Abstract
The blue bottle genus Physalia is one of the well-known siphonophore belonging to the Cnidaria, Hydrozoa. Physalia is also known as a ferocious predator, occasionally stinging and fatally wounding humans, but key details of its life cycle and reproductive biology are unclear. Physalia have separate sexes, and sexual reproduction occurs through the release of complex structures called gonodendra that contain many gonophores that will release either eggs or sperm. It is not known how mature the gonophores are when the gonodendra are released. In this study, we aim to characterize germ cell maturation by conducting histological, cytological, and gene expression analyses of the gonodendron of Physalia utriculus from Japan. We found a layered structure of the gonophore, consistent with other studies; however, gametes were not found even in gonophores that were within the released gonodendra. Moreover, haploid cells were not detected by flow cytometry. Analysis of the expression of putative germ cell marker and meiosis related genes showed high expression in the gonophore. These results strongly suggest that germ cells do not mature until after gonodendra are released. These findings provide valuable insights into the reproductive ecology and life cycle of Physalia.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73611-5.
Keywords: Gonophore, Germ cell, Flow cytometry, Gene expression, Histology
Subject terms: Germline development, Marine biology
Introduction
The genus Physalia, commonly known as the blue bottle or Portuguese Man O’ War is one of the most well-known siphonophores (Cnidaria, Hydrozoa, Cystonectae). Physalia is a member of Cystonectae, a small clade that is sister group to other siphonophore, and has many unique attributes specific to its life at the surface of the ocean1–3. Physalia is pleustonic animal that float on the surface of seawater and is widely distributed in oceans globally, including the Atlantic Ocean, Pacific Ocean, and Indian Ocean4–11. Physalia is a ferocious predator12–14, with occasional reports of stinging humans to deaths15, making its ecology of great interest. However, much of its lifecycle and reproductive ecology are poorly understood, including where and how it reproduces, develops, and drifts to the coastlines.
Members of the order Siphonophorae, to which Physalia belongs, possess a complex life cycle, in which individual bodies (i.e., zooids), with specialized morphology and function, form highly sophisticated colonies. The morphology of individual zooids is so specialized that they function much like “organs”, and the entire colony behaves like a single organism, such that the entire colony is sometimes described as a “superorganism”1,16,17. The zooids within a colony are generated through asexual budding, while new colonies are reproduced sexually1,6,18–20. In some species of Physalia although juveniles have been described3,21, its life cycle is not fully elucidated, and sexual reproduction is poorly characterized.
In siphonophores, sexual reproduction occurs via gonophores. These zooids are reduced medusae that bear gametes but lack structures for other functions such as feeding. In cystonects, including Physalia (Fig. 1), the gonophores are born in complex branching structures known as gonodendra that contain other zooid types as well3. Physalia gonodendra grow from the main colony while it floats at the surface, but then break away and sink before gamete release and fertilization (Supplementary Figure S1; Table 1). Because sexual reproduction occurs away from the mature colony, many features of sexual reproduction in Physalia are relatively inaccessible to study and there are many critical factors that remain unknown. This includes basic facts about whether gametes are generated before or after gonodendra are released, how much time elapses between gonodendron release and spawning, how spawning is coordinated in space and time, and whether eggs are released from gonophores before fertilization.
Fig. 1.
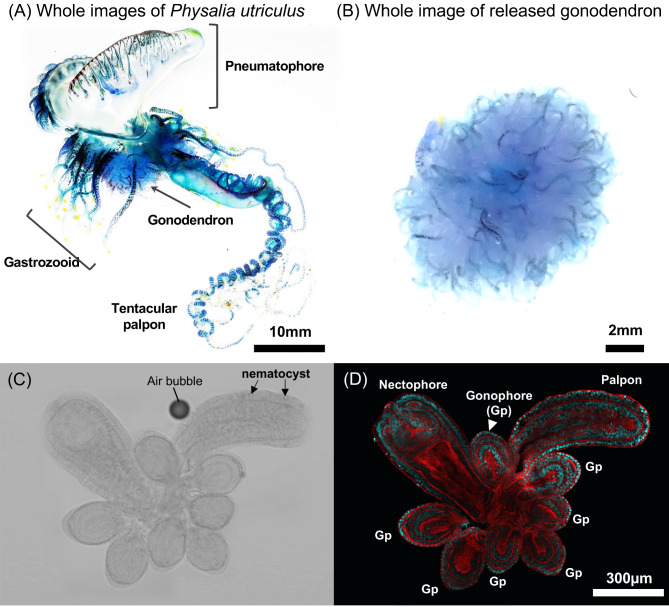
Whole images of Physalia utriculus and anatomical structures of gonodendron. Whole image of P. utriculus (A). In this species, pneumatophore: gas filled float, gastrozooid: feeding zooid, tentaclular poalpon: tentacles attached to foraging zooid, gonodendron: putative reproductive structures. Whole image of gonodendron which released from the colony (B). Fine anatomical structures of gonodendron. nectophore: medusa like structures which involved in locomotion, palpon: digestive zooid, gonophore: putative gonad (C,D). Bright field images (C) and fluorescent images (D). DAPI signals targeting host nuclear DNA (blue), and fluorescent phalloidin signals targeting muscular actin filaments (red), respectively.
