Abstract
Purpose
The bidirectional regulation of macrophages and exosomes provides a meaningful research direction for the treatment of complications arising from both type 1 and type 2 diabetes mellitus. However, there is currently no comprehensive evaluation of the bidirectional regulatory role of macrophages and exosomes in diabetic complications. In this review, we aim to provide the detailed process of the bidirectional regulation mechanism of macrophages and exosomes, and how macrophage-associated exosomes use this mechanism to make it better applied to clinical practice through biotechnology.
Methods
Therefore, we summarized the bidirectional regulation mechanism of macrophages and exosomes and the application based on the bidirectional regulation mechanism from two aspects of inflammation and insulin resistance.
Results
As key regulators of the immune system, macrophages are crucial in the progression of diabetic complications due to their significant impact on the regulation of cellular metabolism, inflammation, and insulin sensitivity. Furthermore, exosomes, as innovative mediators of intercellular communication, transport miRNAs, proteins, and various bioactive molecules, influencing the occurrence and progression of diabetic complications through the regulation of inflammation and insulin resistance. The bidirectional regulation between macrophages and exosomes provides a promising pathway for the treatment of diabetic complications aimed at regulating the immune response and improving insulin sensitivity.
Conclusions
Understanding the complexity of the interaction between macrophages and exosomes can advance the treatment of diabetic complications and drug development, and bringing more innovative and effective treatment strategies for diabetic complications.
Keywords: Macrophage, Exosome, Diabetic complications treatment, Bidirectional mutual regulation
Introduction
Diabetes, as an endocrine metabolic disorder, exists in two primary forms: type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) [1, 2]. Both types share the common characteristic of elevated blood glucose levels. However, persistent hyperglycemia leads to elevated secretion of inflammatory cytokines and reactive oxygen species, increasing oxidative stress both intracellularly and extracellularly. Additionally, hyperglycemia and oxidative stress cause endothelial cell dysfunction, leading to delayed wound healing and the onset of diabetic complications such as diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, diabetic cardiovascular disease, and diabetic foot [3]. In clinical practice, pharmacotherapy remains the primary approach for treating diabetic complications. However, long-term medication use in most patients only delays disease progression and may lead to drug-related side effects such as hypoglycemia, nausea, vomiting, and headaches [4]. Therefore, there is an urgent need to identify new potential targets and therapeutic tools for diabetic complications. The communication networks between cells and organs are essential in the treatment of diabetic complications [5].
Exosomes, as natural nanoparticles, can be released by macrophages and, conversely, can also act on macrophages. This bidirectional regulatory mechanism offers potential advantages in the treatment of diabetic complications [6]. Macrophages participate in intercellular communication and regulation by releasing exosomes carrying bioactive molecules, thereby facilitating information transfer between cells [7]. They can not only regulate diabetes-related inflammatory responses by stimulating the release of inflammatory mediators such as cytokines and chemokines but also release bioactive molecules like miRNAs [8]. These bioactive molecules regulate insulin signaling pathways, influencing insulin sensitivity and improving insulin resistance associated with diabetic complications. Moreover, macrophages regulate their activation states by taking up exosomes from other cells, promoting their polarization towards either classically activated macrophages (M1) or activated macrophages (M2). This process inhibits the inflammatory response of macrophages, alleviates tissue damage and inflammatory diseases associated with diabetes, and aids in the clearance and repair of damaged islets [9]. Consequently, this improves the treatment outcomes for complications such as diabetic nephropathy, diabetic peripheral neuropathy, and diabetic cardiovascular diseases [10–14].
Compared to cell-based therapies, exosome administration offers advantages in precise dosing and targeting. Isolating and purifying exosomes from cell cultures allows for accurate control of the therapeutic product’s dose and composition [15]. Additionally, engineering or modifying exosomes can enhance their targeting specificity to desired tissues or cell types, thereby minimizing off-target effects and reducing the risk of unintended intercellular interactions and associated adverse reactions [16]. Exosome carriers provide substantial benefits for cell-based drug delivery and nanotechnology applications. Incorporating exosomes into drug delivery systems like hydrogels and excipients facilitates effective drug transportation, overcoming diverse biological barriers and mitigating the risk of immunogenicity (Fig. 1) [17]. While numerous studies have explored exosomes about macrophages, there is currently a lack of literature fully elucidating the bidirectional regulatory mechanisms of macrophages and exosomes in complications arising from both T1DM and T2DM. Additionally, preclinical research on this topic remains limited. The primary focus of this article is to review the bidirectional regulatory mechanisms between macrophages and exosomes in complications arising from both T1DM and T2DM. The exploration of how exosomes employ biotechnological methods to address diabetic complications offers fresh insights into strategies for their prevention and management.
Fig. 1.
The preclinical testing process of the exosome drug delivery system. Exosomes (including RNA, DNA, or protein) isolated by are encapsulated in various materials (hydrogel, graphene, microneedle, nanoparticle, 3D-printed scaffold), and act on mouse or rat models of various diabetic complications utilizing injection, subcutaneous implantation, or wound dressing for preclinical research
Exosomes: Biogenesis, Isolation, Composition, and Function
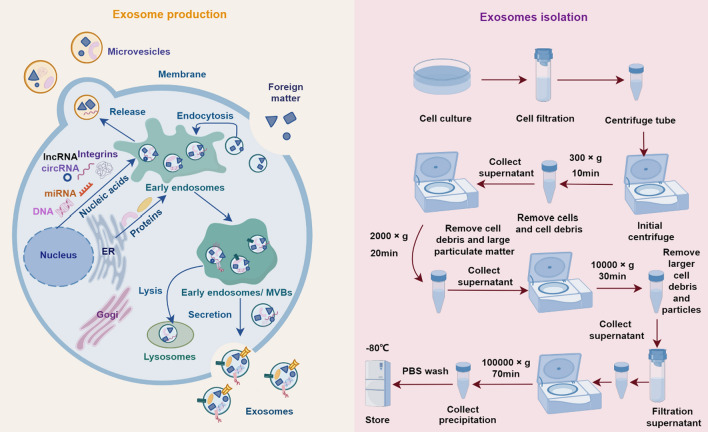
Exosomes originate from the endosomal pathway, initiated by the formation of early endosomes, which subsequently mature into multivesicular bodies (MVBs). Intraluminal vesicles (ILVs) form through the inward budding of the endosomal membrane within the MVB, leading to the encapsulation of cytoplasmic contents within ILVs. MVBs can either fuse with lysosomes for degradation or fuse with the plasma membrane, releasing ILVs as exosomes into the extracellular space [18]. The generated exosomes harbor a diverse array of biomolecules, encompassing various proteins (such as membrane transporters, adhesion molecules, signaling proteins, heat shock proteins, and enzymes involved in various cellular processes), lipids, nucleic acids (including mRNA, miRNA, and other non-coding RNAs), and metabolites [19]. Presently, ultracentrifugation is the predominant technique employed for exosome isolation. Initially, cell culture supernatant is collected and subjected to a pre-clearing step to remove cell debris and nuclei. Subsequently, ultracentrifugation is performed to pellet the exosomes, followed by washing and another round of ultracentrifugation to obtain purified exosomes pellet. Finally, the extracted exosomes are further analyzed, including protein and RNA detection, and stored under appropriate conditions for subsequent experiments (Fig. 2) [20]. Although ultracentrifugation is widely used, it has inherent limitations. Other methods for isolating exosomes, such as ultrafiltration, size exclusion chromatography, immune affinity isolation, polymer-based precipitation, and microfluidic technology are noteworthy alternatives. Researchers can choose the appropriate method based on their specific conditions and requirements (Table 1).
Fig. 2.
Exosome production and isolation. The specific molecules bind to receptors on the cell surface, followed by endocytosis to form early endosomes. These early endosomes migrate within the cytoplasm and gradually mature into late endosomes. During this process, the endosomal membrane invaginates to form intraluminal vesicles (ILVs) within the endosome. Late endosomes, which contain a large number of ILVs, are referred to as multivesicular bodies (MVBs). MVBs are transported through the cytoplasm along microtubules, moving towards the vicinity of the cell membrane. After fusing with the cell membrane, MVBs release the ILVs into the extracellular environment, where they are known as exosomes. Exosomes contain proteins, lipids, and RNA molecules, and they can interact with other cells. In the separation process of exosomes, the precipitate (exosomes) obtained by ultracentrifugation at 100000×g for 70 min can be centrifuged again at 100000×g for 70 min to remove pollutants. Repeat this step twice to ensure the purity of exosomes
Table 1.
Different methods of exosome isolation and their advantages and disadvantages
| Isolation method | Advantages | Disadvantages | References |
|---|---|---|---|
| Ultracentrifugation | Commonly used and mature methods, no special reagents required | Time-consuming, large sampling volume, increased risk of vesicle rupture and loss | [21–23] |
| Ultrafiltration | Easy operation; no need for high-cost equipment; produce highly-purified exosomes | Hamper elution of exosomes | [24–26] |
| Size exclusion chromatography | Efficient isolation of exosomes of different sizes, gentle handling, not easy to destroy exosomes | Possible sample contamination with lipoproteins | [27, 28] |
| Immune affinity isolation | Highly specific, selectively capture specific exosome subpopulations | High reagent costs, specific antibodies | [29, 30] |
| Polymer-based precipitation | Higher yields, acceptable purity, and comparable biophysical properties and biological functions | Non-specific precipitation may be present | [31, 32] |
| Microfluidic technology | Efficient and fast, handles micro-volume samples, high isolation accuracy | High cost of equipment and technology, specialized operating skills | [33, 34] |
Exosomes, as carriers for transferring bioactive molecules between cells, facilitate the exchange of information and signaling molecules, promoting intercellular communication in various physiological processes, and importantly, regulating immune responses [35]. They present antigens, deliver immune-modulatory molecules such as cytokines and anti-inflammatory proteins, and promote immune tolerance or activation. Exosomes regulate the activity of immune cells, modulate the inflammatory microenvironment, and influence the occurrence and progression of inflammatory responses [36, 37]. Exosomes stimulate cell proliferation and migration within damaged tissues, fostering angiogenesis and the formation of new tissue. Consequently, they expedite the repair process of injured tissues [38, 39]. Furthermore, exosomes participate in regulating the body’s metabolic state. They influence the body’s energy balance and metabolic homeostasis by carrying factors that regulate glucose metabolism, lipid metabolism, and protein metabolism [40–42].
