Abstract
Hawthorn special extract WS 1442 has beneficial effects on the cardiovascular system. Experimental studies have shown an antiarrhythmic effect of the substance. In the present study, we investigated antiarrhythmic effects of WS 1442 compared with magnesium/potassium in a large collective of outpatients. Using the IQVIA Disease Analyzer (DA) database, we included 4550 patients with a prescription of WS 1442 and 4550 matched patients with Tromcardin prescriptions (all registered products under the trademark Tromcardin that are magnesium and potassium supplementing foods for special medical purposes) who were followed for 5 years after the index date. The incidence of various cardiac arrhythmias (atrial fibrillation and flutter (AFF), tachycardia, and other cardiac arrhythmias) was recorded. Cox regression models were used to evaluate the potential association between both drugs and arrhythmias. The cumulative incidence of atrial fibrillation and flutter was significantly lower among patients with a prescription of WS 1442 compared to patients with magnesium/potassium prescriptions (10.8% vs. 16.4%, p < 0.001). WS 1442 prescription was significantly associated with a lower incidence of atrial fibrillation and flutter compared to magnesium/potassium (HR 0.71; 95% CI 0.64–0.80; p < 0.001). The cumulative incidence of tachycardia was significantly lower in the WS 1442 group compared to the magnesium/potassium group (8.3% vs. 9.4%, p < 0.001), similarly, the cumulative incidence of other cardiac arrhythmias was significantly lower among patients with WS 1442 compared to patients with magnesium/potassium (10.2% vs. 14.8%, p < 0.001). This study showed that in a large collective of outpatients, intake of hawthorn special extract WS 1442 was associated with a significantly lower incidence of atrial fibrillation, tachycardia, and other cardiac arrhythmias compared to magnesium/potassium, indicating its potential in treating and preventing such conditions.
Keywords: Hawthorn special extract WS 1442, Magnesium/potassium, Cardiac arrhythmias
Subject terms: Cardiology, Health care
Introduction
Cardiovascular disease (CVD) is the most common cause of death worldwide. For example, each year more than 60 million potential years of life are lost to CVD in Europe1. Cardiovascular diseases include hypertension, coronary heart disease (CHD), angina pectoris (AP), atrial fibrillation (AF), heart failure, and others2. There are various evidence-based prevention strategies and treatments for the management of CVD, which include smoking reduction, weight reduction, physical activity, secondary prevention medications, and much more3. Along with synthetic drugs like beta blockers, diuretics, calcium channel blockers, ACE inhibitors, angiotensin II receptor blockers, lipid lowering drugs, and recently sodium glucose transporter 2 (SGLT2) inhibitors, hawthorn (Crataegus) has been playing an important role in the prevention and treatment of various cardiovascular diseases such heart failure for a very long time4,5.
Plant preparations made from hawthorn have various protective effects on the cardiovascular system, which are attributed to flavonoids and oligomeric procyanidins. These include positive inotropic effects, vasorelaxation, and improvement of the blood supply to the heart6. Hawthorn extracts further displayed negative chronotropic effects in a cultured neonatal murine cardiomyocyte assay. The preparations improved rhythmicity of the treated cardiomyocytes. However, it was shown that the chronotropic mechanism of action is not due to beta-adrenergic receptor blockade7. The antiarrhythmic effect of Crataegus extract on digoxin-induced arrhythmias was investigated in anesthetized Wistar rats and an antiarrhythmic effect was documented by shorter durations of atrial and ventricular arrhythmias in the treatment group compared to controls8. For WS 1442, a dry extract from hawthorn leaves with flowers (4-6.6:1), extraction solvent: ethanol 45% (w/w), a prolongation of the action potential and the refractory period as well as pronounced anti-arrhythmic properties could be shown9. Nonclinical studies thus support the traditional medicinal use of hawthorn preparations for the treatment of mild forms of arrythmias.
The positive effects of WS 1442 on the cardiovascular system was shown in various clinical and preclinical studies10,11. It strengthens the contractility of the heart and shows vasodilatative and vasoprotective effects in vitro. As a result, the oxygen supply can be increased12. In vivo studies showed a dose-dependent reduction of ventricular fibrillation and tachycardia in rats treated with WS 144213. Zorniak et al.13 revealed the dose- and time-dependent cardioprotective effect of WS 1442 in rats including mortality index reduction as well as decrease in the incidence and duration of severe ventricular arrhythmias. Furthermore, a prospective, non-interventional study was conducted with patients from general practices14. In this study, the author compared 588 patients with mild heart failure (HF) who received WS 1442 and 364 patients who received therapy without hawthorn (comparative cohort). After 2 years of follow-up, the prevalences of fatigue, stress dyspnoea, and palpitations were significantly lower in the Crataegus cohort than in the comparative cohort.
