Abstract
Background
Alcohol and nicotine interact with the nicotinic acetylcholine receptor system to alter reward-related responses, thereby contributing to the co-use and misuse of these drugs. A missense polymorphism rs16969968 (G>A) in the CHRNA5 gene has shown a strong association with nicotine-related phenotypes. However, less is known about the impact of this variant on alcohol-related phenotypes.
Methods
We assessed the main and interactive effect of smoking and rs16969968 polymorphism on alcohol consumption using the Alcohol Use Disorders Identification Test (AUDIT), Timeline Follow Back (TLFB), and Lifetime Drinking History (LDH) in 980 healthy adults without alcohol use disorder. We further examined the effect of the rs16969968 polymorphism on acute alcohol consumption using a free-access i.v. alcohol self-administration (IV-ASA) human laboratory paradigm in a subset of 153 nonsmoking participants. Subjective alcohol responses, alcohol sensitivity, and expectancy measures were compared between genotype groups (GG; AA/AG).
Results
We observed a significant association of smoking with AUDIT, TLFB, and LDH measures across genotype groups, with smokers showing higher scores compared with nonsmokers. Additionally, we found an association between genotype and TLFB-total drinks in the IV-ASA subset, with the GG group showing higher scores than AA/AG group. Relatedly, the alcohol negative expectancy score was significantly lower in the GG group than the AA/AG group.
Conclusions
Our findings underscore the association of smoking with alcohol measures. We found preliminary evidence for the protective effect of the functional CHRNA5 polymorphism on alcohol consumption and its association with increased negative alcohol expectancies, which highlights the substantial heterogeneity in alcohol responses.
Keywords: Smoking, CHRNA5, alcohol consumption and expectancy, pharmacogenetics
Significance Statement.
The CHRNA5 gene polymorphism rs16969968 has been associated with nicotine addiction, and the α5 subunit encoded by the CHRNA5 gene is expressed in brain regions that also modulate neural responses to alcohol and other drugs. However, human translational studies on the impact of functional CHRNA5 variation on alcohol phenotypes are rare and inconclusive. In this study, we examine the direct and interactive effects of the rs16969968 polymorphism and smoking on alcohol phenotypes in non-AUD drinkers. Additionally, we explored the main effect of the rs16969968 genotype on alcohol self-administration, subjective responses, alcohol sensitivity, and expectancy phenotypes in nonsmokers. Smoking was significantly associated with increases in alcohol measures in individuals without AUD, underscoring the comorbid risk of the co-use of nicotine and alcohol in vulnerable groups. Furthermore, we found preliminary evidence for a protective effect of the functional CHRNA5 polymorphism on alcohol consumption and its association with an increase in negative alcohol expectancies.
INTRODUCTION
Alcohol and tobacco use are leading risk factors contributing to the global burden of disease and preventable deaths (Ezzati et al., 2002; Griswold et al., 2018). Alcohol use disorder (AUD) and excessive alcohol consumption have numerous adverse effects on physical and mental health, including increased risk of various forms of cancers, cardiovascular and liver diseases, and psychiatric comorbidities (Cargiulo, 2007). Nicotine dependence, based on time to first cigarette after waking, is also a major risk factor for cancer, cardiovascular, and upper respiratory disease diseases (Guertin et al., 2015; Selya et al., 2016; Zhu et al., 2019; Thomas et al., 2020) and is highly comorbid with psychiatric conditions, including schizophrenia, attention deficit hyperactivity disorder, anxiety disorders, and depression (Kutlu et al., 2015).
When co-occurring, AUD and nicotine dependence have even greater adverse effects on health and socio-environmental domains by increasing the risk of concurrent psychiatric disease and other substance use disorders (Le Strat et al., 2010; MacLean et al., 2018). Alcohol and nicotine use are highly comorbid and exert a reciprocal influence on the use and misuse of the other substance (Verplaetse and McKee, 2017). Early studies reported the prevalence of smoking among individuals with AUD to be as high as 88%, and more than 90% of smokers with AUD are nicotine dependent (Batel et al., 1995). More recent studies confirm the co-use of nicotine and comorbidity of nicotine dependence in individuals across the spectrum of alcohol drinkers (Pacek et al., 2019). Both occasional and daily smoking increase the risk for hazardous drinking, AUD diagnosis, and relapse to AUD (McKee et al., 2007; Weinberger et al., 2015). Similarly, the level of alcohol consumption, risk of AUD diagnosis (AUDIT score ≥20), and AUD diagnosis increase the rate of nicotine use and dependence (Falk et al., 2006). Moreover, a current or past diagnosis of AUD decreases the likelihood of smoking cessation, while current AUD increases the likelihood of smoking relapse (Weinberger et al., 2013). This high degree of association may be related to shared genetic factors that contribute to both the consumption of alcohol and smoking (Schlaepfer et al., 2008; Cross et al., 2017).
