Abstract
Background and Objective
Asciminib is approved in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) treated with ≥ 2 prior tyrosine kinase inhibitors. Here, we aimed to demonstrate similarity in efficacy/safety of asciminib 80 mg once daily (q.d.) versus 40 mg twice daily (b.i.d.) in patients with CML-CP without T315I mutation and support the use of the 200-mg b.i.d. dosage in patients harboring T315I, using model-informed drug development.
Methods
Data were collected from 199 patients in the phase I (NCT02081378; 10−200 mg b.i.d. or 10−400 mg q.d.) and 154 patients in the phase III (NCT03106779; 40 mg b.i.d.) studies. Evaluations were based on population pharmacokinetics (PopPK) and exposure–response (efficacy/safety) analyses.
Results
PopPK showed comparable exposure (area under the curve, AUC0-24h) for 40 mg b.i.d. and 80 mg q.d. (12,638 vs 12,646 ng*h/mL); average maximum and minimum plasma concentrations for 80 mg q.d. were 1.61- and 0.72-fold those of 40 mg b.i.d., respectively. Exposure–response analyses predicted similar major molecular response rates for 40 mg b.i.d. and 80 mg q.d. (Week 24: 27.6% vs 24.8%; Week 48: 32.3% vs 30.6%). Results also established adequacy of 200 mg b.i.d. in patients with T315I mutation (Week 24: 20.7%; Week 48: 23.7%), along with a similar safety profile for all dose regimens.
Conclusions
Similarity between 40 mg b.i.d. and 80 mg q.d. regimens was investigated, demonstrating similar and substantial efficacy with well-tolerated safety in patients without T315I mutation. The 200-mg b.i.d. dose was deemed safe and effective for patients with T315I mutation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40262-024-01411-1.
Key Points
| Asciminib, a novel BCR::ABL1 inhibitor, has been approved in patients with chronic myeloid leukemia in chronic phase (CML-CP) at a dose of 40 mg twice daily (b.i.d.). A once-daily (q.d.) dosing regimen could provide greater patient compliance in long-term therapy, potentially improving clinical benefit. |
| This exposure–response analysis showed comparable efficacy/safety profiles for asciminib at 80 mg q.d. and 40 mg b.i.d. dosing regimens for patients without the T315I mutation. |
| For patients harboring the T315I mutation, asciminib at 200 mg b.i.d. was effective and safe. |
Introduction
Chronic myeloid leukemia (CML) is driven by the constitutively active BCR::ABL1 tyrosine kinase. The hallmark of CML is the Philadelphia (Ph) chromosome, found in ~ 95% of patients [1]. Evolution of CML involves a chronic phase (CP), accelerated phase (AP), and blast phase [2]. Currently, tyrosine kinase inhibitors (TKIs) are the standard of care in CML treatment, and have greatly improved outcomes in patients with CML. However, there is a serious unmet need in patients with CML-CP due to treatment failure caused by either intolerance or resistance. Factors associated with treatment failure include mutations (particularly the T315I mutation) in the BCR::ABL1 gene [3–5], increasing treatment failure rates with each line of TKI treatment [6–9], limited options in later lines (ponatinib is available with restricted use) [10], and lack of clarity regarding the choice/sequencing of TKIs [11, 12].
Asciminib is a specific, orally bioavailable BCR::ABL1 inhibitor that works by specifically targeting the ABL myristoyl pocket (STAMP), unlike other TKIs which target the ATP-binding site of BCR::ABL1; hence, asciminib is effective against mutations in the ATP-binding site that cause resistance to other TKIs. In addition, asciminib offers potential for improved safety and tolerability when administered as monotherapy [13–16].
The approval of asciminib as third-line therapy in patients was mainly supported by the following two studies: (i) the phase I (first-in-human), open-label, dose-finding study in patients with CML (ClinicalTrials.gov identifier: NCT02081378, hereafter referred to as dose-finding study) [13] designed to determine the recommended dose for expansion (RDE); and (ii) the pivotal phase III, multicenter, open-label, randomized study of asciminib versus bosutinib in patients with CML-CP treated with two or more prior TKIs (NCT03106779, hereafter referred to as ASCEMBL study) [17]. In patients with CML-CP/AP without the T315I mutation, 40 mg twice daily (b.i.d.) was determined as the RDE. The T315I mutation is associated with resistance to all ATP-competitive TKIs except ponatinib and olverembatinib; for patients with CML-CP/AP harboring this mutation, 200 mg b.i.d. was determined as the RDE [18]. Asciminib 40 mg b.i.d. demonstrated statistically significant and clinically meaningful superiority in efficacy compared with bosutinib 500 mg once daily (q.d.), with more than double the proportion of patients achieving a major molecular response (MMR; BCR::ABL1IS ≤ 0.1%) at 96 weeks (37.6% for asciminib vs 15.8% for bosutinib). Asciminib also showed a favorable safety profile, with fewer grade ≥ 3 adverse events (AEs) reported (56.4% for asciminib vs 68.4% for bosutinib) and fewer AEs leading to treatment discontinuation (7.7% for asciminib vs 26.3% for bosutinib) [17].
Considering the requisite long-term compliance to asciminib therapy, b.i.d. dosing under fasting conditions poses practical challenges for many patients with regard to managing their daily meals and schedules, thereby impacting adherence and long-term compliance. One-third or more of patients with CML have been found to be non-compliant to therapy [19]. A patient-led study investigating non-adherence to therapy in patients with CML found that 35.8% of patients taking their medication once daily were highly adherent, whereas only 24.9% of patients taking their medication twice daily were highly adherent and 26.7% were in the low adherence group [20]. In fact, adherence to therapy for chronic health conditions has always been poor, and the findings of a systematic review on adherence to oral medications for chronic conditions indicated that once-daily dosing regimens were associated with an increase in days of adherence versus more frequent dosing (2% to 44% more adherent days than twice-daily regimens and 22% to 41% more adherent days than thrice-daily regimens across individual studies), reaching statistical significance (p < 0.05) in 15 of those 20 studies [21]. Therefore, an asciminib once-daily dosing regimen could be expected to result in improved convenience for patients and better adherence.
