Abstract
Objective
To characterize the experience of people with epilepsy and aligned healthcare workers (HCWs) during the first 18 months of the COVID‐19 pandemic and compare experiences in high‐income countries (HICs) with non‐HICs.
Methods
Separate surveys for people with epilepsy and HCWs were distributed online in April 2020. Responses were collected to September 2021. Data were collected for COVID‐19 infections, the effect of COVID‐related restrictions, access to specialist help for epilepsy (people with epilepsy), and the impact of the pandemic on work productivity (HCWs). The frequency of responses for non‐HICs and HICs were compared using non‐parametric Chi‐square tests.
Results
Two thousand one hundred and five individuals with epilepsy from 53 countries and 392 HCWs from 26 countries provided data. The same proportion of people with epilepsy in non‐HICs and HICs reported COVID‐19 infection (7%). Those in HICs were more likely to report that COVID‐19 measures had affected their health (32% vs. 23%; p < 0.001). There was no difference between non‐HICs and HICs in the proportion who reported difficulty in obtaining help for epilepsy. HCWs in non‐HICs were more likely to report COVID‐19 infection than those in HICs (18% vs 6%; p = 0.001) and that their clinical work had been affected by concerns about contracting COVID‐19, lack of personal protective equipment, and the impact of the pandemic on mental health (all p < 0.001). Compared to pre‐pandemic practices, there was a significant shift to remote consultations in both non‐HICs and HICs (p < 0.001).
Significance
While the frequency of COVID‐19 infection was relatively low in these data from early in the pandemic, our findings suggest broader health consequences and an increased psychosocial burden, particularly among HCWs in non‐HICs. Planning for future pandemics should prioritize mental healthcare alongside ensuring access to essential epilepsy services and expanding and enhancing access to remote consultations.
Plain Language Summary
We asked people with epilepsy about the effects of COVID‐19 on their health and healthcare. We wanted to compare responses from people in high‐income countries and other countries. We found that people in high‐income countries and other countries had similar levels of difficulty in getting help for their epilepsy. People in high‐income countries were more likely to say that their general health had been affected. Healthcare workers in non‐high‐income settings were more likely to have contracted COVID‐19 and have the care they deliver affected by the pandemic. Across all settings, COVID‐19 associated with a large shift to remote consultations.
Keywords: access to healthcare, mental health, pandemic response, seizure, telemedicine
Key points.
The frequency of COVID‐19 infection in people with epilepsy during the acute phase of the pandemic was similar in high‐income countries (HICs) and non‐HICs.
People with epilepsy in HICs were more likely to report that COVID‐19 measures affected their general health.
There was no difference in the proportion of people with epilepsy in non‐HICs and HICs reporting difficulty in accessing treatment.
During the pandemic, there was a general pattern of declining mental health and reduced access to specialist epilepsy care.
A shift to telemedicine consultations appears to have been widely and rapidly adopted in both HICs and non‐HICs.
1. INTRODUCTION
The care of people with epilepsy changed extensively during the COVID‐19 pandemic. 1 , 2 , 3 In the early phase of the outbreak, many neurologists and epilepsy nurses were reassigned to intensive care units and acute medical services. 4 Routine electroencephalogram (EEG) recording, face‐to‐face outpatient clinics, and video‐EEG telemetry monitoring were suspended. 5 Elective surgical procedures, such as intracranial EEG investigations and neuromodulation, were canceled. 5 , 6 Access to antiseizure medications (ASMs) was also disrupted. 7 , 8 Vulnerable populations, including older people, those from ethnic minorities, and people from lower socioeconomic backgrounds, were disproportionately affected by these changes. 9 , 10 , 11 The disruptions caused by COVID‐19 may have had greater impact on people in lower‐income countries than those in more economically developed countries.
Following the rapid development and uptake of effective vaccines for severe acute respiratory syndrome (SARS)‐CoV‐2, beginning in late 2020, coupled with increasing natural immunity, the COVID‐19 crisis has largely subsided. Attention is now being re‐directed towards improving care for people with chronic health conditions in the event of future pandemics. While numerous studies have explored the experiences of people with epilepsy and aligned healthcare workers (HCWs) during the COVID‐19 pandemic in individual countries, 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 there have been few multinational studies in which experiences in high‐income countries (HICs) and lower‐income countries have been directly compared. 23 , 24 , 25 , 26
In the initial phase of the global pandemic, the COVID‐19 and Epilepsy (COV‐E) study group developed and distributed online surveys designed to explore the impact of the virus and the public health measures introduced in response to the crisis on epilepsy‐related factors, general health, and access to treatment and on the personal health and working practices of the clinicians responsible for their care. Questionnaires were deployed on a global scale across countries of varying wealth. Data for the United Kingdom, 27 , 28 United States, 29 and Brazil 30 were previously reported. Here, we present a post‐hoc analysis of the complete data set, with a focus on comparing data for HICs with those for non‐HICs. Given the existing inequalities in epilepsy care globally, it was anticipated that this might reveal similar disparities in the impact of the pandemic between those in non‐HICs and HICs. We hypothesized that the indirect consequences of the pandemic on service reorganization may be as relevant to understanding the overall impact of the pandemic on individual well‐being as the direct effects of COVID‐19 on general and epilepsy‐related health. Combining the findings from the current analysis with those from similar surveys, we explore what changes might be necessary to return services to a more satisfactory level in the “post‐COVID‐19” era and provide some suggestions to help mitigate the risks to people with epilepsy in future pandemics.
2. METHODS
2.1. Study design
Separate surveys were developed for people with epilepsy and HCWs with the involvement of several leading epilepsy organizations and charities, led by the UK charity SUDEP Action and the University of Oxford. Volunteers, including clinicians and people with epilepsy, piloted and iteratively improved the surveys. Once this group agreed on the questionnaires' content and format, they were made available online in April 2020 via the Jisc online survey platform for academic research (https://www.onlinesurveys.ac.uk).
Initial surveys were in English and then translated into 11 other languages. The surveys were piloted by a small test group convened across various global centers. Local language versions were translated and then verified by native speakers. For the comparative analysis, countries were divided into non‐HICs and HICs based on World Bank classifications, which are derived from estimations of per capita gross national income using the World Bank Atlas method. 31 The group of non‐HICs included all countries classified by the World Bank as low‐income, lower‐middle‐income, or upper‐middle‐income 32 (see Tables 1 and 2).
TABLE 1.
