Abstract
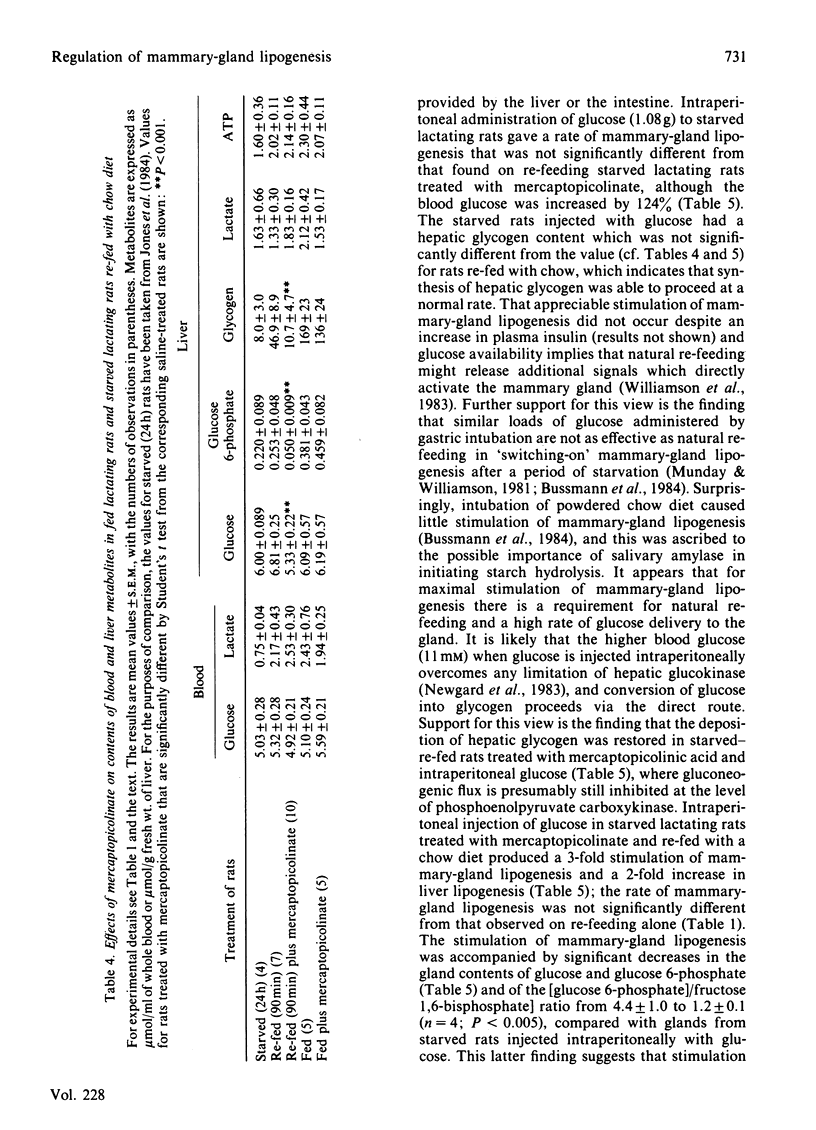
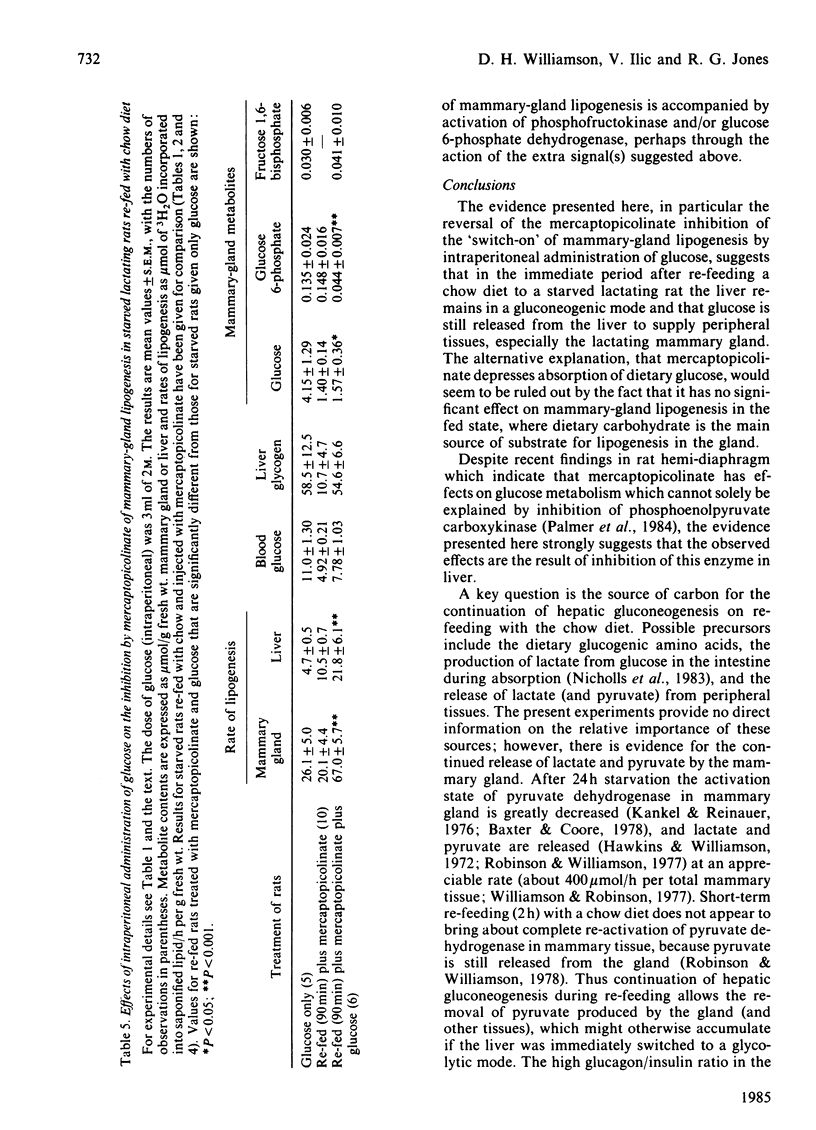
The rapid stimulation of lipogenesis in mammary gland that occurs on re-feeding starved lactating rats with a chow diet was decreased (60%) by injection of mercaptopicolinic acid, an inhibitor of hepatic gluconeogenesis at the phosphoenolpyruvate carboxykinase step. Mercaptopicolinate had no effect on lipogenesis in mammary glands of fed lactating rats. The inhibition of lipogenesis persisted in vitro when acini from mammary glands of re-fed rats treated with mercaptopicolinate were incubated with [1-14C]glucose. Mercaptopicolinate added in vitro had no significant effect on lipogenesis in acini from starved-re-fed lactating rats. Mercaptopicolinate prevented the deposition of glycogen and increased the rate of lipogenesis in livers of starved-re-fed lactating rats, whereas it had no significant effect on livers of fed lactating rats. Administration of intraperitoneal glucose restored the rate of mammary-gland lipogenesis in re-fed rats treated with mercaptopicolinate to the values for re-fed rats. Hepatic glycogen deposition was also restored, and the rate of hepatic lipogenesis was stimulated 5-fold. It is concluded that stimulation of mammary-gland lipogenesis on re-feeding with a chow diet after a period of starvation is in part dependent on continued hepatic gluconeogenesis during the absorptive period. Possible sources of the glucose precursors are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Baxter M. A., Coore H. G. The mode of regulation of pyruvate dehydrogenase of lactating rat mammary gland. Effects of starvation and insulin. Biochem J. 1978 Aug 15;174(2):553–561. doi: 10.1042/bj1740553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann L. E., Ward S., Kuhn N. J. Lactose and fatty acid synthesis in lactating-rat mammary gland. Effects of starvation, re-feeding, and administration of insulin, adrenaline, streptozotocin and 2-bromo-alpha-ergocryptine. Biochem J. 1984 Apr 1;219(1):173–180. doi: 