Abstract
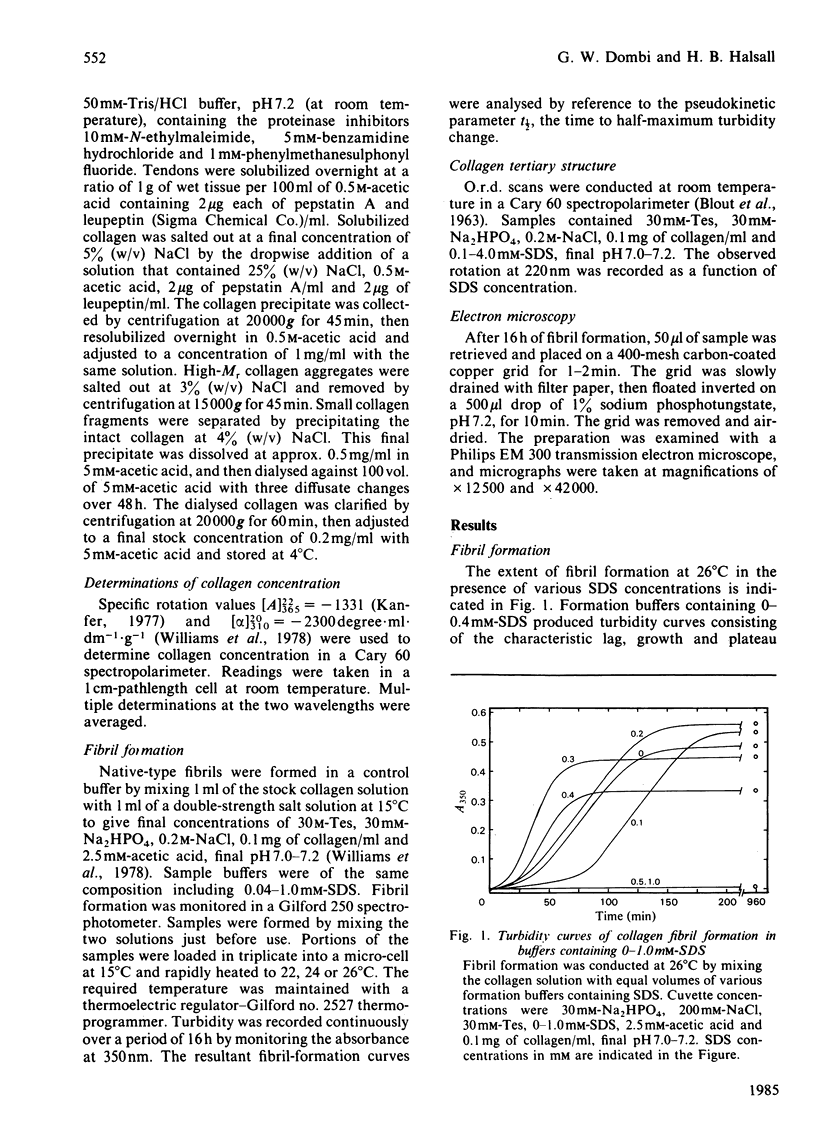
Sodium dodecyl sulphate (SDS) was used to weaken both the electrostatic and the hydrophobic interactions during collagen fibrillogenesis in vitro. The rate and extent of fibril formation as well as fibril morphology were affected by SDS concentration. Both the formation of large fibrils at 0.3 mM-SDS and the complete cessation of fibril formation at 0.5 mM-SDS were considered to be the result of SDS-induced conformational changes in the non-helical telopeptides. A possible mechanism of SDS interaction with the N-terminal and the distal region of the C-terminal telopeptides is offered.