The contents and physiological effects of Exosomes may vary slightly between different subtypes. Taking exosomes derived from M1 and M2 macrophages as examples: under activated conditions, M1 and M2 macrophages secrete exosomes containing different types of bioactive molecules. M1 macrophages primarily secrete pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β), as well as inducible nitric oxide synthase (iNOS) [43]. Meanwhile, M2 macrophages predominantly secrete anti-inflammatory and reparative factors such as interleukin-10 (IL-10), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and tissue repair-promoting molecules. These factors modulate immune responses, imparting a more tolerant and reparative phenotype [44]. Because M1 and M2 macrophages exhibit distinct functional characteristics, the exosomes they produce may also possess different functionalities. Exosomes derived from M1 macrophages are inclined towards promoting pro-inflammatory responses and activating immune cells, exacerbating inflammation and tissue damage. They contribute to fibrosis by enhancing fibroblast activation and extracellular matrix deposition, leading to tissue scarring and functional impairment [45]. In contrast, the exosomes derived from M2, exhibit a propensity for inflammation suppression and tissue repair promotion. They facilitate tissue repair and regeneration by inhibiting pro-inflammatory responses, promoting regulatory T-cell differentiation, reducing fibrosis, stimulating angiogenesis, and facilitating tissue remodeling. These findings underscore their immunomodulatory properties [46, 47].
The Pathology and Mechanisms of Diabetic Complications
Each complication has distinct pathological and pathogenic features. In diabetic retinopathy, the thickening and fibrosis of the retinal capillary basement membrane alter vascular wall structure and disrupt retinal hemodynamics, exacerbating the progression of retinal disease [48]. Diabetic nephropathy involves increased deposition of collagen fibers and other extracellular matrix components in the renal tubulointerstitium, forming fibrotic plaques and impairing tubular function and glomerular structure [49]. Diabetic neuropathy sees high glucose and dyslipidemia affecting peripheral nerve cells, including axons, dorsal root ganglia neurons, and Schwann cells, leading to reduced myelinated nerve fiber density and disrupted nerve conduction [50]. Diabetic foot is characterized by pathologic neuropathy, secondary infections, and occlusive arterial disease [51]. Cardiovascular complications in diabetes feature chronic inflammation, procoagulant states, impaired fibrinolysis, aberrant neovascularization, and microvascular deficits, cumulatively altering blood rheology, arterial structure, and endothelial homeostasis [52].
However, these diabetic complications have a common pathological mechanism driven by chronic hyperglycemia. Hyperglycemia induces oxidative stress and chronic inflammation, leading to cellular and tissue damage [53, 54]. The formation of advanced glycation end-products (AGEs), activation of the protein kinase C (PKC) pathway, and increased production of reactive oxygen species (ROS) can disrupt normal cellular signaling and function, contributing to the development of various complications. Additionally, hyperglycemia directly impairs insulin signaling pathways, further exacerbating insulin resistance [55–58].
Recent studies have highlighted that macrophage-derived exosomes and exosomes derived from cells other than macrophages carry regulatory miRNAs, enzymes, and signaling molecules that regulate glucose metabolism, inflammation, and tissue repair [59]. Macrophage-derived exosomes carrying anti-oxidative enzymes and miRNAs reduce oxidative stress and enhance tissue repair, while exosomes from other cells regulate macrophage lipid metabolism and glucose uptake, further contributing to the treatment of diabetic complications. This bidirectional regulation opens new therapeutic avenues, including targeted drug delivery systems [60].
The Bidirectional Regulation of Macrophages and Exosomes and the Gold Standard for the Treatment of Diabetic Complications
Diabetic complications have established gold standards for treatment. These standards are based on systematic, evidence-based guidelines that provide structured and effective treatment strategies aimed at early detection, slowing disease progression, and improving patient outcomes (Table 2) [61]. As shown in Table 2, the establishment of these therapeutic gold standards has commonalities, most of which are related to inflammatory response, glucose metabolism, and angiogenesis. Importantly, both macrophage-derived exosomes and exosomes acting on macrophages are closely related to these mechanisms. Bidirectional regulation of macrophages and exosomes can act on a variety of pathogenic processes and has advantages in inhibiting the onset and progression of diabetic complications [62]. The development of new exosome-centric therapeutic strategies, such as drug delivery using exosomal carriers, could precisely modulate the pathological process of diabetes and its complications while minimizing side effects [63, 64].
Table 2.
Compendium of gold standards for the treatment of diabetic complications
| Complication types | Gold standard | Indication | Mechanism | Effect | Side effects | References |
|---|---|---|---|---|---|---|
| Diabetic retinopathy | Anti-VEGF therapy | Proliferative diabetic retinopathy, diabetic macular edema | Inhibits VEGF, reducing neovascularization and macular edema | Reduces vision loss, improves visual acuity | Eye pain, intraocular inflammation, vitreous hemorrhage | [75] |
| Laser photocoagulation | Proliferative diabetic retinopathy, severe non-proliferative diabetic retinopathy | Destroys ischemic retinal tissue to prevent neovascularization | Stabilizes retinal structure, prevents vision loss | Eye pain, peripheral vision loss, scarring | [76] | |
| Diabetic nephropathy | Angiotensin converting enzyme inhibitors (ACE inhibitors) | Proteinuria, hypertension | Inhibits angiotensin-converting enzyme, reducing blood pressure and proteinuria | Reduce blood pressure, protects renal function | Hyperkalemia, cough, angioedema | [77] |
| Angiotensin receptor blockers (ARBs) | Proteinuria, hypertension | Blocks angiotensin II receptors, reducing blood pressure and proteinuria | Reduce blood pressure, protects renal function | Hyperkalemia, dizziness, kidney dysfunction | [78] | |
| Sodium-glucose cotransporter 2 inhibitors (SGLT2 inhibitors) | Proteinuria, early diabetic nephropathy | Reduces glucose reabsorption in kidneys, reducing blood sugar and improving renal outcomes | Reduce blood glucose, reduces proteinuria | Genital infections, dehydration, ketoacidosis | [79] | |
| Diabetic neuropathy | Antidepressants | Peripheral neuropathy, pain | Inhibits serotonin and norepinephrine reuptake, modulating pain pathways | Reduces neuropathic pain, improves quality of life | Drowsiness, dizziness, nausea | [80] |
| Anticonvulsants | Peripheral neuropathy, pain | Modulates calcium channel function, reducing neuronal excitability | Reduces neuropathic pain, improves sleep quality | Dizziness, weight gain, peripheral edema | [81] | |
| Diabetic foot | Debridement | Ulcers, necrotic tissue | Removes necrotic tissue, promotes wound healing | Enhances wound healing, prevents infection | Pain, bleeding, infection | [82] |
| Antibiotics | Infected ulcers, soft tissue infections | Eliminates bacterial infection, reducing inflammation | Resolves infection, prevents spread | Antibiotic resistance, gastrointestinal upset | [83] | |
| Offloading devices | Pressure ulcers | Redistributes pressure away from ulcers, reducing mechanical stress | Promotes ulcer healing, prevents new ulcers | Discomfort, skin irritation | [84] | |
| Diabetic cardiovascular disease | Statins | Hyperlipidemia, atherosclerosis | Inhibits HMG-CoA reductase, reducing cholesterol levels | Reduce low density lipoprotein cholesterol, reduces cardiovascular risk | Muscle pain, liver enzyme elevation | [85] |
| Beta-blockers | Hypertension, heart failure | Blocks β-adrenergic receptors, reducing heart rate and blood pressure | Reduce blood pressure, reduces heart failure symptoms | Fatigue, bradycardia, hypotension | [86] | |
| ACE inhibitors | Hypertension, heart failure | Inhibits angiotensin-converting enzyme, reducing blood pressure and myocardial workload | Reduce blood pressure, improves heart function | Hyperkalemia, cough, angioedema | [87] |
Exosomes associated with macrophages can regulate insulin sensitivity and anti-inflammatory factors, thereby helping to reduce blood glucose levels by improving pancreatic β-cell function and insulin signaling [65, 66]. Furthermore, macrophage-derived exosomes transfer anti-atherosclerotic factors, which reduce cholesterol accumulation and slow the progression of atherosclerosis [67]. Exosomes from adipose tissue or the liver modulate macrophage lipid metabolism, thereby reducing blood lipid levels [68]. Macrophage-derived exosomes carry antioxidant enzymes and antioxidant miRNAs. These exosomes help alleviate oxidative stress by reducing the accumulation of free radicals and oxidative products, thus mitigating cellular and tissue damage [69]. Furthermore, exosomes from other cell types carrying antioxidant factors enhance the antioxidant capacity of macrophages, reducing oxidative stress-induced cellular damage [70]. Macrophage-derived exosomes carry angiogenic and endothelial protective factors that regulate macrophage function to protect endothelial cells, reduce vascular injury, and maintain normal vascular function [71, 72]. Not only, do macrophage-derived exosomes mitigate systemic inflammation by modulating inflammatory signaling pathways, but other cell-derived exosomes influence the polarization state of macrophage, thereby reducing chronic inflammation and improving inflammation-related complications of diabetes [73]. Targeting the bidirectional regulation between macrophages and exosomes allows for the use of exosomes derived from macrophages and other cell types as drug delivery systems [74]. These exosomes deliver anti-inflammatory or regulatory factors directly to macrophages, thereby enhancing the therapeutic efficacy of diabetic complications.
