Abstract
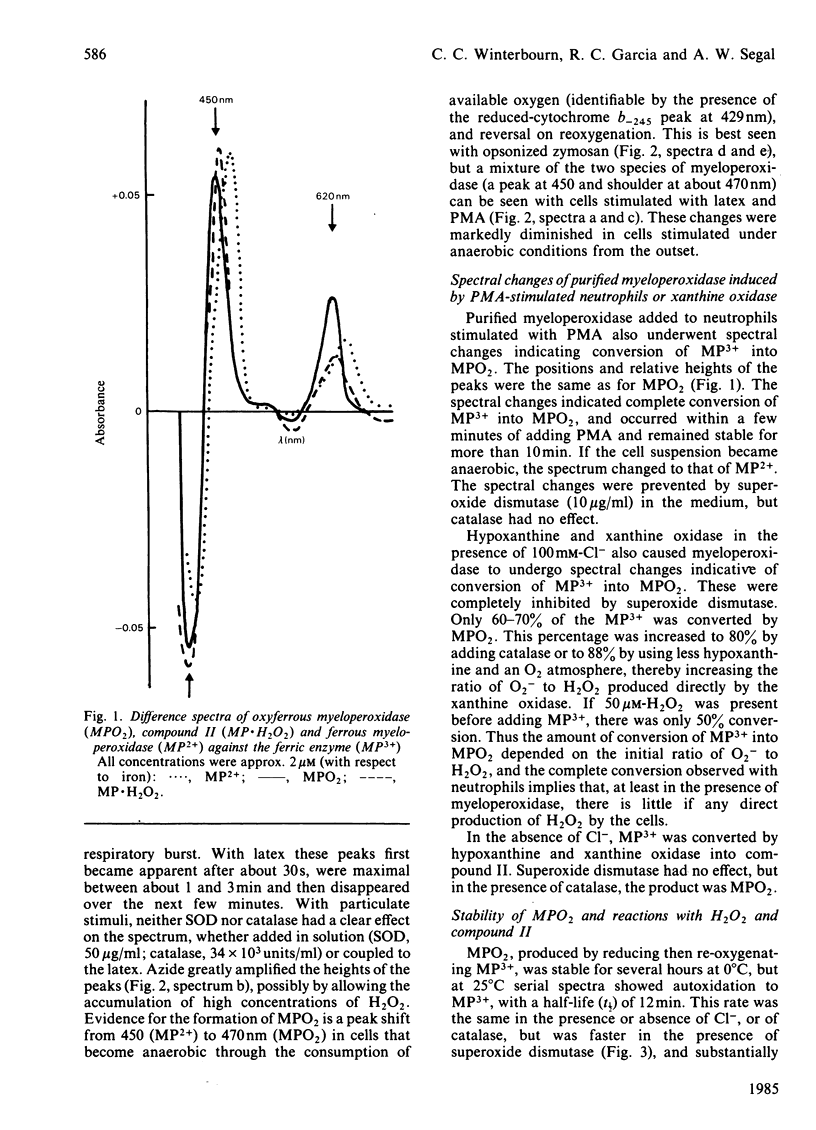
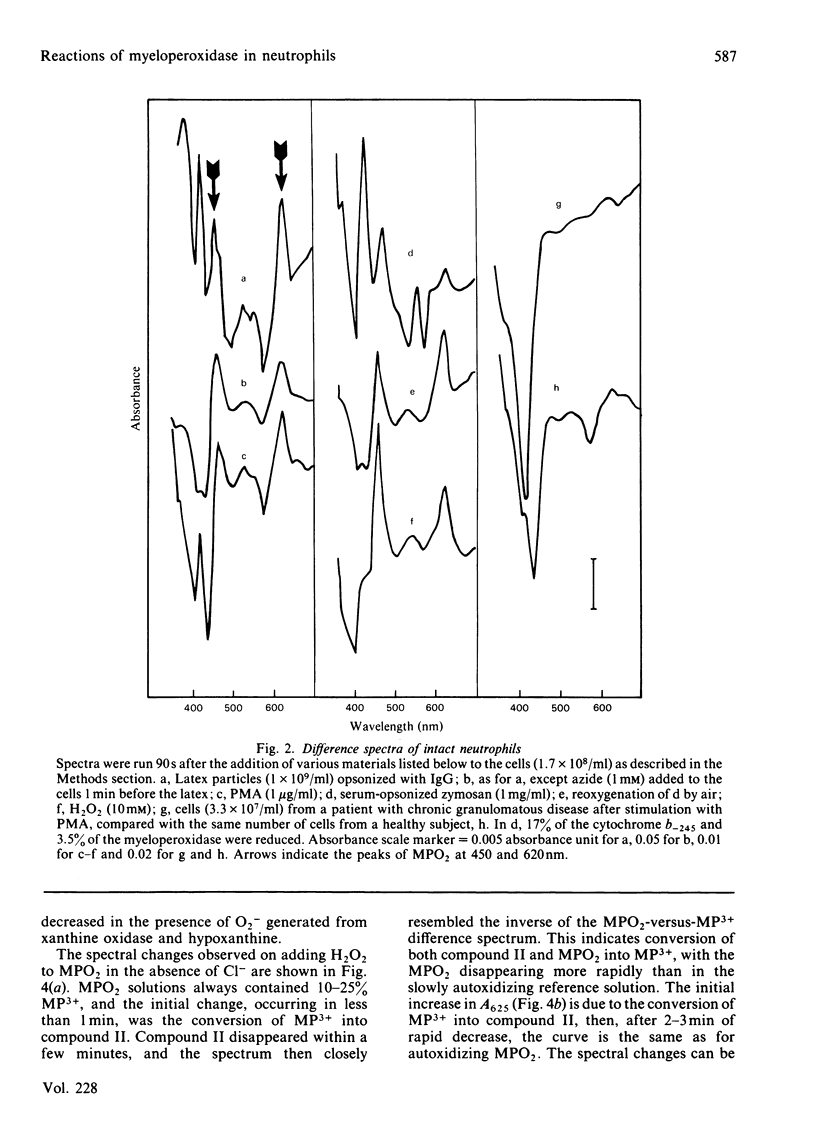
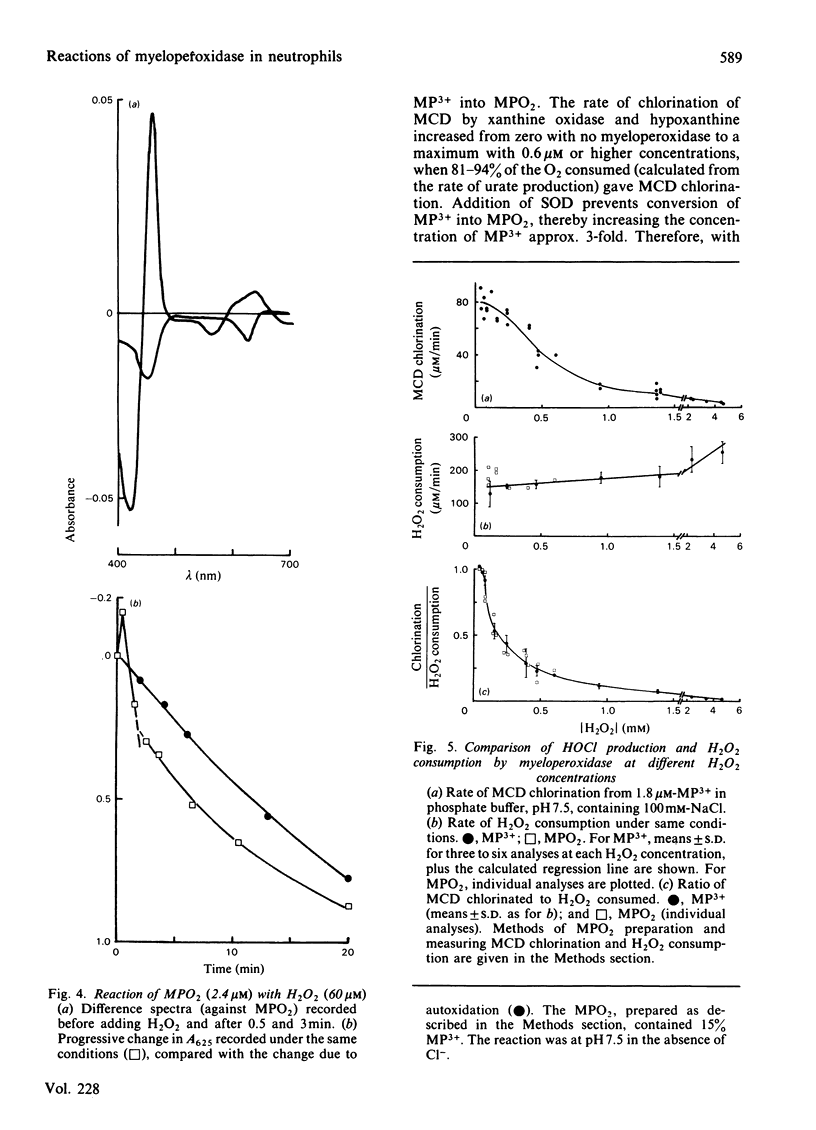
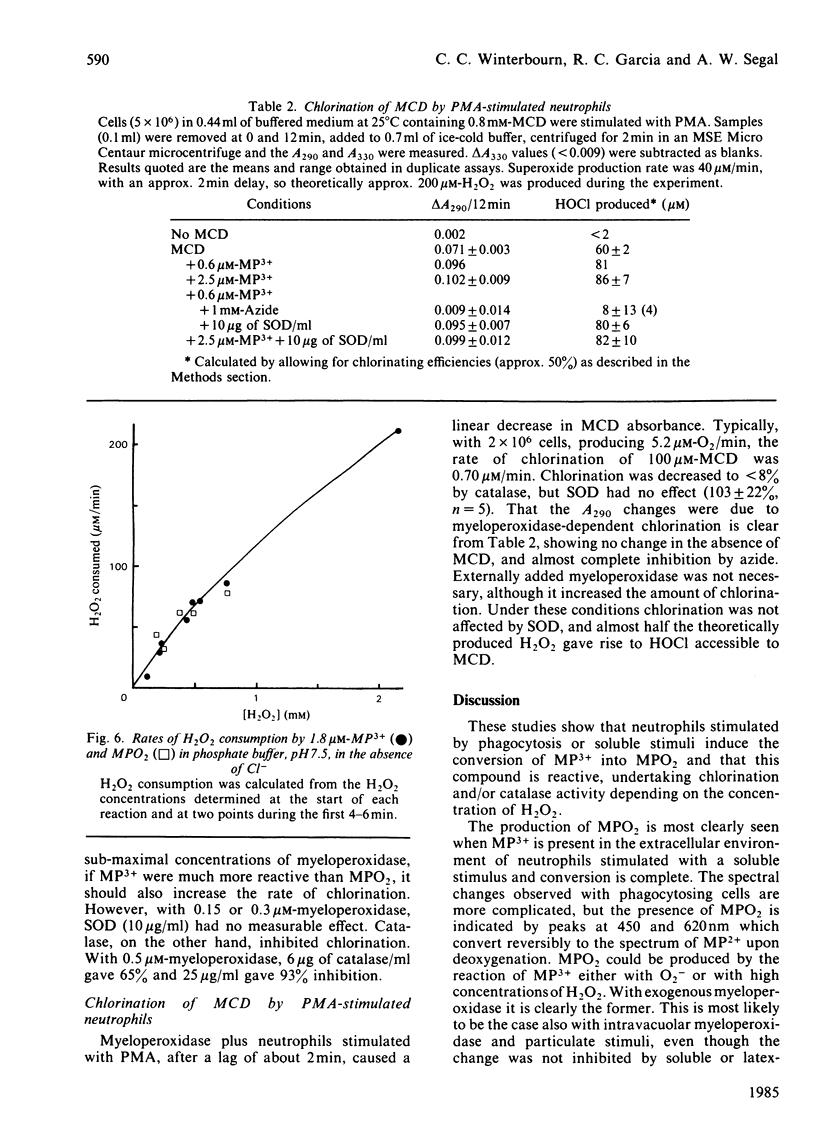
Examination of the spectra of phagocytosing neutrophils and of myeloperoxidase present in the medium of neutrophils stimulated with phorbol myristate acetate has shown that superoxide generated by the cells converts both intravacuolar and exogenous myeloperoxidase into the superoxo-ferric or oxyferrous form (compound III or MPO2). A similar product was observed with myeloperoxidase in the presence of hypoxanthine, xanthine oxidase and Cl-. Both transformations were inhibited by superoxide dismutase. Thus it appears that myeloperoxidase in the neutrophil must function predominantly as this superoxide derivative. MPO2 autoxidized slowly (t 1/2 = 12 min at 25 degrees C) to the ferric enzyme. It did not react directly with H2O2 or Cl-, but did react with compound II (MP2+ X H2O2). MPO2 catalysed hypochlorite formation from H2O2 and Cl- at approximately the same rate as the ferric enzyme, and both reactions showed the same H2O2-dependence. This suggests that MPO2 can enter the main peroxidation pathway, possibly via its reaction with compound II. Both ferric myeloperoxidase and MPO2 showed catalase activity, in the presence or absence of Cl-, which predominated over chlorination at H2O2 concentrations above 200 microM. Thus, although