Abstract
Proteinuria is a biomarker of kidney injury that typically results from glomerular and/or tubulointerstitial disease. Whereas kidney impairment with normal urinary protein excretion is usually less focused and understudied. We conducted a retrospective review of the renal histopathology of the patients with variable degrees of unexplained renal insufficiency but with normal range proteinuria between 2014 and 2024 of three university teaching hospitals in Shenzhen city of Southern China. Patients with kidney dysfunction of undetermined or uncertain etiology and with normal urinary protein excretion (defined by a 24hr urinary protein excretion < 150 mg or spot urinary protein to creatinine ratio [PCR] < 150 mg/g) were enrolled and analyzed. In a total of 2405 patients, 53 (2.2%) fulfilled the inclusion criteria (male/female 40/13, age 47.3 ± 14.3 years) with a mean eGFR of 46.6 ± 16.8 ml/min per 1.73 m2. Glomerular disease (GD) was the most frequent pathological finding identified in 23 (43.4%) patients, while 19 (35.8%) cases showed tubulointerstitial disease (TID) and 11 (20.8%) patients exhibited small vascular disease (SVD). Patients in the TID had the lowest mean eGFR and the highest numerical 24hr urinary protein excretion among the three groups. The incidence of acute kidney injury was significantly higher in TID than in other two groups. The patients in the SVD group had the highest fraction of underlying hypertension. Kidney dysfunction with normal range proteinuria may be related with, in descending order of probablity, glomerular, tubulointerstitial and small vascular diseases. Renal biopsies were proved useful in informing therapeutic choice, long-term management and in predicting prognosis in this setting.
Keywords: Kidney biopsy, Proteinuria, Glomerular disease, Tubulointerstitial disease, Small vascular disease
Introduction
The development of percutaneous renal biopsy (PRB), pioneered by Iversen and Brun in 1951, has profoundly impacted the practice of Nephrology and altered the landscape of renal medicine [1]. Nowadays, kidney biopsy remains essential and irreplaceable in the management of patients with unexplained or unremitting AKI, chronic kidney disease (CKD), and those with kidney allograft dysfunction [2]. Being an invasive procedure however, PRB is used with caution clinically to evaluate the status, activity, and ideally pathophysiology, of kidney injury to inform prognosis and clinical decision. Although a universal guideline has not been found, renal insufficiency, hematuria and excessive proteinuria have routinely served as common indications of kidney biopsy [2, 3]. Proteinuria is a biomarker of renal injury and a driver of CKD as well, which typically arises from glomerular and/or tubulointerstitial disease [4]. A persistently high level of proteinuria associates with irreversible parenchymal damage predicting adverse renal and cardiovascular outcomes [5, 6]. Yet a normal urinary protein excretion does not exclude kidney damage either in its incipient or advanced disease stage [7–11].We herein set to investigate the renal histopathological profile in a group of patients from three tertiary institutions with varying degrees of unexplained renal dysfunction in the absence of overt proteinuria, aiming to provide some experience, reference and insight in the judgement and management in this setting.
Materials and methods
Patient selection and inclusion criteria
All renal biopsies performed between January 2014 and March 2024 in the Nephrology departments of three teaching hospitals in Shenzhen city were reviewed retrospectively. The renal pathological databases were searched to identify any cases meeting the following 3 inclusion criteria: (1) native renal biopsy, (2) normal range of urinary protein (defined by a 24hr urinary protein [24hr-UPr] of less than 150 mg, or spot urine protein to creatinine ratio [PCR] < 150 mg/g [if a 24hr-UPr is unavailable], which is in consistent with the A1 category of proteinuria adopted by KDIGO guideline [12]), and (3) undetermined etiology of kidney functional impairment (using the lowest estimated glomerular filtration rate [eGFR, calculated by 4 index CKD-EPI equation for Chinese: 175 × (Cr Jaffe)−1.234 × (Age)−0.179(× 0.79 if female) [13]] of less than 80 ml/min per 1.73 m2(age- and gender-matched mean eGFR value in the general population) within 1 weeks before biopsy. The exclusion criteria are: (1) younger than 18 years of age, and (2) use of glucocorticoid, immunosuppressive or immunomodulating agent currently or within 6 months before biopsy.
Evaluation of the renal biopsy specimens
All specimens were processed for light microscopy (LM), immunofluorescence (IF) and electronic microscopy (EM). Paraffin sections were cut in 2.5 μm of thickness and stained with hematoxylin and eosin, Masson trichrome, periodic acid–Schiff, Jones methenamine silver, and Congo Red. Snap-frozen fresh tissues cut in 3.5 μm were stained with fluorescein-tagged rabbit anti-human polyclonal antibodies for evaluation of IgG, IgA, IgM, C3, C1q, fibrinogen, and κ and λ light chains. EM samples were processed and assessed per standardized protocol [14].
All candidate biopsies were reevaluated by two pathologists and two nephrologists independent of one another. The following consensus criteria were adopted to describe the pathological changes. Glomerular minor change (GMC) is define as: (1) weak or mild degree focal or segmental mesangial expansion or proliferation, (2) negative or weak IF stain (trace or 1 + intensity on a scale of 0–3), (3) absence or insignificant change in the tubulointerstitial area (< 5–10%), and (4) absence of EM changes characteristic or specific to a known entity (e.g., thrombotic microangiopathy, or monoclonal immunoglobulin deposition disease, etc.). Immunoglobulin A Nephropathy (IgAN) is diagnosed according to the 2016 updated Oxford classification [15]. The diagnosis of diabetic kidney disease (DKD) is made in the light of a history of diabetes mellitus(DM) and in the presence of one or more of the following 4 histopathological changes, given other entity that may potentially lead to similar abnormality is otherwise excluded: (1) glomerular basement membrane (GBM) thickening (> 395 nm in female, and > 430 nm in male), (2) mesangial expansion, (3) arteriolar hyalinosis, and (4) Kimmelstiel-Wilson(K-W) nodule or capsular drop[16, 17]. Small vascular disease (SVD) is defined as the presence of one or more following small arterial or arteriolar changes without significant glomerular or tubulointerstitial lesion: (1) vessel wall thickening or sclerosis, (2) narrowing of vessel lumen, (3) hyalinosis. Tubular interstitial disease (TID) is defined by the presence of one or more following three features: (1) tubular cell degeneration, necrosis, or atrophy, (2) inflammatory hypercellularity and edema of the interstitium, and (3) interstitial fibrosis in the absence of significant glomerular or small arterial disease. The degree of podocyte foot process effacement (PFPE) observed on EM was graded into 3 classes: segmental, < 50%; major, 50–90%; and extensive, > 90% of the podocytic area. After the pathologists and nephrologists reviewed the cases independently, the histopathologies were discussed and ascertained. If there had been any dissention, discussions were held to reach a consensus. The final renal pathological category was classified according to the major histological changes that deemed contributing most to its kidney dysfunction.