10.1042/bj2190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick D. T., Kuhn N. J. Diurnal variation and response to food withdrawal of lactose synthesis in lactating rats. Biochem J. 1978 Jul 15;174(1):319–325. doi: 10.1042/bj1740319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTullio N. W., Berkoff C. E., Blank B., Kostos V., Stack E. J., Saunders H. L. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974 Mar;138(3):387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H. Measurements of substrate uptake by mammary gland of the rat. Biochem J. 1972 Oct;129(5):1171–1173. doi: 10.1042/bj1291171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. G., Ilic V., Williamson D. H. Regulation of lactating-rat mammary-gland lipogenesis by insulin and glucagon in vivo. The role and site of action of insulin in the transition to the starved state. Biochem J. 1984 Oct 15;223(2):345–351. doi: 10.1042/bj2230345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankel K. F., Reinauer H. Activity of pyruvate dehydrogenase complex in the mammary gland of normal and diabetic rats. Diabetologia. 1976 May;12(2):149–154. doi: 10.1007/BF00428981. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J., Grewe B. K. Inhibition of phosphoenolpyruvate carboxykinase, glyceroneogenesis and fatty acid synthesis in rat adipose tissue by quinolinate and 3-mercaptopicolinate. Biochim Biophys Acta. 1981 Jan 26;663(1):302–313. doi: 10.1016/0005-2760(81)90216-2. [DOI] [PubMed] [Google Scholar]
- Munday M. R., Williamson D. H. Role of pyruvate dehydrogenase and insulin in the regulation of lipogenesis in the lactating mammary gland of the rat during the starved-refed transition. Biochem J. 1981 Jun 15;196(3):831–837. doi: 10.1042/bj1960831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Nicholls T. J., Leese H. J., Bronk J. R. Transport and metabolism of glucose by rat small intestine. Biochem J. 1983 Apr 15;212(1):183–187. doi: 10.1042/bj2120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner C. B., Gilboe D. P., Nuttall F. Q. Metabolic effects of oral glucose in the liver of fasted rats. Am J Physiol. 1984 Jan;246(1 Pt 1):E89–E94. doi: 10.1152/ajpendo.1984.246.1.E89. [DOI] [PubMed] [Google Scholar]