the reaction of neutrophil myeloperoxidase with superoxide does not appear to impair its chlorinating ability, the H2O2 concentration in its environment will determine whether the enzyme acts primarily as a catalase or peroxidase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J Biol Chem. 1982 Nov 25;257(22):13240–13245. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., de Boer J. E., Plat H., Wever R. The halide complexes of myeloperoxidase and the mechanism of the halogenation reactions. Biochim Biophys Acta. 1980 Jun 13;613(2):337–348. doi: 10.1016/0005-2744(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Cech P., Lehrer R. I. Phagolysosomal pH of human neutrophils. Blood. 1984 Jan;63(1):88–95. [PubMed] [Google Scholar]
- Foote C. S., Goyne T. E., Lehrer R. I. Assessment of chlorination by human neutrophils. Nature. 1983 Feb 24;301(5902):715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Hager L. P., Morris D. R., Brown F. S., Eberwein H. Chloroperoxidase. II. Utilization of halogen anions. J Biol Chem. 1966 Apr 25;241(8):1769–1777. [PubMed] [Google Scholar]
- Hamers M. N., Bot A. A., Weening R. S., Sips H. J., Roos D. Kinetics and mechanism of the bactericidal action of human neutrophils against Escherichia coli. Blood. 1984 Sep;64(3):635–641. [PubMed] [Google Scholar]