As a common target for both alcohol and nicotine, the neuronal nicotinic acetylcholine receptor (nAChR) system has garnered specific interest in understanding the mechanisms behind the co-use of these drugs (Schlaepfer et al., 2008; Cross et al., 2017). nAChRs are pentameric ligand-gated ion channels widely distributed throughout the peripheral and central nervous systems (Lê et al., 2000; De Biasi, 2002; Hogg et al., 2003; Rahman et al., 2014; Dawson et al., 2018; Wittenberg et al., 2020). These channels, composed of homo- and heteromeric combinations of α (α2–α10) and β (β2–β4) subunits that are mechanistically involved in nicotine- and alcohol-induced reward, addiction, and withdrawal (Lê et al., 2000; De Biasi, 2002; Hogg et al., 2003; Rahman et al., 2014; Dawson et al., 2018; Wittenberg et al., 2020). Nicotine’s binding to α4β2-containing nAChRs is particularly important in initiating nicotine addiction (Wittenberg et al., 2020). Nicotine exerts reward properties upon binding to these receptors, and chronic nicotine exposure results in brain neuroadaptations (Sutherland et al., 2016) that lead to nicotine dependence (Fedota and Stein, 2015; Wittenberg et al., 2020). The α4β2 nAChRs are also implicated in reducing ethanol consumption and reward in both rodent and human models (Ericson et al., 2009; Mitchell et al., 2012). For instance, administration of α4β2 nAChRs partial agonist varenicline attenuate ethanol-induced dopamine (DA) release in the nucleus accumbens (NAc) (Ericson et al., 2009).
The cholinergic receptor nicotinic alpha 5 (CHRNA5) gene, which encodes the α5 nAChR subunit, has been investigated for its association with nicotine and alcohol use and related phenotypes in both humans and animal models (Icick et al., 2020). Genome-wide association studies, meta-analyses, as well as several candidate gene studies have found significant association for a specific missense single-nucleotide polymorphism (SNP) rs16969968 in CHRNA5 with nicotine use disorder and smoking behaviors (Bierut et al., 2008; Stevens et al., 2008; Thorgeirsson et al., 2008; Saccone et al., 2010; Marees et al., 2018), increased risk for nicotine addiction, reduced aversive effect of nicotine, and delayed smoking cessation (Saccone et al., 2007; Chen et al., 2015; Jensen et al., 2015; Icick et al., 2020). Human candidate gene studies have examined the effect of rs16969968 polymorphism on alcohol phenotypes and have yielded discordant results (Sherva et al., 2010; Hällfors et al., 2013). One study found a significant association of the ‘G’ allele of the rs16969968 SNP with the symptoms of AUD (Chen et al., 2009). However, another study identified an effect of ‘AA’ genotype of rs16969968 on increased hazardous drinking using an additive SNP model approach (Brown-Rice et al., 2018).
The rs16969968 SNP is located in exon 5 of CHRNA5 and results in an amino acid substitution (aspartic acid to asparagine, D398N) in the protein (Bierut et al., 2008). Functional analyses of the α5 protein with substituted amino acid 398N demonstrated lower calcium permeability, increased short-term desensitization, and reduced response to nicotinic agonist in α4β2α5 receptors (Bierut et al., 2008; Kuryatov et al., 2011). The α5 SNP increases nicotine self-administration by altering the reward properties of nicotine in the ventral tegmental area (VTA) DAergic neurons (Morel et al., 2014) and modifies the adaptations of those neurons to long-term nicotine exposure and withdrawal (Yang et al., 2023). Although the mechanism of alcohol’s action on this α5 subunit is not clear, preclinical studies have implicated a loss of function of the α5 nAChR subunit with several alcohol-induced phenotypes like hypothermia, hypnosis, anxiolysis, reduced conditioned place preference, and sedation (Santos et al., 2013; Dawson et al., 2018). Additionally, recent alcohol self-administration studies demonstrated increased alcohol consumption in rodents carrying the minor allele ‘A’ or lacking the α5 subunit compared with the wild types (Besson et al., 2019; Quijano Cardé et al., 2022).