Clinically, the antiarrhythmic and palpitation reducing effects of WS 1442 were first observed in heart failure patients during clinical studies5,15,16. Studies based on real-world data showing the antiarrhythmic effect of WS 1442 as active substance in terms of clinical outcomes have not been published yet. Another non-synthetic therapy used for cardiovascular prevention is Tromcardin. Under the tradename Tromcardin several products are marketed that contain magnesium and potassium and are regulatory classified as either complementary foods for special medical purposes or food supplements. The combination of magnesium and potassium may improve the electrolyte balance of the heart and may have an anti-arrhythmic effect by stabilizing the cell membrane and have thus a positive influence on contractility17. Since magnesium/potassium is prescribed as treatment of arrhythmia, it is used as a reference.
The present study aims to get insights on the impact of WS 1442 prescriptions on the incidence of atrial fibrillation and flutter (AFF), tachycardia, and other cardiac arrhythmias in a real-world setting.
Methods
Data source
This retrospective cohort study was based on the IQVIA Disease Analyzer database (DA), which contains case-based information provided by office-based physicians (both general practitioner (GPs) and specialists) in Germany. Information is available on patient demographics, drug prescriptions, concomitant medication, comorbid conditions, sick leave, and referrals to hospitals. Information is provided by nearly 3,000 office-based physicians, representing approximately 3% of all German practices. The database appears to be suitable for pharmaco-epidemiological and pharmaco-economic studies18.
Ethical statement
German law allows the use of anonymous de-identified electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Therefore, no waiver of ethical approval can be obtained from an Institutional Review Board (IRB) or ethics committee. The company and the authors involved had no access to any identifying information at any moment during the analysis of the data.
Study population
This study included patients in GP practices with a prescription of the phytopharmaceutical WS 1442 or magnesium/potassium from January 2000 to December 2020. Patients with a diagnosis of atrial fibrillation and flutter (AFF) (ICD-10 I48), tachycardia and abnormalities of heart beat (ICD-10: I47 including re-entry ventricular arrhythmia, supraventricular tachycardia, ventricular tachycardia, and unspecified paroxysmal tachycardia, R00 including unspecified tachycardia, unspecified bradycardia, palpitations, other and unspecified abnormalities of heart beat), other cardiac arrhythmias (ICD-10: I49 including ventricular fibrillation and flutter, atrial premature depolarization, junctional premature depolarization, ventricular premature depolarization, other and unspecified premature depolarization, Sick sinus syndrome, extrasystoles, long-QT-syndrome and unspecified cardiac arrhythmia) in full patient history prior to the index date were excluded.
Study outcomes and statistical analyses
Study outcomes were temporal associations between WS 1442 prescriptions and incidence of AFF, tachycardia, and other cardiac arrhythmias in the time period 30 days until 5 years following the index date as compared to magnesium/potassium as a reference group. Each patient was retrospectively followed for up to five years after index date till the first outcome diagnosis documentation, last patient’s visit, or end of database (December 31st, 2020). Patients prescribed with WS 1442 were matched to patients with a prescription of magnesium/potassium by sex and age.
To estimate the association between WS 1442 prescriptions and incidence of the above defined outcomes, Cox regression analyses were performed. Results of the Cox regression analysis are displayed using Hazard Ratios (HR) with 95% confidence intervals. These models were adjusted for diagnoses of obesity (ICD-10: E66), diabetes (ICD-10: E10-E14), lipid metabolism disorders (ICD-10: E78), heart failure (ICD-10: I50), renal failure (ICD-10: N18, N19), hypertension (ICD-10: I10), ischemic heart diseases (ICD-10: I20-I25), ischemic stroke (ICD-10: I63, I64, G45), tobacco addiction (ICD-10: F17), alcohol related disorders (ICD-10: F10), and chronic obstructive pulmonary disease (COPD) (ICD-10: J44), as well as co-prescriptions of diuretics (ATC: C03), betablockers (ATC: C08), calcium channel blockers (ATC: C08), and agents acting on the renin-angiotensin system (ATC: C09). As a sensitivity analysis, patients with WS 1442 prescription were matched to patients with a prescription of magnesium/potassium by sex, age, co-morbidities, and co-prescriptions. The association between WS 1442 prescriptions and incidence of the above defined outcome was analyzed using univariable Cox-regression analysis based on fully matched cohorts.