- Harrison J. E., Araiso T., Palcic M. M., Dunford H. B. Compound I of myeloperoxidase. Biochem Biophys Res Commun. 1980 May 14;94(1):34–40. doi: 10.1016/s0006-291x(80)80183-5. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hurst J. K., Albrich J. M., Green T. R., Rosen H., Klebanoff S. Myeloperoxidase-dependent fluorescein chlorination by stimulated neutrophils. J Biol Chem. 1984 Apr 25;259(8):4812–4821. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system I. Analysis of a novel method of intralysosomal iodination. J Cell Biol. 1980 Jul;86(1):292–303. doi: 10.1083/jcb.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M., Metcalf J. A., Root R. K. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983 Mar;61(3):483–492. [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloneperoxidase of the leukocyte of normal blood. 3. The reaction of ferric myeloperoxidase with superoxide anion. Biochim Biophys Acta. 1972 Oct 12;284(2):355–359. doi: 10.1016/0005-2744(72)90130-1. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. I. Reaction of myeloperoxidase with hydrogen peroxide. Biochim Biophys Acta. 1970 Apr 22;206(1):71–77. doi: 10.1016/0005-2744(70)90083-5. [DOI] [PubMed] [Google Scholar]
- Phelps C. F., Antonini E., Giacometti G., Brunori M. The kinetics of oxidation of ferroperoxidase by molecular oxygen. A model of a terminal oxidase. Biochem J. 1974 Jul;141(1):265–272. doi: 10.1042/bj1410265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ J., SHMUKLER H. W. MYELOPEROXIDASE OF THE LEUCOCYTE OF NORMAL HUMAN BLOOD. II. ISOLATION, SPECTROPHOTOMETRY, AND AMINO ACID ANALYSIS. Biochemistry. 