Table 1.
Observed examples of released gonodendron.
| Date | Sites | Observers | Latitude, longitude | References |
|---|---|---|---|---|
| December 28, 2018 | Wollongong NSW, Australia | Lou De Beuzeville | -34.593209, 150.900495 | https://www.inaturalist.org/photos/29791487 |
| December 19, 2019 | Port Macquarie-Hastings - Pt B, New South Wales, Australia | Thomas Mesaglio | -31.622383, 152.834835 | https://www.inaturalist.org/photos/59465554 |
| June 29, 2022 | Miura, Kanagawa, Japan | Kohtsuka Hisanori | 35.159252, 139.611907 | This study (Fig. S3A) |
| November 11, 2023 | Miura, Kanagawa, Japan | Kohei Oguchi | 35.159252, 139.611907 | This study (Fig. S3B) |
| December 10, 2023 | South Pacific Ocean, New Plymouth City Ward, Taranaki, NZ | paritutu_beach_comber | -39.063856, 174.019687 | https://www.inaturalist.org/photos/340267386 |
Like other species of Cystonectae, Physalia is dioecious. This means that sexes are separate and each Physalia produces all male gonophores or all female gonophores. This is the ancestral state in siphonophores, with subsequent shifts to monoecy (colonies that produce both male gonophores and female gonophores) in some siphonophore groups2,3.
However, previous work has characterized the gross morphology of gonodendra and gonophores4,11,16,22,23. These studies confirm that, as in other siphonophores, the sex of Physalia can be established based on gonophore morphology. Male gonophores have a conspicuous centrally located spadix22. In female gonophores, the spadix takes up nearly the entire gonophore, so is not apparent as a distinct internal structure22. However, gametogenesis and gametes remain poorly described, and little is known about sexual maturation.
In this study, in addition to the histological observation of gonodendra, we characterized germ cells of Physalia utriculus from the coast of Sagami Bay in Kanagawa prefecture, Japan based on the diagnosis of the nuclear phase using flow cytometry, and further clarified their localization by gene expression analysis. Genes encoding vasa and piwi are widely conserved in metazoans and are markedly expressed in germ and stem cells24. In hydrozoans, these genes are expressed not only in germ cells, but also in stem cells called i-cells, which are widely distributed in epithelial cells25,26. Particularly in Nanomia bijuga, a species of siphonophore, these genes are strongly localized in both female and male gonophores27, and were used as markers for putative germ cells in this study. Moreover, to characterize stage of germ cell development, expression level of conserved meiosis related genes Dmc1, Mnd1, Msh4/5 and Sycp330,31,32,33 was focused. Additionally, Agalma okenii (Physonectae) was used to ensure technical validity in the diagnosis of karyotypic phases by flow cytometry. Species of the genus Agalma form hermaphroditic colonies, with distinct sexes of gonophore2,28, and cells in the process of gametogenesis were easily detectable.
Results and discussion
Morphological and histological observations of a gonodendron in Physalia uticulus
Morphological and histological observations were performed on gonodendra to examine presence of gametes and germ cells. We observed an ovoid gonophore, a pouch-like digestive zooid as the palpon, and a nectophore (Fig. 1C, D), consistent with other studies3,4,22. Histological observation using confocal laser-scanning microscope (CLSM) revealed that the palpon has a simple two-layered structure, whereas the gonophore has at least three layers (Fig. 1D) as shown in previous studies22. The apical region of the nectophore was complex and intricate (Fig. 1D). Some of the collected samples showed release of gonodendron within a day immediately after collection (Supplementary Table S1). Among the five released gonodendra samples, the minimum size of gonophore was 151 μm, the maximum was 1037 μm, and the average was 599 ± 188 μm (Supplementary Figure S2).
Detailed histological structures were observed in various sizes of gonophores (Fig. 2). The gonophore was approximately oval in shape, extending in the direction of the apical axis as it developed (Fig. 2). Gonophores with diameters of 200 μm and 250 μm contained an outer epithelial cell layer, an inner gastrovascular cavity, and gastrodermic cells (Fig. 2A, B, C). A distinct two or three layers of cells was present between epithelial and gastrodermic cells, referred to as the germ cells in other studies4,11,16,22,23. Gonophores with major axis diameters of 450 μm had a three-layered structure, consisting of epidermal epithelial cells, putative germ cells, and gastrodermic cells, but had developed a gastrovascular cavity and lumen (Fig. 2D). Gonophores with major axis diameters of 800 μm had a thickened gastrodermic cell layer and a structure with an intricate lumen (Fig. 2E). In all gonophores studied, we observed no variation in the morphology of putative germ cell nuclei, as well as no oocytes or spermatocytes.
Fig. 2.