Measurement of urinary protein and creatinine
Urinary protein and albumin level were measured by using the turbidimetry method, serum and urinary creatinine concentration by the Jaffe method. All measurements were carried out on chemical autoanalyzers in the central clinical laboratories of the three hospitals.
Statistics analysis
Results are expressed using mean ± SD or median or interquartile ranges as appropriate. To assess significance, the categorical data were compared between groups with the χ2 test or Fisher’s exact test. The quantitative data were compared with t tests or ANOVA. All tests were two-tailed, a p value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 26.0 for Windows (SPSS, Chicago, IL, USA).
Outcomes
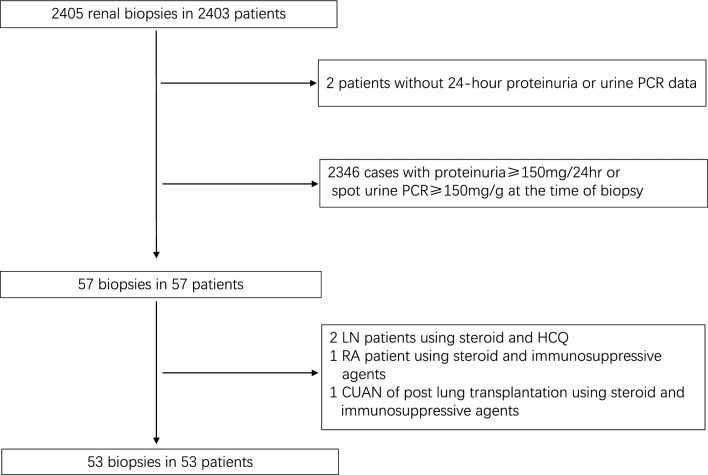
A total of 2405 ultrasound-guided native PRBs were performed during the study period in the three institutions (the university of Hong Kong-Shenzhen Hospital, Shenzhen University General Hospital, and Shenzhen Hospital of Southern Medical University). 53 patients fulfilled the inclusion criteria which accounted for 2.8%, 1.7% and 1.23% of PRB patients of the respective three centers (the rates between the first and the third unit had a significant difference only, p = 0.015). The 53 cases comprised 40 male and 13 female with a mean age of 47.3 ± 14.3 years. 17 (32.1%) and 15 (28.3%) patients had background hypertension (HT) and DM respectively, and nine (15.5%) had intercurrent HT and DM. Seven patients had a history of gout, and one patient was diagnosed monoclonal gammopathy of undetermined significance (MGUS) before biopsy. 12 out of 53 patients were taking an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for durations of one month to four years. No patient was using sodium glucose cotransporter-2 (SGLT2) inhibitor, glucagon-like peptide-1 (GLP-1), dipeptidyl peptidase 4 (DDP4) inhibitor, or mineralocorticoid receptor antagonist before renal biopsy. The flow chart of patient enrollment is shown in Fig. 1, and the demographic and clinical characteristics of the 53 patients are shown in Table 1. The average peak serum creatinine level within 1 week before biopsy was 168.6 ± 108.9 μmol/l, coupling a trough eGFR of 46.6 ± 16.8 ml/min per 1.73 m2. The 24hr-UPr and 24hr urinary albumin levels were 101.3 ± 28.8 mg (Table 1) and 13.5 mg (3.3, 46.8) respectively. Nearly three-fourth (39/53, 73.6%) of patients had an eGFR < 60 ml/min per 1.73 m2 (range 6.7–59.5). The rest 14 (26.4%) patients had a reduced eGFR (range 60.8–77.3 ml/min per 1.73 m2) comparing to gender- and age-matched normal population [18]. Microscopic urinary erythrocyte were present in only five of the 53 patients (4 in the GD group, and 1 in the SVD group, Table 2).
Fig. 1.
Flow chart of patient recruitment (PCR, protein to creatinine ratio; LN, lupus nephritis; HCQ, hydroxychloroquine; RA, rheumatoid arthritis; CUAN, chronic urate acid nephropathy.)
Table 1.
Basic characteristics of the 53 patients.
| Items | Values |
|---|---|
| Gender (M/F) | 40/13 |
| Age (year) | 47.3 ± 14.3 |
| Serum creatinine (μmol/L) | 168.6 ± 108.9 |
| eGFR (ml/min -1.73m2) | 46.6 ± 16.8 |
| 24hr proteinuria (mg) | 101.3 ± 28.8 |
| 24hr albuminuria (mg) | 13.5 (3.3, 46.8) |
| Hematuria ( n/%) | 5/9.43 |
| Duration of renal dysfunction 1 days to 6 yrs | |
| Comorbidities (n/%) | |
| HT | 17/32.1 |
| DM | 15/28.3 |
| HT + DM | 8/15.1 |
| Gout | 7/13.2 |
| MGUS | 1/1.9 |
HT Hypertension; DM Diabetes mellitus; MGUS Monoclonal gammopathy of undetermined significance.
Table 2.
The patient characteristics, eGFR, urinary protein and major pathological change of the 53 patients.
