Abstract
The evolution of the COVID-19 pandemic has been associated with variations in clinical presentation and severity. Similarly, prediction scores may suffer changes in their diagnostic accuracy. The aim of this study was to test the 30-day mortality predictive validity of the 4C and SEIMC scores during the sixth wave of the pandemic and to compare them with those of validation studies. This was a longitudinal retrospective observational study. COVID-19 patients who were admitted to the Emergency Department of a Spanish hospital from December 15, 2021, to January 31, 2022, were selected. A side-by-side comparison with the pivotal validation studies was subsequently performed. The main measures were 30-day mortality and the 4C and SEIMC scores. A total of 27,614 patients were considered in the study, including 22,361 from the 4C, 4,627 from the SEIMC and 626 from our hospital. The 30-day mortality rate was significantly lower than that reported in the validation studies. The AUCs were 0.931 (95% CI: 0.90–0.95) for 4C and 0.903 (95% CI: 086–0.93) for SEIMC, which were significantly greater than those obtained in the first wave. Despite the changes that have occurred during the coronavirus disease 2019 (COVID-19) pandemic, with a reduction in lethality, scorecard systems are currently still useful tools for detecting patients with poor disease risk, with better prognostic capacity.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73664-6.
Keywords: COVID-19 pandemic, Scoring systems, 4C mortality score, SEIMC score, Mortality, Emergency department
Subject terms: Biomarkers, Diseases, Medical research
Introduction
The identification of mortality risk in COVID-19 patients is a critical step in deciding the most appropriate clinical management or level of care1. Hence, since the onset of the pandemic, the validity of preexisting scoring systems for COVID-19, such as the national early warning score (NEWS)2,3, modified early warning score (MEWS), quick sequential organ failure assessment score (qSOFA), sequential organ failure assessment score (SOFA), and scores applied to bacterial pneumonia (Prognostic Severity Index or CURB-65)4 or viral pneumonia (MuLBSTA)5, has been analyzed. In parallel, additional scores, such as the COVID-19 SEIMC score (SEIMC)6, the Quick COVID-19 Severity Index (qCSI)7, the COVID-19 Home Safely Now (CHOSEN) risk score for COVID-198 and the 4C mortality score (4C)9,10, were developed specifically for COVID-19 disease. Among the latter, 4C has been the most widely used worldwide, having demonstrated validity not only as a tool for screening patients with an increased likelihood of poor outcome11 but also as a useful tool to select the most efficient level of healthcare for a patient or as an assistant to guide decisions on specific treatment12.
Development, validation, and cross-comparison of the scores discussed above were carried out primarily in early pandemic waves13,14. However, COVID-19 has evolved in many ways. Particularly significant has been the appearance of new variants, such as Omicron, predominant in Spain in the sixth wave, with a behavior and pathogenicity different from those of the original variants. Additionally, massive vaccination has had a direct influence on reducing lethality15,16, and treatments, such as antiviral drugs and monoclonal antibodies, have shown completely different effects, especially in terms of improving the results of hospitalization.
This situation may have affected the behavior of the scoring systems, and it is necessary to verify their validity and accuracy under the current circumstances17. Therefore, the main objective of this study was to assess the 30-day mortality predictive value of the 4C and SEIMC scores for COVID-19 during the sixth wave of the Spanish pandemic and to compare the results with those of the initial validation studies.
Methods
Study design
This was a longitudinal retrospective observational monocentric study on COVID-19 patients who were admitted to the ED of a tertiary medical center, providing services to a baseline population of 235,000 beneficiaries, by medical record review in adults (≥ 18 years), from December 15, 2021, to January 31, 2022, and subsequent side-by-side comparisons with pivotal validation studies.
The institutional review board of the Hospital Clinico de Valladolid approved the study protocol (reference: PI 22-2575). This study was performed in accordance with the Declaration of Helsinki, and all methods were carried out in accordance with the approved guidelines and following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (supplementary material p3). Informed consent was obtained from all participants and/or their legal guardians.
Population
Patients were recruited via medical records management (Jimena-4 SACYL) database software throughout the inclusion period by selecting patients with a COVID-19 diagnosis at discharge from the ED. The search was supplemented with information provided by the Admitting Department of Patients Hospitalized in the same period with a main diagnosis related to coronavirus disease 2019 and with the list of patients discharged from the hospital until February 1st, 2022. For correct data linkage and to avoid duplication, the following data extractors were checked: medical record number, first and last name, age, and sex.
COVID-19 cases were defined as patients presenting to the ED with evidence of active infection positive by polymerase chain reaction (PCR), rapid antigen test, or 48 h prior to their visit to the ED.
Predictors and data abstraction
A retrospective review of electronic medical records, data referring to immunization status (previous illness and vaccination level), and the variables necessary to calculate 4C and SEIMC scores (see details in supplementary p5) were performed. Finally, 30-day impairment-related data were collected, including noninvasive mechanical ventilation or invasive mechanical ventilation, intensive care unit (ICU) admission, and mortality. The latter was the primary outcome variable (i.e., all-cause 30-day mortality), and the others were secondary outcomes.