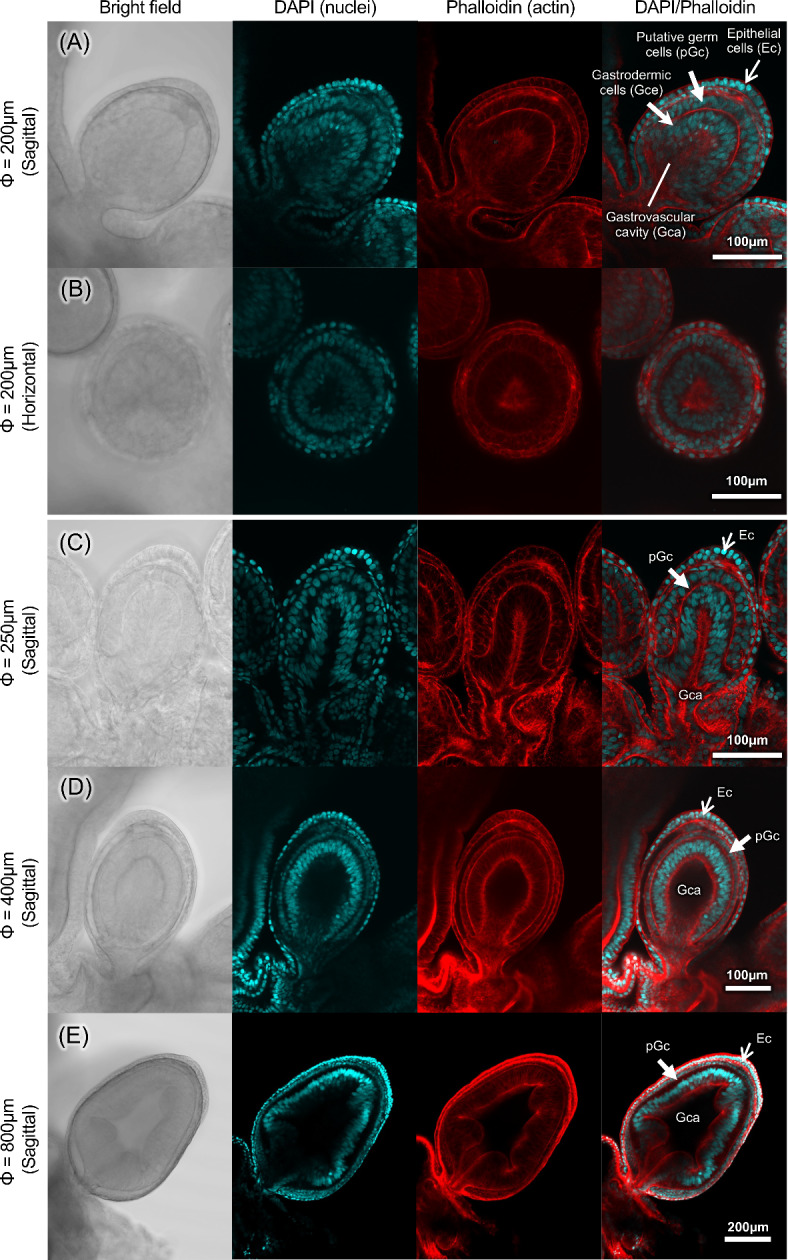
Histology of gonophore in various developmental stages of Physalia utriculus. Sagittal section of a gonophore with a major axis diameter of 200 μm (A). Horizontal section of a gonophore with a major axis diameter of 200 μm (B). Sagittal section of a gonophore with a major axis diameter of 250 μm (C), 400 μm (D) and 800 μm (E). From left to right: brightfield, DAPI fluorescence image, phalloidin fluorescence image, and DAPI/phalloidin stacked image. DAPI signals targeting host nuclear DNA (blue), and fluorescent phalloidin signals targeting muscular actin filaments (red), respectively. Epithelial cells (Ec), gastrovascular cavity (Gca), gastrodermic cells (Gce) and putative germ cells (pGc) were seen in every developmental stages.