Although the CHRNA5 rs16969968 polymorphism has been strongly associated with alcohol use and related phenotypes in preclinical models, human studies investigating the effect of the SNP on alcohol use phenotypes have generally been inconclusive. Given the role of CHRNA5 in alcohol and nicotine use disorder and the high comorbidity of alcohol use and smoking, we sought to examine the direct and interactive effects of the rs16969968 polymorphism and smoking on alcohol consumption and related phenotypes in a sample of healthy adults without AUD. We also explored the main effect of the rs16969968 genotype on alcohol self-administration and subjective responses, alcohol sensitivity, and expectancy phenotypes in a subset of nonsmokers.
MATERIALS AND METHODS
The participants were healthy volunteers (n = 980), 18 to 65 years of age, who were alcohol drinkers with no history of AUD and who were recruited through newspaper advertisements and the National Institutes of Health (NIH) Healthy Volunteer Office. Participants were enrolled in the NIAAA Natural History Study (NCT02231840), which was reviewed and approved by the NIH Intramural Institutional Review Board and conducted at The NIH Clinical Center in Bethesda, Maryland. All participants provided written informed consent for their participation. A subset of the study sample (n = 153) also participated in a laboratory i.v. alcohol self-administration (IV-ASA) session, which was conducted under a separate IRB-approved clinical protocol at the NIH (NCT 03294460). Details of eligibility (inclusion and exclusion) criteria for the Natural History study and the IV-ASA study are provided in the supplementary Methods.
Genotyping
Genomic DNA was extracted from peripheral blood samples using the QIAmp DNA Blood Maxi Kit (Qiagen Hilden, Germany) and run on an Illumina OmniExpress BeadChip array (Illumina, San Diego, CA, USA). Genotyping of the CHRNA5 polymorphism rs16969968 was performed using assay-on-demand from Applied Biosystems (Foster City, CA, USA). The alleles were discriminated by post-polymerase chain reaction plate read on an ABI Prism 7900HT Sequence Detection System. Population-based ancestry information was derived from a panel of 2500 SNPs tested using the CEPH diversity panel to generate 6 Ancestry Informative Markers (AIMs) scores (Hodgkinson et al., 2008; Wiers et al., 2018).
Phenotypic Measures
Alcohol consumption was assessed using the Alcohol Use Disorders Identification Test (AUDIT) (Babor, 2001), Timeline Follow Back (TLFB) (Sobell, 1992), and Lifetime Drinking History (LDH) (Russell et al., 1997) questionnaires. Alcohol sensitivity and expectancy measures were assessed by the Self-Rating of the Effects of Alcohol (SRE) (Schuckit et al., 1997), Alcohol Sensitivity Questionnaire (ASQ) (Fleming et al., 2016), and Alcohol Effects Questionnaire (AEFQ) (Rohsenow, 1983). The Fagerström Test for Nicotine Dependence (FTND) questionnaire and pack-years (Bernaards et al., 2001) was used to evaluate the quantity of cigarette consumed, the compulsion to use, and severity of nicotine dependence (Heatherton et al., 1991). These measures are described in detail in the supplementary Methods.
IV-ASA Study
The IV-ASA session procedure was conducted following 48-hour verified alcohol abstinence via an Alcotest 7410 handheld breathalyzer (Drager Safety Diagnostics, Telford, PA, USA). The session lasted 150 minutes, during which breath alcohol concentrations (BrAC) were measured at 15-minute intervals. Additionally, there was an intoxication safety limit of 100 mg% for the free access session. The IV-ASA study procedure was previously described (Stangl et al., 2017). A summary of the procedures is included in the supplementary Methods.