| No | Gender (M/F) |
Age (year) |
Category | SCr (μmol/L) |
AKI | eGFR (ml/min-1.73 m2) |
24hr-Upr (mg) |
24hr-UAE (mg) |
Concurrent disease | |
|---|---|---|---|---|---|---|---|---|---|---|
| HT | DM | |||||||||
| 1 | M | 42 | GD | 120.3 | − | 61.3 | 58.9 | 3.4 | + | + |
| 2 | M | 31 | GD | 124 | − | 62.3 | 66.7 | 5.2 | − | − |
| 3 | M | 45 | GD | 115.8 | − | 63.4 | 96.3 | 20.7 | + | − |
| 4 | M | 42 | GD | 123 | − | 59.6 | 61.7 | 1.5 | − | − |
| 5 | F | 29 | GD | 151 | − | 39.1 | 46.9 | 1.0 | − | − |
| 6 | F | 68 | GD | 177 | + | 27.9 | 113.6 | 50.2 | + | + |
| 7 | M | 35 | GD | 123 | − | 62.0 | 64.2 | 1.0 | − | − |
| 8▲ | M | 42 | GD | 114 | − | 65.5 | 129.0 | 66 | + | − |
| 9▲ | M | 41 | GD | 151 | − | 46.5 | 50.9 | 5.1 | − | − |
| 10 | M | 48 | GD | 149 | − | 46.0 | 98.2 | 12.2 | + | + |
| 11 | M | 38 | GD | 121 | − | 61.9 | 144.4 | 101 | − | − |
| 12▲ | F | 37 | GD | 87 | − | 73.9 | 120.0 | 69.3 | − | − |
| 13● | M | 81 | GD | 161 | − | 38.0 | 83.6 | 13.9 | + | + |
| 14 | F | 56 | GD | 100 | − | 57.8 | 120.0 | 69.3 | − | − |
| 15 | M | 31 | GD | 114 | − | 73.4 | 87.7 | 1.7 | − | − |
| 16 | F | 58 | GD | 133 | − | 38.0 | 113.31 | 56.8 | + | − |
| 17 | M | 36 | GD | 172 | − | 35.5 | 142.97 | 70.5 | − | − |
| 18 | M | 41 | GD | 123 | − | 62.4 | 126 | NA | − | - |
| 19 | F | 62 | GD | 134 | − | 32.5 | 73.2 | NA | − | + |
| 20▲ | M | 58 | GD | 112 | − | 62 | 142.5 | NA | − | − |
| 21 | M | 61 | GD | 120 | − | 55.9 | 143.25 | NA | − | − |
| 22 | F | 42 | GD | 100.7 | − | 59.3 | 141.5 | NA | + | − |
| 23 | M | 55 | GD | 156.8 | − | 42.11 | 90.39 | NA | − | + |
| 24 | M | 64 | TID | 122 | − | 53.6 | 110 | NA | − | + |
| 25 | M | 40 | TID | 125.2 | − | 58.8 | 71.82 | NA | − | − |
| 26 | M | 26 | TID | 260 | + | 25.8 | 100.1 | NA | − | − |
| 27 | M | 38 | TID | 225 | + | 28.8 | 129.0 | 22.9 | − | − |
| 28 | M | 50 | TID | 141.5 | − | 48.6 | 92.9 | 0.7 | − | − |
| 29 | M | 62 | TID | 187.5 | + | 33.1 | 118.7 | 18.3 | − | − |
| 30※ | M | 55 | TID | 124 | − | 56.3 | NA | NA | − | − |
| 31 | F | 21 | TID | 663 | + | 6.7 | 130.7 | 77 | − | − |
| 32◆ | M | 27 | TID | 112 | − | 72.4 | 130.1 | 1.3 | − | − |
| 33 | M | 64 | TID | 214 | − | 28.1 | 71.3 | 1 | − | + |
| 34 | M | 72 | TID | 216 | + | 27.2 | 59.2 | 8.2 | + | + |
| 35 | M | 63 | TID | 665 | + | 6.9 | 101.4 | 34.0 | + | + |
| 36 | F | 21 | TID | 286 | − | 18.8 | 130.4 | 13.2 | − | − |
| 37 | M | 46 | TID | 126 | − | 58.5 | 77.84 | 7.0 | − | − |
| 38 | F | 60 | TID | 161 | + | 29.7 | 149.78 | 29.3 | + | − |
| 39 | M | 22 | TID | 213 | + | 36.7 | 78 | NA | − | − |
| 40 | M | 66 | TID | 160 | − | 38.1 | 123.2 | NA | − | + |
| 41※ | F | 45 | TID | 239 | + | 24.7 | NA | NA | − | − |
| 42 | M | 33 | TID | 125.3 | − | 60.8 | 75.94 | NA | − | − |
| 43 | M | 49 | SVD | 191 | − | 33.7 | 113.0 | NA | + | − |
| 44 | M | 33 | SVD | 119 | − | 64.9 | 80.77 | NA | + | − |
| 45 | M | 60 | SVD | 139 | − | 48.1 | 118.0 | 13.8 | + | + |
| 46 | F | 47 | SVD | 141 | − | 39.0 | 124.4 | 45.7 | + | − |
| 47▲ | M | 34 | SVD | 152 | − | 47.7 | 118.68 | 39.2 | + | − |
| 48 | M | 40 | SVD | 107 | − | 71.4 | 92.4 | 2.9 | + | − |
| 49 | M | 63 | SVD | 261 | − | 21.9 | 71.84 | 4.4 | − | + |
| 50 | M | 52 | SVD | 115 | − | 47.2 | 79.25 | 5.3 | − | − |
| 51※ | F | 60 | SVD | 95 | − | 52.2 | NA | NA | − | − |
| 52※ | M | 57 | SVD | 128 | − | 53.2 | NA | NA | + | + |
| 53※ | M | 59 | SVD | 138 | − | 47.9 | NA | NA | + | − |
| No | Pathology | |||||||
|---|---|---|---|---|---|---|---|---|
| Major diagnosis | Glom/sGlom | IF | ICI | TA/IFib | SAWT | SAH | PFPE | |
| 1 | GMC | 11/0 | IgA1 + , IgM 1 + ,C1q ± | Neg. | Focal/Neg. | Neg. | Neg. | Segmental |
| 2 | IgAN(M0E0S0T0C0) | 52/4 | IgA2-3 + , IgM 1 + , C3 ± | < 5% | Neg./Neg. | Neg. | Neg. | Major |
| 3 | GMC | 16/0 | IgM ± | ± | Neg./Neg. | Isolate | Neg. | Segmental |
| 4 | GMC | 41/3 | Neg. | ± | Focal/Neg. | Neg. | Neg. | Segmental |
| 5 | GMC | 8/2 | C3 ± -1 + , IgM ± ,C1q ± | ± | Focal/Neg. | Neg. | Neg. | Major |
| 6 | DPGN(CryoGN) | 17/1 | IgG 1 + ,IgM 2 + ,C3 ± ,C1q 1 + ,κ 1–2 + ,λ 1 + | Focal | 10%/Focal | Present | Neg. | Major |
| 7 | GMC | 45/8 | IgA 1 + , IgM ± | Neg. | Neg./Neg. | Present | Present | Diffuse |
| 8▲ | IgAN(M0E0S0T0C0) | 16/0 | IgA 1–2 + ,C3 ± -1 + ,IgM 1 + | < 3% | < 3%/ < 3% | Isolate | Neg. | Major |
| 9▲ | GMC | 39/7 | Neg. | 5% | 10%/Focal | Neg. | Neg. | NA |
| 10 | DKD | 13/6 | Neg. | ± | 10%/ < 10% | Present | Present | Segmental |
| 11 | GMC | 16/3 | IgA ± -1 + | Neg. | < 5%/ < 5% | Isolate | Neg. | Segmental |
| 12▲ | IgAN(M0E0S1T0C0) | 15/2 | IgA 3 + , C3 1–2 + , IgM ± -1 + | ± | 5%/5% | Neg. | Neg. | Major |
| 13● | GMC | 26/2 | Neg. | ± | < 10%/ < 10% | Present | Neg. | Segmental |