Global distribution of responses (people with epilepsy).
| High‐income | Upper‐middle‐income | Lower‐middle‐income | Low‐income | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | N | % HIC | % total | Country | N | % UMIC | % total | Country | N | % LMIC | % total | Country | N | % LIC | % total |
| United States | 574 | 34.4 | 27.3 | Brazil | 200 | 72.5 | 9.5 | India | 132 | 84.6 | 6.3 | Uganda | 2 | 50.0 | 0.1 |
| United Kingdom | 436 | 26.1 | 20.7 | China | 33 | 12.0 | 1.6 | Kenya | 11 | 7.1 | 0.5 | Madagascar | 1 | 25.0 | <0.1 |
| Australia | 308 | 18.5 | 14.6 | South Africa | 26 | 9.4 | 1.2 | Nigeria | 4 | 2.6 | 0.2 | Malawi | 1 | 25.0 | <0.1 |
| France | 100 | 6.0 | 4.8 | Argentina | 3 | 1.1 | 0.1 | Algeria | 2 | 1.3 | 0.1 | Total | 4 | 100 | 0.2 |
| Canada | 63 | 3.8 | 3.0 | Paraguay | 3 | 1.1 | 0.1 | Cambodia | 1 | 0.6 | <0.1 | ||||
| Ireland | 48 | 2.9 | 2.3 | Venezuela | 3 | 1.1 | 0.1 | Egypt | 1 | 0.6 | <0.1 | ||||
| Germany | 35 | 2.1 | 1.7 | Mexico | 2 | 0.7 | 0.1 | Morocco | 1 | 0.6 | <0.1 | ||||
| Belgium | 29 | 1.7 | 1.4 | Bosnia and Herzegovina | 1 | 0.3 | <0.1 | Nepal | 1 | 0.6 | <0.1 | ||||
| Italy | 29 | 1.7 | 1.4 | Colombia | 1 | 0.3 | <0.1 | Philippines | 1 | 0.6 | <0.1 | ||||
| Switzerland | 9 | 0.5 | 0.4 | Indonesia | 1 | 0.3 | <0.1 | Tunisia | 1 | 0.6 | <0.1 | ||||
| New Zealand | 6 | 0.4 | 0.3 | Libya | 1 | 0.3 | <0.1 | Zimbabwe | 1 | 0.6 | <0.1 | ||||
| Netherlands | 5 | 0.3 | 0.2 | Malaysia | 1 | 0.3 | <0.1 | Total | 156 | 100 | 7.4 | ||||
| Sweden | 3 | 0.2 | 0.1 | Türkiye | 1 | 0.3 | <0.1 | ||||||||
| Austria | 2 | 0.1 | 0.1 | Total | 276 | 100 | 13.1 | ||||||||
| Czech Republic | 2 | 0.1 | 0.1 | ||||||||||||
| Denmark | 2 | 0.1 | 0.1 | ||||||||||||
| Finland | 2 | 0.1 | 0.1 | ||||||||||||
| Greece | 2 | 0.1 | 0.1 | ||||||||||||
| Norway | 2 | 0.1 | 0.1 | ||||||||||||
| Qatar | 2 | 0.1 | 0.1 | ||||||||||||
| Singapore | 2 | 0.1 | 0.1 | ||||||||||||
| Slovenia | 2 | 0.1 | 0.1 | ||||||||||||
| United Arab Emirates | 2 | 0.1 | 0.1 | ||||||||||||
| Latvia | 1 | <0.1 | <0.1 | ||||||||||||
| Portugal | 1 | <0.1 | <0.1 | ||||||||||||
| Spain | 1 | <0.1 | <0.1 | ||||||||||||
| Total | 1668 | 100 | 79.2 | ||||||||||||
Note: Classification by income based on World Bank data. 33 One respondent did not provide country data.
Abbreviations: HIC, high‐income countries; LIC, low‐income countries; LMIC, lower‐middle‐income countries; UMIC, upper‐middle‐income countries.
TABLE 2.
Global distribution of responses (healthcare workers).
| High‐income | Upper‐middle‐income | Lower‐middle‐income | Low‐income | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | N | % HIC | % total | Country | N | % UMIC | % total | Country | N | % LMIC | % total | Country | N | % LIC | % total |
| United Kingdom | 79 | 60.3 | 20.2 | Brazil | 235 | 96.3 | 60.0 | Kenya | 9 | 56.3 | 2.3 | Liberia | 1 | 100.0 | 0.3 |
| Ireland | 19 | 14.5 | 4.8 | Colombia | 2 | 0.8 | 0.5 | Nigeria | 4 | 25 | 1.0 | Total | 1 | 100.0 | 0.3 |
| United States | 11 | 8.4 | 2.8 | Malaysia | 2 | 0.8 | 0.5 | Bangladesh | 1 | 6.3 | 0.3 | ||||
| Australia | 10 | 7.6 | 2.6 | China | 1 | 0.4 | 0.3 | Honduras | 1 | 6.3 | 0.3 | ||||
| Germany | 5 | 3.8 | 1.3 | Mexico | 1 | 0.4 | 0.3 | Tunisia | 1 | 6.3 | 0.3 | ||||
| Belgium | 1 | 0.8 | 0.3 | North Macedonia | 1 | 0.4 | 0.3 | Total | 16 | 100.0 | 4.1 | ||||
| Estonia | 1 | 0.8 | 0.3 | South Africa | 1 | 0.4 | 0.3 | ||||||||
| Oman | 1 | 0.8 | 0.3 | Türkiye | 1 | 0.4 | 0.3 | ||||||||
| Poland | 1 | 0.8 | 0.3 | Total | 244 | 100.0 | 62.2 | ||||||||
| Portugal | 1 | 0.8 | 0.3 | ||||||||||||
| Singapore | 1 | 0.8 | 0.3 | ||||||||||||
| Spain | 1 | 0.8 | 0.3 | ||||||||||||
| Total | 131 | 100.0 | 33.4 | ||||||||||||
Note: Classification by income based on World Bank data. 33
Abbreviations: HIC, high‐income countries; LIC, low‐income countries; LMIC, lower‐middle‐income countries; UMIC, upper‐middle‐income countries.
Participants were required to be over the age of 18 years and be persons with epilepsy or HCWs involved in epilepsy care. The surveys were primarily designed to gather quantitative data, with the option to provide qualitative data through free‐text responses to some questions.
The study was approved by the University of Oxford Ethics Committee (Reference: R69353/RE001).
2.2. Measurements
2.2.1. Survey of people with epilepsy
2.2.1.1. Demographic data
Demographic data included age, gender, minority ethnic status, country of residence, and postal area.
2.2.1.2. Epilepsy and health background
Respondents were asked to provide information on their epilepsy type, seizure type(s) and frequency, presence of nocturnal seizures, antiseizure medications (ASMs), primary epilepsy care provider, number of specialist epilepsy consultations in the past year, unplanned/emergency hospital admissions due to epilepsy in the past year, epilepsy associated injuries, and comorbidities. Respondents were also asked if they had contracted COVID‐19 or self‐isolated due to possible exposure.
2.2.1.3. Risk factors for epilepsy morbidity and mortality
Respondents were asked about changes in behavior, habits, and circumstances during the pandemic that might have been associated with increased epilepsy risk. Specifically, people were asked about their mental health status, alcohol and drug consumption, sleeping patterns, and changes to seizures.
People were asked whether they lived alone or with someone who could provide first aid.
Additionally, people were asked about aspects relating to communication with their epilepsy clinician in the previous 12 months, specifically whether they had discussed the following: ASM side effects, rescue medication, alcohol, contraception, driving, life changes, employment, mental health, pregnancy (where relevant), recreational drugs, safety aids, first aid, sleep, stigma, and sudden unexpected death in epilepsy (SUDEP).
2.2.1.4. Access to healthcare
People were asked whether they had experienced difficulty in obtaining prescriptions for ASMs, changes to scheduled epilepsy appointments, and communication with clinicians. Where changes in epilepsy care were reported, respondents were asked whether they were satisfied with the changes. Free text was encouraged to contextualize responses.
2.2.2. Survey of healthcare workers
2.2.2.1. Demographic data
Healthcare workers were asked to provide information on their age, gender, clinical role, country, and postal area of their place of work.