The purpose of this analysis was to illustrate the similarities in efficacy and safety of an alternative asciminib dose regimen of 80 mg q.d. to the approved asciminib dose regimen of 40 mg b.i.d. for patients with CML-CP without the T315I mutation using population pharmacokinetics (PopPK) and exposure–response (ER) analyses based on pooled efficacy (ERe analysis) and safety (ERs analysis) data from the dose-finding and ASCEMBL studies. We also aimed to support the use of 200 mg b.i.d. in patients with CML-CP harboring the T315I mutation. The overall aim of this manuscript is to inform clinicians on asciminib dosing; we thereby provide new results on the exposure–safety relationship of asciminib and present them together with previous results from PopPK and exposure–efficacy analyses to support the 80-mg q.d. dosing regimen in patients with CML-CP without T315I mutation and the 200-mg b.i.d. dosing regimen in patients with T315I mutation [22, 23]. Altogether, the results highlight how model-informed drug development (MIDD) was utilized to support the approval of these two dosing regimens in various countries including the United States.
Methods
Study Design and Conduct
This analysis was based on the data from patients with CML-CP (and CML-AP for safety analyses) from the dose-finding and ASCEMBL studies. Both studies, serving as the data sources for this analysis, were approved by the appropriate Institutional Review Board. Patients were administered asciminib at doses of 10−200 mg b.i.d. or 80 mg, 120 mg or 200 mg q.d. (dose-finding study), or 40 mg b.i.d. (ASCEMBL study). The analysis was categorized into PopPK, ERe and ERs. The details for data pooling and software used in the analyses can be found in the electronic supplementary material (ESM, Methods S1 and S2).
Summary of the Population Pharmacokinetics and Exposure–Response Modeling Strategy
A PopPK model was first built [22], enabling prediction of individual daily exposure metrics (area under the curve [AUC], maximum plasma concentration [Cmax], and minimum plasma concentration [Cmin]) based on actual dosing records. Thereafter, an ERe model and an ERs model were built to link individual exposure metrics with observed longitudinal BCR::ABL1IS levels or key safety events including laboratory abnormalities [23].
Exposure–Response Analysis for Efficacy
An ERe analysis was performed to characterize the time-course of BCR::ABL1IS levels in patients with CML-CP. Previously, a pharmacodynamic model, using a pool of patients with CML-CP from the dose-finding and ASCEMBL studies, investigated the overall ER relationships [23]. Briefly, the model had three compartments representing three cell types: (i) quiescent leukemic stem cells (Q), (ii) proliferating drug-susceptible leukemic bone marrow cells (P) and (iii) proliferating resistant leukemic cells (R). The self-proliferation of leukemic cells is governed by the growth rate constant (kgr); transformations from Q to P and from Q to R are represented by kqp and kqr, respectively. In the subset of patients without the T315I mutation, the drug killing effect on the P-cell population is represented by a power model wherein its slope is characterized by the effect magnitude (Effmag). The drug killing effect in the subset of patients with T315I mutation is characterized by a maximum effect (Emax) model. Two analyses were performed to predict the response to asciminib treatment (Fig S1, see ESM). The first analysis aimed at exploring the difference between a q.d. and a b.i.d. regimen with the same total daily dose. We compared the influence of the 40-mg b.i.d. and 80-mg q.d. regimens on BCR::ABL1IS levels using two drug effect models: the same power model based on the three pharmacokinetic metrics (AUC, Cmax, Cmin) as used previously [23] and a categorical effect model based on the dosing regimens (40 mg b.i.d. and 80 mg q.d.) to simplify the structural model by reducing the number of parameters estimated. The findings are presented in this article. The second analysis used an Emax model to assess the effect of asciminib 200 mg b.i.d. on patients harboring the T315I mutation [23]. In this model, the drug effect was modified to allow estimation of asciminib exposure leading to 50% of maximal efficacy (EC50) along with equation-derived parameters such as EC90 and EC95, achieving 90% and 95% maximal efficacy, respectively.
Exposure–Response Simulations for Efficacy
The simulation methods used with the updated models in this article were similar to those used in a previous article [23]. To further explore the impact of asciminib dosing regimens on patients harboring the T315I mutation, the proportions of patients for whom exposure was above the model-estimated EC50 (and derived EC80, EC90, EC95, etc.) were computed to assess which dosing regimen was more likely to achieve MMR.
Exposure–Response Analysis for Safety
The ERs analysis evaluated the relationship between asciminib exposure metrics and key safety events including laboratory abnormalities (assigned as per NCI Common Terminology Criteria for Adverse Events version 4.03 for amylase, lipase, platelets, neutrophils, hemoglobin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], total bilirubin, and triglycerides); vital signs (systolic and diastolic blood pressures) for hypertension; and adverse events (AEs) of fatigue and asthenia. PopPK-predicted individual exposure metrics for the analyses were obtained using the average over the 5 days prior to a safety event. Each laboratory assessment, vital sign, or AE, as well as grade ≥ 3 treatment-emergent AE (TEAE), was analyzed separately based on abnormal occurrences in each 28-day cycle. Since the treatment duration and the number of planned and unplanned clinical visits (for laboratory, vital sign and/or AE assessments) differed between patients, a binary outcome was created based on 28-day cycles. For each cycle, a value equal to 1 was derived if a patient experienced one or more occurrence of the event within the cycle, or zero otherwise. A generalized linear mixed-effects model (repeated measures logistic regression) was fitted using the SAS procedure PROC GENMOD, based on generalized estimating equation methods. The model provided an estimate of the probability of one or more occurrence of an event within a 28-day cycle, and its association with exposure (the 5-day-average PK prior to the event), time (cycle number) and baseline (baseline values for laboratory parameters and blood pressure baseline status for hypertension). Due to the nature of repeated measures of the data, a repeated-measures logistic regression was used assuming observations within the same patient were correlated.
In addition, the exposure–safety relationship for TEAEs leading to dose reductions and/or interruptions was investigated in a time-to-event analysis using a Cox regression model. The log-transformed 5-day-moving-average exposure prior to onset was used as a time-dependent covariate.
