Abstract
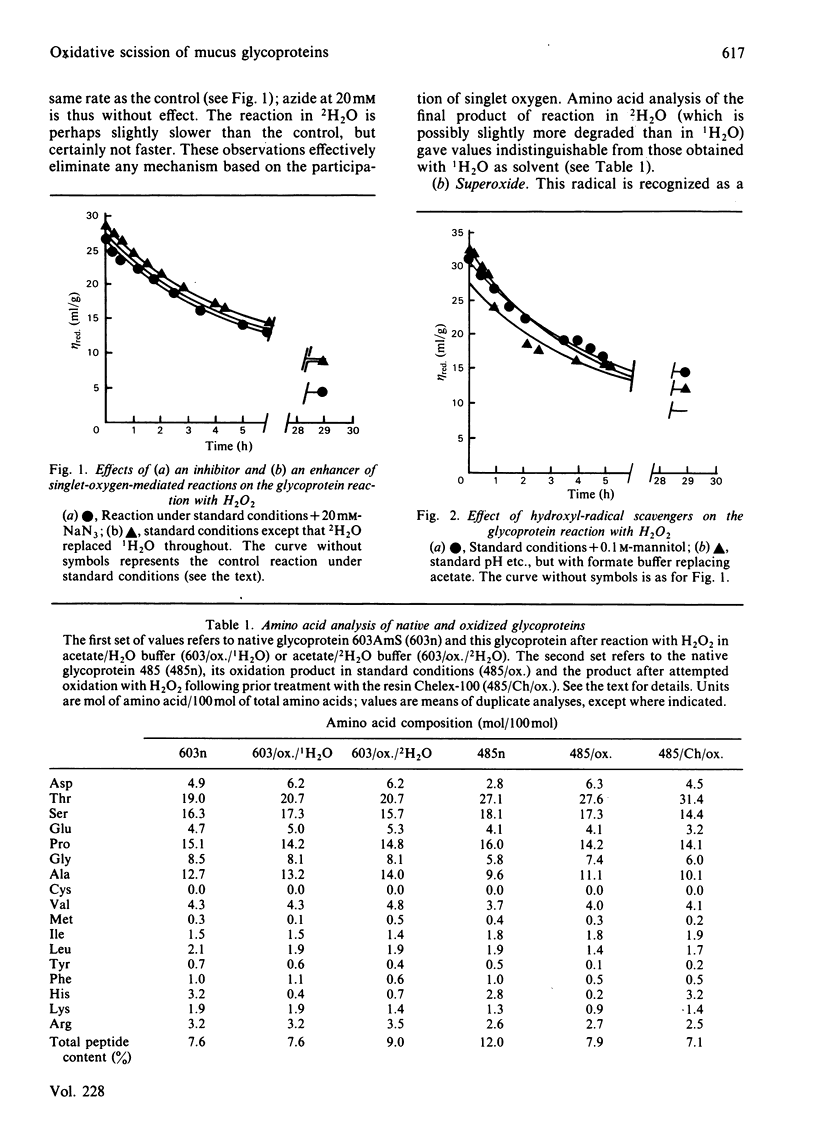
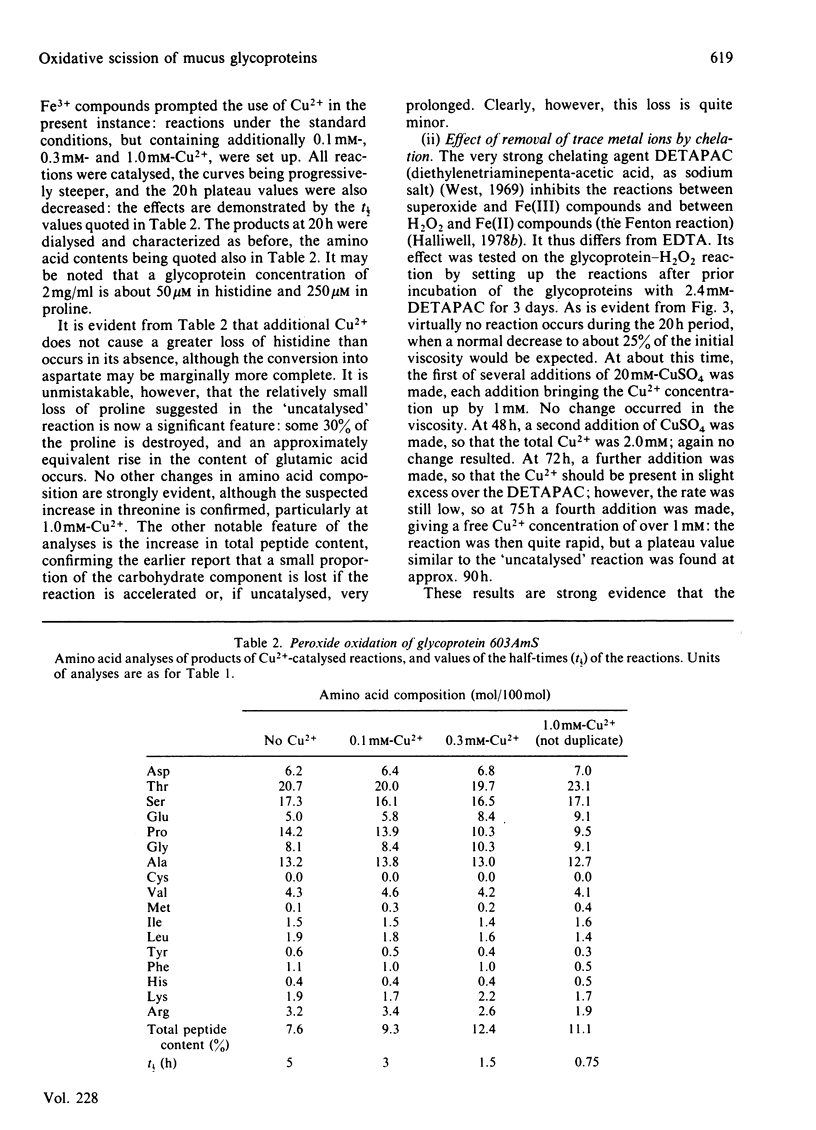
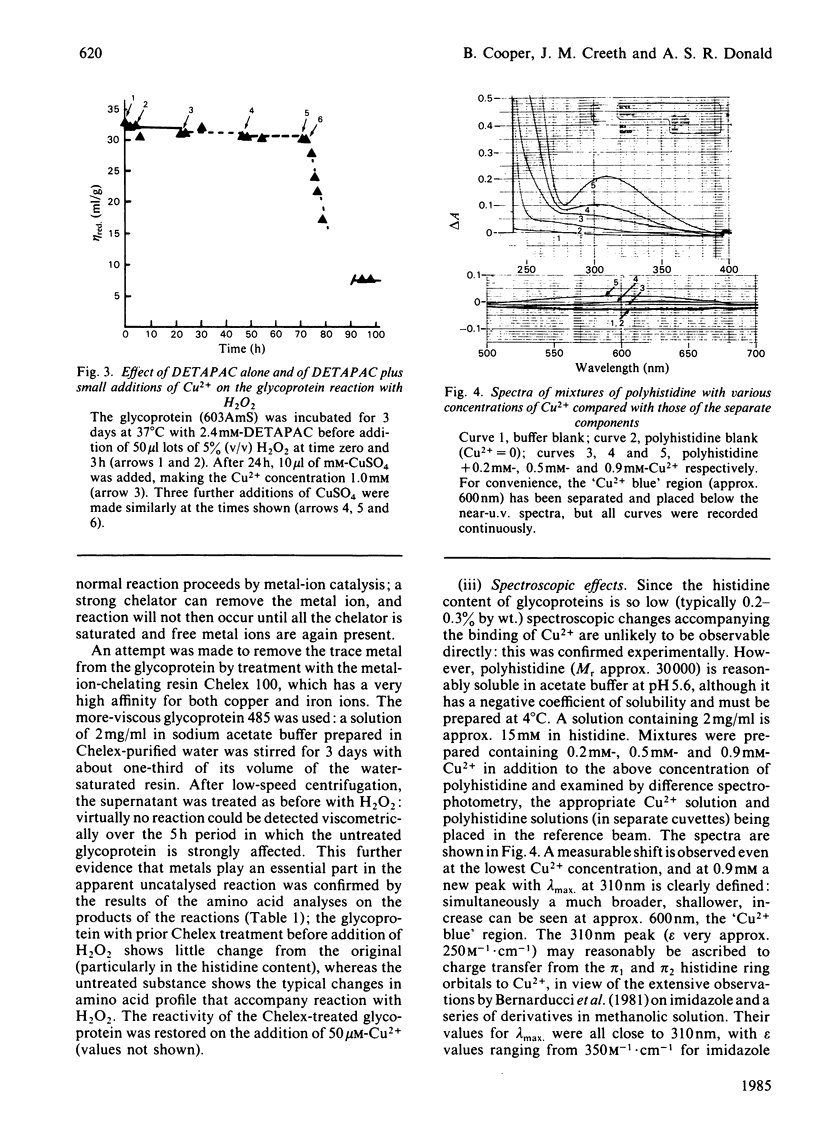
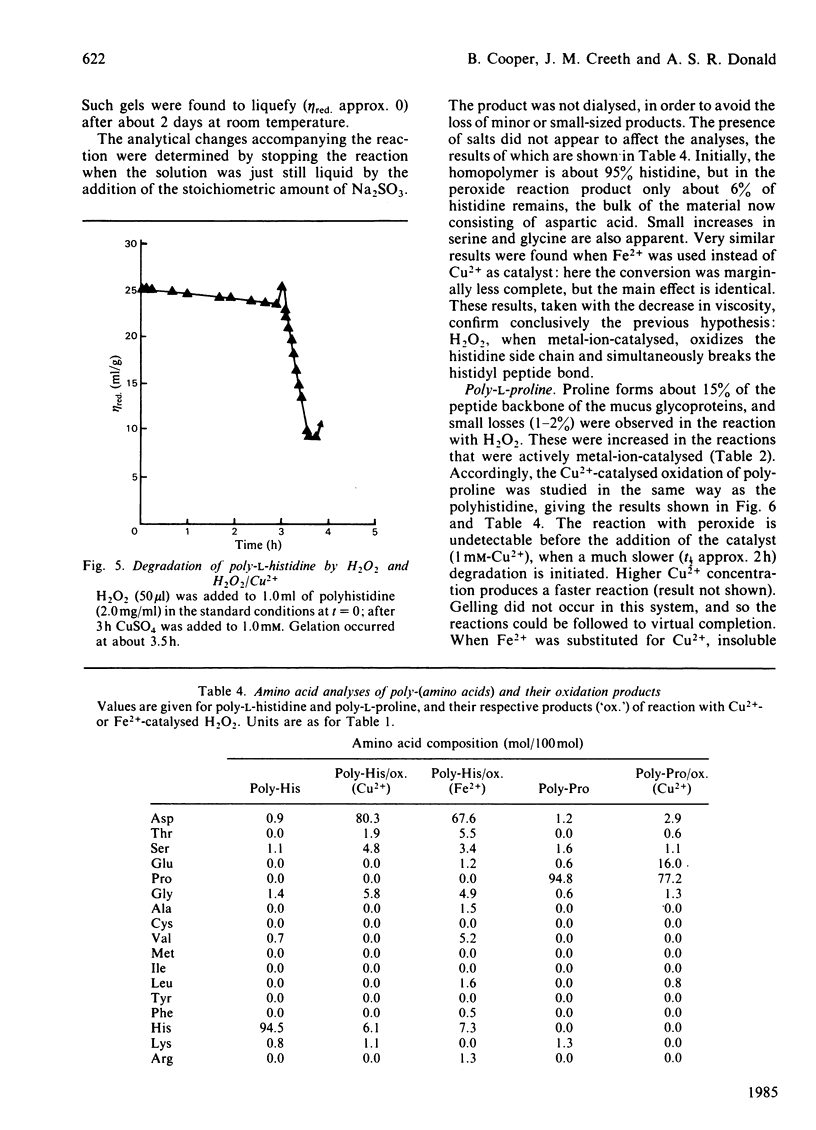
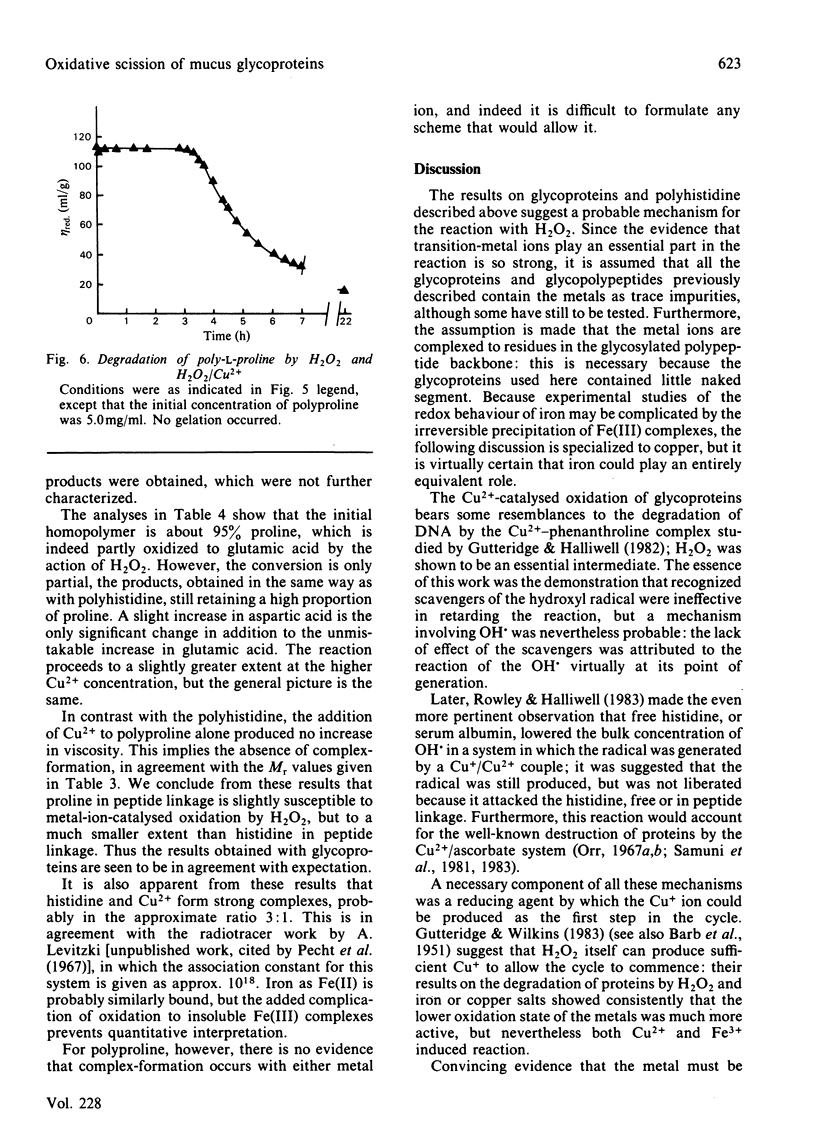
The reaction between ovarian-cyst glycoproteins and H2O2 was investigated in the presence of a number of inhibitors and catalysts. Azide and 2H2O were separately found to have little effect, implying that singlet oxygen was not involved. Superoxide dismutase was destroyed by H2O2, but mannitol had no effect: thus generalized attack by OH., whether originating from HO2.- or more directly, is not indicated. The glycoproteins contained trace quantities of Cu and Fe, amounting to about 2 atoms of metal per glycoprotein molecule. Treatment of the glycoproteins with the strong chelator DETAPAC (diethylenetriaminepenta-acetic acid) or Chelex resin eliminated the reaction with H2O2; activity could be restored by addition of Cu2+ or Fe2+ in millimolar quantities. It was concluded that metal-ion catalysis is an essential step in the attack of H2O2 on glycoproteins. Spectroscopic and other evidence showed that Cu2+ (and probably Fe2+) complexes strongly with poly-L-histidine, and implies that the Cu2+ or Fe2+ in the glycoproteins is complexed with some of the histidine residues in the glycosylated backbone. Neither polyhistidine nor polyproline reacted with H2O2 in the absence of metal ions, but small quantities of Cu2+ or Fe3+ caused degradation. This was rapid with polyhistidine, which was converted largely