2.2.2.2. Health and well‐being
Respondents were asked whether they had been infected with COVID‐19, whether they had to self‐isolate, and the extent to which the pandemic had affected their mental well‐being and their comorbidities. They were also asked about the degree to which concerns for themselves, family members, and colleagues, availability of personal protective equipment (PPE), testing, and social distancing had an impact on their productivity at work.
2.2.2.3. Delivery of services
HCWs were asked about changes to the provision of epilepsy care, including the proportion of clinical consultations that they conducted by telephone or video call before and during the pandemic. They were also asked about the availability of diagnostic tools and interventions, and the degree to which changes during the pandemic had affected their ability to diagnose and treat people with epilepsy.
Survey questions and response options are provided as supplementary material.
2.3. Dissemination
Survey dissemination was led by SUDEP Action. The surveys were shared on social media and promoted by multiple epilepsy support organizations, including, but not limited to: BAND Foundation, Citizens United for Research in Epilepsy (CURE), Epilepsy Action, Epilepsy Foundation America, Epilepsy Research UK, Epilepsy Society, Epilepsy Sparks, the International Bureau for Epilepsy (IBE), and the International League Against Epilepsy (ILAE).
2.4. Data analysis
Survey responses were plotted by country and month along with the global burden of COVID‐19 infections based on data from a public repository. 34 Descriptive statistical analyses and non‐parametric Chi‐square tests were performed to compare the differences in the observed frequency of survey responses between non‐HICs and HICs, as defined by the World Bank. 33 Statistical significance was set at 0.05. All visualizations and analyses were completed in Matlab R2018.
3. RESULTS
3.1. Survey of people with epilepsy
3.1.1. Population demographics
Responses were received from 2105 people with epilepsy in 53 countries spanning Africa, Asia, Australasia, Europe, North America, and South America. One thousand six hundred sixty‐eight responses were from HICs, predominantly from the United States (n = 574); United Kingdom (n = 436); Australia (n = 308); and France (n = 100). The highest numbers of non‐HIC responses were from Brazil (n = 200), India (n = 132), China (n = 33), and South Africa (n = 26). One person did not provide information on their country of residence and was therefore excluded from the comparative analyses. All countries from which at least one response was received are listed in Table 1.
In the combined dataset, the highest number of responses (27%) was in the 30–39 age group. Seventy‐four percent of all respondents were female (see supplementary material).
3.1.2. Exposure to risk during the COVID‐19 pandemic
3.1.2.1. Infection with COVID‐19
The frequency of reported infection with COVID‐19 was the same for non‐HICs and HICs, with only a minority of respondents (7% in each group) indicating that they had been infected during the early phase of the pandemic that our data captures (Figure 1A).
FIGURE 1.
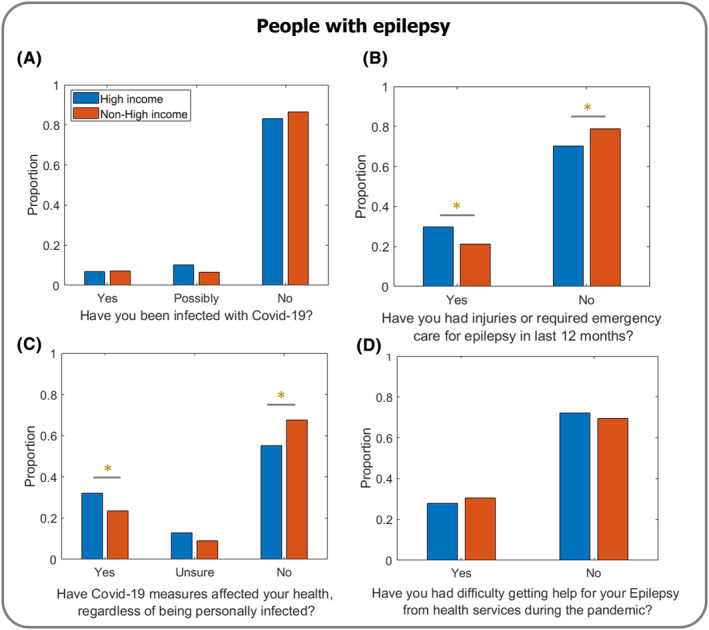
Impact of COVID‐19 on people with epilepsy in non‐HICs versus HICs. (A) Reports of COVID‐19 infection during the study period were comparable between non‐HICs and HICs (χ 2 = 0.11; p = 0.914; N = 1888). (B) Individuals with epilepsy in HICs reported a significantly higher need for emergency care within the last 12 months than those in non‐HICs (χ 2 = 12.56; p < 0.001*; N = 2102), potentially indicative of baseline attendance patterns and what would be considered a necessary indication to attend the Emergency Department. (C) People with epilepsy in HICs reported a greater effect on health, regardless of being personally infected with COVID‐19, than those in non‐HICs (χ 2 = 15.23; p < 0.001*; N = 2102). (D) No difference in accessing help was reported by people with epilepsy in non‐HICs and HICs (χ 2 = 1.28; p = 0.259; N = 2102).
There was no difference in the frequency of COVID‐19 infection between those who identified as belonging to an ethnic minority, and those who did not, in either the HIC group (p = 0.373; Figure 2A) or the non‐HIC group (p = 0.597; Figure 2B).
FIGURE 2.
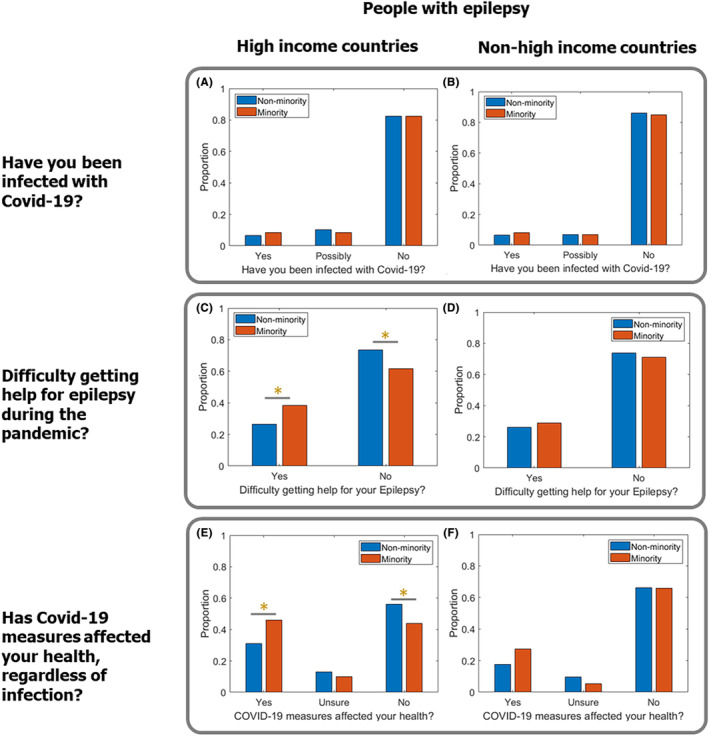
Impact of COVID‐19 on people with epilepsy from minority ethnic groups. (A and B) Reports of COVID‐19 infection were comparable in minority ethnic groups and non‐minority ethnic groups in (A) HICs (p = 0.373) and (B) non‐HICs (p = 0.597). (C) Individuals from minority ethnic groups in HICs had greater difficulty accessing help for epilepsy than those from non‐minority ethnic groups (p = 0.003). (D) There was no difference between minority ethnic groups and non‐minority ethnic groups in the ability to access help for epilepsy in non‐HICs (p = 0.625). (E) Individuals from minority ethnic groups in HICs were more likely to report that COVID‐19 measures affected their health than those from non‐minority ethnic groups (p < 0.001). (F) There was no difference between minority ethnic groups and non‐minority ethnic groups who reported being affected by COVID‐19 measures in non‐HICs (p = 0.655). * indicates p < 0.005.