Bidirectional Regulation of Macrophages and Exosomes
The Mechanism of Multiple Macrophage-Derived Exosomes
Exosomes derived from macrophages exhibit anti-inflammatory functions and are pivotal in regulating inflammation during the progression of diabetic complications, thus impacting the treatment of diabetic complications. For example, macrophage-derived exosomes effectively inhibit inflammation by suppressing inflammatory signaling pathways [88]. Additionally, these exosomes significantly accelerate angiogenesis and enhance tissue repair quality, thereby promoting the healing of diabetic wounds. Moreover, macrophage-derived exosomes are instrumental in the treatment of diabetic fracture healing. Exosomes from M2 macrophages stimulate the PI3K/AKT pathway to induce the conversion of M1 macrophages to M2 macrophages, significantly modulating the bone immune microenvironment and thereby accelerating the healing of diabetic fractures [89]. Furthermore, IL-4-polarized human macrophage exosomes (THP1-IL4-exo) influence cellular signaling by linking energy metabolism in target cells to mitochondrial respiration, with the miR-146b-5p within THP1-IL4-Exo capable of reducing Toll-like receptor (TLR)/ nuclear factor-kappa B (NF-κB) signaling in both macrophages and hematopoietic stem and progenitor cells (HSPCs), thereby controlling inflammatory activity and inhibiting the progression of diabetic atherosclerosis. Therefore, THP1-IL4-Exo can be used to control inflammatory outbreaks in cardiac metabolic tissues during obesity-driven diabetes [90]. However, certain macrophage-derived exosomes can have negative effects on the treatment of diabetic complications. High glucose-treated macrophage-derived exosomes (HG-Exo) exacerbate renal damage in diabetic nephropathy, with elevated expression of TGF-β in the kidney contributing to glomerulosclerosis and renal tubular interstitial fibrosis. Simultaneously, HG-Exo can decrease glomerular filtration rate, enhance the expression of inflammatory cytokine iNOS, induce the polarization of pro-inflammatory M1 macrophages, incite the inflammatory response of renal tissue cells, and exert detrimental effects on the treatment of diabetic nephropathy [91]. High glucose-induced macrophage-derived exosomal TGF-β1 mRNA also leads to the overproduction of fibrosis and inflammatory factors, induces mesangial cell proliferation and activation, and promotes mesangial expansion and renal fibrosis [92]. In summary, exosomes derived from macrophages under non-high glucose conditions can alleviate inflammation induced by diabetes through various mechanisms such as inhibiting the release of inflammatory factors, promoting tissue repair, and modulating cellular signaling pathways. This alleviation of inflammation helps mitigate inflammatory reactions and prevents adverse effects during the treatment of diabetic complications, thereby halting disease progression.
Insulin resistance is not only the initiating factor of T2DM but also elevates the risk of diabetic complications. Adipose tissue macrophage-derived exosomal miR-29a is transported to adipocytes, myocytes, and hepatocytes, impairing their glucose uptake. Moreover, it promotes obesity-induced insulin resistance by overacting the target gene PPAR-d. Therefore, targeting the crucial exosomal miR-29a pathway could be considered a novel approach to improve insulin sensitivity, potentially mitigating obesity-induced insulin resistance by inhibiting its production and release [93]. Additionally, obesity may lead to the suppression of MKP5 expression by bone marrow macrophage-derived exosomal miR-143-5p, thereby modulating the insulin signaling pathway in hepatocytes and resulting in insulin resistance [94]. Cytokines IL-4 and IL-13 stimulate bone marrow-derived macrophages to polarize into M2-type macrophages, which secrete exosomes containing miR-690 to promote insulin sensitivity and enhance insulin’s effect on adipocytes and hepatocytes. Exosomal miR-690 can serve as a novel insulin sensitizer and holds the potential for the treatment of diabetes and its complications [95]. Combining rosiglitazone (Rosi) with adipose tissue macrophage-derived small extracellular vesicles (ATM-sEVs) from lean mice can improve glucose intolerance and insulin sensitivity caused by obesity, without causing adverse effects such as weight gain or hemodilution. Rosi-ATM-sEVs miR-690 can directly enhance insulin sensitivity in insulin-responsive tissues. This provides strong support for the potential of M2-type bone marrow-derived macrophage exosomal miR-690 as an insulin sensitizer [96]. Under high glucose conditions, macrophage-derived exosomal MALAT1 inhibits the expression of miR-150-5p, thereby alleviating insulin resistance and reducing lipid accumulation and vascular inflammation. Macrophage-derived exosomes containing MALAT1 could prevent vascular diseases through cell-free therapy, offering a novel approach to the treatment of diabetic vascular complications [97]. Lipotoxicity-polarized macrophage-derived exosomal miR-27-3p not only reduces glucose tolerance and insulin sensitivity but also triggers an NLRP3-dependent pro-inflammatory response driven by prolonged mtROS generation. Persistent lipotoxicity impairs mitophagy, which in turn exacerbates insulin resistance, establishing a harmful feedback loop between mitophagy dysfunction and insulin resistance, which further worsens diabetic complications. MiR-27-3p antagomir can ameliorate insulin resistance in insulin-sensitive tissues, thereby having a positive impact on preventing the progression of diabetic complications [98]. Exosomes derived from macrophages affect insulin sensitivity by carrying inflammatory factors and molecules that regulate intracellular lipid metabolism, thereby contributing to the occurrence and exacerbation of insulin resistance. Therefore, the development of inhibitors targeting specific pathways and signaling mechanisms may become a significant focus of scientific research, aiming to mitigate the impact of macrophage-derived exosomes on insulin resistance (Table 3).
Table 3.
The mechanisms of macrophage-derived exosomes
| Action pathway | Source of exosomes | Exosome content | Experimental model | Route of administration | Biological function | Molecular mechanism | Application | References |
|---|---|---|---|---|---|---|---|---|
| Inflammation | Macrophages | Exos | Hyperglycemic skin injury SD rat | Subcutaneous injection | Inhibits inflammatory signaling in vivo | TNF-α, IL-6↓ | Diabetic wound healing | [88] |
| M2 macrophages | M2-Exos | Osteotomized db/db mice (T2DM) | Introduce the fracture site | Macrophages polarized from M1 to M2 | PI3K/AKT↑ | Diabetic bone wound healing | [89] | |
| IL-4 polarized human macrophages | MiR-146b-5p, miR-33-5p, miR-21-5p | Apoeh/h Ldlr-/- mice | Intraperitoneal injection | Drive mitochondrial cell signal transduction | TLR/NF-κB↓ | Diabetic atherosclerosis | [90] | |
| High glucose-treated macrophages | HG-treated Raw264.7 | High glucose-induced male C57BL/6 mice | Tail vein injection | Glomerulosclerosis and tubulointerstitial fibrosis | TGF-β, TNF-α, IL-1β↑ | Aggravate diabetic nephropathy | [91] | |
| High glucose-treated macrophages | Raw264.7 | Male-specific pathogen-free C57BL/6 mice | Tail vein injection | Promote mesangial expansion and renal fibrosis | TGF-β/SMAD3↑ | Aggravate diabetic nephropathy | [92] | |
| Insulin resistance | Adipose tissue macrophages | MiR-29a | High fat-induced male C57BL/6 mice | Intravenous injection | Damage insulin sensitivity | PPAR-d↑ | Deterioration of condition | [93] |
| Bone marrow macrophages | MiR-143-5p | High fat-induced mice | Lipopolysaccharide-induced RAW264.7 exosomes | Regulation of the insulin signaling pathway in hepatocytes results in insulin resistance | MKP5↓ | Deterioration of condition | [94] | |
| M2 bone marrow-derived macrophages | MiR-690 | High fat-induced male C57BL/6 mice | Tail vein injection | Promote cell insulin sensitivity | NADK↑ | Improve insulin resistance | [95] | |
| Macrophages containing MALAT1 | MALAT1 | Male Wistar rat with an intraperitoneal injection of streptozotocin | Intracarotid injection | Reduce balloon injury | MiR-150-5p↓ | Diabetic angiopathies | [97] | |
| Lipotoxicity-polarised macrophages | MiR-27-3p | High fat-induced male C57BL/6 mice | Intravenous injection | Reduce glucose tolerance and insulin sensitivity | MIRO1↓ | Deterioration of condition | [98] |
The Mechanism of Various Exosomes Acting on Macrophages
Exosomes exert significant therapeutic effects on diabetic complications by modulating macrophage polarization and participating in intercellular signal transduction. For example, Urinary stem cell-derived exosomal circRNA ATG7 promoted M2 macrophage polarization while inhibiting MI macrophage polarization to reduce the release of pro-inflammatory factors and regulated the SOCS1/STAT3 signaling pathway through miR-4500 to attenuate the renal injury in diabetic nephropathy and alleviate the deteriorating trend of diabetic nephropathy [99]. Human umbilical cord mesenchymal stem cells-derived exosomal miR-146a-5p emerged as a crucial contributor to the therapeutic approach to diabetic nephropathy. It regulated the imbalance of M1/M2 macrophages by targeting the TRAF1-STAT2 pathway, promoting M2 macrophage polarization to inhibit renal inflammation in diabetic nephropathy patients, thereby restoring renal function in diabetic nephropathy [100]. Additionally, miRNA-17-3p contained in exosomes derived from human umbilical cord mesenchymal stem cells plays a crucial role in the targeted treatment of diabetic retinopathy. Overexpression of miRNA-17-3p reduces the levels of TNF-α, IL-1β, IL-6, MDA, VEGF, and ROS while enhancing the activity of SOD and GSH-Px. Targeting STAT1 alleviates inflammation and oxidative damage in mice with diabetic retinopathy [101]. Exosomes derived from endometrial mesenchymal stem cell / stromal cell subsets contributed to the treatment of diabetic inflammation. Four commonly found miRNAs (has-miR-320e, has-miR-182-3p, has-miR-378 g, has-let-7e-5p), presented in exosomes derived from a subset of CD146 + endometrial-derived mesenchymal stem/stromal cells, they contributed to the regulation of macrophage polarization, T-cell activation, and the transcription of inflammatory cytokines. This modulation increased the secretion of anti-inflammatory factors, coupled with a decrease in pro-inflammatory molecules. Such exosomes displayed the potential to locally exert therapeutic anti-inflammatory effects by influencing the function of macrophages and peripheral blood monocytes [102]. In addressing inflammation induced by T2DM, pancreatic β-cell-derived exosomal miR-29 targets TRAF3, promoting the release of CXCL10 from β-cells, leading to increase inflammatory response to diabetic complications. Blocking miR-29 could protect β-cells from the harmful effects of these inflammatory mediators [103]. Furthermore, miR-29 can suppress both innate and adaptive immune responses. It interacts with NF-κB, resulting in β-cell dysfunction and exacerbating the progression of T1DM. Consequently, blocking the transport of miR-29 represents a potential therapeutic strategy for preventing diabetes-related inflammation [104]. In summary, exosomes from different sources have slightly different effects on macrophages because of their different contents. These exosomes trigger the anti-inflammatory potential of macrophages through different pathways and affect different types of diabetic complications.