Subjective Measures
Subjective response measures were collected during the IV-ASA session using the Drug Effects Questionnaire (DEQ), the Biphasic Alcohol Effects Scale (BAES), and the Alcohol Urge Questionnaire (AUQ). These questionnaires were administered at baseline, during the priming phase (at the 10-minute and 20-minute time points), every 15 minutes during the free-access phase, and 15 minutes post free access. The DEQ measures the acute subjective response to alcohol by assessing the extent to which participants experience 5 potential outcomes of alcohol intoxication: “feel”, “like”, “want more”, “high”, and “intoxicated”. Each response was rated on a 100-mm visual analog scale (Morean et al., 2013). Details of these measures are presented in supplementary Table 1. Briefly, BAES is used to measure the stimulative and sedative effects of alcohol (Martin et al., 1993), and the AUQ assesses alcohol drinking urges (Bohn et al., 1995).
Data Analysis
Differences in demographic variables were assessed using chi-square tests by classifying study participants based on the rs16969968 homozygous genotype and smoking status. Hardy-Weinberg equilibrium was tested for the rs16969968 polymorphism in both smokers and nonsmokers in the full sample. As there were relatively few individuals with homozygous genotype for rs16969968 polymorphism (n = 49 AA), the genotype was dichotomized (GG vs AA/AG) to enhance statistical power for detecting a potential difference between groups. We tested for the main and interactive effect of the rs16969968 genotype and smoking on AUDIT, TLFB, and LDH measures using 2-way ANCOVAs of group (2; smokers, nonsmokers) × genotype (2; GG, AA/AG). Due to the absence of a smoking × genotype interaction and substantial differences in effect sizes for the smoking and genotype main effects, additional exploratory analyses were conducted to separately examine the effect of the rs16969968 genotype and smoking on alcohol outcome measures. All ANCOVA analyses included age, sex, and AIMs score for Africa, Europe, and Asia ancestries as covariates. Bonferroni correction was performed to correct for multiple comparison, and the P value significance level was set at .05. We also examined the main effects of smoking, genotype, and their interaction on alcohol measures separately in individuals with White and African ancestry using age and gender as covariates. The data for total AUDIT and TLFB scores was available for all 980 participants; AUDIT-C and AUDIT-P data were missing for 1 participant, while LDH data were available for 868 participants. We also explored the correlation between all the alcohol and smoking measures using Pearson correlation coefficients test. P values ≤.05 were not corrected for multiple comparisons because of smaller sample size of smokers.
Additional exploratory analyses were conducted in the IV-ASA study sample, where we investigated the effect of the rs16969968 genotype on total ethanol consumption (in grams) and average and peak BrAC levels. We also looked at average and peak BrAC level over time intervals to better understand the time-dependent effects (0–30, 30–60, 60–90, and 90–120 minutes). The effect of the rs16969968 genotype was also tested on subjective responses, sensitivity phenotypes, and alcohol expectancy measures. All ANCOVA analyses included age, sex, and AIMs scores as covariates. We also conducted a repeated-measures ANOVA to examine changes in BrAC measures over time and to assess the effect of time × genotype within participants. The association between AEFQ-negative expectancy and alcohol phenotypes was further assessed by linear regression analyses controlling for age, sex, and AIMs scores. The AEFQ-negative expectancy was used as the independent variable, and alcohol measures (AUDIT, TLFB, LDH, and IV-ASA) were used as the dependent variable. To understand the effect of genotype on alcohol SA measures based on real-world alcohol consumption, we conducted linear regression analyses using AUDIT-C as the independent variable and IV-ASA measures as the dependent variables. P values ≤.05 were not corrected for multiple comparisons considering these were exploratory analyses. All analyses were conducted using SPSS software, Version 22.0 (IBM Corporation, Armonk, NY).