3.1.2.2. Emergency care
A higher proportion of respondents in HICs than non‐HICs indicated that they had incurred injury or required emergency care for their epilepsy within the previous 12 months (p < 0.001; Figure 1B).
3.1.2.3. General health and well‐being
Respondents in HICs were more likely to report that their health had been affected by measures implemented in response to the COVID‐19 pandemic, regardless of whether they had personally contracted COVID‐19 (p < 0.001; Figure 1C). Respondents in HICs who identified as belonging to a minority ethnic group were also more likely to report that their health had been affected compared to non‐minority ethnic groups in HICs (p < 0.001; Figure 2E). In non‐HICs, though, there was no difference in the proportion of respondents from minority ethnic groups and non‐minority ethnic groups who reported that COVID‐19 measures had affected their health (p = 0.655; Figure 2F).
There were no statistically significant differences between non‐HICs and HICs in the proportions reporting changes in specific health outcomes related to increased mental health problems (non‐HICs 5%; HICs 25%), disrupted sleep patterns (non‐HICs 3%; HICs 20%), change in seizures (non‐HICs 2%; HICs 10%), increased alcohol consumption (non‐HICs 0%; HICs 3%), or increased recreational drug use (non‐HICs 0%; HICs 1%).
3.1.2.4. First aid
Most people with epilepsy in non‐HICs (64%) and HICs (71%) were living with at least one other person during the isolation/lockdown period, proximate to when they completed the survey. A similar proportion of respondents (64% in non‐HICs and 72% in HICs) indicated that they were living with someone who was aware of their epilepsy and who could provide first aid. Overall, people in HICs were more likely to respond affirmatively to questions on these topics than people in non‐HICs (p ≤ 0.001 and p ≤ 0.01, respectively).
3.1.2.5. Access to healthcare for epilepsy
There was no difference in the proportion of respondents in non‐HICs (30%) and HICs (28%) who reported difficulty accessing healthcare services for their epilepsy during the pandemic (p = 0.259; Figure 1D).
Respondents in HICs who identified as belonging to a minority ethnic group were more likely to report difficulty in accessing epilepsy care than those from non‐minority groups (p = 0.003; Figure 2C). There was no difference in accessing epilepsy care between minority and non‐minority groups in non‐HICs (p = 0.625; Figure 2D).
3.2. Survey of HCWs
3.2.1. Population demographics
Surveys were completed by 392 HCWs in 26 countries. Ninety percent of the responses were from non‐HICs, and 60% were from Brazil. HCWs in the UK provided 60% of the responses received from HICs and 20% of the total responses for all HCWs. All countries from which at least 1 response was received are listed in Table 2.
HCWs in non‐HICs tended to be younger than those in HICs; 49% in non‐HICs were under 40 compared with 18% under 40 in HICs. Fifty‐eight percent of respondents were female (see supplementary material).
3.2.2. Exposure to risk during the COVID‐19 pandemic
3.2.2.1. Infection with COVID
Most HCWs (total 74%: non‐HICs 69%; HICs 83%) had not been infected with COVID‐19 when they completed the survey. Only 14% of all respondents reported infection with COVID‐19, with a significantly higher percentage in non‐HICs (18%) than in HICs (6%; p = 0.001). Approximately 12% of HCWs said they had “possibly” been infected (non‐HICs 13%; HICs 10% Figure 3A).
FIGURE 3.
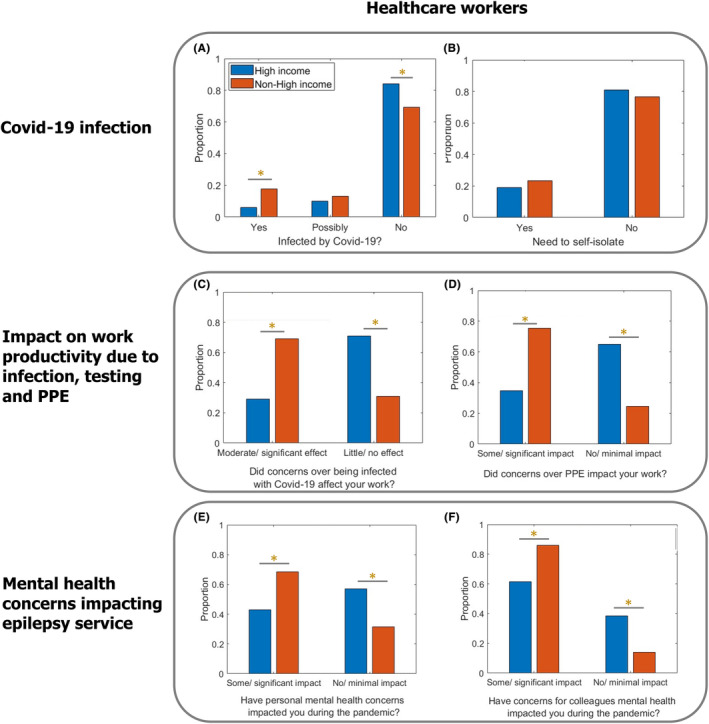
Direct impact of COVID‐19 on HCWs in non‐HICs versus HICs. (A) The frequency of infection with COVID‐19 was significantly higher in HCWs from non‐HICs than HICs (χ 2 = 10.69; p = 0.001; N = 345) despite (B) no significant difference in reported self‐isolation (χ 2 = 0.936; p = 0.333; N = 392). Concern over COVID‐19 infection (C) and concern over availability of COVID‐19 testing services or personal protective equipment (D) led to significantly greater impact on reported work productivity in non‐HICs than HICs (χ 2 = 56.41; p < 0.001*; N = 392 and χ 2 = 35.11; p < 0.001*; N = 392, respectively). Concern regarding personal mental health (E) and the mental health of colleagues (F) led to a greater impact on reported work productivity in non‐HICs compared to HICs (χ 2 = 22.88, p < 0.001*; N = 392 and χ 2 = 30.25, p < 0.001; N = 392, respectively).
3.2.2.2. Need to self‐isolate
Most HCWs (total 77%; non‐HICs 76%; HICs 79%) had not needed to self‐isolate or take time off work owing to a household member showing symptoms of COVID‐19. Overall, 22% indicated that they had needed to self‐isolate (non‐HICs 23%; HICs 19%). There was no difference between non‐HICs and HICs (Figure 3B), despite a significantly higher percentage of HCWs in non‐HICs reporting infection.
3.2.3. Effect on work
3.2.3.1. Concern over infection with COVID‐19
A significantly higher proportion of HCWs in non‐HICs reported that concerns about developing COVID‐19 had at least a moderate effect on their productivity at work (non‐HICs 69%; HICs 29%; p < 0.001; Figure 3C).