Nuclear phase diagnosis by flow cytometry
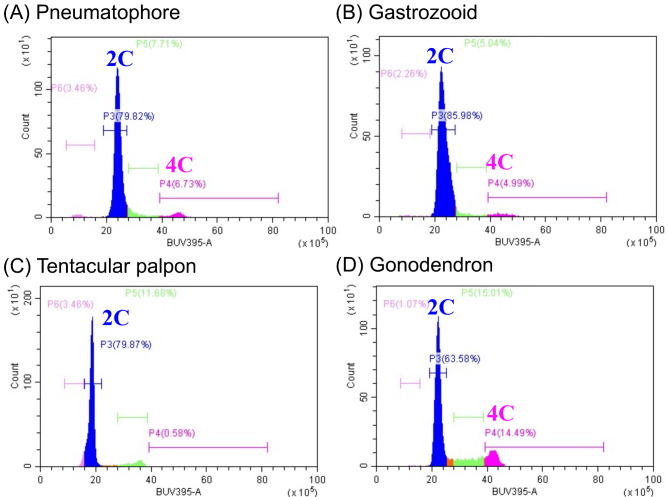
Since morphologically distinct gametes were not identified in histological surveys (Fig. 2), a diagnosis of the nuclear phase was performed using flow cytometry to clarify whether haploid cells (gametes) were actually absent. In P. utriculus, somatic cells derived from the pneumatophore, gastrozooid, and tentacular palpon showed a large peak in cell count at ~ 20 of the BUV395 signal and a smaller peak at ~ 40 (Fig. 3A–C). We considered cells at the large and small peaks to be diploid (2 C) and tetraploid (4 C), respectively, in which DNA had been replicated for cell division. In addition, only two peaks were detected in the gonodendron (Fig. 3D). Haploid cells (1 C) should be detected as a peak at approximately one-half of the 2 C signal. Therefore, no haploid cells were detected in the gonodendron of P. utriculus in this study. Interestingly, more tetraploid cells were detected in gonodendron than in other parts (Fig. 3). Considering that no distinct meiotic pairing chromosome or dividing cells were observed (Figs. 2 and 3), gonophores might be in the early stages of meiosis.
Fig. 3.
Diagosis of karyotypes in the several structures of Physalia utriculus. Pneumatophore (A), gastrozooid (B), tentacular palpon (C) and gonodendron (D). Diploid (2 C) and tetraploid (4 C) cells were seen in every structure, while monoploid cells (1 C) were not detected.
To ensure technical validity and to confirm these results, the nuclear phase of each part was analyzed using A. okenii, in which species gametes and germ cells are clearly present (Supplementary Figure S5). In A. okenii, two peaks (diploid and tetraploid cells) were detected in the tentacle, and feeding zooid: gastrozooid (Supplementary Figure S5B, C), whereas three distinct peaks (diploid, tetraploid, and haploid cells) were detected in the female-gonozooid and male-gonozooid (Supplementary Figure S5D, E). This result indicates that the experimental system accurately detects nuclear ploidy in these species.
Gene expression analysis for putative germ cell markers and meiosis related genes
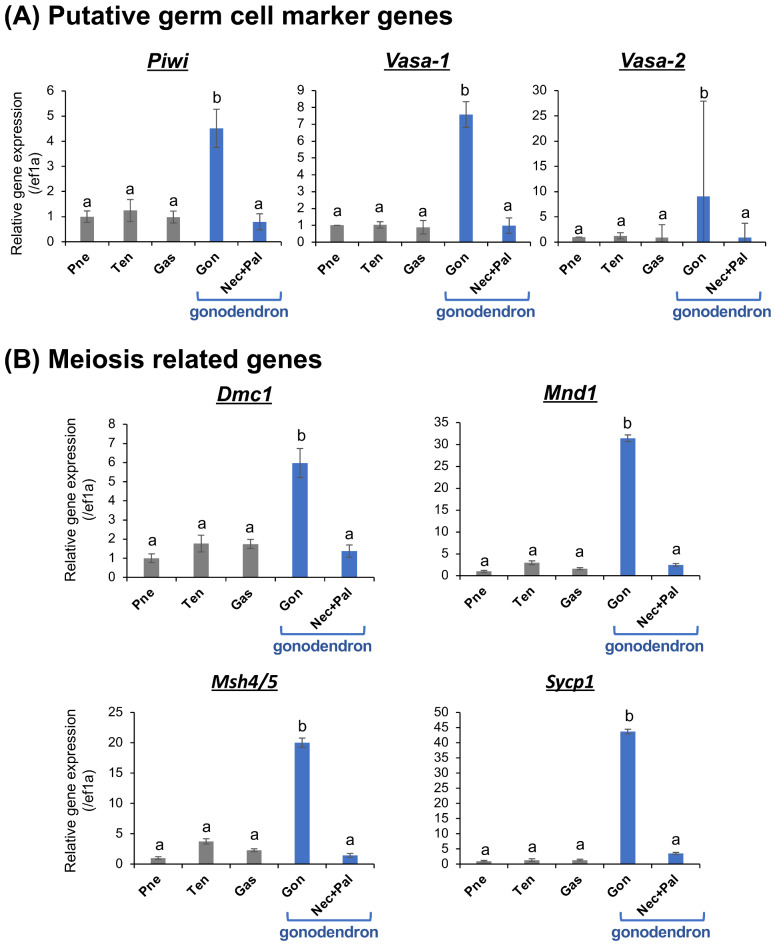
Both histological observations and nuclear phase diagnostics were unable to detect gamete, however, tetraploid cells were more frequently detected in gonodendra. We therefore sought to characterize earlier stage germ cells in the gonophores. We performed gene expression analysis of putative germ cell markers (piwi, vasa-1, and vasa-2) and meiosis related genes (Dmc1, Mnd1, Msh4/5 and Sycp1) (Fig. 4). Real-time qPCR analysis showed significantly higher expression of piwi, vasa-1, and vasa-2 in the gonophore than other structures (Fig. 4A). Detailed gene expression localization visualized by whole-mount in situ hybridization (WISH) revealed clear piwi, vasa-1, and vasa-2 signals in the gonophore, whereas no signals were detected in the nectophore, palpon, etc. (Supplementary Figure S3). These genes were expressed at a relatively superficial level of the gonophore (Supplementary Figure S3), confirming that the cell layer near the surface is the germ cell layer. Both real-time qPCR and WISH results strongly suggest that the putative germline marker genes are expressed in the gonophore inside the gonodendron, and the germ cells are distributed in the gonophores (Fig. 4, Supplementary Figure S3). Additionally, high expression of meiosis-related genes was observed only in gonophores (Fig. 4B). These genes were highly conserved in eukaryota, involved in interhomolog recombination, stable heteroduplex DNA formation after double strand break and expressed in the early stages of prophase I29–32. It was therefore considered that the germ cells were in prophase I stage and that development of germ cells might be arrested at this stage until the gonodendron were released.