Exosomes acting on macrophages regulate their activity while also modulating insulin signaling pathways and lipid metabolism, significantly impacting insulin resistance. Macrophage exposure to adipocyte-derived exosomes resulted in elevated production of the proinflammatory cytokines, heightened macrophage migration to adipose tissue and liver, and facilitated the development of insulin resistance. Inhibition of TLR4 gene expression prevented the adipose tissue-derived exosomal RBP4 protein from inducing the production of pro-inflammatory cytokine by macrophages in a TLR4-dependent manner, thereby suppressing insulin resistance and relieving diabetic complications [105]. The exosomal microRNA miR-1249-3p, derived from natural killer cells, suppresses the TLR4/NF-κB signaling pathway by facilitating the interaction between SKOR1 and SMAD6. This regulatory mechanism attenuates insulin resistance and inflammation, thereby fostering glucose homeostasis. Consequently, targeting this pathway represents a promising therapeutic strategy for managing diabetic complications [106]. The conditioned medium released by Kupffer cells, loaded with lipotoxic hepatocyte- or circulating-derived exosomes, diminished insulin-induced hepatocyte phosphorylation of AKT. The hepatocyte-macrophage/Kupffer cell-hepatocyte paracrine crosstalk was mediated by hepatocyte or circulating-derived exosomes containing saturated fatty acids. Selective depletion of Kupffer cells has been shown to ameliorate steatosis and insulin resistance. Saturated fatty acids released by hepatocytes constitute crucial components of the cargoes within hepatocyte- or circulating-derived small extracellular vesicles. These cargoes are translocated to macrophages and Kupffer cells, initiating insulin resistance through target genes. Lipotoxic saturated fatty acids encapsulated in hepatocyte or circulating-derived small extracellular vesicles represent potential targets for the treatment of diabetic complications [107]. Additionally, research has shown that adipocyte-derived exosomes carrying Sonic Hedgehog (Shh) stimulate M1 macrophage polarization through the Ptch and PI3K pathways, resulting in insulin resistance in adipocytes. Blocking Shh signaling may be a therapeutic approach to inhibit the development of insulin resistance [108]. In summary, exosomes exert a dual role on macrophages: they can both ameliorate and induce insulin resistance. Blocking the pathways associated with exosome-induced insulin resistance or inhibiting the target genes within these pathways could serve as effective strategies for diabetic complications (Table 4).
Table 4.
The mechanism of various exosomes acting on macrophages
| Action pathway | Source of exosomes | Exosome content | Experimental model | Route of administration | Biological function | Molecular mechanism | Application | References |
|---|---|---|---|---|---|---|---|---|
| Inflammation | Urinary stem cells | CircRNA ATG7 | Diabetic nephropathy rat | Intraperitoneal injection | Inhibit M1 macrophage polarization and promote M2 macrophage polarization | SOCS1↑ | Diabetic nephropathy | [99] |
| Human umbilical cord mesenchymal stem cells | MiR-146a-5p | Diabetic nephropathy rat | Tail vein injection | Promote M2 macrophage polarization | TRAF6, STAT1↓ | Diabetic nephropathy | [100] | |
| Human umbilical cord mesenchymal stem cells | MiRNA-17-3p | A mouse diabetes model | Injection | Reduce inflammatory factors and reduce oxidative damage | TNF-α, IL-1β, IL-6, MDA, VEGF, ROS↓ | Diabetic retinopathy | [101] | |
| CD146 + endometrial-derived mesenchymal stem/stromal cells | Hsa-miR-320e, hsa-miR-182-3p, hsa-miR-378 g, hsa-let-7e−5p | Human peripheral blood mononuclear cells | THP-1/eMSC Exos and PBMC/eMSC Exos co-culture | Promote M2 macrophage polarization | IL-10, IL-13↑ | Diabetes-related inflammation | [102] | |
| Pancreatic β Cells | MiR-29 | β-cell specific miR-29a/b/c transgenic mice, High fat-induced C57BL6/J mice | Intraperitoneal injection | Polarized M1 macrophages | CXCL10↑ | Aggravate T2DM | [103] | |
| Pancreatic β Cells | MiR-29 | NOD mice | RNA purification of NOD mice | Increased pro-inflammatory | IFN-γ↓ | Aggravate T1DM | [104] | |
| Insulin resistance | Adipose tissue | ObELV | TLR4 knockout mice | Intravenous injection | Inhibition of macrophage activation | IL–6, TNF–α↓ | Relief insulin resistance | [105] |
| Natural killer cells | MiR-1249-3p | High fat-induced male C57B/6 mice | Tail vein injection | Regulation of the insulin signaling pathway in hepatocytes results in insulin resistance | SKOR1, TLR4/NF-κB↓ | Relief insulin resistance and inflammation | [106] | |
| Hepatocytes | Saturated fatty acid | High fat-induced male C57B/6j mice | Nanoparticle tracking analysis | Activate macrophage proinflammatory response | CCL2, JNK, p38MAPK, p65-NF-κB↑ | Deterioration of condition | [107] | |
| Adipocyte | Sonic hedgehog | 3T3-L1 adipocytes and RAW 264.7 macrophages were co-cultured | Lipofectamine method | M1 macrophage polarization | Ptch/PI3K↑ | Deterioration of condition | [108] |
The Bidirectional Regulation Mechanism of Macrophages and Exosomes
M1 macrophages are crucial inflammatory cells capable of phagocytosing pathogens. However, under hyperglycemic conditions, M1 macrophage polarization induces the secretion of pro-inflammatory cytokines, which intensify cellular inflammation and disrupt insulin signaling pathways, ultimately leading to insulin resistance. Addressing the chronic inflammatory response in diabetic complications necessitates the promotion of M2 macrophage polarization. M2 macrophages release anti-inflammatory cytokines, support angiogenesis, and mitigate insulin resistance [109]. Recent studies have shown that the way in which macrophage-derived exosomes polarize macrophages is affected by glucose concentration. Macrophage-derived exosomes from macrophages treated with 15 mM glucose induce an M2-like phenotype in recipient macrophages, increase the protein levels of major mitochondrial complexes, and elevate IL-6 expression levels, thereby improving insulin sensitivity in the body. Therefore, developing new drugs targeting macrophage-derived exosomes treated with 15 mM glucose holds great potential for the treatment of diabetic complications [110]. The bidirectional regulation between macrophages and exosomes is not limited to macrophage-derived exosomes acting on macrophages. Exosomes derived from other cell types can also act on macrophages to exert therapeutic effects on diabetic complications. To alleviate the chronic inflammatory response caused by diabetic complications, it is also possible to inhibit M1 macrophage polarization. Exosomes derived from bone marrow mesenchymal stem cells mediate miR-25-3p, which not only restricts M1 macrophage polarization but also promotes the conversion of M1 macrophages to M2 macrophages, thereby exerting a reparative effect on cardiac inflammation. It will be instrumental in the treatment of diabetic cardiovascular disease [111]. In addition to inhibiting M1 macrophage polarization, identifying specific targets that can suppress M1 macrophage polarization is also a viable approach for treating diabetic complications. In diabetic nephropathy, exosome-derived miRNA-34a from renal tissue negatively regulates PPARG Coactivator 1 Alpha (PPARGC1A), promoting the activation of M1 macrophages and tubular cell fibrosis. Inhibitors of miRNA-34a can increase PPARGC1A expression, thereby inhibiting M1 macrophage polarization and tubular cell fibrosis, offering a therapeutic strategy for diabetic nephropathy [112].
Treatment of Diabetic Complications
Application of Macrophage-Derived Exosomes
In the treatment of diabetic complications, engineered exosomes demonstrate superior characteristics compared to natural exosomes. These attributes include prolonged circulation, enhanced stability, and targeting capabilities, which effectively aid in drug delivery and release within cells. Engineering of gene-modified M2-like macrophage-derived exosomes through Casein kinase 2 interacting protein-1 (CKIP-1) could be utilized for alveolar bone regeneration under porphyromonas gingivalis (Pg)-dominant inflammatory conditions. CKIP-1 silenced M2-like macrophages transfer exosome Let-7f-5p to cementoblasts, promoting mineralization via targeted activation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-dependent mitochondrial biogenesis. This presents a novel therapeutic strategy for the future management of diabetes-induced periodontitis [113]. Ultracentrifugation and cyclic sonication were employed to successfully engineer Melatonin into M2 macrophage-derived exosomes. The released Melatonin from these engineered exosomes promoted macrophage repolarization through immune reprogramming. Furthermore, these engineered exosomes demonstrated the capacity to rescue the osteogenic and cementogenic differentiation abilities of inflammatory human periodontal ligament cells by mitigating excessive endoplasmic reticulum stress and unfolded protein response. Ultimately, Melatonin-engineered M2 macrophage-derived exosomes effectively mitigate the exacerbation of diabetes mellitus, stemming from the decline in anti-infection ability and the increase in inflammatory factors [114]. Compared to synthetic nanoparticles, macrophage-derived exosomes offer advantages such as biocompatibility and resistance to degradation. They can effectively target and deliver exogenous nucleic acids and therapeutic drugs to diabetes-related tissues, thereby accelerating recovery.