3.2.3.2. Concern over the availability of personal protective equipment
A significantly higher proportion of HCWs in non‐HICs indicated that the availability of PPE had “some” or “significant” impact on their productivity at work (non‐HICs 69%; HICs 34%; p < 0.001; Figure 3D).
3.2.4. Mental health
3.2.4.1. Impact of personal mental health and mental health of colleagues
HCWs in non‐HICs were more likely to report that personal mental health concerns during the pandemic had “some” or “significant” impact on them than HCWs in HICs (non‐HICs 68%; HICs 43%; p < 0.001; Figure 3E). Similarly, the proportion of HCWs reporting that concern for the mental health of colleagues had “some” or “significant” impact was higher in non‐HICs (non‐HICs 85%; HICs 61%; p < 0.001; Figure 3F).
3.2.5. Changes in method of communication
During the pandemic, there was a trend in both HICs and non‐HICs towards more clinical consultations by telephone or video conferencing (Figure 4).
FIGURE 4.
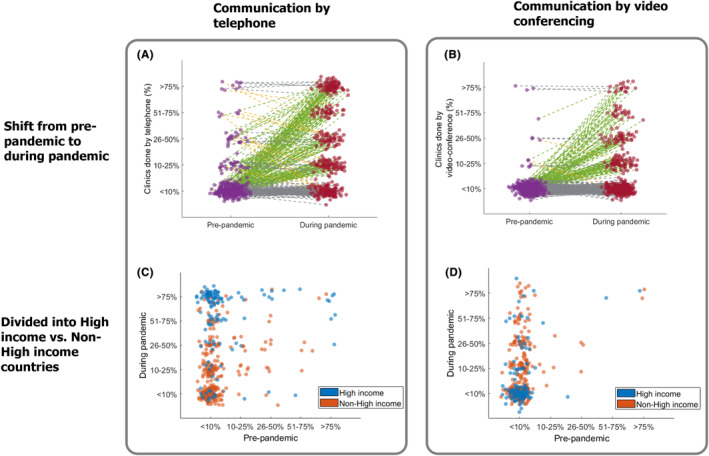
Impact of COVID‐19 on epilepsy clinic communication from pre‐ to during pandemic and visualized for non‐HICs versus HICs. Visualizing the increased frequency of telephone clinics (A) and video conferencing clinics (B) from pre‐pandemic (purple) to during the pandemic (red) for all HCWs, where each dot is a survey respondent (green lines indicate increased use, gray lines indicate same frequency of use, and yellow lines indicate less use). The majority of HCWs reported less than 10% of clinics were conducted by telephone or video‐conferencing pre‐pandemic compared with markedly higher usage during the pandemic. The shift in communication by telephone and video‐conferencing is displayed in (C and D), respectively, for non‐HICs (orange) and HICs (purple) by plotting pre‐pandemic use on the x‐axis and during pandemic use on the y‐axis. Respondents from HICs reported a proportionately greater shift to telephone clinics than those in non‐HICs whereas those from non‐HICs were more likely to utilize video‐conferencing.
Before the pandemic, 2% of HCWs in non‐HICs indicated that at least 50% of consultations were conducted by telephone, compared with 13% during the pandemic (p < 0.001). A greater shift was observed in HICs, where the percentage of HCWs who reported conducting at least 50% of clinical consultations by telephone increased from 7% before the pandemic to 70% during the pandemic (p < 0.001; Figure 4A).
In non‐HICs, 1% of HCWs indicated that at least 50% of clinical consultations were conducted by video call before the pandemic, compared with 11% during the pandemic (p < 0.001). A similar percentage increase was observed for HCWs in HICs, among whom those who reported conducting at least 50% of consultations by video call increased from 2% before the pandemic to 10% during the pandemic (p < 0.001; Figure 4B).
4. DISCUSSION
The current work represents one of the most extensive assessments of the effects of the COVID‐19 pandemic on people with epilepsy and aligned HCWs. It is one of very few analyses to compare data from HICs and non‐HICs. Our findings highlight the challenges to maintaining specialist epilepsy care during the COVID‐19 pandemic and build upon previously reported individual country data from the US, UK, and Brazil 27 , 28 , 29 , 30 and that from other studies. 23 , 24 , 25 , 26 We identified a pattern of declining mental health; increased stress, anxiety, and depression; reduced access to specialist medical care; and interruption to more basic care provision such as dependable access to ASMs. We also present further evidence for the far‐reaching consequences of the pandemic on people with epilepsy beyond being directly infected with COVID‐19.
In considering these impacts in the sections that follow, we make a number of recommendations for changes in healthcare provision for people with epilepsy. These align broadly with the actions proposed in the World Health Organization Intersectional global action plan on epilepsy and other neurological disorders (IGAP), which includes provision for better access to epilepsy services; closure of the treatment gap within countries; and better parity between high‐income and lower‐income countries. 35
4.1. Effect on epilepsy, general health, and psychological well‐being
Our results suggest that people with epilepsy in HICs were more likely to report that COVID‐19 measures had affected their health despite similar reports of changes in seizures, disrupted sleep, and mental health problems with those in non‐HICs. In tandem with other studies, we would suggest that overlapping factors around reduced access to healthcare, reorganization of community services, and general societal changes all contribute to these findings. 36
A systematic review and meta‐analysis of studies examining the psychological impact of COVID‐19 on people with epilepsy (28 studies with 7959 patients/caregivers) reported that 38.9% of individuals experienced anxiety, 30.9% depression or adverse effects on mood, and 36.5% sleep disturbance. 37 Those findings are very similar to HIC responses in the current study. Changes in each of these factors were lower in non‐HIC settings. It is unclear why more people in HICs reported poorer mood and sleep outcomes. Appreciably, these were subjective responses relative to an individual's situation before the pandemic. It is possible, therefore, that there was an overall greater expectation at a group level in HICs and perhaps more resilience to mental and physical stresses in non‐HICs. It is, though, apparent that planning epilepsy care in future pandemics should prioritize mental health management for all people with epilepsy to improve holistic well‐being and reduce seizure risk.
Notably, respondents in HICs who identified as belonging to a minority ethnic group were more likely to report both an overall effect of COVID‐19 measures on their general health, and difficulty in getting help for their epilepsy during the pandemic (see Section 4.2). Whether these findings might reflect existing disparities in access to general and specialized epilepsy healthcare, 38 , 39 the greater impact of COVID‐19 on minority ethnic groups than other members of the population, 40 , 41 or both of these factors is unclear from the data collected. Our findings do, though, underline the importance of addressing healthcare inequalities within countries as well as between countries.
4.2. Access to epilepsy treatment
A surge in canceled outpatient appointments, epilepsy‐related investigations, and inpatient treatments was characteristic of the early stages of the pandemic. As a result, many people with epilepsy were unable to access necessary medical care. 42 Future pandemics may require a similar reallocation of resources to frontline healthcare, and it is essential to consider how best to maintain access to routine investigations such as EEG and MRI and elective surgical procedures such as intracranial EEG investigations, resective surgery, and neuromodulation.