RESULTS
Demographic- and Alcohol-Related Measures
The demographic characteristics classified by the rs16969968 genotype and smoking status are shown in Table 1. Among the total participants, 66.22% were GG homozygotes, 28.78% were GA heterozygotes, and 5% were AA homozygotes. According to dbSNP data, the prevalence of the minor allele ‘A’ is 0.023 among African American populations and 0.366 among European populations. In our cohort, the frequency of the minor allele ‘A’ was 0.0556 among individuals of African American ancestry and 0.4832 among individuals of European ancestry, representing a higher frequency among European ancestry, consistent with the dbSNP data. The GG and AA/AG groups significantly differed in age and race (P ≤ .001). The distribution of genotypes significantly differed between smokers and nonsmokers (P ≤ .001). Among the study participants, 80.1% were nonsmokers, with 63.8% of them carrying the GG genotype and 36.2% with the AA/AG genotype. Of the 19.19% of the participants who were smokers, 76.6% had the GG genotype and 23.4% had the AA/AG genotype. The number of males and the mean age of the participants was higher in the smoking group than in the nonsmoking group (P ≤ .001). There were significant differences in race and ethnicity between the smoking and nonsmoking groups (P ≤ .05), with the number of nonsmokers being higher in all the races and ethnic groups compared with smokers. The genotype distribution in nonsmokers and smokers did not deviate from Hardy–Weinberg expectations (supplementary Table 2). The IV-ASA study participant demographics stratified by the rs16969968 genotype groups are presented in supplementary Table 3.
Table 1.
Demographics Characteristics Classified by the CHRNA5 rs16969968 SNP Genotype and Smoking Status (n = 980)
| Nonsmoker (n = 792) |
Smoker (n = 188) |
P-value (smoker vs nonsmoker) |
GG (n = 649) |
AA/AG (n = 331) |
P-value (GG vs AA/AG) |
|
|---|---|---|---|---|---|---|
| Age (SD) | 33.82 (11.65) | 40.47 (12.105) | <.001 | 36.15 (11.981) | 33.03 (11.85) | .00011 |
| Gender, N (female: male) | 348:444 | 47:141 | <.001 | 271:378 | 124:207 | .1949 |
| Race, N (% of total) | ||||||
| Asian | 56 (7.07) | 4 (2.12) | <.001 | 46 (7.08) | 14 (4.22) | <.0001 |
| Black/African American | 293 (36.99) | 124 (65.95) | 373 (57.47) | 44 (13.29) | ||
| White | 394 (49.74) | 48 (25.53) | 199 (30.66) | 243 (73.41) | ||
| Other | 49 (6.18) | 12 (6.38) | 31 (4.77) | 30 (9.06) | ||
| Ethnicity, n (% of total) | ||||||
| Hispanic or Latino | 60 (7.57) | 4 (2.12) | .0153 | 38 (5.85) | 26 (7.85) | 0.478 |
| Not Hispanic | 723 (91.28) | 180 (95.74) | 602 (92.75) | 301 (90.93) | ||
| Unknown | 9 (1.13) | 4 (2.12) | 9 (1.38) | 4 (1.20) | ||
All data are reported as mean and SE or mean and SD. Others represent multiracial and unknown or unspecified race. P values in bold text indicate statistically significant (P < .05) differences between groups (smoking vs nonsmoking, GG vs AA/AG).
Interaction and Main Association of rs16969968 Genotype and Smoking With Alcohol Measures
Initially, we analyzed the full factorial model, that is, interaction and main association of the rs16969968 genotype and smoking with alcohol measures. There was a significant interaction between the rs16969968 genotype and smoking on TLFB–total drinks (F = 7.773, df = 1971, P = .005), LDH–total lifetime drinks (F = 7.436, df = 1971, P = .007), and LDH–binge drinking years (F = 7.316, df = 1859, P = .007; supplementary Table 4). However, only the TLFB–total drinks association remained significant after Bonferroni correction (P = .045).
Subsequently, we examined various alcohol measures between smokers and nonsmokers across the rs16969968 genotype groups. Smokers in the GG genotype group reported significantly higher scores for all the AUDIT sub scores, TLFB measures, and LDH scores compared with nonsmokers (P ≤ .001). Similar associations were observed in the AA/AG genotype group, whereby smokers showed significantly higher scores for all the alcohol measures compared with nonsmokers (P ≤ .001). All effects remain significant following Bonferroni correction (Table 2).
Table 2.