Concentration-QTcF Analysis
The effect of asciminib concentration on electrocardiogram (ECG) parameters (QT interval corrected using the Fridericia method [QTcF]) was analyzed separately. A linear mixed-effects model was used to characterize QTcF change from baseline versus time-matched observed plasma asciminib concentrations, including baseline QTcF as a covariate and patient as a random effect. Changes in QTcF from baseline were estimated at relevant asciminib concentrations achieved at doses including 40 mg b.i.d., 80 mg q.d., and up to twice the highest clinically relevant exposure (HCRE). The HCRE was determined based on the geometric mean Cmax at 200 mg b.i.d. at steady state and considering a 1.59-fold increase in Cmax of asciminib when taken in combination with imatinib [23]. This was regarded as the worst-case scenario for Cmax as this increment was even higher than the increases in Cmax of asciminib observed in patients with severe renal and hepatic impairment compared with patients with normal renal/hepatic function [24].
Results
Data Pooling
The PopPK and ERs analyses were conducted using data from 353 patients (199 and 154 from the dose-finding and ASCEMBL studies, respectively, including 188 patients following a 40-mg b.i.d. dosing regimen, 18 with 80 mg q.d., and 132 patients with a total daily dose > 80 mg), with 6603 asciminib PK concentrations. Laboratory abnormalities and vital sign analyses used data from 349 patients from both studies, while the QT/QTc analysis used data from 239 patients from the dose-finding study. For the ERe analysis, subsets of this pool were used for the full analysis (303 patients), the first subgroup analysis comparing the effects of the 40-mg b.i.d. and 80-mg q.d. dosing regimens (194 patients), and the second subgroup analysis studying the effect of asciminib 200 mg b.i.d. on patients with the T315I mutation (67 patients who harbored the T315I mutation) [22].
PopPK Analysis
Both 40-mg b.i.d. and 80-mg q.d. dosing regimens showed comparable steady-state AUC0-24h (12,638 ng*h/mL and 12,646 ng*h/mL, respectively), while the average steady-state Cmax and Cmin of 80 mg q.d. were 1.61-fold and 0.72-fold that of 40 mg b.i.d., respectively. Detailed results can be found in our previous publication [22].
Exposure–Response Analysis for Efficacy
Dosing Regimen Analysis: 40 mg b.i.d. versus 80 mg q.d.
To evaluate whether asciminib dosing regimens of 80 mg q.d. and 40 mg b.i.d. led to similar effects on the time-course of BCR::ABL1IS, the final ER model [23] was slightly modified and refitted to better describe a subset of the original dataset, which consisted of patients with starting-dose regimens of either 80 mg q.d. or 40 mg b.i.d. Since the Cmin of asciminib is about 30% lower for the 80-mg q.d. dose compared with that of the 40-mg b.i.d. dose and given that this difference in Cmin may be a contributor for differences in efficacy between the two regimens, the effect of asciminib on BCR::ABL1IS was estimated as a function of Cmin. In an additional model, a function of the dosing regimen (ARM) treated as a categorical covariate was used to further assess the difference between the two dosing regimens. Equations describing the final models have previously been published and are summarized in the ESM (Methods S3) [23].
The results of the two pharmacodynamic models are presented in Table 1.
Table 1.
Parameter estimates of the pharmacodynamic model for asciminib 80 mg q.d. versus 40 mg b.i.d. in the subset of patients without the T315I mutation
| Cmin (RSE) | Regimen (RSE) | |
|---|---|---|
| Population means | ||
| Q (t = 0) | 0.0260 (45.4%) | 0.0156 (46.4%) |
| P (t = 0) | 0.0246 (4.9%) | 0.0253 (4.0%) |
| R (t = 0) | 2.05 × 10–9 (75.4%) | 8.94 × 10–6 (48.4%) |
| kgr (1/y) | 28 (fixed) | 28 (fixed) |
| kqp (1/y) | 0.53 (fixed) | 0.53 (fixed) |
| kqr (1/y) | 6.5 (fixed) | 6.5 (fixed) |
| Effmag | 42.1 (3.4%) | 39.8 (3.18%) |
| Gamma | 0.0399 (7.2%) | |
| Standard deviation of random effects (IIV) | ||
| Q (t = 0) | 3.49 (7.6%) | 3.72 (8.3%) |
| P (t = 0) | 0.147 (20.5%) | 0.174 (17.2%) |
| R (t = 0) | 2.36 (27.4%) | 3.37 (11.7%) |
| Effmag | 0.265 (7.1%) | 0.277 (6.3%)† |
| Cov (Q [t = 0], Effmag) | − 0.647 (11.1%) | − 0.718 (7.1%) |
| Covariate effect magnitude†† | ||
| L10BA0 on Q (t = 0) | 2.53 (14.7%) | 1.97 (19.8%) |
| L10BA0 on P (t = 0) | 2.51 (1.7%) | 2.55 (1.5%) |
| Regimen on Effmag (80 mg q.d.) | − 0.0447 (148%) | |
| NUMTTRT (3,4,5) on Effmag | − 0.0725 (56.7%) | − 0.0692 (55.4%) |
| Residual error | ||
| a (additive) | 0.23 (2.7%) | 0.19 (2.6%) |
| b (proportional) | 0.0975 (7.1%) | 0.081 (6.3%) |
| Fit statistics | ||
| – 2 log-likelihood | 1316.21 | 998.68 |
| Corrected BIC | 1410.42 | 1090.72 |
BIC Bayesian information criteria, b.i.d. twice daily, Cmin minimum plasma concentration, Cov covariate, Effmag effect of drug, IIV inter-individual variability, kgr growth rate of resistant and proliferating cells, kqp and kqr transfer rate constants between proliferating, resistant and quiescent compartments, L10BA0 baseline log10-transformed BCR::ABL1IS, NUMTTRT number of prior TKI treatments, P proliferating leukemic bone marrow cells, Q quiescent stem cells, q.d. once daily, R proliferating resistant cells, RSE relative standard error, t time, TKI tyrosine kinase inhibitor, y year
†This value is for the 40-mg b.i.d. dosing regimen
††Covariate effect magnitude is reported as log. The linear effect is exp(value)
In the Cmin model, the effect of Cmin as estimated by the gamma parameter is small (0.0365), such that a lower Cmin by 30% would lead to a decrease in drug effect by < 2%. Varying Cmin magnitude on the drug killing effect of asciminib on the susceptible P cell population resulted in a plateau. In the regimen model, the Effmag of the 40-mg b.i.d. regimen was 39.8, while the estimated regimen effect was − 0.045 (not statistically significant), giving an Effmag for the 80-mg q.d. regimen close to 38. These two models thus estimated a negligible difference in the drug killing effect on the susceptible leukemic P-cell population between the two regimens. Since the regimen model is associated with better model diagnostics as it has a higher log likelihood and lower Bayesian information criteria (BIC), model diagnostics are presented only for the regimen model (Figs. S1 and S2, see ESM), and the regimen model was used to perform simulations.