into aspartic acid, but slower with polyproline, where limited conversion into glutamic acid occurs. These findings confirm the original hypothesis that peroxide attack on glycoproteins occurs largely at the histidine residues, with simultaneous peptidolysis. The mechanism most probably involves the liberation of OH. by an oxidation-reduction cycle involving, e.g. Cu+/Cu2+: specificity of attack at histidine is due to the location of the metal at these residues only.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A. Quantitative studies of the avidity of naturally occurring substances for trace metals; amino-acids having only two ionizing groups. Biochem J. 1950 Nov-Dec;47(5):531–538. doi: 10.1042/bj0470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almassy R., Zil J. S., Lum L. G., Ifft J. B. Buoyant and potentiometric titrations of synthetic polypeptides. I. Six ionizable homopolypeptides in CsCl solutions. Biopolymers. 1973 Dec;12(12):2713–2729. doi: 10.1002/bip.1973.360121207. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., Donald A. S., Morgan W. T., Creeth J. C. The macromolecular properties of blood-group-specific glycoproteins. Characterization of a series of fractions obtained by solvent fractionation. Biochem J. 1974 Oct;143(1):159–170. doi: 10.1042/bj1430159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Cockle S. A., Fielden E. M., Roberts P. B., Rotilio G., Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J. 1974 Apr;139(1):43–48. doi: 10.1042/bj1390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Cheng L., Kellogg E. W., 3rd, Packer L. Photoinactivation of catalase. Photochem Photobiol. 1981 Jul;34(1):125–129. [PubMed] [Google Scholar]