| 14 | IgAN(M1E0S1T0C0) | 17/3 | IgA 2–3 + ,C3 1 + ,IgM ± | ± | < 5%/ < 5% | Present | Present | Major |
| 15 | ICMGN | 53/0 | IgA ± ,C3 ± ,IgM ± -1 + | Neg. | Neg./Neg. | Neg. | Neg. | Segmental |
| 16 | IgAN (M1E0S1T0C1) | 23/4 | IgA 3 + ,C3 2 + ,IgM– ± | Focal | < 5%/ < 5% | Neg. | Present | Major |
| 17 | MPGN | 12/1 | Neg. | Focal | Focal/Focal | Neg. | Neg. | Segmental |
| 18 | FSGS | 20/1 | IgM1 + | Focal | 5%/Neg. | Present | Present | Segmental |
| 19 | DKD | 15/10 | Neg. | Multifocal | 40%/Multifocal | present | Present | NA |
| 20▲ | IgAN(M1E0S1T0C1) | 28/9 | IgA3 + , IgM 1 + ,C3 1 + | Focal | 20%/Focal | Present | Present | Segmental |
| 21 | IgAN(M1E0S1T0C1) | 19/3 | IgA3 + , C3 ± | Focal | 10%/Focal | Present | Neg. | Diffuse |
| 22 | MPGN | 6/2 | IgM1 + | Multifocal | 35%/Multifocal | Present | Present | Segmental |
| 23 | DKD/CIN | 15/1 | IgG -, IgM + , IgA -, C3 -, C1q- | Focal | 20%/Focal | Significant | Present | Segmental |
| 24 | AIN | 33/0 | IgM ± | Focal | Neg./Neg. | Present | Present | NA |
| 25 | AIN | 17/0 | IgG -, IgM ± , IgA -, C3 -, C1q- | Focal | Focal/Neg. | Mild | Neg. | Segmental |
| 26 | AIN | 35/4 | Neg. | ± | Focal/Neg. | Neg. | Neg. | Segmental |
| 27 | AIN | 30/0 | IgA ± , IgM ± | Focal | Focal/Neg. | Neg. | Neg. | Segmental |
| 28 | AIN | 20/2 | IgM 1 + | Focal | Focal/Neg. | Present | Neg. | Segmental |
| 29 | CIN | 13/5 | IgM ± | Multifocal | 70%/Multifocal | Significant | Present | Segmental |
| 30※ | CIN | 13/2 | IgM 1 + | Multifocal | 40%/Multifocal | Present | Neg. | Major |
| 31 | AIN/IgAN(M1E0S0T0C0)★ | 15/0 | IgA 2 + ,C3 ± | Multifocal | Neg./Neg. | Isolate | Neg. | Segmental |
| 32◆ | AIN | 18/1 | Neg. | Neg. | Neg./Neg. | Neg. | Neg. | Segmental |
| 33 | CIN | 10/3 | Neg. | Focal | 35%/20% | Isolate | Present | Major |
| 34 | AIN | 9/0 | Neg | Extensive | 10%/5% | Present | Neg. | Segmental |
| 35 | AIN | 10/4 | IgG ± | Moderate | < 5%/ < 5% | Significant | Present | Diffuse |
| 36 | AIN | 20/4 | Neg. | Extensive | 5%/Neg. | Neg. | Neg. | Major |
| 37 | CIN | 28/5 | Neg. | Focal | < 3%/Neg. | Present | Present | Major |
| 38 | AIN | 14/1 | Neg. | Multifocal | < 5%/ < 5% | Neg. | Neg. | Segmental |
| 39 | AIN | 45/1 | Neg. | Focal | 5%/Neg. | Neg. | Neg. | Neg. |
| 40 | CIN/DKD | 13/4 | Neg. | Multifocal | 45%/Multifocal | Present | Present | Segmental |
| 41※ | AIN | 11/0 | Neg. | Multifocal | Neg./Neg. | Present | Neg. | Segmental |
| 42 | AIN/IgAN(M0E0S0T0C0)★ | 15/2 | IgA2 + ,IgM ± | Focal | 5%/Focal | Neg. | Neg. | Segmental |
| 43 | SVD | 17/7 | Neg. | Multifocal | Multifocal/Focal | Significant | Present | Segmental |
| 44 | SVD | 34/5 | C3/C1q ± | Focal | Focal/Focal | Present | Present | Segmental |
| 45 | SVD | 78/30 | IgM ± | Multifocal | Focal/Focal | Significant | Present | Segmental |
| 46 | SVD | 10/4 | IgM ± | Focal | Focal/Focal | Significant | Present | Segmental |
| 47▲ | SVD | 37/0 | Neg. | Focal | Trace/Neg. | Significant | Present | Segmental |
| 48 | SVD | 43/17 | IgM ± | Focal | 20%/Neg. | Significant | Present | Segmental |
| 49 | SVD | 17/3 | IgG ± | Focal | 5%/5% | Significant | Present | NA |
| 50 | SVD | 33/10 | IgM ± | Focal | 5%-10%/5%-10% | Present | Present | Major |
| 51※ | SVD | 25/7 | Neg. | Focal | < 3%/Neg. | Present | Neg. | Segmental |
| 52※ | SVD/DKD■ | 37/10 | Neg. | Focal | 20%/Focal | Present | Present | Segmental |
| 53※ | SVD | 17/11 | IgM1 + | Multifocal | 40%/Multifocal | Present | Present | Neg. |
GD, glomerular disease; TID, tubulointerstitial disease; SVD, small vascular disease; SCr, serum creatinine; AKI, acute kidney injury; 24hr-UPr, 24hr urinary protein; 24hr-UAE, 24hr urinary albumin excretion; HT, hypertension; DM, diabetes mellitus; Glom, number of glomerulus; sGlom, number of sclerosed glomerulus; IF, immunofluorescence; ICI, interstitial cellular infiltration; TA, tubular atrophy, IFib, interstitial fibrosis; SAWT, small arterial wall thickening; SAH, small arterial hyalinosis; PFPE, podocyte foot process effacement; GMC, minor glomerular change; IgAN, IgA nephropathy; DPGN, diffuse proliferative glomerular nephritis; CryoGN, cryoglobulinemic glomerular nephritis; DKD, diabetic kidney disease; MGUS, monoclonal gammopathy of undetermined significance; ICMGN, Immune complex mediated glomerulonephritis; ATN, acute tubular necrosis; AIN, acute interstitial nephritis; CIN, chronic interstitial nephritis; SVD, small vascular disease; Neg., negative; NA, not available.
★ AIN on top of IgAN, with AIN as the major cause of kidney dysfunction.
■ SVD on top of DKD,SVD is more evident to be the major cause of kidney dysfunction.
▲ the patient had microscopic hematuria.
● the patient was diagnosed MGUS.