Fig. 4.
Gene expression profiles using real-time qPCR of three putative germ cell marker genes (piwi, vasa-1 and vasa-2) (A) and four meiosis related genes (Dmc1, Mnd1, Msh4/5 and Sycp3) (B) in several structures of P. utriculus. Relative expression levels of targeting genes (mean 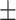 S.D., n = 4) were calibrated using an internal control (ef1a) (A). Different letters shown above the bars indicate significant differences among categories (Tukey’s test, p < 0.05). Pneumatophore (Pne), tentacular palpon (Ten), gastrozooid (Gas), gonophore (Gon) and other structure in gonodendron i.e., nectophore and palpon (Nec+Pal).
S.D., n = 4) were calibrated using an internal control (ef1a) (A). Different letters shown above the bars indicate significant differences among categories (Tukey’s test, p < 0.05). Pneumatophore (Pne), tentacular palpon (Ten), gastrozooid (Gas), gonophore (Gon) and other structure in gonodendron i.e., nectophore and palpon (Nec+Pal).
Conclusion
Where and how do they reproduce? — insights into the reproductive ecology and life cycle
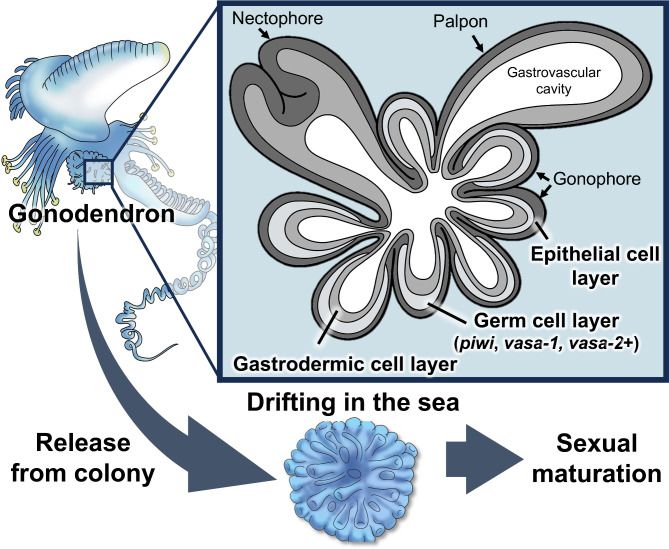
In this study, (i) we observed no morphologically distinct gametes in the gonodendron and gonophore (Figs. 1 and 2), (ii) we observed no haploid cells (gametes) in the gonodendron (Fig. 3), and (iii) we did find putative germ cell marker genes expressed in the gonophore (Figs. 4 and 5), (iv) we revealed germ cells were in prophase I stage of meiosis.
Fig. 5.
Schematic diagram showing the localization of germ cells and hypothetical sexual maturation processes in Physalia. Whole anatomy of Physalia and detailed histological structures of gonodendra.
These findings indicate that all the gonodendra observed in this study were not yet sexually mature. These results are consistent with the histological characteristics of other study4, suggesting that sexual maturation of gonophores occurs after gonodendron release.
All of the samples examined in this study were either washed ashore or collected in the intertidal zone of reefs along the coast of Sagami Bay, Japan. Because most of the samples drifted ashore after typhoons or strong winds blowing from the south and only a few cases of immature populations have been reported, gonodendra do not develop in shallow waters, at least not in the Sagami Bay area. The origin of this population is unclear, but because Physalia are often found in tropical and subtropical oceans33, they may reproduce near the equator and drift to the waters around Japan on the wind and waves. The internal structures of the gonodendron are complex and consists of various parts (Fig. 1). The functions of these parts are largely unknown. The palpon contains cnidocytes (Fig. 1C), the nectophore is open to the outside (Fig. 1D), and the gastrovascular system is well-developed and connected to the gonophore (Figs. 1 and 2). Our work here sets the stage for describing the subsequent steps in Physalia reproduction after the gonodendra are released. Many questions remain about how long it takes them to sexually mature, what depths they sink to, if gamete release is coordinated to maximize fertilization success, and whether spawning occurs all at once or is a gradual process.