In addition to our database, data from the SEIMC development and validation studies and the 4C validation cohort were collected from the original or pivotal studies6,10. Detailed information was extracted from the tables within the study and transformed to a database that presented the same characteristics as the original cohorts. In particular, the available information regarding the number of patients at each integer value of the score was used to introduce that value of the score as many times as patients appear to present that integer value, and so on with each integer value.
Statistical analysis
Quantitative variables are described as the means and standard deviations, and categorical variables are described as absolute frequencies and percentages. For quantitative variables, comparisons of means were assessed via the Mann‒Whitney U test. For qualitative variables, the chi-square test (or, if necessary, Fisher’s exact test) was used for 2 × 2 contingency tables or/and contrast of proportions to determine the relationship of association or dependence.
To assess the validity of the models for predicting mortality in both the pivotal and HCUV-derived databases, the outcome and value of each score were adjusted via logistic regression (one for each score and study cutoff). With such adjustment, the area under the receiver operating characteristic (ROC) curve (AUC) and the corresponding 95% confidence intervals (CIs) of the model in the validation cohort were determined. The AUC results were compared via Delong’s test. Additionally, a calibration analysis was performed by calculating the calibration curve, that is, plotting the predicted vs. observed probability of the outcome and determining several metrics associated with calibration.
All the statistical analyses were performed via our own codes and basic functions in R, version 4.2.2 (http://www.R-project.org). The sample size calculation can be found in supplementary data p5.
Results
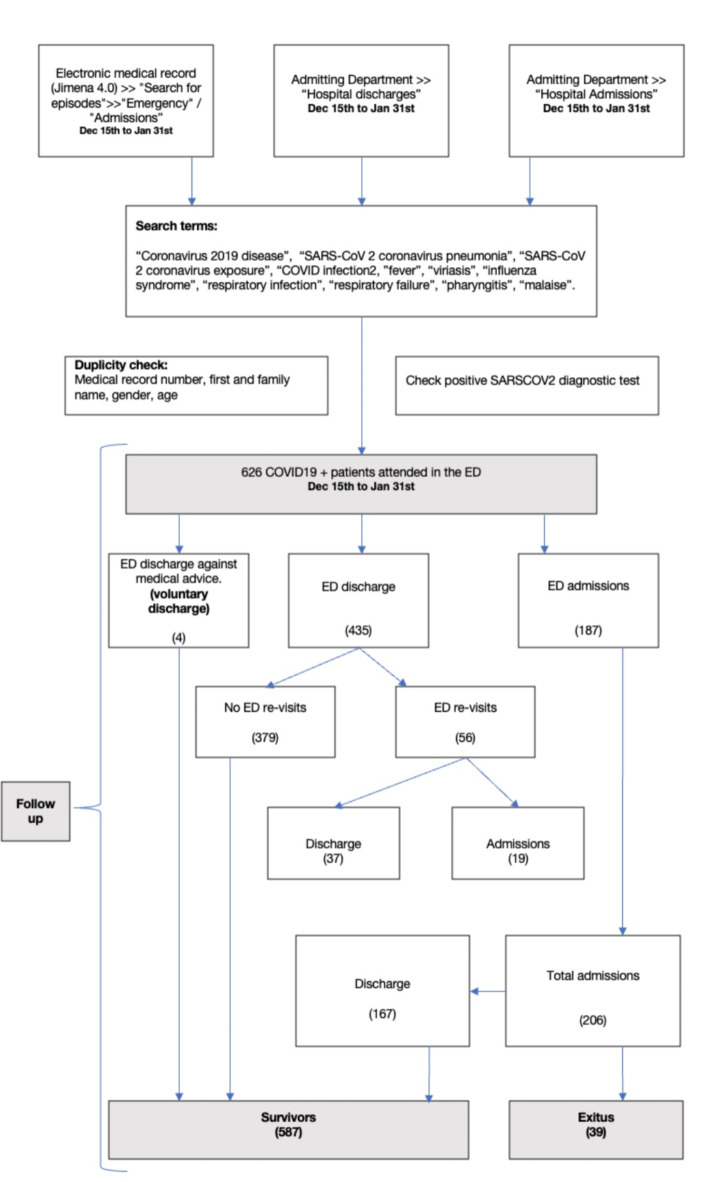
A total of 626 patients met the inclusion criteria (Fig. 1). Globally, 52.4% (328) were female, with a median age of 50 years (IQR: 37–66). Patients presented a Charlson comorbidity index score of 0 points (IQR: 0–1), and those with ≥ 2 comorbidities (including obesity, as indicated by the 4C score) accounted for 20.3% (127 patients). No previous disease and no vaccination shots were present in 21.7% (136 cases) of the patients. A total of 206 (32,9%) patients were hospitalized. The 30-day mortality rate during the follow-up period was 6.2% (39 patients), and 10.1% (63 patients) developed complications, including the use of noninvasive mechanical ventilation or invasive mechanical ventilation and intensive care unit (ICU) admission. Among those admitted, 39 (18.9%) died, and 63 experienced complications.