◆ the patient had past history of gout, with prominent interstitial edema in the absence of glomerular change suggesting acute tubulointerstitial injury as the major cause of kidney dysfunction.
※ the patient had no 24hr-UPr, but urine PCR < 150 mg/g.
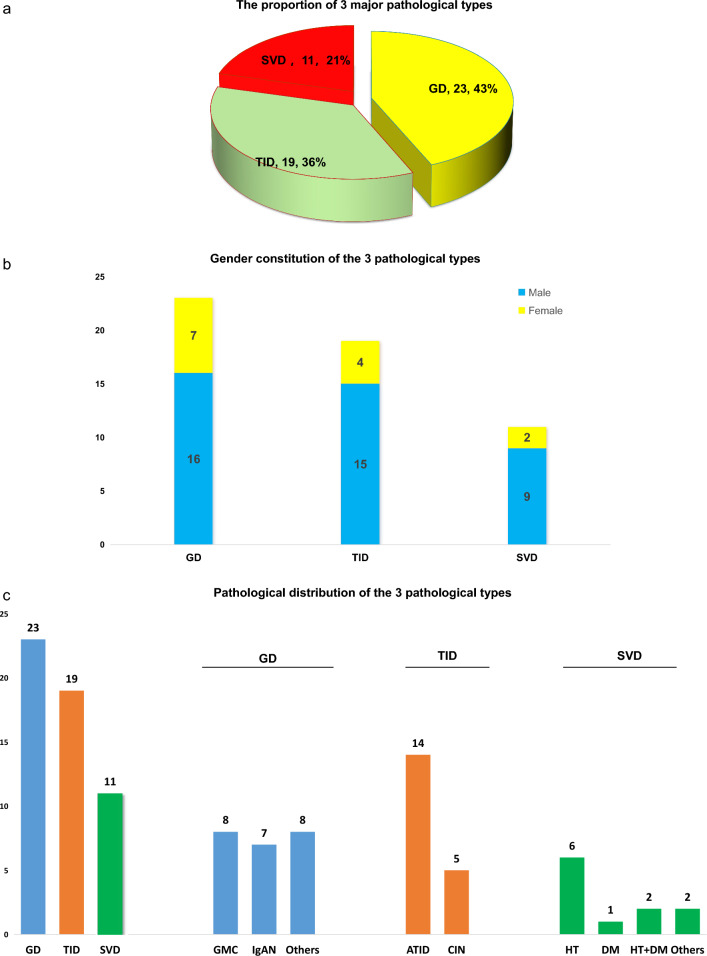
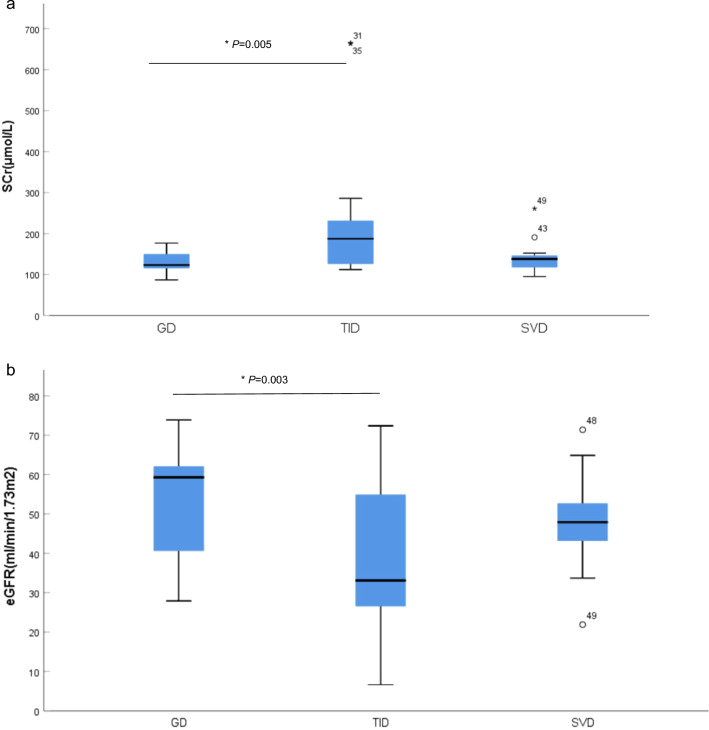
The kidney histopathology of the 53 patients can be classified into three categories: GD, TID and SVD. GD was the most frequent pathological change identified in 23 patients (43.4%); 19 patients (35.8%) displayed TID, and 11 patients (20.8%) exhibited SVD as the major pathological impairment (Fig. 2a–c, Table 2). In the GD group, eight patients displayed GMC, seven had IgAN (2 M0E0S0T0C0, 1 M0E0S1T0C0, 1 M1E0S1T0C0 and 3 M1E0S1T0C1), three revealed DKD, two cases had MPGN, one patient with FSGS and 1 with Immune complex mediated glomerulonephritis. And, there was one case of diffuse proliferative glomerular nephritis (DPGN) secondary to cryoglobulinemia (patient no. 6, Table 2). In the TID group, 14 of the 19 patients observed acute tubulointerstitial injury (2 had underlying IgAN, patient nos. 31,42), 5 patients were diagnosed chronic tubulointerstitial disease (1 case with concurrent DKD, patient no.40). In the SVD group, the renal impairment of six patients were attributed to HT, one patient was linked to DM, and two patients to concurrent HT and DM. Patients in TID had the lowest mean eGFR and the highest numerical 24hr-UPr among the three groups (Fig. 3a, b, Table 3). Ten of the 53 patients presented with AKI (2012 KDIGO definition) within two weeks before renal biopsy, of whom nine were in the TID group, the other one was in the GD group (the case with DPGN). The incidence of AKI was significantly higher in the TID group than in the GD (p = 0.002) and the SVD group (p = 0.011). Patients in SVD group had a higher rate of underling HT versus the TID group (p = 0.004) and GD group (p = 0.066). The pathological characteristics of the 53 patients are shown in Table 2.
Fig. 2 .
The proportion, gender constitution and pathological distribution of 3 pathological types.
Fig. 3.
a The mean SCr in the three pathological types, b The mean eGFR in the three pathological types.
Table 3.