1964 Sep;3:1234–1238. doi: 10.1021/bi00897a009. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Coade S. B. Kinetics of oxygen consumption by phagocytosing human neutrophils. Biochem Biophys Res Commun. 1978 Oct 16;84(3):611–617. doi: 10.1016/0006-291x(78)90749-0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Garcia R. C., Harper A. M., Banga J. P. Iodination by stimulated human neutrophils. Studies on its stoichiometry, subcellular localization and relevance to microbial killing. Biochem J. 1983 Jan 15;210(1):215–225. doi: 10.1042/bj2100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Tamura M., Yamazaki I. Reactions of the oxyform of horseradish peroxidase. J Biochem. 1972 Feb;71(2):311–319. doi: 10.1093/oxfordjournals.jbchem.a129768. [DOI] [PubMed] [Google Scholar]
- Test S. T., Weiss S. J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984 Jan 10;259(1):399–405. [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOTA K., YAMAZAKI I. THE ACTIVITY OF THE HORSERADISH PEROXIDASE COMPOUND 3. Biochem Biophys Res Commun. 1965 Jan 4;18:48–53. doi: 10.1016/0006-291x(65)90880-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Yokota K. Oxidation states of peroxidase. Mol Cell Biochem. 1973 Nov 15;2(1):39–52. doi: 10.1007/BF01738677. [DOI] [PubMed] [Google Scholar]