In the first year of the pandemic, the ILAE published guidance for maintaining video‐EEG investigations based on the necessity and urgency of each case. 43 , 44 These remain valid and can be more generally applied to prioritize in‐person appointments and procedures according to those in greatest need. Equally important is maintaining the safety of clinicians and medical technicians, especially those reassigned to frontline care. Whether specialist epilepsy clinicians should be routinely redeployed to acute medical care is outside of the scope of this study; however, alternative measures may be more efficient. For example, instead of reassigning people from their base specialty, epilepsy clinicians may offer enhanced access through a rapid‐access outpatient clinic to reduce the burden on emergency services. Similarly, in future pandemics, dedicated diagnostic centers distal from hospitals providing acute care might offer a solution to the challenges of continuing to provide diagnostic consultations for people with new‐onset seizures.
Effective healthcare delivery will require providing clinicians with adequate PPE and mental health support to enable them to continue offering optimal treatment. Our study highlights that the effects of COVID‐19 infection, access to PPE, and mental health concerns were a particular worry for HCWs in non‐HICs. This socioeconomic inequity will require ongoing attention.
4.3. Telemedicine
One of the far‐reaching changes to healthcare has been the shift to remote consultations delivered via telephone or video call. Over the past 3 years, most clinicians and those receiving treatment for chronic health conditions have used an increased number of telemedicine consultations, which is expected to continue. There remain, though, barriers to access, including inequity in the availability of requisite technologies and reduced technological aptitude in particularly vulnerable groups. Additionally, clinicians cannot perform physical examinations and specific clinical tests such as EEG or MRI during remote consultations. In the current study, most HCWs expressed lower confidence in diagnosing epilepsy remotely, which may lead to sub‐optimal clinical care. Experiences with telemedicine during the pandemic have, however, shown that routine clinical care can be successfully delivered remotely, 45 , 46 and there are clear positives to telemedicine, including easier review of people who find it challenging to travel to care settings and the ability to review people who need to self‐isolate. In the post‐pandemic context, people who are COVID‐19 positive may not always have to cancel clinical appointments but might instead have the option of telephone or video. Similarly, if clinicians are COVID‐19 positive, but well enough to continue working, they may be able to utilize remote consultations.
Perhaps surprisingly, data from the current study showed a greater shift to telephone, rather than video, consultations in HICs. The exact reasons for this are uncertain but may have been associated with technical considerations or concerns over data protection. Individual or clinician preference, ease of access, and convenience in taking a phone call rather than waiting in a virtual waiting room may also contribute. It is also possible that video consultations were more widely used later in the pandemic, following the study period.
Previous surveys of people with epilepsy have reported greater acceptance of telemedicine in HICs than in lower‐income countries 14 , 18 , 19 , 21 , 47 where access might remain more restricted. 16 , 20 Telemedicine may, though, represent a valuable opportunity to improve the delivery of care in resource‐poor settings, especially regions where attending in person requires traveling long distances, provided the obstacles to uptake can be overcome. This is equally true for disadvantaged groups in HICs. 48 In part owing to the pandemic, smartphone usage is increasing in low and middle income countries, 49 which may lead to expanded access to healthcare and other resources for previously marginalized people. This may, in turn, facilitate a greater degree of self‐management through apps and other technologies.
5. LIMITATIONS
This study had several limitations. Survey data are susceptible to selection and recall bias, and it was impossible to verify that respondents met all the criteria for participation. Some questions may have been misinterpreted as there was no direct supervision when completing the survey. Future studies could try to incorporate standardized scales, for example, a score for depression or anxiety, when feasible.
Participation required internet access and familiarity with using computers, so individuals from resource‐limited settings and those with more severe epilepsy or intellectual impairment may not be adequately represented. This was reflected by the low proportion of responses from people with epilepsy in non‐HICs. Sixty‐three percent of responses from non‐HICs came from countries classified as upper‐middle‐income countries. Data were, therefore, overall skewed to people with epilepsy in more resource‐privileged settings. Despite broad parity in per capita GDP among countries in the same World Bank classifications, there may be important disparities in income levels and access to healthcare between countries in the same income group, between regions within the same country, and between individuals. It is difficult to determine, for example, whether respondents from Brazil and India, the countries that were best represented in their respective per capita GDP groups, were indicative of the general populations of those countries (data on personal income, for example, were not collected), or of other countries within the same World Bank groupings. Both Brazil and India are members of the BRICS (Brazil, Russia, India, China, South Africa) group of major developing nations, and India is one of the world's 10 fastest‐growing economies. 50 In the 2022 World Inequality Report, though, India was also ranked among the most unequal countries in terms of income and wealth distribution, 51 factors which, together with demographic and healthcare disparities, underpinned relative COVID‐19 risk during the first year of the pandemic in that country. 52 The relatively small number of respondents from non‐HICs reduced the statistical power of some of the analyses.
Countries experienced differing effects throughout the pandemic. Data were collected over an extended time window, but comparing responses received at different time points during the pandemic was not feasible. Responses received during a peak time of infection and periods of national lockdown in one country may not have been concurrent with similar experiences in other settings. The data are also relative; for example, respondents were asked to comment on changes to their situation before the emergence of COVID‐19, meaning that pre‐existing disparities will be reflected in our data.
6. CONCLUSIONS
We present the results of one of the largest surveys of people with epilepsy during the time of COVID‐19 and consider how the findings might influence epilepsy management in future pandemics. The frequency of COVID‐19 infection was broadly comparable for people with epilepsy in non‐HICs and HICs. However, those in HICs were more likely to report effects on general health and mental health from pandemic‐related restrictions. Access to healthcare remained similar in non‐HICs during the pandemic early stages, highlighting the resilience of HCWs in countries where limited access to PPE and greater impact on HCW mental health were reported. Positive findings include the rapid uptake of telemedicine, which may have lasting effects on open access to clinical consultations, particularly for those in resource‐limited settings and those from minority ethnic groups.
FUNDING INFORMATION
This study received no specific funding.
CONFLICT OF INTEREST STATEMENT
PP is supported by an Emerging Leadership Investigator Grant from the Australian National Health and Medical Research Council (APP2017651), The University of Melbourne, Monash University, the Weary Dunlop Medical Research Foundation, Brain Australia, and the Norman Beischer Medical Research Foundation. He has received speaker honoraria or consultancy fees to his institution from Chiesi, Eisai, LivaNova, Novartis, Sun Pharma, Supernus, and UCB Pharma, outside the submitted work. He is an Associate Editor for Epilepsia Open. JF has received research support from the Epilepsy Study Consortium, Epilepsy Foundation, GW/FACES, and NINDS. DMA is supported by EpLink, Dravet Syndrome Foundation, and McLaughlin grants. NJ received grant funding paid to her institution for grants unrelated to this work from NINDS (NIH U24NS107201, NIH IU54NS100064, 3R01CA202911‐05S1, R21NS122389, R01HL161847) during the study period. She receives an honorarium for her work as an Associate Editor of Epilepsia. JHC is supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at Great Ormond Street Hospital, NIHR, Engineering and Physical Sciences Research Council, GOSH Charity, Epilepsy Research UK, and the Waterloo Foundation. JWS is based at UCLH/ UCL Comprehensive Biomedical Research Centre, which receives a proportion of funding from the UK Department of Health's NIHR Biomedical Research Centres funding scheme. He receives support from the Dr Marvin Weil Epilepsy Research Fund, the Christelijke Vereniging voor de verpleging van Lijders aan Epilepsie, The Netherlands, and the UK Epilepsy Society. All other authors report no conflicts of interest. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Data S1.
Data S2.
Data S3.