Full text
PDF
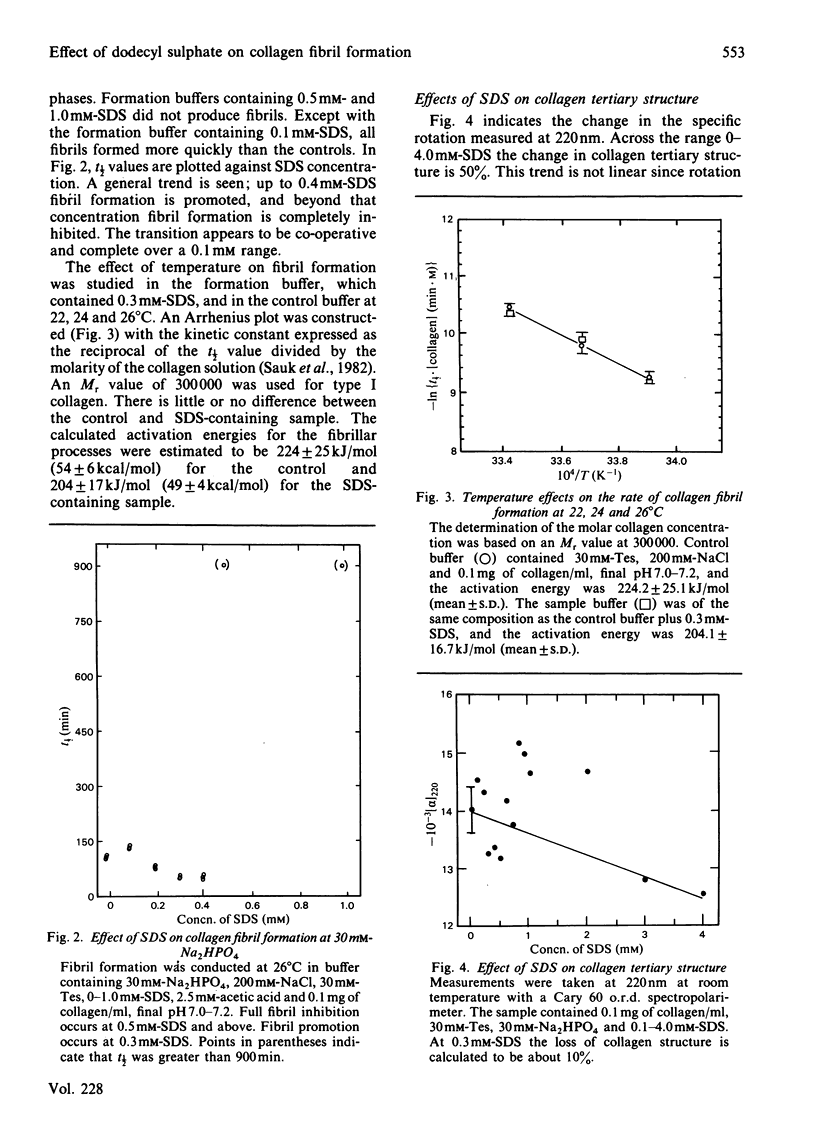

Images in this article
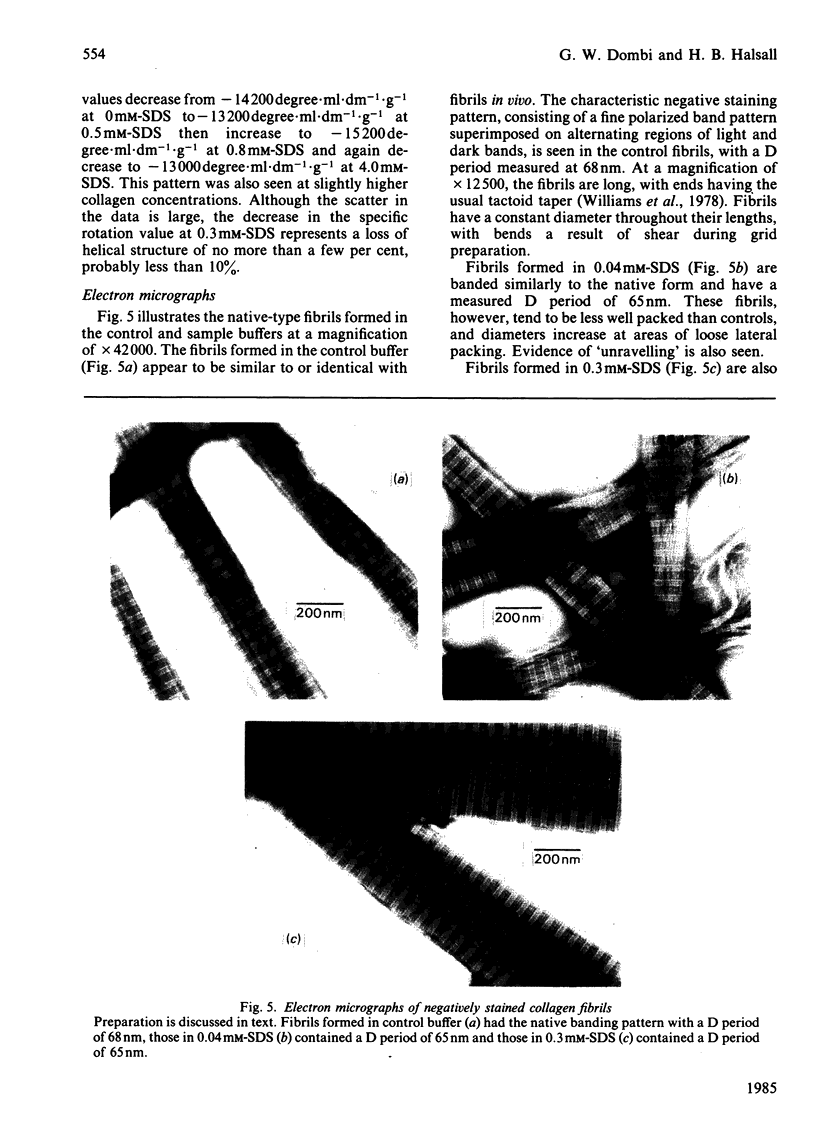
Selected References