Fig. 1.
Patient flowchart of patients from the Hospital Clínico Universitario de Valladolid cohort.
A comparison of the clinical characteristics of the survivors and nonsurvivors can be found in Table 1. Among the HCUV cohort, the 4C score low-risk group with 320 cases (51.1%) and the SEIMC score moderate-risk group with 257 cases (41.4%) were the largest groups.
Table 1.
Clinical baseline patient characteristics and score calculation.
| 30-day mortality | ||||
|---|---|---|---|---|
| Survivors | Nonsurvivors | Odd ratio (95%CI)b | p valuec | |
| No. (%) with dataa | 587 (93.7) | 39 (6.3) | N.A. | N.A. |
| 4Cd and SEIMe score variables | ||||
| Age, year | 50.5 (19.7) | 81.3 (12.5) | 1.09 (1.06–1.11) | < 0.001 |
| Sex, female | 307 (52.3) | 21 (53.8) | 0.94 (0.48–1.81) | 0.855 |
| CCI, point | 0.85 (1.59) | 3.18 (2.42) | 1.54 (1.35–1.75) | < 0.001 |
| Dyspnea | 214 (36.5) | 20 (51.3) | 1.83 (0.95–3.55) | 0.071 |
| RR, breaths/min | 15 (3) | 19 (6) | 1.19 (1.12–1.26) | < 0.001 |
| Oxygen saturation, % | 96 (3) | 90 (7) | 0.81 (0.76–0.87) | < 0.001 |
| Glasgow coma scale, point | 15 (0.12) | 14.4 (1.62) | 0.19 (0.06–0.64) | 0.007 |
| N/L ratio, x103/µl | 4.07 (5.45) | 10.9 (15) | 1.08 (1.05–1.12) | < 0.001 |
| GF, ml/min/1.73 m2 (CKD-EPI) | 83.6 (20.4) | 49.4 (27.8) | 0.95 (0.94–0.97) | < 0.001 |
| Urea, mmol/L | 3.80(4.62) | 13.5 (11.4) | 1.18 (1.13–1.24) | < 0.001 |
| C-reactive protein, mg/dL | 45.3 (6.73) | 103 (10.5) | 1.01 (1.00-1.01) | < 0.001 |
| Immunization condition | ||||
| Immunosuppressive therapy | 66 (16.7) | 10 (35.7) | 2.79 (1.18–6.25) | 0.021 |
| COVID-19 previous | 52 (8.86) | 1 (2.56) | 0.31 (0.01–1.46) | 0.166 |
| Fully immunizedf | 337 (57.4) | 20 (51.3) | 0.78 (0.41–1.51) | 0.459 |
| Hospital outcomes | ||||
| Hospital-inpatient | 167 (28.4) | 39 (100) | 184,2 (11,3-3013,5) | < 0,001 |
| NIMV | 17 (2.9) | 4 (10.3) | 3.92 (1.05–11.4) | 0.043 |
| IMV | 11 (1.9) | 3 (7.7) | 4.50 (0.94–15.5) | 0.059 |
| ICU-admission | 17 (2.9) | 4 (10.3) | 4.88 (1.30–14.4) | 0.022 |
| SEIMC score | ||||
| Rating | 6.1 (5.95) | 17.7 (7.43) | 1.19 (1.14–1.24) | < 0.001 |
| Risk level, point | ||||
| Low | 140 (43.6) | 0 (0) | N.A. | N.A: |
| Moderate | 256 (43.6) | 1 (2.5) | N.A. | N.A. |
| High | 88 (15) | 6 (15.4) | N.A. | N.A: |
| Extra high | 103 (17.5) | 32 (82.1) | N.A. | N.A. |
| 4C score | ||||
| Rating | 4.32 (4.16) | 12.8 (3.02) | 1.53 (1.37-1,71) | < 0.001 |
| Risk level, point | ||||
| Low | 320 (54.5) | (0) | N.A. | N.A: |
| Moderate | 156 (26.6) | 3 (7.7) | N.A. | N.A. |
| High | 98 (16.7) | 22 (56.4) | N.A. | N.A: |
| Extra high | 13 (2.2) | 14 (35.9) | N.A. | N.A. |
CI, confidence interval; NA, not applicable; CCI, Charlson comorbidity index; RR, respiratory rate; N/L, neutrophil/lymphocyte ratio; GF, glomerular filtration; NIMV, noninvasive mechanical ventilation; IMV, invasive mechanical ventilation; Ref, reference.
aValues expressed as total number (percentage) and mean (standard deviation) as appropriate.
bFisher’s exact probability statistic was used.
cThe Mann‒Whitney U test or chi-square test was used as appropriate.
d4C score variables: age, sex, at birth, comorbidities, respiratory rate, oxygen saturation, Glasgow Coma Scale score, urea, and C-reactive protein.
eSEIMC score variables: age, sex, at birth, neutrophil/lymphocyte ratio, glomerular filtration, and dyspnea.
fPatient passed COVID-19 or complied with the current vaccination protocol within the previous six months.