Utilizing fusion technology to load exosomes onto biomaterials or employing cell engineering techniques to construct nanosystems carrying specific exosomes can both be used to treat diabetic complications. The F127/tissue-adhesive o-nitrobenzyl alcohol-modified hyaluronic acid (HA-NB) adhesive dual-responsive hydrogel loaded with M2 macrophage-derived exosomes exhibits a dense network structure, tissue adhesiveness, and dual sensitivity to temperature and light. This hydrogel promotes bone healing by facilitating GSK-3β phosphorylation, which activates the Wnt signaling pathway and the β-catenin signaling pathway. Therefore, it holds significant potential for applications in promoting diabetic bone healing [115]. The PANB/Au/AC hydrogel system, utilizing polyallylamine hydrochloride-modified AuNRs as functionalized crosslinking agents, demonstrated outstanding photothermal antibacterial performance under near-infrared radiation, highlighting its advantages in promoting wound healing. The M2 macrophage-derived exosomes incorporated into the PANB/Au/AC hydrogel exhibit sustained and controlled release, effectively suppressing inflammation and eradicating bacterial infections at diabetic wound sites. The integrated hydrogel system effectively accelerates diabetic wound healing by leveraging antibiosis, antioxidant stress, and anti-inflammatory effects [116]. In addition to the hydrogel system described above, a dual-layer microneedle-based wound dressing system has been developed. This system concurrently suppressed inflammation and enhanced angiogenesis at the wound site by encapsulating M2 macrophage-derived exosomes at the needle tip and polydopamine nanoparticles in the backing layer. The exosomes released by this wound dressing system enhance macrophage polarization toward the M2 phenotype. Macrophage exosomes and the photothermal properties of the system work together to inhibit diabetic inflammation and promote angiogenesis in a cell-free manner by up-regulating the expression of CD31 and VWF [117]. Diabetic foot ulcers treatment remains a significant challenge for clinical practitioners. The multifunctional hyaluronic acid hydrogel loaded with M2 macrophage-derived exosomes exhibits excellent self-healing and tissue adhesion properties. This enables rapid hemostasis and, through continuous release of pre-loaded M2 macrophage-derived exosomes, promotes angiogenesis at the wound site, accelerating the healing of diabetic foot ulcers [118]. Moreover, intraperitoneal administration of cationic liposome-assisted lipid nanoparticles enables stable and specific protein expression in insulin-secreting β-cells of the pancreas. The levels of gene transfer facilitated by peritoneal macrophage-derived exosomes aid ionizable lipid nanoparticles in effectively and specifically delivering mRNA to the pancreas. Peritoneal macrophage-derived exosome gene therapy holds promise for treating stubborn pancreatic diseases such as diabetes-related complications (Table 5) [119].
Table 5.
Engineered exosomes and exosome-loaded drug delivery systems
| Category | Biomaterials | Exosomes source | Experimental model | Route of administration | Disease | Biological function | References |
|---|---|---|---|---|---|---|---|
| Macrophage | Genetically engineered exosomes | M2 macrophages | BALB/c nude male mice | Hypodermic implantation | Diabetic periodontitis | CKIP-1↓, PGC-1α↑ | [113] |
| Melatonin engineered exosomes | M2 Macrophages | Rat ligature periodontitis model | Injection | Diabetic periodontitis | Reduce excessive endoplasmic reticulum stress and unfolded protein response | [114] | |
| F127/HA-NB-adhesive dual-sensitive hydrogel | M2 Macrophages | Pentobarbital was used to drill holes in the skulls of SD rat | Fill with the bone defect site | Diabetic bone healing | Promote GSK-3β phosphorylation to activate the wnt signaling pathway and β-catenin signaling pathway | [115] | |
| PANB/Au/AC-hydrogel | M2 Macrophages | Male C57BL/6J mice were intraperitoneally injected with streptozocin | Bond | Diabetic wound healing | Antibiosis, anti-oxidative stress, anti-inflammatory | [116] | |
| Double-layer microneedle-based wound dressing system | M2 Macrophages | A female SD rat was intraperitoneally injected with streptozocin | Wound dressing | Diabetic wound healing | Improving the expression of CD31 and VWF to promote angiogenesis | [117] | |
| Multifunctional hyaluronic acid hydrogel | M2 Macrophages | Bleeding liver of mouse | Stent dressing adhered to the bleeding site | Diabetic foot ulcers | Self-healing and tissue adhesion | [118] | |
| Ionizable lipid nanoparticles | Peritoneal macrophages | C57BL/6 female mice with a small incision along the midline were made with sterile scissors | Syringe filling | Diabetic wound healing | Promote pancreatic mRNA delivery | [119] | |
| Cells other than macrophages | Engineered exosomes | Adipose stem cells | Male C57BL/6 mice were intraperitoneally injected with streptozotocin and two round full-thickness wounds were made with a skin punch | Subcutaneous injection | Diabetic wound healing | Inhibition of p65 and IκBα phosphorylation | [120] |
| Engineered exosomes | Mesenchymal stromal cells (miR-146a) | Male BKS.Cg-m + / + Leprdb/J (db/db) mice | Tail vein injection | Diabetic neuropathy | Activation of vascular endothelial cells and change of axonal sensory transduction characteristics | [121] | |
| Engineered exosomes | Epidermal stem cells (miR-203a-3p) | Two full-thickness wounds were formed on the back of db/db mice using a punch | Intradermal injection | Diabetic wound healing | Activation of JAK2/STAT3 signaling pathway | [122] | |
| Engineered exosomes | Bone marrow Mesenchymal stromal cells | Female Wistar rat were intraperitoneally injected with streptozotocin | Intramuscular injection | Diabetic neuropathy | Promote pancreatic cell proliferation and inhibit pancreatic β cell apoptosis | [123] | |
| All-natural hydrogel | Protocatechuic aldehyde hybridized collagen | Application of streptozotocin to Sprague Dawley rat and creation of a whole wound | Hydrogel treatment | Diabetic wound healing | M1 macrophages are transformed into M2 macrophages | [125] | |
| Graphene oxide-loaded gelatin-alginate hydrogel | Platelet cells | Establishment of chronic full-thickness dorsal skin wounds in diabetic Wistar rat | Local application | Diabetic wound healing | Promote HSP expression and M2 macrophage polarization | [126] | |
| Injectable hydrogel | Human adipose-derived mesenchymal stem cells | Establishment of round full-thickness wounds in male SD rat diabetic model | Tegaderm film coverage | Diabetic wound healing | Promote M2 macrophage polarization | [127] | |
| Bioinspired adaptable indwelling microneedle | Mesenchymal stem cells | Diabetic rat model to create a circular full-thickness wound | Skin penetration | Diabetic Ulcers | Inhibit M1 macrophage polarization | [128] | |
| Electroconductive nerve dressing | Bone marrow stem cells | A diabetic sciatic nerve crush injury model was established in female SD rat | No.25 needle injection | Diabetic neuropathy | Through NF-κB pathway regulates macrophage polarization | [129] |
Application of Exosomes from Multiple Sources Acting on Macrophages
Engineered exosomes carrying specific nucleic acids such as siRNA, miRNA, or DNA are delivered to target cells by macrophages, thereby regulating gene expression and playing a significant role in treating inflammatory diseases associated with diabetes. MiR-132 is precisely delivered into adipose-derived stem cell-engineered exosomes, forming miR-132-containing engineered exosomes (miR-132-Exo). MiR-132-exo inhibits the phosphorylation of p65 and IκB along the NF-κB signaling pathway while inducing M2 macrophage polarization, thereby reducing inflammation, accelerating angiogenesis, and promoting collagen remodeling, offering a novel approach to enhancing the speed of diabetic wound healing [120]. Moreover, through the inhibition of the TLR4/NF-κB pathway, engineered mesenchymal stromal cell-derived exosomes, enriched with microRNA-146a, suppress macrophage infiltration and M1 macrophage polarization within the sciatic nerve, consequently mitigating inflammation, diminishing cellular inflammatory factor secretion, and fostering vascular endothelial activation and cytokine production modulation. This intervention significantly enhances nerve conduction velocity, and reduces thresholds for thermal and mechanical stimulation, thus aiding in the amelioration of diabetic neuropathy [121]. Epidermal stem cell-derived exosomes, as potential therapeutic agents, exhibited promising prospects in the realm of engineered exosomes. Specifically, exosomal miR-203a-3p derived from epidermal stem cells orchestrated the regulation of inflammatory responses by downregulating SOCS3 expression in macrophages. This molecular modulation activated the JAK2/STAT3 signaling pathway, ultimately inducing M2 macrophage polarization. The application of miR-203a-3p sensitizer additionally amplified cell regeneration across diverse tissues, ultimately leading to improved diabetic wound healing [122]. The engineered exosomes derived from bone marrow mesenchymal stromal cells (BMSCs), obtained by fusing exosomes originating from BMSCs with liposome-containing polypyrrole nanoparticles (PpyNps), possess antioxidant components that could protect cells in oxidative stress environments. Furthermore, these engineered exosomes promote pancreatic cell proliferation and alleviate hyperglycemia by protecting pancreatic β-cells and enhancing insulin sensitivity, thus facilitating the recovery of damaged nerves in diabetic peripheral neuropathy [123].
