6. Other studies, with different methodologies, reported a wide range of 0.01–3% incidence of IBD in asymptomatic patients4,5,7,16–18. Canada continues to have one of the highest rates of IBD in the world2,12,19, including the highest incidence and prevalence of CD20,21, which could also explain the higher incidence of IBD in the current study. The total incidence of IBD in populations screened in a FIT program followed by colonoscopy has been studied by a Spanish group6, and the comparison of our results to the previous study are outlined in Table 3. On closer inspection of this, it appears that more of their cases of colonic disease were assigned to a ‘UC’ diagnosis than we were able to. Their cases of ‘CD’ were also highest for those that had ileal disease despite ileal intubation being much lower than in our screened population (38% vs. 71%). Furthermore, we noted double the proportion of ileocolonic cases (40% vs. 21%), and yet comparable proportions of colonic disease (45% vs. 37%). It is possible that there are different subtypes of colonic IBD in older patients which may have differed between the two groups, hence, leading to this discrepancy. Ultimately, use of ancillary diagnostic measures, such as transabdominal ultrasound (TAUS) may help in distinguishing between the two22.
Table 3.
Data comparison between studies comparing IBD incidence from FIT screening programs.
| n | Rodriguez-Lago et al. | Bedi et al. |
|---|---|---|
| 110 | 191 | |
| Median age | 57 | 58 |
| Smoking history (%) | 10 | 12 |
| Ulcerative Colitis (%) | 71.8 | 30.4 |
| Crohn’s Disease (%) | 21.8 | 57.1 |
| IBDU (%) | 6.4 | 12.5 |
| UC distribution | ||
| Proctitis (%) | 32 | 25.8 |
| Left-sided (%) | 33 | 37.9 |
| Extensive/pancolitis (%) | 35 | 36.2 |
| CD distribution | ||
| Ileal (%) | 42 | 14.7 |
| Colonic (%) | 37 | 45 |
| Ileocolonic (%) | 21 | 40.3 |
| Upper gastrointestinal tract (%) | 0 | 0 |
| Perianal (%) | 0 | 0 |
| TI intubation (%) | 38 | 71.2 |
| Any treatment (%) | 73.6 | 64.9 |
| Oral Mesalamine (%) | 57.2 | 48.7 |
| Rectal Mesalamine (%) | 30.9 | 17.8 |
| Steroids (%) | 14.5 | 18.3 |
| Azathioprine (%) | 5.5 | 6.8 |
| Methotrexate (%) | 0.9 | 2.6 |
| Biologic (%) | 1.8 | 17.8 |
| Surgery (%) | 1.8 | 2.1 |
| Follow-up period, median (months) | 25 | 25 |
Significant values are in bold.
UC ulcerative colitis, CD Crohn’s disease, IBDU Inflammatory Bowel Disease unclassified, TI terminal ileum.
Male predominance has been established for IBD in the older population and our findings are consistent with this sex distribution4,7,23. Molodecky et al. performed a systematic review and compared sex variation in published literature over the last five decades worldwide and found a slightly higher incidence of UC in males and CD in females12. Shah et al. performed a systematic review and meta-analysis of variation between the sexes for UC and CD24. They found similar incidences of UC in males and females until age 45, after which males had a higher incidence of UC. Females were found to have a higher incidence of CD, except under the ages of 10–1424. The biological reasoning for the variation in incidence in males and females remains unknown and could be secondary to role of hormones in addition to environment, genetics, and immune dysregulation. Global variation in the incidence of subclinical IBD in this age category also remains unknown. Whilst the incidence of IBD is increasing worldwide, European and North American countries still have a higher prevalence of IBD, as compared to Asian, African, and South American countries12. The incidence rates of IBD in immigrant populations in Canada to those in children of immigrants who were either born in Canada or moved to Canada at a very young age have been investigated. It was reported that although the risk of IBD in immigrants is low, it was increased in children who were born in Canada or moved there at a young age25. Our study offers a unique perspective as BC, Canada, is home to a wide ethnic diversity with high immigration rates from all over the world. This is likely attributable to the collective effects of genetic factors, environmental triggers, microbiome and diet, and innate and adaptive immunity in the development of IBD.
IBD is classified into UC, CD, and IBDU based on the distinctive endoscopic and histological findings. UC has been identified as the most common IBD subtype in the older population, especially amongst those with subclinical disease4–7,14,26. Contrary to published literature, our study demonstrated a higher incidence of subclinical CD compared to UC in this group. This is consistent with the findings by Coward et al., who compared rates of CD and UC, in all ages, across various Canadian provinces, and found higher rates of CD compared to UC27,28. The proportion of colonoscopies with terminal ileal intubation was high, possibly prompted by findings of segmental colitis, thus leading to a confirmation bias, in the diagnosis of ileitis. However, it is possible that cases of isolated ileitis went undetected in patients who did not have ileal intubation during colonoscopy. Significantly, the incidence of patients diagnosed with IBDU is similar to rates reported in literature.