Differences in the sample characteristics between two therapy cohorts were compared using the Wilcoxon signed-rank test for continuous age, the Stuart-Maxwell test for categorical age, and the McNemar test for sex and comorbidities. P-values of < 0.05 were considered statistically significant. Regression analyses were repeated for several age groups, men, and women.
Results
Baseline characteristics
Initially, 5,304 patients with WS 1442 prescription and 15,515 patients with magnesium/potassium prescriptions were included. After matching of two cohorts, this study included 4,550 patients with WS 1442 prescription and 4,550 matched patients with magnesium/potassium prescriptions. Due to the matching, both cohorts were not significantly different in terms of average age (70.4 years) and sex (70–71% were females). The prevalence of heart failure (22.1% vs. 12.9%) was much higher among patients who started to be treated with WS 1442 than among patients who were put on magnesium/potassium. Diabetes (18.3% vs. 15.7%), lipid metabolism disorders (32.0% vs. 28.7%), and hypertension (52.1% vs. 46.5%) were more frequent among patients in the magnesium/potassium group. Moreover, prevalence of cardiovascular therapies, especially betablockers and agents acting on the renin–angiotensin system were more frequent in the magnesium/potassium group (Table 1).
Table 1.
Characteristics of study patients after 1:1 matching.
| Variable | Patients with WS 1442 (n = 4550) | Patients with magnesium/potassium (n = 4550) | P-value* |
|---|---|---|---|
| Age (mean, standard deviation) | 70.4 (14.7) | 70.4 (14.7) | 1.000 |
| ≤ 60 | 26.8 | 26.8 | 1.000 |
| 61–70 | 19.0 | 19.0 | |
| 71–80 | 31.6 | 31.6 | |
| > 80 | 22.6 | 22.6 | |
| Female | 70.9 | 70.4 | 0.644 |
| Male | 29.1 | 29.6 | |
| Private health insurance | 29.7 | 29.3 | 0.692 |
| Statutory health insurance | 71.3 | 71.7 | |
| Obesity | 5.9 | 6.6 | 0.106 |
| Diabetes | 15.7 | 18.3 | < 0.001 |
| Lipid metabolism disorders | 28.7 | 32.0 | < 0.001 |
| Hypertension | 46.5 | 52.1 | < 0.001 |
| Ischemic heart diseases | 21.0 | 22.6 | 0.055 |
| Heart failure | 22.1 | 12.9 | < 0.001 |
| Ischemic stroke | 4.6 | 4.7 | 0.887 |
| Renal failure | 5.1 | 5.7 | 0.155 |
| COPD | 8.0 | 8.9 | 0.113 |
| Tobacco addiction | 1.4 | 1.8 | 0.076 |
| Alcohol related disorders | 0.4 | 0.6 | 0.215 |
| Drugs prescribed during the study therapy | |||
| Diuretics | 25.9 | 28.5 | 0.004 |
| Betablockers | 29.1 | 41.1 | < 0.001 |
| Calcium channel blockers | 15.1 | 19.7 | < 0.001 |
| Agents acting on the renin–angiotensin system | 39.8 | 49.2 | < 0.001 |
Data are percentages unless otherwise specified.
*P-values were obtained using McNemar tests for categorical variables with two categories, Stuart-Maxwell tests for categorical variables with more than two categories, and Wilcoxon signed-rank tests for continuous variables.
Incidence of atrial fibrillation and flutter
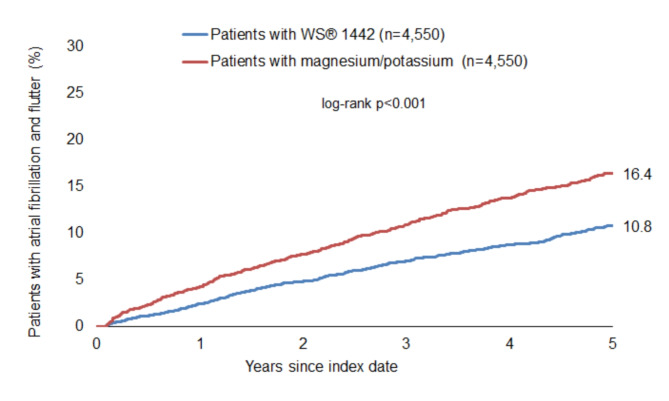
The cumulative incidence of atrial fibrillation and flutter was significantly lower among patients with WS 1442 compared to those with magnesium/potassium (10.8% vs. 16.4%, p < 0.001) (Fig. 1), whereby each patient was retrospectively followed for up to five years after index date till the first outcome diagnosis documentation, last patient’s visit, or end of database. WS 1442 prescription was significantly associated with a lower incidence of atrial fibrillation and flutter compared to magnesium/potassium (HR: 0.71; 95% CI: 0.64–0.80; p < 0.001)—subgroup analyses showed consistent effects. These associations were confirmed in the sensitivity analysis (Table 2).