Exosome-loaded drug delivery systems primarily achieve precise delivery by transporting exosomes to target macrophages. This approach helps to increase the concentration of exosomes within macrophages, thereby enhancing the therapeutic efficacy for diabetic complications [124]. The physical entanglement network formed by fish gelatin (FG) and protocatechuic aldehyde (PA), along with the chemical crosslinking network formed by photopolymerized methacrylate gelatin (FGMA), constitutes a fully natural hydrogel (FGMA/FG/PA), which exhibits excellent biocompatibility and effective antioxidant properties. The physical closure of wounds reduces external infections, while its chemical antibacterial action and ROS scavenging ability can decrease wound inflammation, demonstrating the significant potential for diabetic wound treatment [125]. The gelatin-alginate hydrogel system loaded with reduced graphene oxide, by incorporating platelet-derived exosomes, inhibits M1 macrophage polarization and promotes M2 macrophage polarization while enhancing the expression of heat shock proteins (HSPs) and activating cellular protective signaling pathways. Near-infrared activation experiments have demonstrated that this multimodal hydrogel can completely close wounds in vivo, showing great potential for the biotherapy of diabetic wounds [126]. Additionally, injectable exosome hydrogels with self-healing properties, composed of chitosan-aniline tetramer (CS-AT) and polyethylene glycol diacrylate (PEG-DA) terminated with benzaldehyde, promote M2 macrophage polarization. They also enhance the proliferation, migration, and tube formation ability of human umbilical vein endothelial cells, accelerating diabetic wound healing [127]. The biomimetic adaptive retention microneedles prepared using a combination of template replication and 3D printing techniques, consist of a tunable polyvinyl alcohol hydrogel needle tip encapsulated with mesenchymal stem cell exosomes and a detachable support base made of 3 M medical tape. The microneedles suppress M1 macrophage polarization, promote cell proliferation, induce angiogenesis and neurogenesis, modulate immune responses, and alleviate inflammation, offering significant value in the care of diabetic complications and tissue engineering [128]. Electroconductive nerve dressings, formulated from a blend of tannic acid and polypyrrole, were laden with exosomes derived from bone marrow stem cells. These exosomes were anchored onto the conductive hydrogel through the establishment of reversible, non-covalent hydrogen bonds, enabling the continuous release and accumulation of exosomes at the site of the lesion. This conductive hydrogel not only exhibited anti-inflammatory effects by regulating macrophage polarization through the NF-κB pathway but also enhanced the regeneration of myelinated axons via the MEK/ERK pathway. As a result, this composite material mitigated diabetes-induced muscle denervation, leading to the restoration of muscle function. The comprehensive impact included an amelioration of diabetic peripheral nerve injury, characterized by enhanced nerve regeneration, recovery of motor function, and alleviation of inflammatory pain [129].
Application of Bidirectional Regulation Mechanism Targeting Macrophages and Exosomes
The bidirectional regulation between macrophages and exosomes holds significant therapeutic potential. Exosomes derived from macrophages and other cell types can serve as efficient drug delivery systems, enhancing the therapeutic effects by directly delivering anti-inflammatory factors or regulatory molecules to macrophages. This approach can improve the treatment of diabetic complications by reducing chronic inflammation and promoting tissue repair. Electrospun scaffolds are engineered by anchoring exosomes derived from reparative M2 macrophages onto nanofiber scaffolds with topologically porous structures. These scaffolds facilitate the release of exosomes, which can be internalized by target cells. Due to the inclusion of exosomes extracted from M2 macrophages, this delivery system not only reduces immunogenicity but also enhances cell migration, tube formation, osteogenic differentiation, and M2 macrophage polarization, thereby promoting diabetic fracture healing [130]. Cell-derived exosomes other than macrophages can also be incorporated into hydrogels to enhance the polarization of M1 macrophages to the M2 phenotype. Incorporating bone marrow-derived mesenchymal stem cell exosomes into carboxyethyl chitosan-dialdehyde carboxymethyl cellulose hydrogels can promote the formation of M2 macrophages and modulate human umbilical vein endothelial cells to enhance angiogenesis. This approach can synergistically regulate the wound inflammatory microenvironment in T1DM rats, thereby restoring the structural integrity and functionality of diabetic skin [131].
Conclusion
The bidirectional regulation of macrophages and exosomes is involved in chronic inflammation, and insulin resistance, which directly affects the progression of diabetic complications. A variety of engineering technical means have been used to optimize the nature and function of exosomes, and a variety of drug delivery systems have been developed to load exosomes, to achieve the loading and delivery of exosomes, and to help deliver exosomes accurately to the target organ to ensure their safety and efficacy in clinical applications. Currently, the quality and quantity of exosomes isolated and purified from cells may be limited [132, 133]. More research should be directed toward the identification and targeted modification of useful components of exosomes. In the future, bidirectional regulation of macrophages and exosomes will hopefully provide strong support for clinical practice, and in-depth exploration in this field is expected to lead to more innovative and effective therapeutic strategies for diabetic complications.
Acknowledgement
The figures in this article were drawn by Figdraw, an online drawing website (https://www.figdraw.com).
Author Contributions
Xue Li conducted the literature review, drafted the manuscript and visualized the figures. Lianrong Yang formally analyzed and investigated this manuscript. Shujun Xu formally analyzed this manuscript. Yuan Tian was responsible for resources and supervision. Xin Meng revised this manuscript and performed funding acquisition.
Funding
This work was supported by the Science Foundation of Heilongjiang Administration of Traditional Chinese Medicine (Grant No. ZHY18-021).
Data availability
Data will be made available on request.
Declarations
Conflict of interest
The authors have no conflicts of interest associated with this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daneman, D. Type 1 diabetes. Lancet. 367:847–858, 2006. 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 2.Stumvoll, M., B. J. Goldstein, and T. W. van Haeften. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 365:1333–1346, 2005. 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 3.Tuomi, T. Type 1 and type 2 diabetes: what do they have in common? Diabetes. 54:S40–S45, 2005. 10.2337/diabetes.54.suppl_2.s40. [DOI] [PubMed] [Google Scholar]
- 4.Sterrett, J. J., S. Bragg, and C. W. Weart. Type 2 diabetes medication review. Am J Med Sci. 351:342–355, 2016. 10.1016/j.amjms.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu, M., et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 21:9–17, 2019. 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 6.Sun, Y., et al. The Utility of exosomes in diagnosis and therapy of diabetes mellitus and associated complications. Front Endocrinol. 12:756581, 2021. 10.3389/fendo.2021.756581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song, J., et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J Cachexia Sarcopenia Muscle. 14:915–929, 2023. 10.1002/jcsm.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, J., et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE-/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 510:565–572, 2019. 10.1016/j.bbrc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Liu, X., et al. Which BMI for diabetes patients is better? From the view of the adipose tissue macrophage-derived exosome. Diabetes Metab Syndr Obes. 15:141–153, 2022. 10.2147/DMSO.S345890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong, Y., et al. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv Mater.35:e2212300, 2023. 10.1002/adma.202212300. [DOI] [PubMed] [Google Scholar]
- 11.Li, D., and N. Wu. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res Clin Pract.187:109882, 2022. 10.1016/j.diabres.2022.109882. [DOI] [PubMed] [Google Scholar]
- 12.Li, H. D., et al. Roles and crosstalks of macrophages in diabetic nephropathy. Front Immunol. 13:1015142, 2022. 10.3389/fimmu.2022.1015142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, X., et al. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med.11:e468, 2021. 10.1002/ctm2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, O., C. R. Donnelly, and R. R. Ji. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol. 62:17–25, 2020. 10.1016/j.conb.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran, P. H. L., et al. Exosomes and nanoengineering: a match made for precision therapeutics. Adv Mater. 32:e1904040, 2020. 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- 16.Kang, J.-S. Chapter 20—The potential of exosomes as theragnostics in various clinical situations. Exosomes (A Clinical Compendium). 2020. 10.1016/B978-0-12-816053-4.00020-1. [Google Scholar]
- 17.Saleh, A. F., et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 11:6990–7001, 2019. 10.1039/c8nr08720b. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri, R., and V. S. LeBleu. The biology, function, and biomedical applications of exosomes. Science. 367:eaau6977, 2020. 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwivedi, M., et al. Biochemistry of exosomes and their theranostic potential in human diseases. Life Sci.315:121369, 2023. 10.1016/j.lfs.2023.121369. [DOI] [PubMed] [Google Scholar]
- 20.Wu, X., et al. Exosomes extraction and identification. Methods Mol Biol. 2054:81–91, 2019. 10.1007/978-1-4939-9769-5_4. [DOI] [PubMed] [Google Scholar]
- 21.Street, J. M., et al. Urine exosome isolation and characterization. Methods Mol Biol. 1641:413–423, 2017. 10.1007/978-1-4939-7172-5_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-González, R., et al. A method for isolation of extracellular vesicles and characterization of exosomes from brain extracellular space. Methods Mol Biol. 1545:139–151, 2017. 10.1007/978-1-4939-6728-5_10. [DOI] [PubMed] [Google Scholar]