Most patients in our study with CD were found to have colonic CD, and pancolitis was present in almost the same proportion of patients as left-sided colitis in those with UC. This is consistent with findings of Li et al. who performed polygenetic risk scores (PRS) for patients with CD across various ages and demonstrated low PRS scores for older patients29. They also noted that patients with late onset CD diagnosis were more likely to have colonic CD, with a distinct disease location, disease behaviour, and management compared to younger patients diagnosed with CD29. Therefore, late onset CD could be a distinct disease milieu, which may be more similar to pancolonic UC diagnosis in older patients.
Treatment recommendations for patients with symptomatic IBD have been well-established30–33. A systematic review demonstrated that although the treatment strategies were similar in older patients diagnosed with IBD, use of biologic agents was associated with lower response and higher adverse event rates3. Hence, the strategy of “start low-go slow” was advocated. Early initiation of disease modifying therapy, such as, anti-tumour necrosis factor or other biologic agents, has been beneficial in slowing further disease progression and symptom resolution34. However, little is known regarding the role of treatment or appropriate monitoring in patients with subclinical disease, especially in older patients. Our study had a lower treatment initiation rate of 65% compared to 88% in a similar study performed by Rodrigues-Lagos et al. (Table 3)6. This may be due to a lack of guidelines and drug efficacy trials for treatment of older patients with IBD, who may be more prone to adverse effects from various medications35. Benchimol et al. studied the management of older patients with IBD in Canada, USA, UK, and Denmark and attributed the variability to patient or physician preference, concerns about adverse events and poly-pharmacy, differences in quality of care, adherence to clinical guidelines, or pharmaceutical industry marketing35. Bernstein et al. studied younger patients with IBD and showed a higher comorbidity burden in those with IBD compared to matched controls36. This indicates that the management of potentially co-morbid older patients with an initial subclinical IBD diagnosis is likely more nuanced than for patients with clinically symptomatic disease37. Additionally, 8 patients in our study required 2 or more biologics, with 2 patients requiring 4 biologics for disease management. In addition to medical management, 4 patients required surgical intervention for IBD related stricture or medically refractory colitis in our study. This is substantial given that 3 of the 4 patients who required surgery did not have any symptoms at the time of screening colonoscopy. It is unclear whether early advanced medical treatment could have avoided the need for surgical intervention in those patients.
The strengths of this study include the relatively large sample size and ethnic diversity of the study population. The limitations include a retrospective study design, a retrospective characterization of IBD subtype that may not capture disease evolvement over time, and inability to review the entire cohort of BCCSP patients diagnosed with colitis. As BC does not have a common electronic medical record available for research purposes, it was not feasible to involve all 130 colonoscopy sites. Exclusion of sites from smaller or remote communities introduces potential bias as patient characteristics and management may differ. Finally, lack of demographic data and information regarding generational residency in Canada or North America limited the evaluation of role of genetics in IBD incidence.
Conclusion
A significant incidence of asymptomatic IBD in patients undergoing screening colonoscopy for FIT positivity is reported. Of interest, CD was more prevalent than UC in patients diagnosed in the BC Colon Screening Program, with patients exhibiting a more aggressive disease course requiring biologic therapy, present in higher proportion than previously noted in comparable populations in the literature. As Canada has amongst the higher prevalence of IBD worldwide, and since several Canadian provinces and territories have initiated colon screening programs, the incidence of subclinical IBD will increase. Further studies exploring the natural disease progression, benefits and harms of various treatment modalities, and resource allocation are needed to inform practice guidelines and improve care of these patients. This will be especially important in those individuals with a more aggressive clinical trajectory.
Author contributions
HB, JT, RP, KA, ZK, HW, NF, KR, DS, BS planned and/or conducted the study HB, JT, BS collected and/or interpreted the dataHB, JT and BS drafted the manuscriptHB, JT, RP, KA, ZK, HW, NF, KR, DS, BS edited the manuscript.