Fig. 1.
Cumulative incidence of atrial fibrillation and flutter in patients with WS 1442 and magnesium/potassium prescriptions.
Table 2.
Association between WS 1442 prescription as compared to magnesium/potassium prescription and incidence of atrial fibrillation and flutter.
| Subgroup | Multivariable Cox regression adjusted for co-diagnoses and co-prescriptions* based on age- and sex-matched cohorts | Univariable Cox regression based on fully matched cohorts | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value* | Hazard ratio (95% CI) | P-value* | |
| Total | 0.71 (0.64–0.80) | < 0.001 | 0.69 (0.61–0.77) | < 0.001 |
| ≤ 60 | 0.68 (0.50–0.92) | 0.011 | 0.58 (0.37–0.91) | 0.019 |
| 61–70 | 0.77 (0.59–1.01) | 0.054 | 0.82 (0.64–1.06) | 0.133 |
| 71–80 | 0.62 (0.52–0.75) | < 0.001 | 0.59 (0.49–0.71) | < 0.001 |
| > 80 | 0.78 (0.63–0.97) | 0.026 | 0.76 (0.62–0.94) | 0.010 |
| Female | 0.65 (0.56–0.75) | < 0.001 | 0.59 (0.51–0.68) | < 0.001 |
| Male | 0.86 (0.71–1.05) | 0.129 | 0.94 (0.78–1.15) | 0.555 |
*Adjusted for obesity, diabetes, lipid metabolism disorders, hypertension, ischemic heart diseases, heart failure, ischemic stroke, renal failure, COPD, tobacco addiction, alcohol related disorders, and co-prescriptions of diuretics, betablockers, calcium channel blockers, agents acting on the renin–angiotensin system.
Incidence of tachycardia
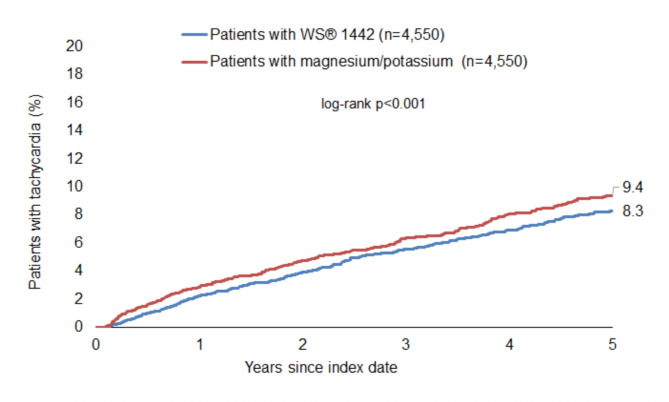
The cumulative incidence of tachycardia was significantly lower among patients with WS 1442 compared to patients with magnesium/potassium (8.3% vs. 9.4%, p < 0.001), however the absolute difference was relatively small (Fig. 2).
Fig. 2.
Cumulative incidence of tachycardia in patients with WS 1442 and magnesium/potassium prescriptions.
WS 1442 prescription was significantly associated with a lower incidence of tachycardia compared to magnesium/potassium (HR 0.83; 95% CI 0.73–0.96; p = 0.011). In subgroup analyses, the associations were consistent, but not always significant due to the reduced sample size. These associations were mostly confirmed in the sensitivity analysis (Table 3).
Table 3.