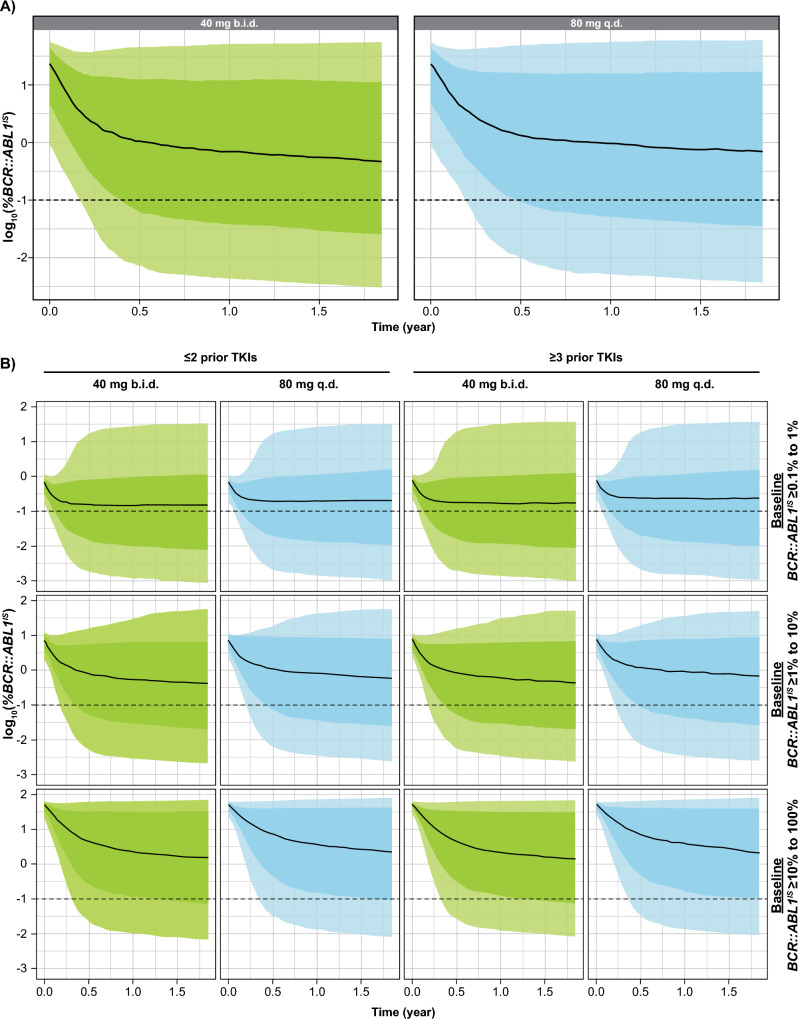
As expected, the predictions from simulations were very similar for 80-mg q.d. and 40-mg b.i.d. dosing regimens (Fig. 1). The resulting predicted MMR rates at Week 24 and Week 48, with or without stratification by baseline BCR::ABL1IS levels and number of prior TKIs are displayed in Table 2.
Fig. 1.
Simulated time-course of log10-transformed BCR::ABL1IS for asciminib 40 mg b.i.d. and 80 mg q.d. in patients not harboring the T315I mutation: all patients (a) and patients stratified by number of prior TKIs and baseline BCR::ABL1IS levels (b). The black line represents the median over the 100 replicates of the 50th percentile of BCR::ABL1IS. The darker shaded area represents the median of 25th and 75th percentiles of BCR::ABL1IS, and the lighter shaded area is the median of 10th and 90th percentiles of BCR::ABL1IS. b.i.d. twice daily, q.d. once daily, TKI tyrosine kinase inhibitor
Table 2.
MMR rate at Weeks 24 and 48 comparing 40-mg b.i.d. versus 80-mg q.d. asciminib regimens from simulations of 100 patients per trial in 100 trials based on a PD model for patients not harboring the T315I mutation
| Category | 40 mg b.i.d. | 80 mg q.d. | ||
|---|---|---|---|---|
| 24-wk MMR rate (%) | 48-wk MMR rate (%) | 24-wk MMR rate (%) | 48-wk MMR rate (%) | |
| Overall | 27.6 ± 4.5 | 32.3 ± 4.8 | 24.8 ± 4.2 | 30.6 ± 4.7 |
| Baseline BCR::ABL1IS > 0.1% to 1%, ≤ 2 prior TKIs | 44.2 ± 5.2 | 45.2 ± 5.6 | 40.5 ± 4.3 | 42.2 ± 4.1 |
| Baseline BCR::ABL1IS > 0.1% to 1%, ≥ 3 prior TKIs | 42.2 ± 4.7 | 43.9 ± 4.5 | 39.2 ± 4.7 | 41.3 ± 4.8 |
| Baseline BCR::ABL1IS > 1% to 10%, ≤ 2 prior TKIs | 29.8 ± 4.3 | 34.9 ± 4.7 | 27.1 ± 4.9 | 33.2 ± 5.2 |
| Baseline BCR::ABL1IS > 1% to 10%, ≥ 3 prior TKIs | 29.4 ± 4.6 | 34.8 ± 4.9 | 25.6 ± 4.3 | 31.7 ± 5.1 |
| Baseline BCR::ABL1IS > 10% to 100%, ≤ 2 prior TKIs | 17.9 ± 3.8 | 23.9 ± 4.2 | 14.9 ± 2.9 | 21.7 ± 3.9 |
| Baseline BCR::ABL1IS > 10% to 100%, ≥ 3 prior TKIs | 17.0 ± 3.7 | 23.5 ± 4.6 | 14.7 ± 3.3 | 21.6 ± 3.6 |
Data reported as mean ± standard deviation
b.i.d. twice daily, MMR major molecular response, PD pharmacodynamic, q.d. once daily, TKI tyrosine kinase inhibitor, wk week
The overall predicted MMR rates for 40 mg b.i.d. versus 80 mg q.d. were 27.6% versus 24.8% and 32.3% versus 30.6% at Weeks 24 and 48, respectively. These rates are very similar for both regimens, though slightly lower for 80 mg q.d., and in close agreement with the observed rates in the ASCEMBL study (25.5% at Week 24) [17]. As baseline BCR::ABL1IS level was a significant covariate in the model, we further stratified the simulations (Fig. 1). While MMR rates were lower for patients with high baseline BCR::ABL1IS for all dosing regimens, the stratified results show that patients with higher baseline BCR::ABL1IS levels would still derive benefit from asciminib treatment. The predicted BCR::ABL1IS levels still decrease after 1 year, suggesting that these patients would benefit from a continuous long-term treatment to further decrease BCR::ABL1IS. Figure 1 and Table 2 show that patients with more lines of prior TKI therapy (≥ 3) would also benefit from asciminib treatment, with an efficacy similar to that predicted for patients treated with fewer prior TKIs.