Data availability
The data generated and analyzed during this study is available from corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical considerations
The study was approved by the University of British Columbia Research Ethics Board and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res.2019 7247238 (2019). [DOI] [PMC free article] [PubMed]
- 2.Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 390, 2769–2778 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Gisbert, J. P. & Chaparro, M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment. Pharmacol. Ther.39, 459–477 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Howarth, G. F., Robinson, M. H. E., Jenkins, D., Hardcastle, J. D. & Logan, R. F. A. High prevalence of undetected ulcerative colitis: data from the Nottingham fecal occult blood screening trial. Am. J. Gastroenterol.97, 690–694 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Mayberry, J. F., Ballantyne, K. C., Hardcastle, J. D., Mangham, C. & Pye, G. Epidemiological study of asymptomatic inflammatory bowel disease: the identification of cases during a screening programme for colorectal cancer. Gut. 30, 481–483 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez-Lago, I. et al. Characteristics and progression of Preclinical Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol.16, 1459–1466 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Sakata, T. et al. Asymptomatic inflammatory bowel disease with special reference to ulcerative colitis in apparently healthy persons. Am. J. Gastroenterol.96, 735–739 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Burisch, J. Crohn’s disease and ulcerative colitis. Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan. Med. J.61, B4778 (2014). [PubMed] [Google Scholar]
- 9.Munkholm, P. Crohn’s disease–occurrence, course and prognosis. An epidemiologic cohort-study. Dan. Med. Bull.44, 287–302 (1997). [PubMed] [Google Scholar]
- 10.Langholz, E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan. Med. Bull.46, 400–415 (1999). [PubMed] [Google Scholar]
- 11.Satsangi, J., Silverberg, M. S., Vermeire, S. & Colombel, J. F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 55, 749–753 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 142, 46–54e42 (2012). quiz e30. [DOI] [PubMed] [Google Scholar]
- 13.Jones, H. W. & Hoare, A. M. Does ulcerative colitis behave differently in the elderly? Age Ageing. 17, 410–414 (1988). [DOI] [PubMed] [Google Scholar]
- 14.Loftus, E. V. et al. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 114, 1161–1168 (1998). [DOI] [PubMed] [Google Scholar]
- 15.van Walree, I. C., van Tuyl, S. C. & Hamaker, M. E. Late-onset inflammatory bowel disease in the very elderly. Neth. J. Med.73, 4–9 (2015). [PubMed] [Google Scholar]
- 16.Greenwald, D. A. & Brandt, L. J. Inflammatory bowel Disease after Age 60. Curr. Treat. Options Gastroenterol.6, 213–225 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Katz, S. & Feldstein, R. Inflammatory bowel disease of the elderly: a wake-up call. Gastroenterol. Hepatol. (N Y). 4, 337–347 (2008). [PMC free article] [PubMed] [Google Scholar]
- 18.Russel, M. G. & Stockbrügger, R. W. Epidemiology of inflammatory bowel disease: an update. Scand. J. Gastroenterol.31, 417–427 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol.12, 720–727 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Benchimol, E. I. et al. The impact of inflammatory bowel disease in Canada 2018: a scientific report from the Canadian Gastro-Intestinal Epidemiology Consortium to Crohn’s and Colitis Canada. J. Can. Assoc. Gastroenterol.2, S1–S5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein, C. N. et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am. J. Gastroenterol.101, 1559–1568 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Ellrichmann, M. et al. Endoscopic ultrasound of the colon for the differentiation of Crohn’s disease and ulcerative colitis in comparison with healthy controls. Aliment. Pharmacol. Ther.39, 823–833 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Charpentier, C. et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 63, 423–432 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Shah, S. C. et al. Sex-based differences in the incidence of inflammatory bowel diseases-pooled analysis of population-based studies from the Asia-Pacific region. Aliment. Pharmacol. Ther.49, 904–911 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Benchimol, E. I. et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol.110, 553–563 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Loftus, E. V. et al. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gut. 46, 336–343 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coward, S. et al. The rising prevalence of inflammatory Bowel Disesae in Canada: analyzing the past to predict the future. J. Can. Assoc. Gastroenterol.1, 47–48 (2018). [Google Scholar]
- 28.Coward, S. et al. Past and Future Burden of Inflammatory Bowel diseases based on modeling of Population-Based Data. Gastroenterology. 156, 1345–1353e4 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Li, D. et al. Late-Onset Crohn’s disease is a subgroup distinct in genetic and behavioral risk factors with UC-Like characteristics. Inflamm. Bowel Dis.24, 2413–2422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bressler, B. et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 148, 1035–1058e3 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Feuerstein, J. D. et al. AGA Clinical Practice guidelines on the management of moderate to severe Ulcerative Colitis. Gastroenterology. 158, 1450–1461 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panaccione, R. et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the management of Luminal Crohn’s Disease. Clin. Gastroenterol. Hepatol.17, 1680–1713 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G. & Long, M. D. ACG Clinical Guideline: Ulcerative Colitis in adults. Am. J. Gastroenterol.114, 384–413 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Her, M. & Kavanaugh, A. Advances in use of immunomodulatory agents–a rheumatology perspective. Nat. Rev. Gastroenterol. Hepatol.12, 363–368 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Taleban, S., Colombel, J. F., Mohler, M. J. & Fain, M. J. Inflammatory bowel disease and the elderly: a review. J. Crohns Colitis. 9, 507–515 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Benchimol, E. I. et al. International variation in medication prescription rates among elderly patients with inflammatory bowel disease. J. Crohns Colitis. 7, 878–889 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Bernstein, C. N., Nugent, Z., Shaffer, S., Singh, H. & Marrie, R. A. Comorbidity before and after a diagnosis of inflammatory bowel disease. Aliment. Pharmacol. Ther.54, 637–651 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed during this study is available from corresponding author on reasonable request.