Association between WS 1442 prescription as compared to magnesium/potassium prescription and incidence of tachycardia.
| Subgroup | Multivariable Cox regression adjusted for co-diagnoses and co-prescriptions* based on age- and sex-matched cohorts | Univariable Cox regression based on fully matched cohorts | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value* | Hazard ratio (95% CI) | P-value* | |
| Total | 0.83 (0.73–0.96) | 0.011 | 0.91 (0.79–1.04) | 0.168 |
| ≤ 60 | 0.73 (0.55–0.97) | 0.031 | 0.76 (0.56–1.03) | 0.075 |
| 61–70 | 0.87 (0.65–1.16) | 0.343 | 0.90 (0.69–1.17) | 0.438 |
| 71–80 | 0.82 (0.65–1.04) | 0.098 | 0.98 (0.77–1.24) | 0.840 |
| > 80 | 0.90 (0.63–1.30) | 0.586 | 0.97 (0.68–1.39) | 0.884 |
| Female | 0.87 (0.74–1.03) | 0.104 | 0.92 (0.78–1.08) | 0.296 |
| Male | 0.72 (0.55–0.95) | 0.021 | 0.87 (0.66–1.15) | 0.322 |
*Adjusted for obesity, diabetes, lipid metabolism disorders, hypertension, ischemic heart diseases, heart failure, ischemic stroke, renal failure, COPD, tobacco addiction, alcohol related disorders, and co-prescriptions of diuretics, betablockers, calcium channel blockers, agents acting on the renin–angiotensin system.
Incidence of other cardiac arrhythmias
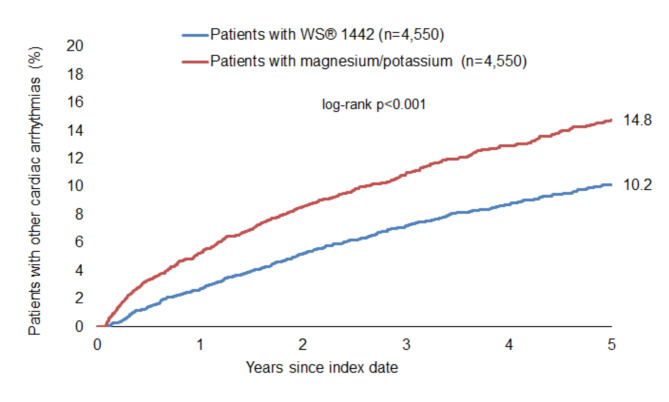
The cumulative incidence of other cardiac arrhythmias was significantly lower among patients with WS 1442 compared to patients with magnesium/potassium (10.2% vs. 14.8%, p < 0.001) (Fig. 3). WS 1442 prescription was significantly associated with a lower incidence of other cardiac arrhythmias compared to magnesium/potassium (HR: 0.68; 95% CI: 0.60–0.77; p < 0.001). This association was stronger among youngest (≤ 60) and oldest (> 80) age groups and also stronger among men compared to women. These associations were confirmed in the sensitivity analysis (Table 4).
Fig. 3.
Cumulative incidence of other cardiac arrhythmias in patients with WS 1442 and magnesium/potassium prescriptions.
Table 4.
Association between WS 1442 prescription as compared to magnesium/potassium prescription and incidence of other cardiac arrhythmias.
| Subgroup | Multivariable Cox regression adjusted for co-diagnoses and co-prescriptions* based on age- and sex-matched cohorts | Univariable Cox regression based on fully matched cohorts | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value* | Hazard ratio (95% CI) | P-value* | |
| Total | 0.68 (0.60–0.77) | < 0.001 | 0.65 (0.58–0.74) | < 0.001 |
| ≤ 60 | 0.50 (0.38–0.65) | < 0.001 | 0.45 (0.33–0.53) | < 0.001 |
| 61–70 | 0.73 (0.57–0.93) | 0.011 | 0.76 (0.61–0.96) | 0.019 |
| 71–80 | 0.78 (0.63–0.97) | 0.022 | 0.70 (0.57–0.85) | < 0.001 |
| > 80 | 0.59 (0.44–0.80) | < 0.001 | 0.61 (0.46–0.80) | < 0.001 |
| Female | 0.72 (0.62–0.83) | < 0.001 | 0.67 (0.58–0.78) | < 0.001 |
| Male | 0.60 (0.49–0.75) | < 0.001 | 0.62 (0.50–0.76) | < 0.001 |
*Adjusted for obesity, diabetes, lipid metabolism disorders, hypertension, ischemic heart diseases, heart failure, ischemic stroke, renal failure, COPD, tobacco addiction, alcohol related disorders, and co-prescriptions of diuretics, betablockers, calcium channel blockers, agents acting on the renin–angiotensin system.
Discussion
In this retrospective cohort study, we investigated antiarrhythmic effects of WS 1442 compared with magnesium/potassium in a large collective of outpatients. Using a matched-pairs design and based on more than 9,000 primary care patients, WS 1442 prescription was significantly associated with a lower incidence of three main heart rhythm disorders (atrial fibrillation and flutter, tachycardia, and other cardiac arrhythmias).