- Clamp J. R., Creeth J. M. Some non-mucin components of mucus and their possible biological roles. Ciba Found Symp. 1984;109:121–136. doi: 10.1002/9780470720905.ch9. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Cooper B., Donald A. S., Clamp J. R. Studies of the limited degradation of mucus glycoproteins. The effect of dilute hydrogen peroxide. Biochem J. 1983 May 1;211(2):323–332. doi: 10.1042/bj2110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Allen A. Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet. 1984 Jun 16;1(8390):1328–1330. doi: 10.1016/s0140-6736(84)91822-1. [DOI] [PubMed] [Google Scholar]
- DOBBIE H., KERMACK W. O., LEES H. Complex-formation between polypeptides and metals. I. Application of various experimental methods to the glycine-copper system. Biochem J. 1955 Feb;59(2):240–246. doi: 10.1042/bj0590240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The role of the superoxide and hydroxyl radicals in the degradation of DNA and deoxyribose induced by a copper-phenanthroline complex. Biochem Pharmacol. 1982 Sep 1;31(17):2801–2805. doi: 10.1016/0006-2952(82)90136-8. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978 Dec 15;96(2):238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The superoxide dismutase activity of iron complexes. FEBS Lett. 1975 Aug 1;56(1):34–38. doi: 10.1016/0014-5793(75)80105-0. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Creeth J. M. Studies of the denaturation and partial renaturation of ovalbumin. Biochem J. 1972 Sep;129(3):665–676. doi: 10.1042/bj1290665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifft J. B. The buoyant titration of native and carbamylated ovalbumin. C R Trav Lab Carlsberg. 1971;38(16):315–338. [PubMed] [Google Scholar]
- Ito T. Cellular and subcellular mechanisms of photodynamic action: the 1O2 hypothesis as a driving force in recent research. Photochem Photobiol. 1978 Oct-Nov;28(4-5):493–508. doi: 10.1111/j.1751-1097.1978.tb06957.x. [DOI] [PubMed] [Google Scholar]
- Kong S., Davison A. J. The role of interactions between O2, H2O2, .OH,e- and O2- in free radical damage to biological systems. Arch Biochem Biophys. 1980 Oct 1;204(1):18–29. doi: 10.1016/0003-9861(80)90003-x. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Anbar M., Berger A. Specific oxidation of peptides via their copper complexes. Biochemistry. 1967 Dec;6(12):3757–3765. doi: 10.1021/bi00864a020. [DOI] [PubMed] [Google Scholar]
- Matheson I. B., Etheridge R. D., Kratowich N. R., Lee J. The quenching of singlet oxygen by amino acids and proteins. Photochem Photobiol. 1975 Mar;21(3):165–171. doi: 10.1111/j.1751-1097.1975.tb06647.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Orr C. W. Studies on ascorbic acid. I. Factors influencing the ascorbate-mediated inhibition of catalase. Biochemistry. 1967 Oct;6(10):2995–3000. doi: 10.1021/bi00862a004. [DOI] [PubMed] [Google Scholar]