Methods
Sample collection and morphological observation
Samples of Physalia utriculus and Agalma okenii were collected by plastic bags from the rocky shores and beaches at Kanagawa prefecture, Japan, in 2020–2024 (Supplementary Table S1). Specimens were immediately dissected using fine tweezers under a stereomicroscope SZX16 (Olympus, Tokyo, Japan) and fixed in 4% paraformaldehyde (PFA). Among the collected Physalia samples, especially those that released gonodendra (Supplementary Figure S1; Table 1), the long axis diameter of the gonophore was measured to determine the degree of maturity.
Histological observation by confocal laser-scanning microscopy
To investigate the histological features, the gonodendron was observed using a confocal laser-scanning microscopy (CLSM). Gonodendrons were dissected out from 10 specimens, fixed in PFA in filtered seawater for > 3 h, and washed for 15 min with 0.3% Triton-X 100 in 1× phosphate-buffered saline (PBST) at least three times. The nuclei (DNA) and cytoskeleton (F-actin) were stained with 40,6-diamidino-2-phenylindole (DAPI) (2 µg/mL; Sigma, St. Louis, MO, USA) and rhodamine-phalloidin (1:40; Invitrogen, Paisley, UK), respectively, for 1 h at room temperature. Then, the samples were washed twice in PBST, mounted in 50% glycerol in PBS, and observed under the CLSM (FV3000; Olympus, Japan).
Cytological examination by flow cytometry
To detect gametes and haploid germ cells, the nuclear phases composing the gonodendron were diagnosed using flow cytometry (P. utriculus: n = 10, A. okenii: n = 3). Using the DNA content of diploid somatic cells derived from several parts of P. utriculus and A. okenii, which have obvious developed gonads (Fig. 4; Supplementary Figure S5), nuclear DNA content and ploidy were evaluated. Briefly, several parts were homogenized with the buffer of a Single Cell Isolation Kit (Invent Biotechnologies, Plymouth, MN, USA), stained with DAPI, and fixed in 4% PFA. Nuclear DNA was quantified using a CytoFLEX flow cytometer and monitoring CytExpert software (Beckman Coulter, CA, USA).
RNA extraction and cDNA synthesis
To identify the location of germ cells, the expression of putative germ cell marker genes was analyzed. Several parts, including the pneumatophore, tentacular palpon, gastrozooid, gonophore, and other structure in gonodendron (i.e., nectophore and palpon) (Fig. 1), were dissected from four specimens. Total RNA was extracted using ISOGEN (NIPPON GENE, Tokyo, Japan) and treated with DNaseI (Thermo Fisher Scientific, Waltham, MA, USA). For each sample, 200 ng of total RNA was reverse transcribed with a HighCapacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA).
Gene identification
For gene expression analysis, orthologs of candidate germ cell marker genes were searched in the transcriptome database of Physalia (Transcriptome Shotgun Assembly of NCBI: accession numbers: SRX5780675- SRX5780684; SRX5780715- SRX5780720)34. Protein sequences of candidate genes (Piwi: AHI50300.1; Vasa-1: AHI50304.1; Vasa-2: AHI50305.1; Mnd1: NP_115493.1; Dmc1: NP_008999.2; Msh4/5: NP_002431.2; Sycp3: NP_001171419.1) were used as queries for tBLASTn searches against the P. physalis dataset. The obtained nucleotide and amino acid sequences in P. physalis were aligned with orthologous sequences in several metazoan lineages with ClustalW. To verify the orthology of candidate genes, phylogenetic analyses were performed with MEGA-X35 (Supplementary Figure S3, S4). Best-fit evolutionary models were determined according to Bayesian information criterion and bootstrap support values were estimated using 1,000 runs. Based on the obtained sequences, primers for real-time quantitative PCR (qPCR) and probes for in situ hybridization were designed using Primer3 software36 (Supplementary Table S2).
Gene expression analysis by real-time qPCR
Real-time qPCR was performed using SYBR Green Master Mix with the sequence detection system ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA), using the relative standard curve method. RefFinder software37 used to analyze gene expression level of glyceraldehyde 3-phosphate dehydrogenase (gapdh), 18 S rRNA, ribosomal protein S9 (rps9), and elongation factor 1 alpha (ef1a) to identify suitable reference genes for quantification. Finally, ef1a was chosen as the best reference gene. Relative gene expression was measured compared with the reference gene ef1a. For statistical analysis, Tukey’s multiple comparisons test (n = 4; p < 0.05) was performed after one-way ANOVA (p < 0.05), using R 4.2.2 (https://www.r-project.org).