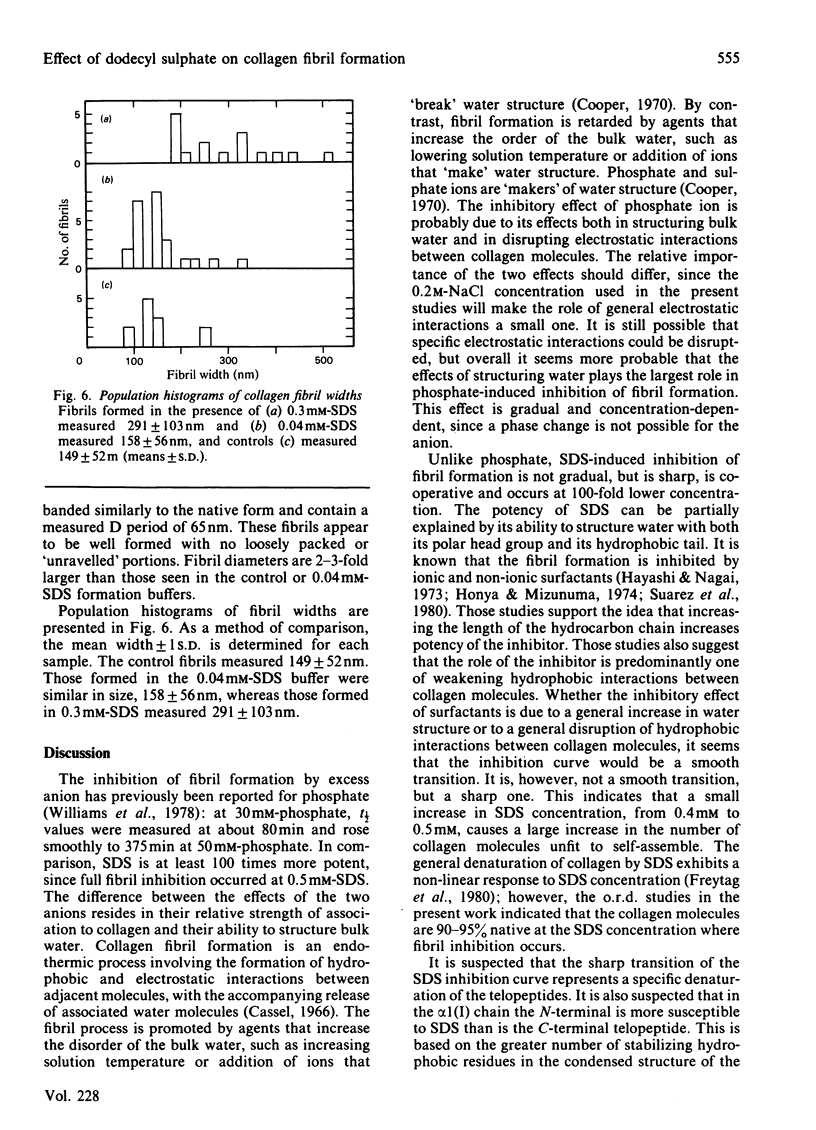
These references are in PubMed. This may not be the complete list of references from this article.