Association of the CHRNA5 rs16969968 SNP Genotype With Alcohol Measures in Smoking and Nonsmoking Groups
| Alcohol Measures | GG Genotype |
df | F |
P-value (Smokers vs nonsmokers in GG group) |
AA/AG Genotype |
df | F |
P-value (Smokers vs nonsmokers in AA/AG group) |
P-value (GG vs AA/AG in smokers) |
P-value (GG vs AA/AG in nonsmokers) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonsmokers (n = 505) | Smokers (n = 144) | Nonsmokers (n = 287) | Smokers (n = 44) | |||||||||
| AUDIT-C | 4.492 (0.131) | 8.338 (0.248) | 1642 | 175.3 | <.001 | 4.731 (0.185) | 7.728 (0.432) | 1323 | 43.84 | <.001 | .692 | .66 |
| AUDIT-P | 2.483 (0.222) | 6.845 (0.422) | 1642 | 72.4 | <.001 | 2.870 (0.314) | 7.147 (0.734) | 1323 | 38.72 | <.001 | .613 | .405 |
| Total AUDIT Score | 6.974 (0.316) | 15.183 (0.601) | 1642 | 132.1 | <.001 | 7.609 (0.447) | 14.875 (1.045) | 1324 | 48.48 | <.001 | .784 | .448 |
| TLFB–Total drinks | 140.58 (12.106) | 491.85 (23.017) | 1642 | 135.6 | <.001 | 156.95 (17.091) | 370.25 (40.045) | 1324 | 69.8 | <.001 | .377 | .998 |
| TLFB–Drinking days | 30.85 (1.16) | 57.599 (2.206) | 1642 | 103.8 | <.001 | 31.704 (1.638) | 56.5 (3.838) | 1324 | 45.8 | <.001 | .489 | .771 |
| TLFB–Average drinks per day | 3.26 (0.152) | 7.395 (0.288) | 1642 | 127.05 | <.001 | 3.452 (0.214) | 6.507 (0.502) | 1324 | 61.03 | <.001 | .722 | .972 |
| TLFB–Binge drinking days | 12.006 (1.022) | 40.719 (1.942) | 1642 | 147.5 | <.001 | 13.91 (1.442) | 34.69 (3.379) | 1324 | 46.43 | <.001 | .7 | .692 |
| LDH–Total lifetime drinks | 9451.49 | 42859.31 (2649.2) | 1569 | 92.7 | <.001 | 12367.34 | 30327.38 (4553.3) | 1285 | 30.8 | <.001 | .446 | .442 |
| LDH–Binge drinking years | 2.703 (0.345) | 10.776 (0.647) | 1569 | 105.8 | <.001 | 3.597 (0.487) | 7.928 (1.112) | 1285 | 17.1 | <.001 | .318 | .591 |
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; LDH, Lifetime Drinking History; TLFB, Timeline Followback. All data are reported as mean and SE. Alcohol measures mean scores are adjusted for covariates sex, ancestry scores, and age. P values in bold text indicate statistically significant (P < .05) differences between groups (smoking vs nonsmoking, GG vs AA/AG) after Bonferroni correction. AUDIT-C and AUDIT-P data was available for 979 individuals and LDH data was available for 868 individuals.
We further examined the effect of the rs16969968 genotype by comparing the alcohol measures between the GG and AA/AG genotype groups in nonsmoking and smoking groups. There was no significant association between the rs16969968 genotype and AUDIT, TLFB, and LDH measures in either nonsmokers or smokers. There was no significant difference in alcohol measures between the GG and AA/AG genotype groups in the smoking or nonsmoking groups (Table 2).
Association Between Smoking and Alcohol Measures
There was a significant association between smoking and alcohol measures, with smokers showing higher scores on all the AUDIT measures, TLFB scores, and LDH measures (P ≤ .001; Table 3). All effects remain significant after Bonferroni correction. Correlation analyses between the AUDIT, TLFB, and LDH alcohol measures with FTND and other smoking measures demonstrated a significant positive correlation, with correlation coefficients ranging from 0.145 to 0.55. Details of these analyses are provided in supplementary Table 5.
Table 3.