Analysis for Patients Harboring the T315I Mutation
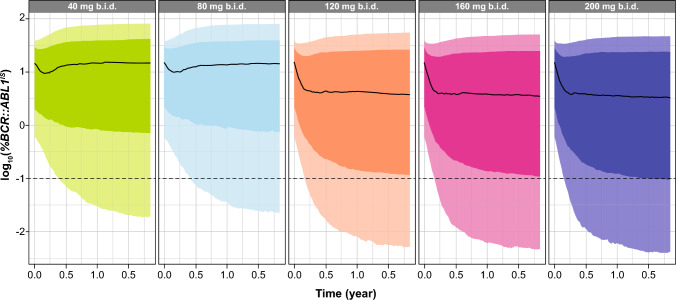
We used the final T315I model [23] to further evaluate the efficacy of asciminib in patients with the T315I mutation following a 200-mg b.i.d. dosing regimen, using an Emax model (Methods S3, see ESM). Compared with the dose of 200 mg b.i.d., this analysis indicated that patients treated with lower dose regimens were less likely to respond to treatment (Fig. 2). Only the three highest doses (120, 160, and 200 mg b.i.d.) showed a general decrease in BCR::ABL1IS levels over time, suggesting that these regimens achieved higher reductions in BCR::ABL1IS as compared with 40 mg b.i.d. and 80 mg q.d. in patients with the T315I mutation (Fig. 2). Findings of the ERe analysis also showed the benefit of using the highest clinical dose to treat patients harboring the T315I mutation: indeed, at 200 mg b.i.d., 99.4% of patients had asciminib exposure (measured as AUC) above the estimated 90% effective concentration (EC90). Lower doses of asciminib also resulted in a lower proportion of patients achieving EC90 than 200 mg b.i.d. (120 mg b.i.d. [84.5% patients] and 160 mg b.i.d. [96.8% patients]) [23].
Fig. 2.
Simulated time-course of log10-transformed BCR::ABL1IS for asciminib 40 mg b.i.d., 80 mg q.d., 120 mg b.i.d., 160 mg b.i.d., or 200 mg b.i.d. in patients (with any number of prior TKI treatments) harboring the T315I mutation. The black line represents the median over the 100 replicates of the 50th percentile of BCR::ABL1IS. The darker shaded area represents the median of 25th and 75th percentiles of BCR::ABL1IS and the lighter shaded area is the median of 10th and 90th percentiles of BCR::ABL1IS. b.i.d. twice daily, q.d. once daily, TKI tyrosine kinase inhibitor
Based on the simulated BCR::ABL1IS, the predicted MMR rates for the 200-mg b.i.d. dose regimen at Weeks 24 and 48 were 20.7% and 23.7%, respectively. Similar MMR rates were obtained for 120 mg b.i.d. (19.3% and 22.9%) and 160 mg b.i.d. (20.2% and 23.8%).
Exposure–Response Analysis for Safety
The efficacy of 80 mg q.d., 40 mg b.i.d. and 200 mg b.i.d. was demonstrated in the earlier publication [22] and in the previous section. Evaluation of a potential link between exposure and safety was analyzed and results are described in this section to justify the benefit/risk between efficacy and safety.
Laboratory Abnormalities
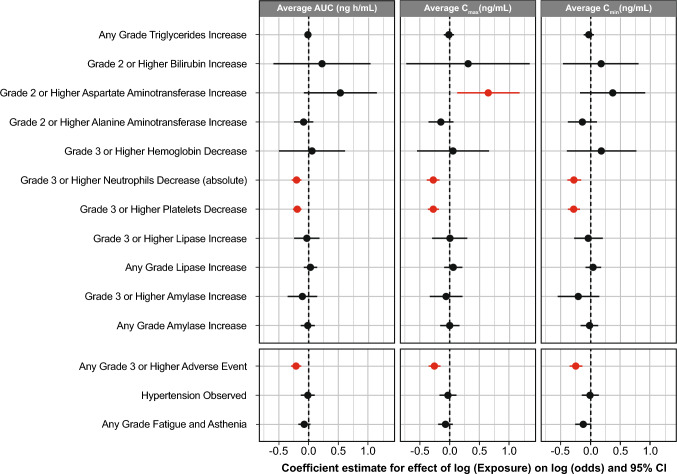
The analysis of laboratory abnormalities showed no statistically significant relationship between asciminib exposure and the majority of the laboratory events analyzed. Figure 3 presents the results of the repeated measures logistic regression analysis. Regression coefficient estimates were negative for the exposure metrics for the majority of laboratory events (including amylase increase [any grade and grade ≥ 3], platelet decrease [grade ≥ 3], neutrophil decrease [grade ≥ 3], ALT increase [grade ≥ 2] and triglyceride increase [any grade]), suggesting no increase in the risk of laboratory events with increased exposure. Exposure metric regression coefficients were positive for several laboratory abnormalities (lipase increase of any grade and grade ≥ 3, hemoglobin decrease of grade ≥ 3, and bilirubin increase of grade ≥ 2), but they were not statistically significant, suggesting minimal impact of increased exposure on the risk of these laboratory abnormalities. Only AST increase of grade ≥ 2 showed a significant positive estimate for the exposure metric, with a p-value of 0.015 for Cmax and 0.088 for AUC. However, the frequency of these events was very low in the ASCEMBL study (1.3% of patients and 0.1% of cycles). For a 5-fold increase in the geometric mean Cmax with the 40-mg b.i.d. dose, the predicted probability of experiencing an event within a cycle increases from 0.3% to 0.9% for the dose-finding study and from 0.1 to 0.4% for the ASCEMBL study, which highlights the rarity of these events (Table S1, see ESM).