In previous clinical trials, WS 1442 has been compared to placebo. Mostly cardiovascular effects of WS 1442 among patients with chronic heart failure were investigated16,19–21. The largest clinical trial contained 2681 adults with NYHA class II/III heart failure (1338 treated with WS 1442). Using WS 1442 (900 mg/day) as an add-on to standard treatment, the authors demonstrated that WS 1442 could reduce the incidence of sudden cardiac death in patients with left ventricular ejection fraction between 25% and 35%20.
The use of WS 1442 and, ultimately, magnesium/potassium should not be seen as an alternative to guideline-based drug options for the prevention of cardiac arrhythmias. Rather, the former in particular seems to have an additional effect even in the presence of existing medication. It is known that WS 1442 is a therapy with few side effects and a favorable interaction profile. In the SPICE (Survival and Prognosis: Investigation of WS 1442 in congestive heart failure) study, administration of WS 1442 in combination with standard therapy for heart failure was associated with an excellent safety and tolerability profile, as the adverse events observed in the WS 1442 patients were of the same types and occurred at comparable rates as those in the placebo group20. It has been shown in vitro that the negative chronotropic effect of WS 1442 is unlikely to be promoted by a β-adrenergic receptor blockade as is the case with beta-blockers22.
The proportion of patients taking beta-blockers is relatively high in both comparison groups. Interestingly, beta-blockers, which have a clearly proven prophylactic effect for tachycardic cardiac arrhythmia of any kind23, were prescribed even less in the WS 1442 group than in the controls (29.1% vs. 41.1%). Furthermore, pre-existing heart failure was more present in the WS 1442 group (22.1% vs. 12.9%). Nevertheless, the incidence of various tachycardic cardiac arrhythmias is lower in this group, which could speak for the effect of WS 1442.
Different mechanisms have been mentioned as possible mediators of the cardioprotective effect of hawthorn: anti-inflammatory action, anti-oxidation, endothelial protection, anti-platelet action and prolongation of refractory period and action potential13,24–27. These are all mechanisms that are important in the pathogenesis of both heart failure and arrhythmias28,29. Interestingly, animal studies also show that WS 1442 may prevent ventricular arrhythmias, e.g., in ischemia-triggered arrhythmias. Oral administration of WS 1442 (10 or 100 mg x kg(-1) x day(-1)) for 7 days before ligation of the left coronary artery significantly decreased the area of myocardial infarction within the ischemic zone in rats30. WS 1442 treatment also attenuated the elevation of the ST-segment in the ECG, diminished the incidence of ventricular fibrillations (control: 67%; 10 mg x kg(-1): 64%; 100 mg x kg(-1): 27%) and reduced the mortality rate (control: 47%; 10 mg x kg(-1): 27%; 100 mg x kg(-1): 9%)13. Further studies would certainly be required to investigate the mechanism of action.
From a clinical perspective, there is a wide range of potential applications of WS 1442 in combination with the entire spectrum of therapeutic procedures, starting with basic measures such as exercise and a healthy nutrition, chemical-synthetic drug therapy, and interventional methods such as pulmonary vein isolation23. In clinical trials, an increase in quality of life was shown for WS 1442 in patients with heart failure NYHA II in combination with standard therapy and training compared to patients only receiving standard therapy and training31.
Retrospective primary care database analyses are generally limited by the validity and completeness of data. First, assessments are relied on ICD codes entered by GPs and no data from specialists or hospitals were available. Diagnosis codes lack deep granularity and do not allow for the differentiation of disease severity stages or outcomes. For example, outcome diagnoses were defined using ICD-10 3d level (i.e. I49) and no analyses of deeper levels (i.e. I49.1) were possible as the most frequent diagnoses by GPs are undefined (i.e. I49.9). Second, results of quality-of-life assessments, results of electrocardiogram (ECG), and data on socioeconomic status and lifestyle-related risk factors (smoking, alcohol, physical activity) are not available. Third, the prevalence of some cardiovascular disorders and therapies was more frequent among patients with magnesium/potassium. Although we used multivariable regression analyses to adjust for possible effects of co-diagnoses and co-therapies on the outcomes, the data may lack detailed diagnosis information that would allow for better adjustment. Fourth, to buy herbal medicines, which are OTC drugs, or dietary supplements, patients do not need a prescription from physicians. The database does not include data on the use of herbal medicines that patients buy without prescriptions. Fifth, since both preparations are OTC, data on adherence to therapy during the follow-up is not available. Finally, patients can only be observed in a single practice; when they receive a diagnosis or prescription by another physician, such prescriptions cannot be analyzed as data from different practices cannot be linked. However, the strengths of this study are the large sample size, the use of data collected in general practices representing a real-life context, and the inclusion of a wide range of physical conditions with adjustment for these in multivariable regression models.