- Orr C. W. Studies on ascorbic acid. II. Physical changes in catalase following incubation with ascorbate or ascorbate and copper (II). Biochemistry. 1967 Oct;6(10):3000–3006. doi: 10.1021/bi00862a005. [DOI] [PubMed] [Google Scholar]
- Pecht I., Levitzki A., Anbar M. The copper-poly-L-histidine complex. I. The environmental effect of the polyelectrolyte on the oxidase activity of copper ions. J Am Chem Soc. 1967 Mar 29;89(7):1587–1591. doi: 10.1021/ja00983a009. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals in the presence of copper salts: a physiologically significant reaction? Arch Biochem Biophys. 1983 Aug;225(1):279–284. doi: 10.1016/0003-9861(83)90031-0. [DOI] [PubMed] [Google Scholar]
- Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur J Biochem. 1983 Dec 1;137(1-2):119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Shinar E., Navok T., Chevion M. The analogous mechanisms of enzymatic inactivation induced by ascorbate and superoxide in the presence of copper. J Biol Chem. 1983 Dec 25;258(24):14778–14783. [PubMed] [Google Scholar]
- Singh A. Chemical and biochemical aspects of superoxide radicals and related species of activated oxygen. Can J Physiol Pharmacol. 1982 Nov;60(11):1330–1345. doi: 10.1139/y82-200. [DOI] [PubMed] [Google Scholar]
- Tomita M., Irie M., Ukita T. Sensitized photooxidation of histidine and its derivatives. Products and mechanism of the reaction. Biochemistry. 1969 Dec;8(12):5149–5160. doi: 10.1021/bi00840a069. [DOI] [PubMed] [Google Scholar]