The mean eGFR and 24hr-UPr in the the three pathological types.
| Age(yr) | SCr(μmol/L) | eGFR(ml/min-1.73m2) | 24hr-UPr(mg) | |
|---|---|---|---|---|
| GD | 46.9 ± 13.3 | 129.7 ± 23.4 | 53.3 ± 13.4 | 100.7 ± 33.0 |
| TID | 46.1 ± 17.4 | 229.8 ± 161.7* | 37.6 ± 18.7** | 103.0 ± 27.3 |
| SVD | 50.4 ± 10.8 | 144.2 ± 46.4 | 47.9 ± 13.6 | 99.8 ± 21.0 |
GD, glomerular disease; TID, tubulointerstitial disease; SVD, small vascular disease
*significantly higher v.s. GD, p = 0.005
**significantly lower v.s. GD, p = 0.003
Discussion
Proteinuria, along with hematuria and serum creatinine and cystatin C, represents the essential biomarker of kidney injury. 24 hr urine proteinuria has been widely accepted as gold standard in urinary protein evaluation, providing accurate sample collection had been made. Spot urine PCR and albumin-creatinine ratio (ACR) have very good correlation with timed urine protein, while its diagnostic performance could be compromised in high-level proteinuria (> 3.0–3.5 g/day) [19, 20] or in low urinary creatinine excretion[21], which may result in under- or over-estimate daily urine protein respectively. Pathological proteinuria typically results from disruption of glomerular filtration barrier and/or the impairment of the renal tubular resorptive function. It is estimated that kidneys may reabsorb as much 3.2 g of albumin and 9.6 g of low molecular protein (given a sieving coefficient of 0.00062 and 0.987 respectively) daily in a normal adult [22]. Under pathological conditions, not including overflow proteinuria as in multiple myeloma, abnormal urinary protein occurs in either of the two situations: (1) glomerular filtration barrier damage leading to excessive protein in the primary urine exceeding the tubular resorption reserve, and (2) impairment of resorptive capability owing to functional or structural damage of the tubule. An extreme example of the latter is Dent disease, in which mutations of the ClCN5 or OCRL gene result in defective membrane traffic of apical megalin and cubilin receptors in the tubular epithelial cells to cause the marked proteinuria consisting of both low molecular protein and albumin [23]. The protein in the primary urine is mainly reabsorbed via megalin and cubilin of the renal tubules, with around 71% albumin retrieved in the proximal tubule and 26% in the distal nephron according to animal model [22]. As such, it seems sensible to suggest that the 2.2% of non-proteinuric patients in our cohort may have preserved tubular resorptive function to trade off the proteinuric leak by glomerular injury. Similar instances had been documented in a number of glomerular or non-glomerular entities [7, 9, 10, 24–27], although the underlying mechanisms could possibly be more complex.
In the GD group, the majority of patients (15/23) had minor or non-severe pathological abnormalities (8 GMC, 7 IgAN, Table 2) that align with low-grade glomerular lesion corresponding to lesser urinary protein leakage. Similar scenarios had been reported in a number of glomerular diseases, such as in IgAN [10, 28–30] and DKD [7, 31, 32]. In 2015, Hoshino et al. reported that 35 out of 56 patients with isolated hematuria (mean eGFR 94.3 ± 18.3 ml/min per 1.73 m2) had low-grade IgAN (gradeIor II of Lee’s grading system) as the major pathological change [10]. Among T2DM patients with histologically confirmed DKD, the incidence of nonproteinuric or normal albuminuric diabetic nephropathy had been reported to be between 16.7 and 19.5% [7, 31]. Compared with their proteinuric counterparts, nonproteinuric DKD patients presented with following clinicopathological characteristics: (1) morphologically insignificant or low-grade glomerular lesion, (2) well-preserved interstitial and arteriolar compartments, (3) better blood pressure control, and (4) relatively well-preserved renal function [7, 24]. Nonetheless, it is noteworthy that a small fraction of DKD patients with significant structural and functional impairment in the kidney have normal albuminuria [7, 33, 34]. Such phenomenon was evident in the three DKD cases in our GD group (patient nos. 10, 19, 23 in Table 2). They were two male and one female with a mean eGFR of 40.2 ml/min per 1.73m2, and a history of DM and kidney dysfunction of about ten years. Their renal biopsies revealed sclerotic glomeruli and typical arteriolar sclerosis and hyalinosis, in the absence of K-W nodule (pathological features consistent with type III of Fioretto classification for diabetic nephropathy [35]), while their mean 24hr-UPr were only 87.2 mg. Comparable pathological-proteinuric dissociation had also been documented in, but not limited to, lupus nephritis [36, 37] and obesity-related glomerulopathy [26]. In our study, a 68-year-old female patient was found to have cryoglobulinemic DPGN. She presented with AKI and her eGFR was 27.9 ml/min per 1.73 m2 at the time of renal biopsy, while the 24hr-urinary protein/albumin was only 113.7 mg/50.2 mg, with no microscopic hematuria. Her renal function significantly improved after treatment with pulse steroid, plasma exchange and rituximab.
Acute interstitial nephritis (AIN) reportedly accounted for 15–27% of renal biopsy findings among subjects of acute kidney injury, for which the causes might involve drug, infection, systemic disease and idiopathic origin [38]. The urinary protein excretion in acute tubulointerstitial diseases is typically modest with median or mean values of 0.7 g (0.39, 1.0)/24hr [39] or 0.9 ± 1.1 g/24hr (range, 0–6 g) [40] in different cohorts. The reported incidence of nonproteinuric AIN was infrequent, ranging between 4.5 [38] and 8% [40], which could be owing to preserved protein resorption function of renal tubules. In our series, ten of the patients presented with AKI, of whom nine (90%) exhibited tubulointerstitial disease; eight out of these nine cases had a history of diarrhea prior to biopsy, implying acute gastroenteritis as the trigger of AKI. In the TID group, a higher incidence of AKI and more extensive tubulointerstitial involvement might account for its lowest eGFR amongst the three groups (Table 2).
The fraction of HT (8/11) in the SVD group is the highest among the three groups. Six of the 11 cases had isolate HT (without DM) and 1 patient with isolate DM (without HT); arteriolar hyalinosis and significant small vascular wall thickening were observed in these seven patients. Whereas, four patients in the GD group with intercurrent HT and DM registered no apparent small vascular change (patient nos. 1, 6, 10, 13, Table 2), which is thought to be related with younger age, shorter disease vintage, or possibly, limited specimen tissue. The resistant blood vessels (small artery and arterioles, with lumen diameters in-between 15 and 300 μm) are early targets of HT and DM. Endothelial cell is now recognized as the primary site of tissue injury in both conditions. HT and DM, via multiple pathways, induce endothelial dysfunction and subsequent vasospasm and mural remodeling of small vessels [41, 42]. Both HT and DM are known to cause arterial wall thickening and luminal narrowing. Another feature of hypertensive and diabetic vascular disease is small artery hyalinosis, which is associated impaired vascular autoregulation and is a marker of reduced blood flow and hypoxemia of renal interstitium [43]. Similar histological changes can also be seen in the elderly due to vascular ageing. The typical pathological abnormalities of hypertensive nephrosclerosis (HTN) include glomerular ischemia (induced by afferent arteriolar sclerosis) and glomerular hypertrophy and sclerosis (secondary to hyperperfusion). Whereas DM may induce pathological damage in every compartment of the kidney, the most characteristic of which is (K-W) nodular glomerular sclerosis that results from excessive extracellular matrix accumulation and mesangial expansion. But the degree of the renal damage depends as well on the disease severity, duration, and the genetic predisposition of individual HT and DM patient [44–46]. It is notable that either small arterial sclerosis or hyalinosis is not pathognomic in HTN or DKD [47]. Renal vascular lesions are observable in a variety of nephropathies that are independent of systemic hypertension [48]. This phenomenon is thought to be attributable to the activation of RAAS (renin angiotensin and aldosterone system) of local tissue [47]. As shown in our series, six cases (26.1%, patient nos. 7, 11, 14, 18, 20 and 21) of the GD group and seven cases (35%, patient nos. 28–31, 37, 40, 43) in the TID group had no background HT or DM, but arteriolar hyalinosis and/or small vascular wall thickening of variable degree were identified (Table 2).