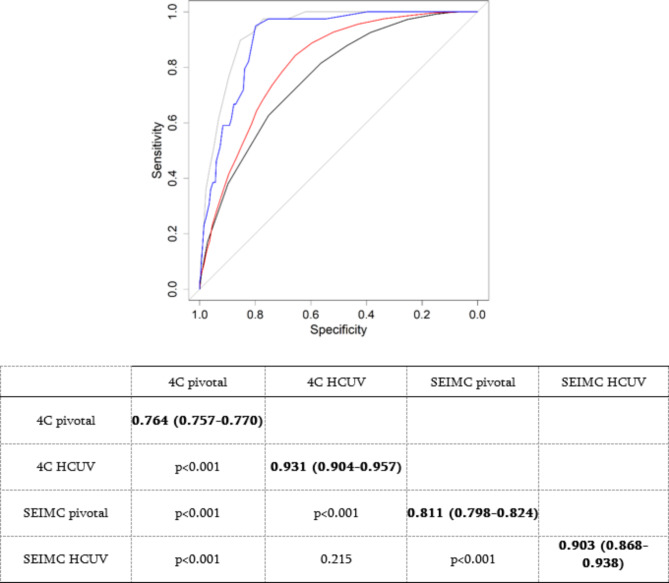
Table 2 depicts the distribution of patients by risk category in both cases with respect to the original studies, which presented statistically significant differences (p < 0.001). The worst performing group in the current cohort was the extrahigh-risk group in terms of both the 4C and SEIMC scores, with 51.9% and 23.7% 30-day mortality, respectively, which are considerably lower than those reported in pivotal studies (Table 2). The 4C score had an AUC of 0.931 (95% CI: 0.904–0.957) and a SEIMC score of 0.903 (95% CI: 0.868–0.938) (p < 0.001 in both models), with a predictive performance significantly superior to that of pivotal studies (Fig. 2).
Table 2.
Distribution and mortality compared by risk level of the 4C and SEIMC scores between the pilot study cohort and the HCUV cohort.
| 4C score, Knight et al.9 | HCUV | |||
|---|---|---|---|---|
| Total | Nonsurvivors | Total | Nonsurvivors | |
| No. (%) with dataa | 22,361 (100) | 6371 (30.1) | 626 (100) | 39 (6.2) |
| Risk level | ||||
| Low, 0–3 points | 1650 (7.4) | 20 (1.2) | 320 (51.1) | 0 (0) |
| Moderate, 4–8 points | 4889 (21.9) | 486 (9.9) | 159 (25.4) | 3 (1.9) |
| High, 9–14 points | 11,664 (52.2) | 3665 (31.4) | 120 (19.2) | 22 (18.3) |
| Extra high, ≥ 15 points | 4158 (18.6) | 2560 (61.6) | 27 (4.3) | 14 (51.9) |
| SEIMC score, Berenguer et al.5 | HCUV | |||
|---|---|---|---|---|
| Total | Nonsurvivors | Total | Nonsurvivors | |
| No. (%) with dataa | 4627 (100) | 1037 (22.4) | 626 (100) | 39 (6.2) |
| Risk level | ||||
| Low, 0–2 points | 572 (12.4) | 4 (0.7) | 140 (22.4) | 0 (0) |
| Moderate, 3–5 points | 1018 (22) | 42 (4.1) | 257 (41.4) | 1 (0.4) |
| High, 6–8 points | 927 (20) | 117 (12.6) | 94 815) | 6 (6.4) |
| Extra high, ≥ 9 points | 2110 (45.6) | 874 (41.4) | 135 (21.5) | 32 (23.7) |
HCUV, University Clinical Hospital of Valladolid.
aValues expressed as total number (percentage). The percentages of nonsurvivors were calculated from the total number of participants in each group (horizontally).
Fig. 2.
AUC comparisons between different mortality cohorts and scores. HCUV: University Clinical Hospital of Valladolid. The black line = 4C pivotal; the gray line = 4C HUCV; the red line = SEIMC pivotal; the blue line = SEIMC HUCV. Delong’s test (p value) for each comparison is shown in the table. The diagonal (bold values) shows the AUC and the 95% confidence interval.