- 23.Caradec, J., et al. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin Biochem. 47:1286–1292, 2014. 10.1016/j.clinbiochem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Momen-Heravi, F., et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 3:162, 2012. 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheruvanky, A., et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 292:F1657–F1661, 2007. 10.1152/ajprenal.00434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson, M. F., et al. Integrated systems for exosome investigation. Methods. 87:31–45, 2015. 10.1016/j.ymeth.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Monguió-Tortajada, M., et al. Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci. 76:2369–2382, 2019. 10.1007/s00018-019-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witwer, K. W., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013. 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal, S. K., and T. L. Whiteside. Immunoaffinity-based isolation of melanoma cell-derived and T cell-derived exosomes from plasma of melanoma patients. Methods Mol Biol. 2265:305–321, 2021. 10.1007/978-1-0716-1205-7_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altıntaş, Ö., and Y. Saylan. Exploring the versatility of exosomes: a review on isolation, characterization, detection methods, and diverse applications. Anal Chem. 95:16029–16048, 2023. 10.1021/acs.analchem.3c02224. [DOI] [PubMed] [Google Scholar]
- 31.Gao, M., et al. Comparison of yield, purity, and functional properties of large-volume exosome isolation using ultrafiltration and polymer-based precipitation. Plast Reconstr Surg. 149:638–649, 2022. 10.1097/PRS.0000000000008830. [DOI] [PubMed] [Google Scholar]
- 32.Coughlan, C., et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr Protoc Cell Biol.88:e110, 2022. 10.1002/cpcb.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J., et al. Towards microfluidic-based exosome isolation and detection for tumor therapy. Nano Today.37:101066, 2021. 10.1016/j.nantod.2020.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, P., et al. Progress in exosome isolation techniques. Theranostics. 7:789–804, 2017. 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang, C., and T. Thum. Exosomes: new players in cell–cell communication. Int J Biochem Cell Biol. 44:2060–2064, 2012. 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Kumari, P., S. S. Wright, and V. A. Rathinam. Role of extracellular vesicles in immunity and host defense. Immunol Invest. 53:10–25, 2024. 10.1080/08820139.2024.2312896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, J., et al. M1 macrophage-derived exosome lncRNA PVT1 promotes inflammation and pyroptosis of vascular smooth muscle cells in abdominal aortic aneurysm by inhibiting miR-186-5p and regulating HMGB1. Cardiovasc Toxicol. 24:302–320, 2024. 10.1007/s12012-024-09838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long, R., and S. Wang. Exosomes from preconditioned mesenchymal stem cells: tissue repair and regeneration. Regen Ther. 25:355–366, 2024. 10.1016/j.reth.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, Z., et al. M2 macrophage-derived exosomal lncRNA MIR4435-2HG promotes progression of infantile hemangiomas by targeting HNRNPA1. Int J Nanomed. 18:5943–5960, 2023. 10.2147/IJN.S435132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Göran Ronquist, K. Extracellular vesicles and energy metabolism. Clin Chim Acta. 488:116–121, 2019. 10.1016/j.cca.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 41.Castaño, C., et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci USA. 115:12158–12163, 2018. 10.1073/pnas.180885511539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng, L., Y. Wang, and L. Huang. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 25:1665–1675, 2017. 10.1016/j.ymthe.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arabpour, M., A. Saghazadeh, and N. Rezaei. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol.97:107823, 2021. 10.1016/j.intimp.2021.107823. [DOI] [PubMed] [Google Scholar]
- 44.Coulis, G., et al. Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis. Sci Adv. 9:eadd9984, 2023. 10.1126/sciadv.add9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, B. W., et al. The role of macrophage polarization and cellular crosstalk in the pulmonary fibrotic microenvironment: a review. Cell Commun Signal. 22:172, 2024. 10.1186/s12964-024-01557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiseleva, V., et al. Biochemical and molecular inducers and modulators of M2 macrophage polarization in clinical perspective. Int Immunopharmacol.122:110583, 2023. 10.1016/j.intimp.2023.110583. [DOI] [PubMed] [Google Scholar]
- 47.Hao, M. M., et al. Macrophage-derived exosome promotes regulatory T cell differentiation in malignant pleural effusion. Front Immunol. 14:1161375, 2023. 10.3389/fimmu.2023.1161375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lechner, J., O. E. O’Leary, and A. W. Stitt. The pathology associated with diabetic retinopathy. Vision Res. 139:7–14, 2017. 10.1016/j.visres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Rajiv, Agarwal. Pathogenesis of diabetic nephropathy. ADA Clinical Compendia. 1:2–7, 2021. 10.2337/db20211-2. [Google Scholar]
- 50.Elafros, M. A., et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 21:922–936, 2022. 10.1016/S1474-4422(22)00188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mponponsuo, K., R. G. Sibbald, and R. Somayaji. A comprehensive review of the pathogenesis, diagnosis, and management of diabetic foot infections. Adv Skin Wound Care. 34:574–581, 2021. 10.1097/01.ASW.0000791876.10485.d4. [DOI] [PubMed] [Google Scholar]
- 52.Nishizawa, T., and K. E. Bornfeldt. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann Med. 44:555–563, 2012. 10.3109/07853890.2011.585346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forbes, J. M., and M. E. Cooper. Mechanisms of diabetic complications. Physiol Rev. 93:137–188, 2013. 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 54.Kuroki, T., K. Isshiki, and G. L. King. Oxidative stress: the lead or supporting actor in the pathogenesis of diabetic complications. J Am Soc Nephrol. 14:S216–S220, 2003. 10.1097/01.asn.0000077405.07888.07. [DOI] [PubMed] [Google Scholar]
- 55.Dandona, P., et al. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes. J Clin Endocrinol Metab. 88:2422–2429, 2003. 10.1210/jc.2003-030178. [DOI] [PubMed] [Google Scholar]
- 56.Ighodaro, O. M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 108:656–662, 2018. 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 57.Garg, S. S., and J. Gupta. Polyol pathway and redox balance in diabetes. Pharmacol Res.182:106326, 2022. 10.1016/j.phrs.2022.106326. [DOI] [PubMed] [Google Scholar]
- 58.Dewanjee, S., et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 833:472–523, 2018. 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 59.Louiselle, A. E., et al. Macrophage polarization and diabetic wound healing. Transl Res. 236:109–116, 2021. 10.1016/j.trsl.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Castaño, C., A. Novials, and M. Párrizas. Exosomes and diabetes. Diabetes Metab Res Rev.35:e3107, 2019. 10.1002/dmrr.3107. [DOI] [PubMed] [Google Scholar]
- 61.Bellary, S. The changing character of diabetes complications. Lancet Diabetes Endocrinol. 10:5–6, 2022. 10.1016/S2213-8587(21)00313-2. [DOI] [PubMed] [Google Scholar]
- 62.Calcutt, N. A., et al. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 8:417–429, 2009. 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashrafizadeh, M., et al. Exosomes as promising nanostructures in diabetes mellitus: from insulin sensitivity to ameliorating diabetic complications. Int J Nanomed. 17:1229–1253, 2022. 10.2147/IJN.S350250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, C., et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 9:65–76, 2019. 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xing, Y., et al. The immuno-modulation effect of macrophage-derived extracellular vesicles in chronic inflammatory diseases. Front Immunol.12:785728, 2021. 10.3389/fimmu.2021.785728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su, W., et al. Diabetic microenvironment preconditioning of adipose tissue-derived mesenchymal stem cells enhances their anti-diabetic, anti-long-term complications, and anti-inflammatory effects in type 2 diabetic rats. Stem Cell Res Ther. 13:422, 2022. 10.1186/s13287-022-03114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu, X. The role of exosomes and exosome-derived microRNAs in atherosclerosis. Curr Pharm Des. 23:6182–6193, 2017. 10.2174/1381612823666170413125507. [DOI] [PubMed] [Google Scholar]
- 68.Yu, H., et al. Diabetes is accompanied by secretion of pro-atherosclerotic exosomes from vascular smooth muscle cells. Cardiovasc Diabetol. 22:112, 2023. 10.1186/s12933-023-01833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han, L., X. Cai, and H. Zhou. Exosomal microRNAs: potential nanotherapeutic targets for diabetic kidney disease. Nanomedicine. 18:1669–1680, 2023. 10.2217/nnm-2023-0023. [DOI] [PubMed] [Google Scholar]
- 70.Zhou, X., et al. The bone mesenchymal stem cell-derived exosomal miR-146a-5p promotes diabetic wound healing in mice via macrophage M1/M2 polarization. Mol Cell Endocrinol.579:112089, 2024. 10.1016/j.mce.2023.112089. [DOI] [PubMed] [Google Scholar]
- 71.Luo, G., et al. M2 macrophage-derived exosomes induce angiogenesis and increase skin flap survival through HIF1AN/HIF-1α/VEGFA control. Arch Biochem Biophys.751:109822, 2024. 10.1016/j.abb.2023.109822. [DOI] [PubMed] [Google Scholar]
- 72.Daneshvar, A., et al. M2 macrophage-derived exosomes for bone regeneration: a systematic review. Arch Oral Biol. 2024. 10.1016/j.archoralbio.2024.106034. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, Y., et al. Islet-resident macrophage-derived miR-155 promotes β cell decompensation via targeting PDX1. iScience.27:109540, 2024. 10.1016/j.isci.2024.109540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dini, L., et al. Microvesicles and exosomes in metabolic diseases and inflammation. Cytokine Growth Factor Rev. 51:27–39, 2020. 10.1016/j.cytogfr.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Osaadon, P., et al. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye. 28:510–520, 2024. 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Everett, L. A., and Y. M. Paulus. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 21:35, 2021. 10.1007/s11892-021-01403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnett, A. H. The role of angiotensin II receptor antagonists in the management of diabetes. Blood Press Suppl. 1:21–26, 2001. 10.1080/080370501750066471. [DOI] [PubMed] [Google Scholar]
- 78.Cantarovich, F., and B. Rangoonwala. Therapeutic effects of angiotensin II inhibition or blockade on the progression of chronic renal disease. Int J Clin Pract. 57:801–822, 2003. 10.1111/j.1742-1241.2003.tb10618.x. [PubMed] [Google Scholar]
- 79.Günes-Altan, M., et al. Is GFR decline induced by SGLT2 inhibitor of clinical importance? Cardiovasc Diabetol. 23:184, 2024. 10.1186/s12933-024-02223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Røikjer, J., C. D. Mørch, and N. Ejskjaer. Diabetic peripheral neuropathy: diagnosis and treatment. Curr Drug Saf. 16:2–16, 2021. 10.2174/1574886315666200731173113. [DOI] [PubMed] [Google Scholar]
- 81.Peltier, A., S. A. Goutman, and B. C. Callaghan. Painful diabetic neuropathy. BMJ.348:g1799, 2014. 10.1136/bmj.g1799. [DOI] [PubMed] [Google Scholar]