Podocyte foot process and slit diaphragm are the key components to maintain glomerular basement membrane (GBM) selectivity. 46 of the 50 patients with available EM data invariably showed different degrees of PFPE (segmental in 31 [67.4%], major in 12 [26.1%], and extensive effacement in three [6.5%], Table 2). PFPE results from fusion and flattening deformation of foot process, due to the disruption and reorganization of its actin cytoskeleton. It is an energy-consuming adaptive response of podocyte to trade off excessive damage under various pathological stressors [49, 50]. PFPE is potentially reversible if stressful insult is resolved [50]. The relative moderate PFPE change observed in our series is a reflection of nonsevere podocyte injuries in general.
Admittedly there were limitations in this study that include pathological methodology, retrospective nature and a relative small patient number. The classification of our patients are mainly based upon morphological study in conjunction with clinical data and judgements of the reviewers, which may potentially compromise accuracy. Since molecular pathology, given available, is more informative and specific, capable of differentiating overlapping zone or even disproving the diagnosis made by a routine approach [51]. Some other factors, such as PRB indication adopted, clinician’s input, geography, disease epidemiology and patient consent, among others, may swing patient selection and study outcome [52]. The difference in the rate of enrollment of the three centers provided some thought in this connection. It is our hope that this study may serve to provide experience, reference and insight to assist clinical decision-making.
Conclusions
In summary, we conducted a retrospective investigation of the renal histopathology in patients presenting with non-proteinuric kidney dysfunction, a situation by no means rarely encountered in practice. Analyses indicated GD, followed by TID, accounted for around 80% of 53 cases enrolled; SVD was the pathological subtype in the rest of 20% patients. Renal dysfunction of normal-range proteinuria may possibly, but not necessarily, be related with histologically nonsevere glomerular disease, or tubular-interstitial involvement. In a patient with a history of hypertension, small arterial or arteriolar sclerosis could be considered as the underlying cause of his/her kidney dysfunction.
Acknowledgements
The authors of this article would like to thank Mr. T.C. Pai for his kind contribution to grammar check of the manuscript.
Authors’ contributions
X.L., Y.R. and X.G., conceptualization. H.H., L.F. and L.Y., data collection and analysis. H.H. and X.L., statistical analysis and initial writing. P.P. and X.L., oversaw and revised the manuscript. All authors contributed to critical review and approved the final version of the manuscript.
Funding
None declared.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hai-Yan He, Ling Feng and Yong-Ke You have contributed equally to this work.
References
- 1.D’Agati VD, Mengel M. The rise of renal pathology in nephrology: structure illuminates function. Am J Kidney Dis. 2013;61(6):1016–25. [DOI] [PubMed] [Google Scholar]
- 2.Dhaun N, Bellamy CO, Cattran DC, Kluth DC. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85(5):1039–48. [DOI] [PubMed] [Google Scholar]
- 3.Ubara Y, Kawaguchi T, Nagasawa T, Miura K, Katsuno T, Morikawa T, Ishikawa E, Ogura M, Matsumura H, Kurayama R, et al. Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol. 2021;25(4):325–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63(3):809–25. [DOI] [PubMed] [Google Scholar]
- 5.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17(11):2974–84. [DOI] [PubMed] [Google Scholar]
- 6.Writing Group for the CKDPC, Grams ME, Coresh J, Matsushita K, Ballew SH, Sang Y, Surapaneni A, Alencar de Pinho N, Anderson A, Appel LJ, et al. Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. 2023;330(13):1266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanouchi M, Furuichi K, Hoshino J, Toyama T, Hara A, Shimizu M, Kinowaki K, Fujii T, Ohashi K, Yuzawa Y, et al. Nonproteinuric versus proteinuric phenotypes in diabetic kidney disease: a propensity score-matched analysis of a nationwide. Biopsy-Based Cohort Study Diabetes Care. 2019;42(5):891–902. [DOI] [PubMed] [Google Scholar]
- 8.Hobeika L, Hunt KJ, Neely BA, Arthur JM. Comparison of the rate of renal function decline in nonproteinuric patients with and without diabetes. Am J Med Sci. 2015;350(6):447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshadri L, Venkataraman I. Hypertension in pregnancy. Outcomes, proteinuric vs. nonproteinuric. J Reprod Med. 1997;42(2):88–90. [PubMed] [Google Scholar]
- 10.Hoshino Y, Kaga T, Abe Y, Endo M, Wakai S, Tsuchiya K, Nitta K. Renal biopsy findings and clinical indicators of patients with hematuria without overt proteinuria. Clin Exp Nephrol. 2015;19(5):918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iimori S, Naito S, Noda Y, Sato H, Nomura N, Sohara E, Okado T, Sasaki S, Uchida S, Rai T. Prognosis of chronic kidney disease with normal-range proteinuria: the CKD-ROUTE study. PLoS ONE. 2018;13(1): e0190493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, Inker LA, Levin A, Mehrotra R, Palevsky PM, et al. Nomenclature for kidney function and disease: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2020;97(6):1117–29. [DOI] [PubMed] [Google Scholar]
- 13.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44. [DOI] [PubMed] [Google Scholar]
- 14.Liapis H. Electron microscopy in kidney research: seeing is believing. Ultrastruct Pathol. 2013;37(5):340–5. [DOI] [PubMed] [Google Scholar]
- 15.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 2017;91(5):1014–21. [DOI] [PubMed] [Google Scholar]
- 16.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63. [DOI] [PubMed] [Google Scholar]
- 17.Najafian B, Fogo AB, Lusco MA, Alpers CE. AJKD atlas of renal pathology: diabetic nephropathy. Am J Kidney Dis. 2015;66(5):e37-38. [DOI] [PubMed] [Google Scholar]
- 18.Delanaye P, Jager KJ, Bokenkamp A, Christensson A, Dubourg L, Eriksen BO, Gaillard F, Gambaro G, van der Giet M, Glassock RJ, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane C, Brown M, Dunsmuir W, Kelly J, Mangos G. Can spot urine protein/creatinine ratio replace 24 h urine protein in usual clinical nephrology? Nephrology (Carlton). 2006;11(3):245–9. [DOI] [PubMed] [Google Scholar]