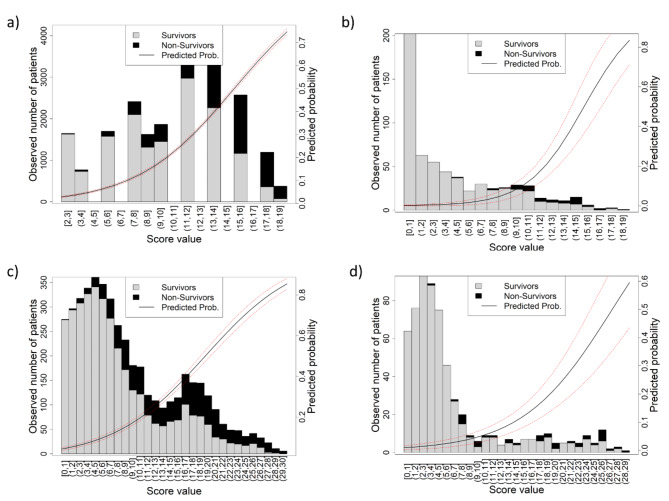
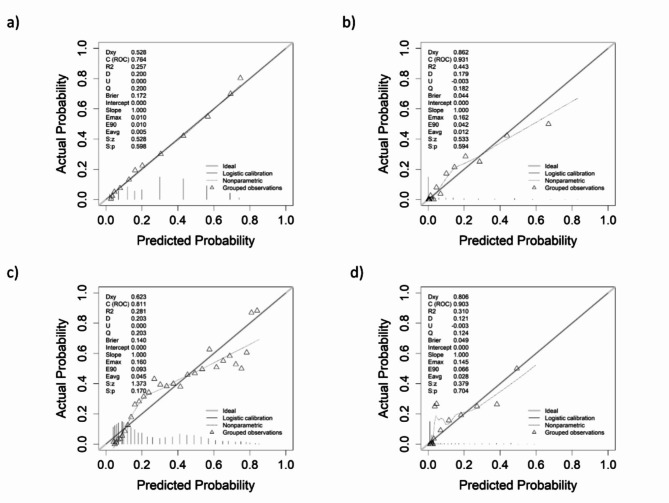
The predicted probability curves in both pivotal studies (Fig. 3a, c) described a sigmoidal shape. In contrast, in the HCUV plots (Fig. 3b, d), an exponential curve was observed. Nonsurviving variables (black bars in the graph) are concentrated in the upper ranges and are practically nonexistent in the lower ranges. Similarly, analysis of the calibration curve (Fig. 4) clearly revealed that the HCUV cohort (Fig. 4a, c) presented the best calibration compared with pivotal studies (Fig. 4b, d). On the basis of Somers’ Dxy measure, the HCUV values for both calibrations were closer to 1 than those of the pivotal studies were. That is, the closer the value is to 1, the greater the predictive capacity of the score. The remaining values from the HCUV cohort presented the derived calibrations with the best match and performance.
Fig. 3.
Observed distribution of mortality according to scores. (a) 4C pivotal, (b) 4C HCUV, (c) SEIMC pivotal, (d) SEIMC HCUV. The solid line shows the predicted probability of the outcome variable, and the dashed lines show the 95% confidence interval.
Fig. 4.
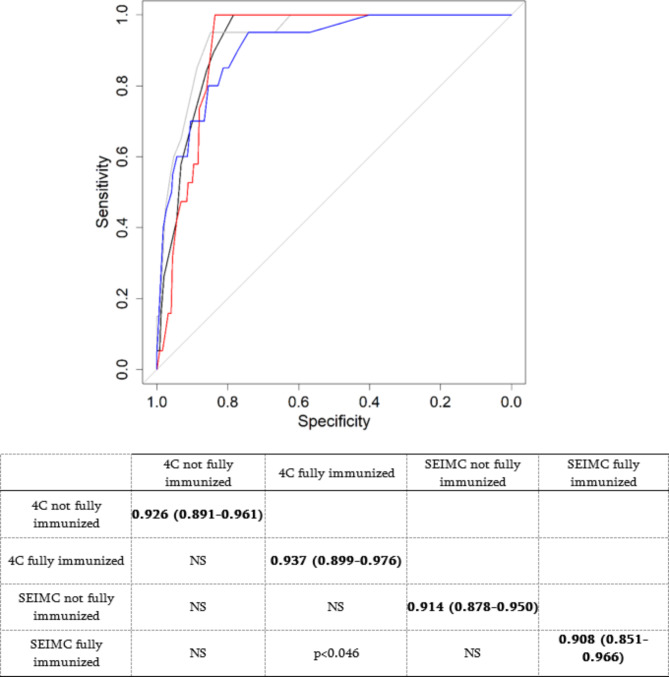
Calibration curves according to scores. (a) 4C pivotal, (b) 4C HCUV, (c) SEIMC pivotal, (d) SEIMC HCUV. NS: not statistically significant. Black line = 4C not fully immunized; gray line = 4C fully immunized; red line = SEIMC not fully immunized; blue line = SEIMC fully immunized. Delong’s test (p value) for each comparison is shown in the table. The diagonal (bold values) shows the AUC and the 95% confidence interval.
The cutoff points for 4C and SEIMC in the current cohort were 9.5 and 7.5 points, indicating sensitivities of 95% and 97%, respectively. In the pivotal studies, the cutoff points were 13 and 8.5 points, respectively (Table 3).