Conclusion
The findings of this retrospective cohort study showed that patients prescribed with WS 1442 exhibited a significantly lower incidence of atrial fibrillation and flutter, tachycardia, and other cardiac arrhythmias compared to those prescribed magnesium/potassium. These findings underscore the previously established use of WS 1442 for varying heart diseases as well as the importance of further exploration of WS 1442 as a promising adjunct in the treatment and prevention of cardiac arrhythmias.
Author contributions
KK contributed to the design of the study, performed the statistical analyses, and wrote the first draft of the manuscript. PWG and CW contributed to the design of the study and corrected the manuscript. All authors contributed to and have approved the manuscript.
Funding
This study was funded by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany.
Data availability
The data that support the findings of this study are not openly available due to reasons of privacy and are available from the corresponding author upon reasonable request.
Declarations
Competing interests
CW received speakers fee from Dr. Willmar Schwabe GmbH & Co. KG, Novartis, Medtronic, Abbott, Sanofi, and Daiichi Sankyo in the past. PWG received speaker’s fees from Dr. Willmar Schwabe GmbH & Co. KG in the past. KK declares to have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Townsend, N. et al. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 19, 133–143. 10.1038/s41569-021-00607-3 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Olvera Lopez, E., Ballard, B. D. & Jan, A. Cardiovascular Disease. https://www.ncbi.nlm.nih.gov/books/NBK535419/ (2022). [PubMed]
- 3.Beauchamp, A., Peeters, A., Tonkin, A. & Turrell, G. Best practice for prevention and treatment of cardiovascular disease through an equity lens: a review. Eur. J. Cardiovasc. Prev. Rehabil. 17, 599–606. 10.1097/HJR.0b013e328339cc99 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Chang, W. T., Dao, J. & Shao, Z. H. Hawthorn: potential roles in cardiovascular disease. Am. J. Chin. Med. 33, 1–10. 10.1142/s0192415x05002606 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Tauchert, M., Gildor, A. & Lipinski, J. Einsatz Des Hochdosierten Crataegusextraktes WS 1442 in Der Therapie Der Herzinsuffizienz Stadium NYHA II. Herz 24, 465–474 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Martinelli, F. et al. Botanical, phytochemical, anti-microbial and pharmaceutical characteristics of hawthorn (Crataegus monogyna Jacq). Rosaceae Mol. 26, 7266 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabavi, S. F. et al. Polyphenolic composition of Crataegus monogyna Jacq.: from chemistry to medical applications. Nutrients 7, 7708–7728. 10.3390/nu7095361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alp, H., Soner, B. C., Baysal, T. & Sahin, A. S. Protective effects of Hawthorn (Crataegus oxyacantha) extract against digoxin-induced arrhythmias in rats. Anatol. J. Cardiol. 15, 970–975. 10.5152/akd.2014.5869 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krzeminski, T. & Chatterjee, S. S. Ischemia and early reperfusion induced arrhythmias: beneficial effects of an extract of Crataegus oxyacantha L. Pharm. Pharmacol. Lett. 3, 45–48 (1993). [Google Scholar]
- 10.Holubarsch, C. J. F., Colucci, W. S. & Eha, J. Benefit-risk assessment of Crataegus extract WS 1442: an evidence-based review. Am. J. Cardiovasc. Drugs 18, 25–36. 10.1007/s40256-017-0249-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegener, T. et al. Significance of hawthorn extract in general practice—a current positioning. MMW Fortschr. Med. 160, 1–7. 10.1007/s15006-018-0725-4 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Zorniak, M., Szydlo, B. & Krzeminski, T. F. Crataegus special extract WS 1442: up-to-date review of experimental and clinical experiences. J. Physiol. Pharmacol. 68, 521–526 (2017). [PubMed] [Google Scholar]