- 20.Bökenkamp A. Proteinuria-take a closer look! Pediatr Nephrol. 2020;35(4):533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siwach SB, Kalra OP, Sharma R, Singh V, Chopra JS. Estimation of 24 hour protein excretion from single random urine specimen. Indian J Med Res. 1990;92:105–8. [PubMed] [Google Scholar]
- 22.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol. 2012;2012: 481520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Berkel Y, Ludwig M, van Wijk JAE, Bökenkamp A. Proteinuria in Dent disease: a review of the literature. Pediatr Nephrol. 2017;32(10):1851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanouchi M, Furuichi K, Hoshino J, Ubara Y, Wada T. Nonproteinuric diabetic kidney disease. Clin Exp Nephrol. 2020;24(7):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toto RD. Proteinuria and hypertensive nephrosclerosis in African Americans. Kidney Int Suppl. 2004;92:S102-104. [DOI] [PubMed] [Google Scholar]
- 26.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, Alastrue A, Ariza A. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73(8):947–55. [DOI] [PubMed] [Google Scholar]
- 27.Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A, et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49(1):69–82. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Liu J, Duan S, Chen P, Tang L, Zhang L, Feng Z, Cai G, Wu J, Chen X. Clinicopathological features to predict progression of IgA nephropathy with mild proteinuria. Kidney Blood Press Res. 2018;43(2):318–28. [DOI] [PubMed] [Google Scholar]
- 29.Tan M, Fang J, Xu Q, Zhang C, Zou G, Wang M, Li W. Outcomes of normotensive IgA nephropathy patients with mild proteinuria who have impaired renal function. Ren Fail. 2019;41(1):875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He L, Cao X, Yang D, Zhuo H, Peng X, He L, Liu H, Peng Y. Clinicopathological analysis for IgA nephropathy with isolated hematuria and/or mild proteinuria. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2019;44(6):642–8. [DOI] [PubMed] [Google Scholar]
- 31.Ekinci EI, Jerums G, Skene A, Crammer P, Power D, Cheong KY, Panagiotopoulos S, McNeil K, Baker ST, Fioretto P, et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care. 2013;36(11):3620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316(6):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fufaa GD, Weil EJ, Lemley KV, Knowler WC, Brosius FC 3rd, Yee B, Mauer M, Nelson RG. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol. 2016;11(2):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, Lovato T, Richardson M, Myers BD, Nelson RG. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012;82(9):1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, Sambataro M, Abaterusso C, Baggio B, Crepaldi G, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39(12):1569–76. [DOI] [PubMed] [Google Scholar]
- 36.Chedid A, Rossi GM, Peyronel F, Menez S, Atta MG, Bagnasco SM, Arend LJ, Rosenberg AZ, Fine DM. Low-level proteinuria in systemic lupus erythematosus. Kidney Int Rep. 2020;5(12):2333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on Lupus Nephritis: core curriculum 2020. Am J Kidney Dis. 2020;76(2):265–81. [DOI] [PubMed] [Google Scholar]
- 38.Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–61. [DOI] [PubMed] [Google Scholar]
- 39.Clarkson MR, Giblin L, O’Connell FP, O’Kelly P, Walshe JJ, Conlon P, O’Meara Y, Dormon A, Campbell E, Donohoe J. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19(11):2778–83. [DOI] [PubMed] [Google Scholar]
- 40.González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P, Bernis C, Parra EG, Delgado R, Sanz M, Ortiz M, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73(8):940–6. [DOI] [PubMed] [Google Scholar]
- 41.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endemann DH, Pu Q, De Ciuceis C, Savoia C, Virdis A, Neves MF, Touyz RM, Schiffrin EL. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension. 2004;43(2):399–404. [DOI] [PubMed] [Google Scholar]
- 43.Bazzi C, Stivali G, Rachele G, Rizza V, Casellato D, Nangaku M. Arteriolar hyalinosis and arterial hypertension as possible surrogate markers of reduced interstitial blood flow and hypoxia in glomerulonephritis. Nephrology (Carlton). 2015;20(1):11–7. [DOI] [PubMed] [Google Scholar]
- 44.Kopp JB. Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens. 2013;22(3):266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Rao F, Wen G, Gayen JR, Zhang K, Vaingankar SM, Biswas N, Mahata M, Friese RS, Fung MM, et al. Naturally occurring genetic variants in human chromogranin A (CHGA) associated with hypertension as well as hypertensive renal disease. Cell Mol Neurobiol. 2010;30(8):1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dastgheib SA, Najafi F, Shajari A, Bahrami R, Asadian F, Sadeghizadeh-Yazdi J, Akbarian E, Emarati SA, Neamatzadeh H. Association of plasminogen activator inhibitor-1 4G5G polymorphism with risk of diabetic nephropathy and retinopathy: a systematic review and meta-analysis. J Diabetes Metab Disord. 2020;19(2):2005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyrier A. Nephrosclerosis: update on a centenarian. Nephrol Dial Transplant. 2015;30(11):1833–41. [DOI] [PubMed] [Google Scholar]
- 48.Tracy RE, Bhandaru SY, Oalmann MC, Guzman MA, Newmann WP 3rd. Blood pressure and nephrosclerosis in black and white men and women aged 25 to 54. Mod Pathol. 1991;4(5):602–9. [PubMed] [Google Scholar]
- 49.Kopp JB, Anders HJ, Susztak K, Podesta MA, Remuzzi G, Hildebrandt F, Romagnani P. Podocytopathies. Nat Rev Dis Primers. 2020;6(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89(6):1221–30. [DOI] [PubMed] [Google Scholar]
- 51.Patel J, Torrealba JR, Poggio ED, Bebiak J, Alpers CE, Grewenow SM, Toto RD, Eadon MT. Molecular signatures of diabetic kidney disease hiding in a patient with hypertension-related kidney disease: a clinical pathologic molecular correlation. Clin J Am Soc Nephrol. 2022;17(4):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiorentino M, Bolignano D, Tesar V, Pisano A, Van Biesen W, D’Arrigo G, Tripepi G, Gesualdo L. Group E-EIW: renal biopsy in 2015–from epidemiology to evidence-based indications. Am J Nephrol. 2016;43(1):1–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.