ACKNOWLEDGMENTS
This study was funded by SUDEP Action Registered charity 1 164 250 (England & Wales) and supported by the Oxford NIHR Biomedical Research Centre. We are grateful to all the following organizations and many individuals whom we are unable to name individually, for promoting this work through online platforms. We also very much appreciate the valuable input of all the respondents who have completed the surveys. BAND Foundation (https://bandfdn.org). Dravet Syndrome UK (https://www.dravet.org.uk). Epilepsy Action (https://www.epilepsy.org.uk). Epilepsy Connections (https://www.epilepsyconnections.org.uk). Epilepsy Foundation America (https://www.epilepsy.com). Epilepsy Research UK (https://epilepsy‐institute.org.uk). Epilepsy Society (https://epilepsysociety.org.uk). Epilepsy Sparks (https://www.epilepsysparks.com). International Bureau for Epilepsy (IBE) (https://www.ibe‐epilepsy.org). International League Against Epilepsy (ILAE)—British Branch (https://ilaebritish.org.uk). Matthew's Friends (https://www.matthewsfriends.org). Neurological Alliance (https://www.neural.org.uk). SUDEP Action (https://sudep.org).
Vasey MJ, Tai XY, Thorpe J, Jones GD, Ashby S, Hallab A, et al. The impact of COVID‐19 on people with epilepsy: Global results from the coronavirus and epilepsy study. Epilepsia Open. 2024;9:1931–1947. 10.1002/epi4.13035
Michael J. Vasey and Xin You Tai—joint first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Kendzerska T, Zhu DT, Gershon AS, Edwards JD, Peixoto C, Robillard R, et al. The effects of the health system response to the COVID‐19 pandemic on chronic disease management: a narrative review. Risk Manag Healthc Policy. 2021;14:575–584. 10.2147/rmhp.S293471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adan GH, Mitchell JW, Marson T. Epilepsy care in the COVID‐19 era. Clin Med (Lond). 2020;20:e104–e106. 10.7861/clinmed.2020-0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chudasama YV, Gillies CL, Zaccardi F, Coles B, Davies MJ, Seidu S, et al. Impact of COVID‐19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr Clin Res Rev. 2020;14:965–967. 10.1016/j.dsx.2020.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mummery CJ, Kipps CM. UK neurology response to the COVID‐19 crisis. Clin Med (Lond). 2020;20:266–269. 10.7861/clinmed.2020-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Granata T, Bisulli F, Arzimanoglou A, Rocamora R. Did the COVID‐19 pandemic silence the needs of people with epilepsy? Epileptic Disord. 2020;22:439–442. 10.1684/epd.2020.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahrens SM, Ostendorf AP, Lado FA, Arnold ST, Bai S, Bensalem‐Owen MK, et al. Impact of the COVID‐19 pandemic on epilepsy center practice in the United States. Neurology. 2022;98:e1893–e1901. 10.1212/wnl.0000000000200285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albert DVF, Das RR, Acharya JN, Lee JW, Pollard JR, Punia V, et al. The impact of COVID‐19 on epilepsy care: a survey of the American Epilepsy Society membership. Epilepsy Curr. 2020;20:316–324. 10.1177/1535759720956994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller WR, Von Gaudecker J, Tanner A, Buelow JM. Epilepsy self‐management during a pandemic: experiences of people with epilepsy. Epilepsy Behav. 2020;111:107238. 10.1016/j.yebeh.2020.107238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burneo JG, Jette N, Theodore W, Begley C, Parko K, Thurman DJ, et al. Disparities in epilepsy: report of a systematic review by the north American Commission of the International League against Epilepsy. Epilepsia. 2009;50:2285–2295. 10.1111/j.1528-1167.2009.02282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiltz NK, Koroukian SM, Singer ME, Love TE, Kaiboriboon K. Disparities in access to specialized epilepsy care. Epilepsy Res. 2013;107:172–180. 10.1016/j.eplepsyres.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shallcross AJ, Becker DA, Singh A, Friedman D, Jurd R, French JA, et al. Psychosocial factors associated with medication adherence in ethnically and socioeconomically diverse patients with epilepsy. Epilepsy Behav. 2015;46:242–245. 10.1016/j.yebeh.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abokalawa F, Ahmad SF, Al‐Hashel J, Hassan AM, Arabi M. The effects of coronavirus disease 2019 (COVID‐19) pandemic on people with epilepsy (PwE): an online survey‐based study. Acta Neurol Belg. 2022;122:59–66. 10.1007/s13760-021-01609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casassa C, Moss R, Goldenholz DM. Epilepsy during the COVID‐19 pandemic lockdown: a US population survey. Epileptic Disord. 2021;23:257–267. 10.1684/epd.2021.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fonseca E, Quintana M, Lallana S, Luis Restrepo J, Abraira L, Santamarina E, et al. Epilepsy in time of COVID‐19: a survey‐based study. Acta Neurol Scand. 2020;142:545–554. 10.1111/ane.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedrich L, Sruk A, Bielen I. Responses of people with epilepsy to the COVID‐19 pandemic in the time of national lockdown. Epilepsy Behav. 2021;116:107790. 10.1016/j.yebeh.2021.107790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guilhoto LM, Mosini AC, Susemihl MA, Pinto LF. COVID‐19 and epilepsy: how are people with epilepsy in Brazil? Epilepsy Behav. 2021;122:108115. 10.1016/j.yebeh.2021.108115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puteikis K, Jasionis A, Mameniškienė R. Recalling the COVID‐19 lockdown: insights from patients with epilepsy. Epilepsy Behav. 2021;115:107573. 10.1016/j.yebeh.2020.107573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly C, Muggeridge A, Cross JH. The perceived impact of COVID‐19 and associated restrictions on young people with epilepsy in the UK: young people and caregiver survey. Seizure. 2021;85:111–114. 10.1016/j.seizure.2020.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosengard JL, Donato J, Ferastraoaru V, Zhao D, Molinero I, Boro A, et al. Seizure control, stress, and access to care during the COVID‐19 pandemic in new York City: the patient perspective. Epilepsia. 2021;62:41–50. 10.1111/epi.16779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah U, Rajeshree S, Ravat P, Kalika M, Mehta S, Sapre A, et al. Challenges for low middle‐income people with epilepsy during the COVID‐19 pandemic: lessons learnt, call for action. Epilepsia Open. 2022;7:665–673. 10.1002/epi4.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trivisano M, Specchio N, Pietrafusa N, Calabrese C, Ferretti A, Ricci R, et al. Impact of COVID‐19 pandemic on pediatric patients with epilepsy—the caregiver perspective. Epilepsy Behav. 2020;113:107527. 10.1016/j.yebeh.2020.107527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wanigasinghe J, Jayawickrama A, Hewawitharana G, Munasinghe J, Weeraratne CT, Ratnayake P, et al. Experience during COVID‐19 lockdown and self‐managing strategies among caregivers of children with epilepsy: a study from low middle income country. Seizure. 2021;84:112–115. 10.1016/j.seizure.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cross JH, Kwon C‐S, Asadi‐Pooya AA, Balagura G, Gómez‐Iglesias P, Guekht A, et al. Epilepsy care during the COVID‐19 pandemic. Epilepsia. 