Fig. 3.
Regression coefficient estimates of repeated-measures logistic regression model for asciminib exposure metrics versus laboratory events, vital signs (hypertension) and AEs of fatigue/asthenia. AE adverse event, AUC area under the concentration–time curve, CI confidence interval, Cmax maximum plasma concentration, Cmin minimum plasma concentration
Adverse Events and Vital Signs
The results of the repeated measures logistic regression analysis showed a negative coefficient estimate (range, − 0.075 to − 0.008) for the asciminib exposure metrics (AUC, Cmax, and Cmin) for hypertension or fatigue and asthenia (any grade), as well as for TEAEs of grade ≥ 3, (AUC, − 0.211; Cmax, − 0.256; Cmin, − 0.245) (Fig. 3). These results suggest no clinically relevant relationship between exposure and these safety events. TEAEs leading to dose reduction or dose interruption were reported in 152 of 351 patients (43.3%). A time-dependent Cox regression analysis provided positive coefficient estimates of 0.032 for AUC (p = 0.715), 0.074 for Cmax (p = 0.449), and 0.016 for Cmin (p = 0.834), but these results were not statistically significant.
QT/QTc Analysis
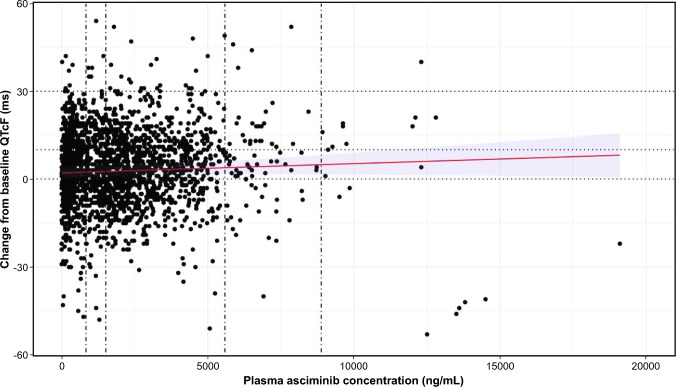
The estimated mean QTcF changes from baseline were 3.35 (90% CI 2.28–4.43), 3.64 (90% CI 2.60–4.68), 5.37 (90% CI 3.97–6.77), and 6.77 (90% CI 4.67–8.87) ms for Cmax at 40 mg b.i.d., 80 mg q.d., 200 mg b.i.d., and the HCRE, respectively, below the regulatory threshold of 10 ms and therefore not clinically significant according to the regulatory guidance (Fig. 4).
Fig. 4.
Scatterplot, regression line (red), and 90% CI (blue shaded area) of change from baseline QTcF versus plasma asciminib concentration. Data are from 239 patients from the dose-finding study (patients with CML and Ph+ acute lymphoblastic leukemia). Each circle represents a data point (PK sample with time-matched QTc assessment) any time post-asciminib dose. Vertical lines represent median Cmax for 40 mg b.i.d., 80 mg q.d., 200 mg b.i.d. and HCRE. Scatterplot includes only the matched ECG records. The model was QTcF change from baseline = concentration + baseline QTcF are fixed effects, and patient is a random effect. b.i.d. twice daily, CI confidence interval, Cmax maximum plasma concentration, HCRE highest clinically relevant exposure, PK pharmacokinetic, q.d. once daily, QTcF QT interval corrected using the Fridericia method, TKI tyrosine kinase inhibitor
ECG Analysis From Clinical Data
In the dose-finding study, new QTcF > 500 ms was noted in 3/241 (1.2%) patients (of whom two also had increase in QTcF > 60 ms from baseline). However, none of these abnormalities were associated with cardiac-related symptoms. In the ASCEMBL study, new QTcF > 500 ms with increase in QTcF > 60 ms from baseline was noted in 1/156 (0.6%) patients, which was reported as a treatment-related grade 3 ECG QT prolongation. This event was managed with treatment interruption and dose reduction, and no subsequent episodes of QTcF > 500 ms were observed in this patient.
Discussion
Based on the preclinical and clinical findings, 40 mg b.i.d. has been found to be an efficacious and well tolerated dosing regimen for asciminib [14]. The results from the ASCEMBL study in patients with Ph+ CML-CP without the T315I mutation showed a superior benefit/risk profile of asciminib 40 mg b.i.d. compared with bosutinib 500 mg q.d. [13]. The 80-mg q.d. regimen was proposed as an alternative regimen to 40 mg b.i.d. to encourage better patient compliance. MIDD, based on integrated data across dose regimens and studies, is a key approach to support the development and regulatory review of novel drugs [25–27]; we have used it in this study to assess and justify the comparability of 80-mg q.d. with 40-mg b.i.d. dosing regimens.
The ERe analysis indicated similar efficacy of asciminib 40 mg b.i.d. and 80 mg q.d. The predicted MMR rates were very similar between the two regimens and were in close agreement with clinical data from the ASCEMBL study [17]. While arguably data for the 80-mg q.d. dose regimen were limited (N = 18), MIDD provided a strong rationale for the similar efficacy between the two regimens, justifying the approval of both dosing regimens by several countries, including the United States.
The asciminib dose of 200 mg b.i.d. for patients with CML-CP harboring the T315I mutation was selected based on the preclinical findings (showing that 4- to 13-fold higher asciminib concentrations were required for sufficient inhibition of BCR::ABL1T315I versus non-mutated BCR::ABL1) [14], together with safety, efficacy, and PK data from the dose-finding study [13]. The approval of this dosing regimen for patients with the T315I mutation in the United States and other countries was supported by modeling and simulation strategies. Simulations based on the time-course of BCR::ABL1IS over 2 years using PopPK-simulated PK metrics demonstrated that a dose of 200 mg b.i.d. was effective and adequate in this heavily pretreated patient population with limited treatment options. Asciminib has the advantage of being active against the T315I mutation, one of the most frequently identified BCR::ABL1 mutations (20% of all point mutations in later-line CML-CP) [14].