Whole-mount in situ hybridization
To examine the detailed localization of piwi, vasa-1, and vasa-2, whole-mount in situ hybridization (WISH)27 was performed on the gonodendron of P. utriculus. First, the target genes were amplified by specific primers with T7 and SP6 sequences (Supplementary Table 2). DIG-labeled sense and antisense RNA probes were prepared by in vitro transcription using a DIG Labeling Kit (Roche Diagnostics, Mannheim, Germany). The gonodendron was dissected and fixed with 4% PFA/PBS. The samples were treated sequentially with PBST for 10 min (three times), 10 µg/mL protenase K (20 min), and 4% PFA/PBS (20 min), then the samples were prehyribidized for 1 h at 60° C in a hybridization buffer [5× SSC, 50% formamide, 0.1% Tween 20, 5× Denhardt’s solution (Nacalai tesque, Kyoto, Japan), 750 µg/mL Salmon sperm DNA (Roche Diagnostics, Mannheim, Germany)], samples were hybridized with the digoxigenin-labeled RNA probes at 60 °C for approximately 24 h.
To visualize signals, hybridized samples were rinsed two times in buffer I (50% formamide, 2× SSC with 0.1% SDS) for 60 min, washed in buffer II (2× SSC with 0.1% SDS), incubated in blocking buffer (1% blocking reagent in buffer II) for 60 min, and then incubated with the anti-DIG-AP conjugate (Roche Diagnostics, Mannheim, Germany) (500× dilution) in buffer II for 60 min. The samples were washed two times with PBST for 15 min, and the hybridized probe was detected by incubating with BM-purple (Roche Diagnostics, Mannheim, Germany) solution for 2 h at room temperature. The reaction was stopped with 4% PFA/PBS. The samples were washed with PBST, mounted with methanol solution, and images were captured using a stereomicroscope SZX16 (Olympus, Tokyo, Japan) connected to a DP26 CCD camera (Olympus, Tokyo, Japan).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to Kazuhisa Hori, Mitsugu Kitada, Aya Adachi, Michiyo Kawabata, Itaru Kobayashi, Izumi Komori, Yukio Kurihara, Hiromi Kurihara and Miura Fishery Cooperative Association for their help with the sampling. We especially thank Katsumi Takamura, Takato Izumi and Hiroshi Namikawa for their valuable comments on histological observations, and Catriona Munro for their helpful advice on gene expression analysis of meiosis related genes. Especially, we would like to thank Soma Chiyoda for providing the photographs and Mikako Oguchi for the illustrations (Fig. 5). We also thank Toru Miura for the use of CLSM and qPCR systems and the IMSUT FACS Core Laboratory for their help with flow cytometry analysis. This work was supported by Grant-in-Aid for Research Activity Start-up (No. 22K20662) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Research Institute of Marine Invertebrates to KO.
Author contributions
K.O. and C.W.D. conceptualized and designed the study. K.O. performed histological, cytological and gene expression analysis. K.O., G.Y., and H.K., performed field sampling and morphological observations. All authors wrote the manuscript and approved the final version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mańko, M. K., Munro, C. & Leclère, L. Establishing bilateral symmetry in hydrozoan planula larvae, a review of siphonophore early development. Integ Comp. Biol. icad 081 (2023). [DOI] [PubMed]
- 2.Dunn, C. W., Pugh, P. R. & Haddock, S. H. D. Molecular phylogenetics of the Siphonophora (Cnidaria), with implications for the evolution of functional specialization. Syst. Biol. 54, 916–935 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Munro, C., Vue, Z., Behringer, R. R. & Dunn, C. Morphology and development of the Portuguese man of war, Physalia physalis. Sci. Rep. 9, 15522 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardi, J. & Marques, A. C. Taxonomic redescription of the Portuguese man-of-war, Physalia physalis (Cnidaria, Hydrozoa, Siphonophorae, Cystonectae) from Brazil. Iheringia Sér. Zool.. 97, 425–433 (2007). [Google Scholar]
- 5.Karunarathne, K. D. & De Croos, M. D. S. T. Pleustonic colonies of cnidarians (Physalia physalis, Porpita porpita and Velella velella) found along the coastal belt of Sri Lanka. Indian J. Geo-Mar. Sci. (IJMS). 51, 45–55 (2022). [Google Scholar]
- 6.Chun, C. Histologie der Hydromedusen. In Klassen und Ordnungen des Tierreichs, Zweiter Band, II Abteilung (Bronn, H. G. ed.). 295–326 (1897).
- 7.Bigelow, H. B. The Siphonophorae. Reports of the scientific research expedition to the tropical Pacific Albatross XXIII. Mem. Museum Comp. Zool. Harv. Coll. 38, 173–402 (1911). [Google Scholar]
- 8.Bigelow, H. B. Hydromedusae, siphonophores and ctenophores of the Albatross Philippine expedition. Bull. United States Natl. Museum. 1 (5), 279–362 (1919). pl. 39–42. [Google Scholar]
- 9.Fenner, P. J., Williamson, J. A., Burnett, J. W. & Rifkin, J. A newly differentiated species of Physalia physalis in Australia. Aust. Med. J. 158, 500 (1993). [DOI] [PubMed] [Google Scholar]
- 10.Linnaeus, C. Systema Naturae per regna tria Naturae. ed. 10. 823 (British Museum (Natural History), 1758).