Table 3.
Further details of AUCs.
| 4C pivotal | 4C HCUV | SEIMC pivotal | SEIMC HCUV | |
|---|---|---|---|---|
| Youden cut-off, point | 13 (11–13) | 9.5 (7.5–10.5) | 8.5 (7.5–9.5) | 7.5 (6.5–7.5) |
| Specificity | 0.75 (0.56–0.76) | 0.83 (0.75–0.89) | 0.66 (0.62–0.71) | 0.79 (0.73–0.83) |
| Sensitivity | 0.64 (0.62–0.82) | 0.95 (0.87-1) | 0.84 (0.79–0.87) | 0.97 (0.9-1) |
| Positive predictive value | 0.28 (0.27–0.36) | 0.06 (0.06–0.07) | 0.24 (0.23–0.25) | 0.07 (0.06–0.07) |
| Negative predictive value | 0.98 (0.98–0.99) | 0.88 (0.88–0.89) | 0.97 (0.97–0.98) | 0.89 (0.88–0.90) |
| Positive likelihood ratio | 2.52 (1.40–3.43) | 5.63 (3.43–9.03) | 2.45 (2.05-3) | 4.73 (3.36–6.05) |
| Negative likelihood ratio | 0.48 (0.23–0.68) | 0.06 (0-0.17) | 0.24 (0.18–0.35) | 0.03 (0-0.14) |
Bracketed number indicate 95% confidence interval.
HCUV, Hospital Clínico Universitario de Valladolid.
The effect on mortality prediction of fully immunized patients (patients who passed COVID-19 or complied with the current vaccination protocol within the previous six months) was studied by comparing the AUC of the HCUV by splitting the cohort into fully immunized or not fully immunized patients; the results are shown in Fig. 5. No statistically significant difference was found between the scores of fully immunized patients and those of fully immunized patients (all AUCs > 0.9), except for the comparison between the SIMC patients and the 4C patients, in which there were significantly greater AUCs for the 4C patients (p < 0.046).
Fig. 5.
AUC comparisons between different mortality rates considering fully immunized factors and scores.
Discussion
The present study assessed the ability of the two most commonly used scores in our clinical context (4C and SEIMC) to predict 30-day mortality at the current time of the pandemic compared with data obtained in the original validation studies. Both scoring systems showed high predictive ability, with AUCs of 0.93 and 0.90 for 4C and SEIMC, respectively. These data substantially improve not only those obtained in two-source studies (0.76 for 4C and 0.81 for SEIMC) but also those of the most current studies, such as that of De Vito et al.18 (AUC = 0.78). Therefore, we consider that the usefulness of these scores to assess risk in patients with COVID-19 is ongoing.
The COVID-19 pandemic has undergone several changes over the course and evolution of the pandemic. First, according to the scores, high or extrahigh mortality risk decreased from 70.8% for 4C10 or 65.6% for SEIMC6 to 23.5% and 26.6%, respectively, in the current cohort. As a result, the 30-day mortality rate was significantly reduced. In our study, the mortality rates were 6.2% and 30.1% for Knight et al.10 and 22.4% for Berenguer et al.6. The data obtained were in line with those of comparable studies in the vaccinated population. Hippisley-Cox et al.19 reported a ratio of 4% 14-day mortality, and Yek et al.20 reported a mortality rate of 1.6%. An important question arises from this important decrease in mortality: clinical management should be based primarily on mortality, as in the past, or should include other major complications, e.g., mechanical ventilation (invasive or noninvasive) or intensive care unit (ICU) admissions21. In this context, Gupta et al.9 demonstrated the usefulness of the 4C score at the beginning of the pandemic, although current data may need to be confirmed.
However, mortality among hospitalized patients remains high (18.9%), similar to that reported in the SEMI-COVID-19 Network study22, which included 17.9% of patients admitted during the vaccination period (January 1 to December 5, 2021). This same study revealed that in-hospital mortality declined only slightly during the different phases of the pandemic, with 19.4% in the first wave (up to June 10, 2020), 16.5% in the prevaccination period (June 11 to December 31, 2020), and 17.9% in the vaccination period. These data suggest that changes in the COVID-19 pandemic have led to a greater proportion of patients at low risk, which reduces overall mortality, but that mortality remains very high in patients who, according to the scores used, are at high or very high risk. Moreover, a study of the effect on fully immunized patients revealed no difference in mortality prediction.
Therefore, despite the reduction in overall mortality, identifying patients at high risk of mortality and/or complications by means of risk scores continues to be a key strategy.