- Palmer T. N., Caldecourt M. A., Warner J. P., Sugden M. C. The role of phosphoenolpyruvate carboxykinase in muscle alanine synthesis. Biochem J. 1984 Dec 15;224(3):971–976. doi: 10.1042/bj2240971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Control of glucose metabolism in isolated acini of the lactating mammary gland of the rat. The ability of glycerol to mimic some of the effects of insulin. Biochem J. 1977 Dec 15;168(3):465–474. doi: 10.1042/bj1680465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Control of lactating rat mammary-gland metabolism by insulin [proceedings]. Biochem Soc Trans. 1978;6(6):1316–1318. doi: 10.1042/bst0061316. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Palmer T. N., Myles D. D. Direction of carbon flux in starvation and after refeeding: in vitro and in vivo effects of 3-mercaptopicolinate. Biochem Int. 1983 Sep;7(3):329–337. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis in the rat, and the effects of litter size and malnutrition. Biochem J. 1979 Aug 15;182(2):287–294. doi: 10.1042/bj1820287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., McKeown S. R., Ilic V. Interactions of glucose, acetoacetate and insulin in mammary-gland slices of lactating rats. Biochem J. 1975 Aug;150(2):145–152. doi: 10.1042/bj1500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Munday M. R., Jones R. G., Roberts A. F., Ramsey A. J. Short-term dietary regulation of lipogenesis in the lactating mammary gland of the rat. Adv Enzyme Regul. 1983;21:135–145. doi: 10.1016/0065-2571(83)90012-2. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Robinson A. M. Control of glucose metabolism in lactating-rat mammary gland: effects of other substrates, insulin and starvation [proceedings]. Biochem Soc Trans. 1977;5(4):829–834. doi: 10.1042/bst0050829. [DOI] [PubMed] [Google Scholar]