- 11.Totton, A. K. Studies on Physalia physalis (L.). Part 1. Natural history and morphology. Discov. Rep. 30, 301–368 (1960). [Google Scholar]
- 12.Damian-Serrano, A., Haddock, S. H. & Dunn, C. W. The evolution of siphonophore tentilla for specialized prey capture in the open ocean. Proc. Natl. Acad. Sci. USA. 118: e2005063118 (2021). [DOI] [PMC free article] [PubMed]
- 13.Damian-Serrano, A. et al. Characterizing the secret diets of siphonophores (Cnidaria: Hydrozoa) using DNA metabarcoding. Plos One. 17, e0267761 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hetherington, E. D., Damian-Serrano, A., Haddock, S. H., Dunn, C. W. & Choy, C. A. Integrating siphonophores into marine food‐web ecology. Limnol. Oceanogr. Lett. 7, 81–95 (2022). [Google Scholar]
- 15.Burnett, J. W. & Gable, W. D. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 27, 823–824 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Mackie, G. O. Studies on Physalia physalis (L.). Part 2. Behavior and histology. Discov. Rep. 30, 371–407 (1960). [Google Scholar]
- 17.Wilson, E. O. & Sociobiology The New Synthesis (Belknap Press of Harvard University, 1975).
- 18.Carré, D. Étude Histologique Du développement De Nanomia bijuga (Chiaje, 1841), siphonophore physonecte agalmidae. Cah Biol. Mar. 10, 325–341 (1969). [Google Scholar]
- 19.Carré, C. & Carré, D. A complete life cycle of the calycophoran siphonophore Muggiaea kochi (Will) in the laboratory, under different temperature conditions: Ecological implications. Philos. Trans. R Soc. Lond. B Biol. Sci. 334, 27–32 (1991). [Google Scholar]
- 20.Russell, F. S. On the development of Muggiaea atlantica. J. Mar. Biol. Ass. 441, 22 (1938). [Google Scholar]
- 21.Okada, Y. K. Dévelopement post-embryonnaire de la Physalie Pacifique. Mem. Coll. Sci. Kyoto Imp Univ. Ser. B. 8, 1–27 (1932). [Google Scholar]
- 22.Stech, O. Die Genitalanlagen Der Rhizophysanen. Ztschr. Wissensch Zool. Leipz. lxxxvi, 134–171 (1907). [Google Scholar]
- 23.Perez, С. Division directe des noyaux dans le spadice des gonophores chez la Physalie. Arch. Anat. Micr Paris. XXV, 548–554 (1929). [Google Scholar]
- 24.Extavour, C. G. & Akam, M. Mechanisms of germ cell specification across the metazoans: Epigenesis and preformation. Development. 130, 5869–5884 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Rebscher, N., Volk, C., Teo, R. & Plickert, G. The germ plasm component Vasa allows tracing of the interstitial stem cells in the cnidarian Hydractinia echinata. Dev. Dyn. 237, 1736–1745 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Gahan, J. M., Bradshaw, B., Flici, H. & Frank, U. The interstitial stem cells in Hydractinia and their role in regeneration. Curr. Opin. Genet. Dev. 40, 65–73 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Siebert, S. et al. Stem cells in Nanomia bijuga (Siphonophora), a colonial animal with localized growth zones. EvoDevo. 6, 22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mańko, M. K. & Pugh, P. R. AClausiclausi (Bedot, 1888) (Siphonophora: Physonectae)–Complementary description with notes on species distribution and ecology. Zootaxa. 4441, 311–331 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Malik, S. B., Pightling, A. W., Stefaniak, L. M., Schurko, A. M. & Logsdon Jr J.M. An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis. PloS One. 3, e2879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schurko, A. M. & Logsdon, J. M. Jr Using a meiosis detection toolkit to investigate ancient asexual scandals and the evolution of sex. BioEssays. 30, 579–589 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Fraune, J. et al. Hydra meiosis reveals unexpected conservation of structural synaptonemal complex proteins across metazoans. PNAS. 109, 16588–16593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loidl, J. Conservation and variability of meiosis across the eukaryotes. Annu. Rev. Genet. 50, 293–316 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Minemizu, R., Kubota, S., Hirano, Y. & Lindsay D.J. A photographic guide to the jellyfishes of Japan. Heibonsha Tokyo 1–360 (2015).
- 34.Munro, C., Zapata, F., Howison, M., Siebert, S. & Dunn, C. W. Evolution of gene expression across species and specialized zooids in Siphonophora. Mol. Biol. Evol. 39, msac027 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. In Bioinformatics Methods and Protocols. Methods in Molecular Biology. Vol. 132 (eds Misener, S. & Krawetz, S. A.) 365–386 (Humana Press, 2000). [DOI] [PubMed]
- 37.Xie, F., Wang, J., & Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genomics. 23, 125 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.