2021;62:2322–2332. 10.1111/epi.17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Millevert C, Van Hees S, Siewe Fodjo JN, Wijtvliet V, Faria de Moura Villela E, Rosso B, et al. Impact of COVID‐19 on the lives and psychosocial well‐being of persons with epilepsy during the third trimester of the pandemic: results from an international, online survey. Epilepsy Behav. 2021;116. 10.1016/j.yebeh.2021.107800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Hees S, Siewe Fodjo JN, Wijtvliet V, Van den Bergh R, Faria de Moura Villela E, da Silva CF, et al. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID‐19 pandemic: a multicountry online survey. Epilepsy Behav. 2020;112:107350. 10.1016/j.yebeh.2020.107350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Gaudecker JR, Clarke DF, Perkins S, Ali A, Sanjuan D, Vidaurre J. Epilepsy care delivery during COVID‐19 in resource‐limited countries: a survey in collaboration with international epilepsy equity group. Epilepsy Behav. 2023;138:108998. 10.1016/j.yebeh.2022.108998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorpe J, Ashby S, Hallab A, Ding D, Andraus M, Dugan P, et al. Evaluating risk to people with epilepsy during the COVID‐19 pandemic: preliminary findings from the COV‐E study. Epilepsy Behav. 2021;115:107658. 10.1016/j.yebeh.2020.107658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thorpe J, Ashby S, Cross JH, Sander JW, Newton C, Hanna J, et al. The impact of COVID‐19 on epilepsy care: Perspectives from UK healthcare workers. Epilepsy Behav Rep. 2021;16:100487. 10.1016/j.ebr.2021.100487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dugan P, Carroll E, Thorpe J, Jette N, Agarwal P, Ashby S, et al. Impact of the COVID‐19 pandemic on people with epilepsy: findings from the US arm of the COV‐E study. Epilepsia Open. 2022;7:645–656. 10.1002/epi4.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andraus M, Thorpe J, Tai XY, Ashby S, Hallab A, Ding D, et al. Impact of the COVID‐19 pandemic on people with epilepsy: findings from the Brazilian arm of the COV‐E study. Epilepsy Behav. 2021;123:108261. 10.1016/j.yebeh.2021.108261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The World Bank . The World Bank atlas method ‐ detailed methodology. Accessed April 16, 2024. https://datahelpdesk.worldbank.org/knowledgebase/articles/378832‐what‐is‐the‐world‐bank‐atlas‐method. [Google Scholar]
- 32. The World Bank . World Bank country and lending groups. Accessed April 16, 2024. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519‐world‐bank‐country‐and‐lending‐groups (2024). [Google Scholar]
- 33. The World Bank . The World by Income and Region. Accessed August 7, 2023. https://datatopics.worldbank.org/world‐development‐indicators/the‐world‐by‐income‐and‐region.html 2023.
- 34. Our World in Data . Coronavirus Pandemic (COVID‐19). Accessed August 7, 2023. https://ourworldindata.org/coronavirus 2023.
- 35. World Health Organization (WHO) . Draft Intersectional global action plan on epilepsy and other neurological disorders 2022–2031. Accessed May 9, 2024. https://www.who.int/news/item/28‐04‐2022‐draft‐intersectoral‐global‐action‐plan‐on‐epilepsy‐and‐other‐neurological‐disorders‐2022‐2031 2022.
- 36. Kuroda N. Epilepsy and COVID‐19: updated evidence and narrative review. Epilepsy Behav. 2021;116:107785. 10.1016/j.yebeh.2021.107785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuroda N, Kubota T. Psychological impact of the COVID‐19 pandemic for patients with epilepsy: a systematic review and meta‐analysis. Epilepsy Behav. 2021;124:108340. 10.1016/j.yebeh.2021.108340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szaflarski M, Szaflarski JP, Privitera MD, Ficker DM, Horner RD. Racial/ethnic disparities in the treatment of epilepsy: what do we know? What do we need to know? Epilepsy Behav. 2006;9:243–264. 10.1016/j.yebeh.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 39. Miller JS, Oladele F, McAfee D, Adereti CO, Theodore WH, Akinsoji EO. Disparities in epilepsy diagnosis and management in high‐income countries. Neurology Clinical Practice. 2024;14:e200259. 10.1212/CPJ.0000000000200259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mathur R, Rentsch CT, Morton CE, Hulme WJ, Schultze A, MacKenna B, et al. Ethnic differences in SARS‐CoV‐2 infection and COVID‐19‐related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. 10.1016/S0140-6736(21)00634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morales DR, Ali SN. COVID‐19 and disparities affecting ethnic minorities. Lancet. 2021;397:1684–1685. 10.1016/S0140-6736(21)00949-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuroda N, Kubota T, Horinouchi T, Ikegaya N, Kitazawa Y, Kodama S, et al. Impact of COVID‐19 pandemic on epilepsy care in Japan: a national‐level multicenter retrospective cohort study. Epilepsia Open. 2022;7:431–441. 10.1002/epi4.12616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. International League Against Epilepsy (ILAE) . Video‐EEG telemetry for epilepsy surgery during COVID‐19 restrictions. 2020.
- 44. Beniczky S, Husain A, Ikeda A, Alabri H, Helen Cross J, Wilmshurst J, et al. Importance of access to epilepsy monitoring units during the COVID‐19 pandemic: consensus statement of the international league against epilepsy and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132:2248–2250. 10.1016/j.clinph.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fesler JR, Stanton S, Merner K, Ross L, McGinley M, Bena J, et al. Bridging the gap in epilepsy care: a single‐center experience of 3700 outpatient tele‐epilepsy visits. Epilepsia. 2020;61:e95–e100. 10.1111/epi.16619 [DOI] [PubMed] [Google Scholar]
- 46. Kristoffersen ES, Sandset EC, Winsvold BS, Faiz KW, Storstein AM. Experiences of telemedicine in neurological out‐patient clinics during the COVID‐19 pandemic. Ann Clin Transl Neurol. 2021;8:440–447. 10.1002/acn3.51293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lallana S, Fonseca E, Restrepo JL, Quintana M, Seijo‐Raposo I, Abraira L, et al. Medium‐term effects of COVID‐19 pandemic on epilepsy: a follow‐up study. Acta Neurol Scand. 2021;144:99–108. 10.1111/ane.13439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yardi R, McLouth CJ, Mathias S, et al. Telemedicine as a path to bridging inequities in patients with epilepsy. Epilepsia. 2023;64:3238–3245. 10.1111/epi.17793 [DOI] [PubMed] [Google Scholar]
- 49. (ITU) ITU . Mobile cellular subscriptions (per 100 people). Accessed August 7, 2023. https://data.worldbank.org/indicator/IT.CEL.SETS.P2 2023.
- 50. International Monetary Fund (IMF) . Real GDP growth: Annual percent change. Accessed June 15, 2024. https://www.imf.org/external/datamapper/NGDP_RPCH@WEO/OEMDC/ADVEC/WEOWORLD 2024.
- 51. Chancel L, Piketty T, Saez E, Zucman G. World inequality report 2022. World Inequality Lab 2021.
- 52. Pathak PK, Singh Y, Mahapatro SR, Tripathi N, Jee J. Assessing socioeconomic vulnerabilities related to COVID‐19 risk in India: a state‐level analysis. Disaster Med Public Health Prep. 2022;16:590–603. 10.1017/dmp.2020.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data S2.
Data S3.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