The lack of clinically meaningful relationships between asciminib exposure and the majority of the laboratory events analyzed supports the acceptability of the safety profiles of the 80-mg q.d. regimen in patients with CML-CP without the T315I mutation and the 200-mg b.i.d. regimen in patients with CML-CP harboring the T315I mutation, especially given the consistent results between AUC, Cmax, and Cmin as exposure metrics. Similarly, the negative coefficient estimates obtained by the repeated-measures logistic regression model, which assessed the relationship between the probability of occurrence of safety events (hypertension or fatigue and asthenia) and asciminib exposure, suggest that an increase in asciminib exposure was not associated with an increased risk of these events. Furthermore, safety results demonstrated that an increase in asciminib exposure was not associated with a higher probability of occurrence of grade ≥ 3 TEAEs. Additionally, the risk of TEAE-related dose reduction or dose interruption was not associated with asciminib exposure. These results are in agreement with a recent publication showing that after a median exposure of 2 years to asciminib 200 mg b.i.d., only four (8.3%) patients with CML-CP harboring the T315I mutation discontinued asciminib due to adverse events [28].
These observations from the ERs analyses suggest that asciminib has a similar safety profile across all dose regimens (and associated exposure), whether at 40 mg b.i.d., 80 mg q.d., or 200 mg b.i.d. The number of patients with safety data obtained at an 80-mg q.d. dosing regimen may be considered small (N = 18); however, the small sample size at 80 mg q.d. is more than counterbalanced by the total amount of data collected over several years of treatment, showing the favorable safety profile of asciminib administered at a total daily dose of ≥ 80 mg (N = 150). Even though a 60% increase in Cmax (based on PopPK) was observed with an 80-mg q.d. dosing regimen compared with 40 mg b.i.d., the 80-mg q.d. dosing regimen was considered to have a similar safety profile to 40-mg b.i.d. dosing, as there was a lack of association between the occurrence of AEs, laboratory abnormalities or vital sign alterations with increasing exposure (and up to 5-fold the observed Cmax in patients receiving asciminib at 40 mg b.i.d.).
In summary, the ERe analysis suggests similar efficacy between 80 mg q.d. and 40 mg b.i.d., whereas the ERs analysis indicates no correlation between increase in asciminib exposure and increase in probability of occurrence of a safety event when using any PK metrics for 40 mg b.i.d. versus 80 mg q.d. These results are in line with the existing clinical data [29, 30]. Overall, the dose of 80 mg q.d. is considered as efficacious and safe as 40 mg b.i.d. and can be used as an alternative asciminib dosing regimen for patients with CML not harboring the T315I mutation. Asciminib has a negative food effect, with exposure decreased by approximately 30% when administered with a low-fat meal and by approximately 65% with a high-fat meal compared with the fasted state [30]; therefore, poor compliance with fasted conditions would reduce its oral bioavailability. The 80-mg q.d. dosing provides a more convenient regimen that could improve patients’ treatment compliance [20]. Both 40-mg b.i.d. and 80-mg q.d. dose regimens have been approved in the United States [31], ACCESS countries (UK, Australia, Singapore, Switzerland), and South Korea, among others, while Japan and the European Union approved only the b.i.d. dosing regimen (40 mg b.i.d.).
Conclusions
The asciminib clinical trials provided confirmatory evidence that a total daily asciminib dose of 80 mg (administered as 40 mg b.i.d.) has a favorable benefit/risk profile in heavily pre-treated patients with CML without the T315I mutation, displaying potent anti-leukemic activity with a well-tolerated safety profile. Exposure-response modeling showed that the 80-mg q.d. and 40-mg b.i.d. regimens are comparable with regard to safety and efficacy. The 80-mg q.d. dose regimen is a patient-centric regimen which is likely to support better compliance, considering asciminib is administered under fasting conditions, thus potentially deriving enhanced benefit from the therapy. Moreover, based on the available clinical data and the results of the present research, the 200-mg b.i.d. regimen is also shown to have a positive risk–benefit profile in patients with CML harboring the T315I mutation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Wenping Wang and Farkad Ezzet for their scientific expertise/contribution during the model development and interpretation along with Amrit Singh, PhD, and Twarita Chakraborty, PhD, Novartis Healthcare Private Limited, Hyderabad, and Vanesa Martinez Lopez, PhD, Novartis Ireland, for providing medical writing/editorial support in accordance with Good Publication Practice (GPP2022) guidelines (https://www.ismpp.org/gpp-2022). This study was sponsored by Novartis Pharmaceuticals.
Data availability
Anonymized patient-level data from clinical trials may be shared by Novartis in a consortium called ClinicalStudyDataRequest.com (CSDR) in accordance with Novartis’ policy for sharing clinical trial data (https://www.clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Novartis.aspx).
Code availability
Not applicable.
Declarations
Funding
This study was sponsored by Novartis Pharmaceuticals.
Conflict of Interest
Francois Pierre Combes (FPC), Sherwin K. B. Sy (SS), Ying Fei Li (YFL), Kohinoor Dasgupta (KD), Sebastien Lorenzo (SL), Shruti Kapoor (SK), Matthias Hoch (MH), and Yu-Yun Ho (YYH) are employees of Novartis. FPC and MH are also Novartis stockholders. FPC is also a shareholder of Simulation Plus. None of the authors declares any other conflict of interest.
Data Sharing Statement
Anonymized patient‐level data from clinical trials may be shared by Novartis in a consortium called ClinicalStudyDataRequest.com (CSDR) in accordance with Novartis’ policy for sharing clinical trial data (https://www.clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Novartis.aspx).
Consent to Participate
Not applicable.
Consent for publications
Not applicable.
Ethics Approval
This analysis was based on the data from patients enrolled in two different clinical trials. Both trials, serving as the data sources for this analysis, were approved by the appropriate Institutional Review Board.
Author Contributions
All the authors contributed to conception and design of the research, data collection, analysis, and interpretation, as well as drafting, reviewing, and providing final approval of the manuscript. Conceptualization: SS, FPC, MH, SL, YFL, YYH. Formal analysis: SS, FPC, SL, YFL. Validation: SS, FPC, SL, YFL. Investigation: SS, SL, FPC, YFL. Visualization: SL, FPC, YFL. Methodology: SS, SL, FPC, YFL, YYH. Writing—original draft: SS, SL, FPC, YFL. Writing—review and editing: SS, FPC, YFL, SL, MH, YYH, KD, SK.