- 13.Zorniak, M., Porc, M. P. & Krzeminski, T. F. Hawthorn revisited: time- and dose-dependent cardioprotective action of WS-1442 special extract in the reperfusion-induced arrhythmia model in rats in vivo. J. Physiol. Pharmacol. 70, 14. 10.26402/jpp.2019.2.14 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Habs, M. Prospective, comparative cohort studies and their contribution to the benefit assessments of therapeutic options: heart failure treatment with and without hawthorn special extract WS 1442. Forsch. Komplementarmed Klass Nat. 11 (Suppl 1), 36–39. 10.1159/000080574 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Koller, M. et al. Weißdorn-Spezialextrakt in Der Therapie früher Stadien Der KHK-assoziierten Herzinsuffizienz. Ergebnisse Einer Prospektiven Kohortenstudie Zur Wirtschaftlichkeit Und Zum Einfluss auf die Lebensqualität Der Patienten. MMW Fortschr. Med. 147, 159–164 (2005). [Google Scholar]
- 16.Eggeling, T., Regitz-Zagrosek, V., Zimmermann, A. & Burkart, M. Baseline severity but not gender modulates quantified Crataegus extract effects in early heart failure—a pooled analysis of clinical trials. Phytomedicine 18, 1214–1219. 10.1016/j.phymed.2011.06.022 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Weglicki, W., Quamme, G., Tucker, K., Haigney, M. & Resnick, L. Potassium, magnesium, and electrolyte imbalance and complications in disease management. Clin. Exp. Hypertens. 27, 95–112. 10.1081/ceh-200044275 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Rathmann, W., Bongaerts, B., Carius, H. J., Kruppert, S. & Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 56, 459–466. 10.5414/cp203320 (2018). [PubMed] [Google Scholar]
- 19.Tauchert, M. Efficacy and safety of crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am. Heart J. 143, 910–915. 10.1067/mhj.2002.121463 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Holubarsch, C. J. F., Colucci, W. S., Meinertz, T., Gaus, W. & Tendera, M. The efficacy and safety of Crataegus extract WS® 1442 in patients with heart failure: the SPICE trial. Eur. J. Heart Fail. 10, 1255–1263. 10.1016/j.ejheart.2008.10.004 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Zick, S. M., Vautaw, B. M., Gillespie, B. & Aaronson, K. D. Hawthorn extract randomized blinded chronic heart failure (HERB CHF) trial. Eur. J. Heart Fail. 11, 990–999. 10.1093/eurjhf/hfp116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long, S. R., Carey, R. A., Crofoot, K. M., Proteau, P. J. & Filtz, T. M. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine 13, 643–650. 10.1016/j.phymed.2006.01.005 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Hindricks, G. et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The task torce for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 42, 373–498. 10.1093/eurheartj/ehaa612 (2021). [DOI] [PubMed]
- 24.Rieckeheer, E., Schwinger, R. H., Bloch, W. & Brixius, K. Hawthorn special extract WS® 1442 increases red blood cell NO-formation without altering red blood cell deformability. Phytomedicine 19, 20–24. 10.1016/j.phymed.2011.08.059 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Pöpping, S., Rose, H., Ionescu, I., Fischer, Y. & Kammermeier, H. Effect of a hawthorn extract on contraction and energy turnover of isolated rat cardiomyocytes. Arzneimittelforschung 45, 1157–1161 (1995). [PubMed] [Google Scholar]
- 26.Joseph, G., Zhao, Y. & Klaus, W. Pharmacologic action profile of crataegus extract in comparison to epinephrine, amirinone, milrinone and digoxin in the isolated perfused guinea pig heart. Arzneimittelforschung 45, 1261–1265 (1995). [PubMed] [Google Scholar]
- 27.Müller, A., Linke, W. & Klaus, W. Crataegus extract blocks potassium currents in guinea pig ventricular cardiac myocytes. Planta Med. 65, 335–339. 10.1055/s-1999-13997 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Hu, Y. F., Chen, Y. J., Lin, Y. J. & Chen, S. A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 12, 230–243. 10.1038/nrcardio.2015.2 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Van Wagoner, D. R. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J. Cardiovasc. Pharmacol. 52, 306–313. 10.1097/FJC.0b013e31817f9398 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Veveris, M., Koch, E. & Chatterjee, S. S. Crataegus special extract WS® 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 74, 1945–1955. 10.1016/j.lfs.2003.09.050 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Härtel, S. et al. Effects of endurance exercise training and Crataegus extract WS® 1442 in patients with heart failure with preserved ejection fraction—a randomized controlled trial. Sports 2, 59–75. 10.3390/sports2030059 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of privacy and are available from the corresponding author upon reasonable request.