- 82.Game, F. L. Osteomyelitis in the diabetic foot: diagnosis and management. Med Clin North Am. 97:947–956, 2013. 10.1016/j.mcna.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Peppard, W. J., and J. A. Weigelt. Role of linezolid in the treatment of complicated skin and soft tissue infections. Expert Rev Anti Infect Ther. 4:357–366, 2006. 10.1586/14787210.4.3.357. [DOI] [PubMed] [Google Scholar]
- 84.Jarl, G., et al. Personalized offloading treatments for healing plantar diabetic foot ulcers. J Diabetes Sci Technol. 17:99–106, 2023. 10.1177/19322968221101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galicia-Garcia, U., et al. Statin treatment-induced development of type 2 diabetes: from clinical evidence to mechanistic insights. Int J Mol Sci. 21:4725, 2020. 10.3390/ijms21134725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grossman, E., and F. H. Messerli. Long-term safety of antihypertensive therapy. Prog Cardiovasc Dis. 49:16–25, 2006. 10.1016/j.pcad.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 87.James, P. A., et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 311:507–520, 2014. 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 88.Li, M., et al. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol. 47:3793–3803, 2019. 10.1080/21691401.2019.1669617. [DOI] [PubMed] [Google Scholar]
- 89.Wang, Y., et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact Mater. 28:273–283, 2023. 10.1016/j.bioactmat.2023.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phu, T. A., et al. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol Ther. 30:2274–2297, 2022. 10.1016/j.ymthe.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu, M., et al. Exosomes from high glucose-treated macrophages activate macrophages andinduce inflammatory responses via NF-κB signaling pathway in vitro and in vivo. Int Immunopharmacol.84:106551, 2020. 10.1016/j.intimp.2020.106551. [DOI] [PubMed] [Google Scholar]
- 92.Zhu, Q. J., et al. Exosomes fromhigh glucose-treated macrophages activate glomerularmesangial cells via TGF-B1/Smad3 pathway in vivo and in/vitro. FASEBJ. 4:9279–9290, 2019. 10.1096/fj.201802427RRR. [DOI] [PubMed] [Google Scholar]
- 93.Liu, T., et al. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 515:352–358, 2019. 10.1016/j.bbrc.2019.05.113. [DOI] [PubMed] [Google Scholar]
- 94.Li, L., et al. Bone marrow macrophage-derived exosomal miR-143-5p contributes to insulin resistance in hepatocytes by repressing MKP5. Cell Prolif.54:e13140, 2021. 10.1111/cpr.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ying, W., et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 33:781-790.e5, 2021. 10.1016/j.cmet.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rohm, T. V., et al. Adipose tissue macrophages secrete small extracellular vesicles that mediate rosiglitazone-induced insulin sensitization. Nat Metab. 2024. 10.1038/s42255-024-01023-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shyu, K. G., et al. Exosomal MALAT1 derived from high glucose-treated macrophages up-regulates resistin expression via miR-150-5p downregulation. Int J Mol Sci. 23:1095, 2022. 10.3390/ijms23031095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li, J. M., et al. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. 66:2368–2386, 2023. 10.1007/s00125-023-05992-7. [DOI] [PubMed] [Google Scholar]
- 99.Sun, Y., et al. Urinary stem cell-derived exocrine circRNA ATG7 regulates the SOCS1/STAT3 signaling pathway through miR-4500, inhibits M1 macrophage polarization, and alleviates the progression of diabetes nephropathy. Int Urol Nephrol. 56:1449–1463, 2024. 10.1007/s11255-023-03819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang, Y., et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res Ther. 13:171, 2022. 10.1186/s13287-022-02855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li, W., et al. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int Immunopharmacol.90:107010, 2021. 10.1016/j.intimp.2020.107010. [DOI] [PubMed] [Google Scholar]
- 102.Leñero, C., et al. CD146+ endometrial-derived mesenchymal stem/stromal cell subpopulation possesses exosomal secretomes with strong immunomodulatory miRNA attributes. Cells. 11:4002, 2022. 10.3390/cells11244002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun, Y., et al. Expression of miRNA-29 in pancreatic β cells promotes inflammation and diabetes via TRAF3. Cell Rep.34:108576, 2021. 10.1016/j.celrep.2020.108576. [DOI] [PubMed] [Google Scholar]
- 104.Roggli, E., et al. Changes in microRNA expression contribute to pancreatic β-cell dysfunction in prediabetic NOD mice. Diabetes. 61:1742–1751, 2012. 10.2337/db11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deng, Z. B., et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 58:2498–2505, 2009. 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang, Y., et al. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct Target Ther. 6:409, 2021. 10.1038/s41392-021-00805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Martinez, I., et al. Saturated fatty acid-enriched small extracellular vesicles mediate a crosstalk inducing liver inflammation and hepatocyte insulin resistance. JHEP Rep.5:100756, 2023. 10.1016/j.jhepr.2023.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song, M., et al. Adipocyte-derived exosomes carrying sonic hedgehog mediate M1 macrophage polarization-induced insulin resistance via Ptch and PI3K pathways. Cell Physiol Biochem. 48:1416–1432, 2018. 10.1159/000492252. [DOI] [PubMed] [Google Scholar]
- 109.Liu, Y., et al. Macrophage-derived exosomes promote activation of NLRP3 inflammasome and autophagy deficiency of mesangial cells in diabetic nephropathy. Life Sci.330:121991, 2023. 10.1016/j.lfs.2023.121991. [DOI] [PubMed] [Google Scholar]
- 110.Tacconi, S., et al. M1-derived extracellular vesicles polarize recipient macrophages into M2-like macrophages and alter skeletal muscle homeostasis in a hyper-glucose environment. Cell Commun Signal. 22:193, 2024. 10.1186/s12964-024-01560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Du, J., et al. BMSC-derived exosome-mediated miR-25-3p delivery protects against myocardial ischemia/reperfusion injury by constraining M1-like macrophage polarization. Mol Med Rep. 30:142, 2024. 10.3892/mmr.2024.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zheng, S., et al. Renal tissue-derived exosomal miRNA-34a in diabetic nephropathy induces renal tubular cell fibrosis by promoting the polarization of M1 macrophages. IET Nanobiotechnol. 2024:5702517, 2024. 10.1049/2024/5702517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang, X., et al. Genetically engineered M2-like macrophage-derived exosomes for P. gingivalis-suppressed cementum regeneration: from mechanism to therapy. Bioact Mater. 32:473–487, 2023. 10.1016/j.bioactmat.2023.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cui, Y., et al. Melatonin engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and immune reprogramming for periodontitis therapy. Adv Sci.10:e2302029, 2023. 10.1002/advs.202302029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao, X., et al. A novel adhesive dual-sensitive hydrogel for sustained release of exosomes derived from M2 macrophages promotes repair of bone defects. Mater Today Bio.23:100840, 2023. 10.1016/j.mtbio.2023.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li, W., et al. Development of an antiswelling hydrogel system incorporating M2-exosomes and photothermal effect for diabetic wound healing. ACS Nano. 17:22106–22120, 2023. 10.1021/acsnano.3c09220. [DOI] [PubMed] [Google Scholar]
- 117.Zeng, J., et al. M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing. Mater Today Bio.20:100649, 2023. 10.1016/j.mtbio.2023.100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu, P., et al. Angiogenesis-based diabetic skin reconstruction through multifunctional hydrogel with sustained releasing of M2 Macrophage-derived exosome. Chem Eng J.410:132413, 2022. 10.1016/j.cej.2021.132413. [Google Scholar]
- 119.Melamed, J. R., et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci Adv. 9:eade1444, 2023. 10.1126/sciadv.ade1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ge, L., et al. Engineered exosomes derived from miR-132-overexpresssing adipose stem cells promoted diabetic wound healing and skin reconstruction. Front Bioeng Biotechnol. 11:1129538, 2023. 10.3389/fbioe.2023.1129538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fan, B., et al. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp Neurol.341:113694, 2021. 10.1016/j.expneurol.2021.113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang, H., et al. Analysis of miR-203a-3p/SOCS3-mediated induction of M2 macrophage polarization to promote diabetic wound healing based on epidermal stem cell-derived exosomes. Diabetes Res Clin Pract.197:110573, 2023. 10.1016/j.diabres.2023.110573. [DOI] [PubMed] [Google Scholar]
- 123.Singh, A., et al. Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation. Bioact Mater. 6:2231–2249, 2021. 10.1016/j.bioactmat.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kar, R., et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. 9:577–594, 2023. 10.1021/acsbiomaterials.2c01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fu, Y. J., et al. All-natural immunomodulatory bioadhesive hydrogel promotes angiogenesis and diabetic wound healing by regulating macrophage heterogeneity. Adv Sci.10:e2206771, 2023. 10.1002/advs.202206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hao, P. C., et al. Enhanced diabetic wound healing using platelet-derived extracellular vesicles and reduced graphene oxide in polymer-coordinated hydrogels. J Nanobiotechnology. 21:318, 2023. 10.1186/s12951-023-02068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang, K., et al. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem Eng J.430:132664, 2022. 10.1016/j.cej.2021.132664. [Google Scholar]
- 128.Zhang, X., et al. Bioinspired adaptable indwelling microneedles for treatment of diabetic ulcers. Adv Mater.35:e2210903, 2023. 10.1002/adma.202210903. [DOI] [PubMed] [Google Scholar]
- 129.Yang, Q., et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact Mater. 26:194–215, 2023. 10.1016/j.bioactmat.2023.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jin, S., et al. M2 macrophage-derived exosome-functionalized topological scaffolds regulate the foreign body response and the coupling of angio/osteoclasto/osteogenesis. Acta Biomater. 177:91–106, 2024. 10.1016/j.actbio.2024.01.043. [DOI] [PubMed] [Google Scholar]
- 131.Geng, X., et al. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv.133:112613, 2022. 10.1016/j.msec.2021.112613. [DOI] [PubMed] [Google Scholar]
- 132.Lin, B., et al. Microfluidic-based exosome analysis for liquid biopsy. Small Methods.5:e2001131, 2021. 10.1002/smtd.202001131. [DOI] [PubMed] [Google Scholar]
- 133.Morarad, R., W. Naeowong, and A. Sirivat. Iontophoretically controlled insulin delivery via water-soluble conductive polymer PANI: PSS and thermoplastic polyurethane matrix. Drug Deliv Transl Res. 14(1):280–293, 2024. 10.1007/s13346-023-01399-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.