- Capaldi M. J., Chapman J. A. The C-terminal extrahelical peptide of type I collagen and its role in fibrillogenesis in vitro. Biopolymers. 1982 Nov;21(11):2291–2313. doi: 10.1002/bip.360211115. [DOI] [PubMed] [Google Scholar]
- Capaldi M. J., Chapman J. A. The C-terminal extrahelical peptide of type I collagen and its role in fibrillogenesis in vitro: effects of ethylurea. Biopolymers. 1984 Feb;23(2):313–323. doi: 10.1002/bip.360230210. [DOI] [PubMed] [Google Scholar]
- Chandrakasan G., Torchia D. A., Piez K. A. Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J Biol Chem. 1976 Oct 10;251(19):6062–6067. [PubMed] [Google Scholar]
- Comper W. D., Veis A. Characterization of nuclei in in vitro collagen fibril formation. Biopolymers. 1977 Oct;16(10):2133–2142. doi: 10.1002/bip.1977.360161005. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic studies of the assembly in vitro of native collagen fibrils. Biochem J. 1970 Jul;118(3):355–365. doi: 10.1042/bj1180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag J. W., Noelken M. E., Hudson B. G. Physical properties of collagen--sodium dodecyl sulfate complexes. Biochemistry. 1979 Oct 16;18(21):4761–4768. doi: 10.1021/bi00588a042. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Gelman R. A., Poppke D. C., Piez K. A. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J Biol Chem. 1979 Nov 25;254(22):11741–11745. [PubMed] [Google Scholar]
- Gelman R. A., Williams B. R., Piez K. A. Collagen fibril formation. Evidence for a multistep process. J Biol Chem. 1979 Jan 10;254(1):180–186. [PubMed] [Google Scholar]
- Hayashi T., Nagai Y. Factors affecting the interactions of collagen molecules as observed by in vitro fibril formation. II. Effects of species and concentration of anions. J Biochem. 1973 Aug;74(2):253–262. [PubMed] [Google Scholar]
- Hayashi T., Nagai Y. Factors affecting the interactions of collagen molecules as observed by in vitro fibril formation. III. Non-helical regions of the collagen molecules. J Biochem. 1974 Jul;76(1):177–186. doi: 10.1093/oxfordjournals.jbchem.a130543. [DOI] [PubMed] [Google Scholar]
- Honya M., Mizunuma H. Collagen fibrillogenesis in vitro: an accelerative effect of surfactants on collagen fibril formation. J Biochem. 1974 Jan;75(1):113–121. doi: 10.1093/oxfordjournals.jbchem.a130365. [DOI] [PubMed] [Google Scholar]
- Kanfer I. Binding of betamethasone and dexamethasone by collagen. J Pharm Pharmacol. 1977 May;29(5):314–316. doi: 10.1111/j.2042-7158.1977.tb11323.x. [DOI] [PubMed] [Google Scholar]
- Sauk J. J., Johnson D., Roszkowski M. The effect of some varying lipid A structures on the inhibition of fibrillogenesis in basement membrane collagen. J Oral Pathol. 1982 Feb;11(1):64–71. doi: 10.1111/j.1600-0714.1982.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Suarez G., Veliz M., Nagel R. L. Role of hydrophobic interactions in collagen fibril formation: effect of alkylureas in vitro. Arch Biochem Biophys. 1980 Dec;205(2):422–427. doi: 10.1016/0003-9861(80)90125-3. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Gross J. Collagen fibrillogenesis: intermediate aggregates and suprafibrillar order. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4027–4031. doi: 10.1073/pnas.73.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD G. C., KEECH M. K. The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: kinetic and electron-microscope studies. Biochem J. 1960 Jun;75:588–598. doi: 10.1042/bj0750588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD G. C. The formation of fibrils from collagen solutions. 2. A mechanism of collagen-fibril formation. Biochem J. 1960 Jun;75:598–605. doi: 10.1042/bj0750598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R., Gelman R. A., Poppke D. C., Piez K. A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem. 1978 Sep 25;253(18):6578–6585. [PubMed] [Google Scholar]