On the other hand, the two scores displayed excellent sensitivity for the prediction of 30-day mortality (95% for the 4C and 97% for the SEIMC), considerably exceeding the initial studies (64% and 84%, respectively). This is especially relevant in emergency care to avoid treating mild patients who later develop complications. In contrast to other studies, even recent ones, such as that of De Vito et al.18, the cutoff points for predicting high mortality are inferior to those of the original studies (9.5 for 4C and 7.5 for SEIMC). Above these cutoff points, mortality ratios are well above 10%, but below these cutoff points, mortality is practically nil (0–1%). To our knowledge, this dichotomous distribution of mortality is unprecedented18,23. We found only 2 studies with comparable data to ours (with a clinical situation similar to the present one and assessing mortality according to risk groups). First, according to De Vito et al.19, mortality continues to rise progressively (0.7%, 4.3%, 13.9% and 41.7% in group 4C from lowest to highest risk, respectively). The other, in Japan, by Baba et al.24, during the sixth wave (from November 23, 2021, to February 1, 2022), presented such low mortality ratios in all groups (0%, 0.3%, 2.4% and 0%, respectively), which makes any comparison difficult. The specificity results were lower than those presented by the sensitivity, that is, 83% for the 4C cohort and 79% for the SEIMC cohort in the HCUV cohort and 75% and 66%, respectively, in the initial studies. This lower specificity than the excellent sensitivity is a putative downside associated with these scores. Therefore, this should be considered when using them.
Despite being a single-center study, over one and a half months, the number of patients recruited was notable. To revalidate the efficacy of both the 4C and SEIMC scores, updated multicenter studies should be conducted at the current time of the pandemic to assess the efficacy of the scoring systems in real time. To reduce potential bias, the results from the current HCUV cohort were compared with original data from the pivotal studies used to validate the 4C and SEIMC scores. The AUC values obtained via the data derived from these pivotal studies were equivalent to the data presented in previous publications; thus, our results are robust.
This work has several limitations. In our series, age (50 years) and the comorbidity rate (0 points) were much lower than those reported in other similar studies24,25. Patients were included through multiple hospital registries with comprehensive inclusion criteria. Consequently, the distribution was a reflection of the patients seen in the emergency department at the time of the pandemic and the immunization status of the community. In our cohort, once the critical outbreak was over, several factors influenced the fact that mild patients began to come and overcrowd to the emergency department, which is not strictly clinical: a high frequency of young patients due to the difficulty of access to primary care, the need to justify the disease for possible sick leave, more expensive home diagnostic tests and more supply problems than at present, excessive fear of the disease, better resources, care and protocols for hospital referrals from nursing homes, etc., resulting in a clear change in the characteristics of the patients and an overinflation of mild patients. Mortality data from the HCUV cohort, however, were not directly influenced by younger age or fewer comorbidities; indeed, for both scores, the high- or extrahigh-risk categories continued to have high mortality, with results analogous to those of other studies in vaccinated populations26. Comparisons between cohorts were not strictly matched, but neither was the timing nor evolution of the pandemic. The HCUV cohort was composed of patients managed in the ED, whereas the original validation cohorts of the 4C and SEIMC scores were primarily inpatients. This difference can be explained by the evolution of COVID-19 diagnosis and treatment. In the first phase of the pandemic, due to the severity of the infection, lack of adequate therapy and/or absence of vaccines, the vast majority of patients diagnosed with COVID-19 are hospitalized depending on hospital capacity. As knowledge of disease progression and vaccination status has improved, admission rates have decreased exponentially, resulting in hospital admission for complicated cases or particularly fragile persons. These data explain the difference between the cohorts compared. Finally, these scores were designed to predict mortality at 30 days. It could be interesting to study whether these scores can also predict other complications, such as revisiting patients to the ED. However, revisits to the emergency department depend on many conditions, not always clinical, and can be variable depending on the health system, so it cannot be considered a complication strictly.
In summary, despite the major changes that have occurred during the COVID-19 pandemic, with a significant reduction in lethality, scorecard systems such as the 4C and SEIMC are currently still very useful tools for detecting patients with poor evolution risk, with better prognostic capacity in patients in the sixth wave than in patients in the original wave, with which the scores were derived and validated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Pedro Ángel de Santos Castro and Carlos del Pozo Vegas conceptualized, managed, and coordinated the project, assisted with the design of methodology, analyzed the data, and prepared the initial and final drafts of the manuscript. Ancor Sanz-García, Francisco Martín-Rodríguez and Pedro de Santos Castro took responsibility for the data and their analysis. Leyre Teresa Pinilla Arribas, Daniel Zalama Sánchez, Tony Giancarlo Vásquez del Águila, Pablo González Izquierdo, Sara de Santos Sánchez, Carlos del Pozo Vegas and Pedro Ángel de Santos Castro assisted with management and coordination of the project, assisted with the design of methodology, and helped to review the manuscript. Pedro Ángel de Santos Castro and Francisco Martín-Rodríguez conceptualized the project and helped to review and comment on the initial and final drafts of the manuscript. All authors performed a critical review and approved the final manuscript for interpretation of the data and important intellectual input.
Funding
This study has not received any funding for its implementation.
Data availability
The data that support the findings of this study are available on request from the corresponding author ASG. The data are not publicly available due to restrictions, and their containing information could compromise the privacy of research participants.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Health Research Ethics Board of all participating centers (ref. PI 22-2575).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carlos del Pozo Vegas, Email: cpozove@saludcastillayleon.es.