Footnotes
Francois Pierre Combes, Sherwin K. B. Sy have contributed equally.
References
- 1.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93(3):442–59. [DOI] [PubMed] [Google Scholar]
- 2.Apperley JF. Chronic myeloid leukaemia. The Lancet. 2015;385(9976):1447–59. [DOI] [PubMed] [Google Scholar]
- 3.Jabbour EJ, Cortes JE, Kantarjian HM. Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk. 2013;13(5):515–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolini FE, Ibrahim AR, Soverini S, Martinelli G, Müller MC, Hochhaus A, et al. The BCR-ABLT315I mutation compromises survival in chronic phase chronic myelogenous leukemia patients resistant to tyrosine kinase inhibitors, in a matched pair analysis. Haematologica. 2013;98(10):1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12(24):7374–9. [DOI] [PubMed] [Google Scholar]
- 6.Akard LPAM, Hill CE, Pinilla-Ibarz J. The, “Hit Hard and Hit Early” Approach to the Treatment of Chronic Myeloid Leukemia: Implications of the Updated National Comprehensive Cancer Network Clinical Practice Guidelines for Routine Practice. Clin Adv Hematol Oncol. 2013;11(7):421–32. [Google Scholar]
- 7.Bosi GR, Fogliatto LM, Costa TEV, Grokoski KC, Pereira MP, Bugs N, et al. What happens to intolerant, relapsed or refractory chronic myeloid leukemia patients without access to clinical trials? Hematol Transfus Cell Ther. 2019;41(3):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015;15(6):323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes J, Kantarjian H. Chronic myeloid leukemia: sequencing of TKI therapies. Hematology Am Soc Hematol Educ Program. 2016;2016(1):164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauro MJ, Talpaz M, Radich JP. Sequential therapy in chronic myelogenous leukemia: where do emerging therapies fit within current treatment regimens? Clin Adv Hematol Oncol. 2013;11(11 Suppl 17):1–15. [PubMed] [Google Scholar]
- 13.Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manley PW, Barys L, Cowan-Jacob SW. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 2020;98: 106458. [DOI] [PubMed] [Google Scholar]
- 15.Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61(18):8120–35. [DOI] [PubMed] [Google Scholar]
- 16.Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543(7647):733–7. [DOI] [PubMed] [Google Scholar]
- 17.Hochhaus A, Réa D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, et al. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023;37(3):617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mian AA, Rafiei A, Haberbosch I, Zeifman A, Titov I, Stroylov V, et al. PF-114, a potent and selective inhibitor of native and mutated BCR/ABL is active against Philadelphia chromosome-positive (Ph+) leukemias harboring the T315I mutation. Leukemia. 2015;29(5):1104–14. [DOI] [PubMed] [Google Scholar]
- 19.Jabbour E, Saglio G, Radich J, Kantarjian H. Adherence to BCR-ABL inhibitors: issues for CML therapy. Clin Lymphoma Myeloma Leuk. 2012;12(4):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geissler J, Sharf G, Bombaci F, Daban M, De Jong J, Gavin T, et al. Factors influencing adherence in CML and ways to improvement: results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017;143(7):1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-33. [PubMed] [Google Scholar]
- 22.Li YF, Combes FP, Hoch M, Lorenzo S, Sy SKB, Ho YY. Population pharmacokinetics of asciminib in tyrosine kinase inhibitor-treated patients with philadelphia chromosome-positive chronic myeloid leukemia in chronic and acute phases. Clin Pharmacokinet. 2022;61(10):1393–403. [DOI] [PubMed] [Google Scholar]
- 23.Combes FP, Li YF, Hoch M, Lorenzo S, Ho YY, Sy SKB. Exposure-efficacy analysis of asciminib in philadelphia chromosome-positive chronic myeloid leukemia in chronic phase. Clin Pharmacol Ther. 2022;112(5):1040–50. [DOI] [PubMed] [Google Scholar]
- 24.Manley PW, Stiefl N, Cowan-Jacob SW, Kaufman S, Mestan J, Wartmann M, et al. Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem. 2010;18(19):6977–86. [DOI] [PubMed] [Google Scholar]
- 25.Madabushi R, Seo P, Zhao L, Tegenge M, Zhu H. Review: role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharm Res. 2022;39(8):1669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sy SKB, Zhuang L, Sy S, Derendorf H. Clinical pharmacokinetics and pharmacodynamics of ceftazidime-avibactam combination: a model-informed strategy for its clinical development. Clin Pharmacokinet. 2019;58(5):545–64. [DOI] [PubMed] [Google Scholar]
- 27.Venkatakrishnan K, van der Graaf PH. Toward project optimus for oncology precision medicine: multi-dimensional dose optimization enabled by quantitative clinical pharmacology. Clin Pharmacol Ther. 2022;112(5):927–32. [DOI] [PubMed] [Google Scholar]
- 28.Cortes JE, Sasaki K, Kim DW, Hughes TP, Etienne G, Mauro MJ, et al. Asciminib monotherapy in patients with chronic-phase chronic myeloid leukemia with the T315I mutation after ≥ 1 prior tyrosine kinase inhibitor: 2-year follow-up results. Leukemia. 2024;38(7):1522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoch M, Sato M, Zack J, Quinlan M, Sengupta T, Allepuz A, et al. Pharmacokinetics of asciminib in individuals with hepatic or renal impairment. J Clin Pharmacol. 2021;61(11):1454–65. [DOI] [PubMed] [Google Scholar]
- 30.Hoch M, Zack J, Quinlan M, Huth F, Forte S, Dodd S, et al. Pharmacokinetics of asciminib when taken with imatinib or with food. Clin Pharmacol Drug Dev. 2022;11(2):207–19. [DOI] [PubMed] [Google Scholar]
- 31.The Food and Drug Administration U. Highlights of asciminib prescribing information. 2021 [cited 2022 November 10]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215358s000Orig1lbl.pdf. Accessed 21 Aug 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized patient-level data from clinical trials may be shared by Novartis in a consortium called ClinicalStudyDataRequest.com (CSDR) in accordance with Novartis’ policy for sharing clinical trial data (https://www.clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Novartis.aspx).
Not applicable.