Ancor Sanz-García, Email: ancor.sanz@gmail.com.
References
- 1.Fried, S., Bar-Shai, A., Frydman, S. & Freund, O. Transition of care interventions to manage severe COVID-19 in the ambulatory setting: A systematic review. Intern. Emerg. Med.19(3), 765–775 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Scott, L. J. et al. Prognostic value of National Early warning scores (NEWS2) and component physiology in hospitalised patients with COVID-19: A multicentre study. Emerg. Med. J.39(8), 589–594 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myrstad, M. et al. National early warning score 2 (NEWS2) on admission predicts severe disease and in-hospital mortality from Covid-19—A prospective cohort study. Scand. J. Trauma. Resusc. Emerg. Med.28(1), 66 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas, B. et al. Prognostic accuracy of emergency department triage tools for adults with suspected COVID-19: The PRIEST observational cohort study. Emerg. Med. J.38(8), 587–593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George, R. et al. Validation of MuLBSTA score to derive modified MuLB score as mortality risk prediction in COVID-19 infection. PLOS Glob Public. Health2(8), e00005 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenguer, J. et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: The COVID-19 SEIMC score. Thorax76(9), 920–929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haimovich, A. D. et al. Development and validation of the Quick COVID-19 Severity Index: A prognostic tool for early clinical decompensation. Ann. Emerg. Med.76(4), 442–453 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine, D. M. et al. Derivation of a clinical risk score to predict 14-day occurrence of hypoxia, ICU admission, and death among patients with Coronavirus disease 2019. J. Gen. Intern. Med.36(3), 730–737 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta, R. K. et al. Development and validation of the ISARIC 4C deterioration model for adults hospitalised with COVID-19: A prospective cohort study. Lancet Respir Med.9(4), 349–359 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C mortality score. BMJ370, m3339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong, V. M. T. et al. Clinical prediction models for mortality in patients with covid-19: External validation and individual participant data meta-analysis. BMJ378, e069881 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghani, H. et al. Relevance of prediction scores derived from the SARS-CoV-2 first wave, in the evolving UK COVID-19 second wave, for safe early discharge and mortality: A PREDICT COVID-19 UK prospective observational cohort study. BMJ Open12(12), 054469–e054469 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu, H., Yao, N. & Qiu, Y. Predictive value of 5 early warning scores for critical COVID-19 patients. Disaster Med. Public. Health Prep. 16(1), 232–239 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martín-Rodríguez, F. et al. One-on-one comparison between qCSI and NEWS scores for mortality risk assessment in patients with COVID-19. Ann. Med.54(1), 646–654 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyberg, T. et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet399(10332), 1303–1312 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis.22(9), 1293–1302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González, D. & Castillo, J. Importance of assessing risk for a poor COVID-19 outcome in the post-vaccination era. Emergencias35(1), 1–3 (2023). [PubMed] [Google Scholar]
- 18.De Vito, A. et al. Is the 4C score still a valid item to predict in-hospital mortality in people with SARS-CoV-2 infections in the Omicron variant era? Life Basel13(1), 1–10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippisley-Cox, J. et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: National prospective cohort study. BMJ374, n2244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yek, C. et al. Risk factors for severe COVID-19 outcomes among persons aged ≥ 18 years who completed a primary COVID-19 vaccination series —65 Health Care facilities, United States, December 2020-October 2021. MMWR Morb Mortal. Wkly. Rep.71(1), 19–25 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai, S. et al. Assessment of respiratory support decision and the outcome of invasive mechanical ventilation in severe COVID-19 with ARDS. J. Intensive Med.2(2), 92–102 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos-Rincón, J. M. et al. Validation of the RIM Score-COVID in the Spanish SEMI-COVID-19 Registry. Intern. Emerg. Med.18(3), 907–915 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba, H. et al. Statistical analysis of mortality rates of coronavirus disease 2019 (COVID-19) patients in Japan across the 4C mortality score risk groups, age groups, and epidemiological waves: A report from the nationwide COVID-19 cohort. Open Forum Infect. Dis.10(1), ofac638 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fericean, R. M. et al. COVID-19 clinical features and outcomes in elderly patients during six pandemic waves. J. Clin. Med.11(22), 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín-Conty, J. L. et al. COVID-19 as a risk factor for long-term mortality in patients managed by the emergency medical system: A prospective, multicenter, ambulance-based cohort study. Front. Public. Health10, 1076627 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali, A. M., Tofiq, A. M., Rostam, H. M., Ali, K. M. & Tawfeeq, H. M. Disease severity and efficacy of homologous vaccination among patients infected with SARS-CoV-2 Delta or Omicron VOCs, compared to unvaccinated using main biomarkers. J. Med. Virol.94(12), 5867–5876 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author ASG. The data are not publicly available due to restrictions, and their containing information could compromise the privacy of research participants.