Association of the CHRNA5 rs16969968 SNP Genotype and Smoking With Alcohol Measures
| Alcohol Measures | Nonsmokers (n = 792) | Smokers (n = 188) | df | F | P -value (Smokers vs nonsmokers) | GG (n = 649) |
AA/AG (n = 331) | df | F | P-value (GG vs AA/AG group) |
|---|---|---|---|---|---|---|---|---|---|---|
| AUDIT-C | 4.611 (0.108) | 8.033 (0.251) | 1970 | 153.061 | <.001 | 6.415 (0.142) | 6.230 (0.236) | 1970 | 0.431 | .512 |
| AUDIT-P | 2.677 (0.184) | 6.996 (0.425) | 1970 | 84.662 | <.001 | 4.664 (0.241) | 5.009 (0.401) | 1970 | 0.519 | .472 |
| Total AUDIT Score | 7.292 (0.262) | 15.029 (0.606) | 1971 | 134.03 | <.001 | 11.079 (0.343) | 11.242 (0.571) | 1971 | 0.057 | .812 |
| TLFB–Total drinks | 148.77 (10.025) | 431.056 (23.22) | 1971 | 121.417 | <.001 | 316.22 (13.149) | 263.606 (21.88) | 1971 | 4.068 | (.044) .396 |
| TLFB–Drinking days | 31.281 (0.961) | 57.049 (2.22) | 1971 | 110.138 | <.001 | 44.229 (1.26) | 44.102 (2.097) | 1971 | 0.003 | .96 |
| TLFB–Average drinks per day | 3.358 (0.126) | 6.951 (0.291) | 1971 | 125.346 | <.001 | 5.33 (0.165) | 4.979 (0.274) | 1971 | 1.151 | .284 |
| TLFB–Binge drinking days | 12.958 (0.846) | 37.705 (1.96) | 1971 | 131.035 | <.001 | 26.362 (1.11) | 24.301 (1.847) | 1971 | 0.877 | .349 |
| LDH–Total lifetime drinks | 10909.41 (1173.86) | 36593.34 (2643.85) | 1859 | 76.879 | <.001 | 26155.4 (1517.04) | 21347.36 (2502.60) | 1859 | 2.576 | .109 |
| LDH–Binge drinking years | 3.15 (0.287) | 9.352 (0.645) | 1859 | 75.198 | <.001 | 6.740 (0.37) | 5.762 (0.611) | 1859 | 1.785 | .182 |
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; LDH, Lifetime Drinking History; TLFB, Timeline Followback. All data are reported as mean and SE. Alcohol measures mean scores are adjusted for covariates sex, ancestry scores, and age. P values in bold text indicate statistically significant (P < .05) differences between groups (smoking vs nonsmoking, GG vs AA/AG) after Bonferroni correction. TLFB–Total drinks P = .044 in parentheses represents a significant P value before Bonferroni correction.
Association Between rs16969968 Genotype and Alcohol Measures
There was a significant association of the rs16969968 genotype with total drinks consumed in the past 90 days (P = .044), with the GG genotype group showing higher mean score than the AA/AG genotype group. However, this association did not remain significant after Bonferroni correction and could be considered as preliminary indication of the rs16969968 genotype effect (supplementary Fig 1). We did not find an association between the genotype and other TLFB measures, AUDIT, and LDH scores (Table 3). Supplementary Table 6 provides the mean scores of alcohol measures across CHRNA5 rs16969968 SNP genotype groups, determined by the additive model.
Race-Specific Association of rs16969968 Genotype and Smoking on Alcohol Measures
In individuals with African American ancestry, we observed a significant association between the rs16969968 genotype and TLFB–total drinks consumed in the past 90 days (P = .017) ,with the GG genotype group showing higher mean score than the AA/AG genotype group. The association was nonsignificant after Bonferroni correction. However, in individuals with White ancestry, a significant association of the rs16969968 genotype was observed with TLFB–drinking days (P = .05), with the AA/AG genotype group showing a higher mean score than the GG genotype group. There was no association of the genotype on other TLFB measures, AUDIT, and LDH scores across ancestry groups. The association between smoking and alcohol measures remained significant across ancestry groups, with smokers showing higher scores on all the alcohol measures than nonsmokers (supplementary Table 7).
IV-ASA Subgroup Analyses
Association Between rs16969968 Genotype and IV-ASA Measures, Subjective Responses, and Alcohol Sensitivity
Consistent with the larger cohort, we observed a significant association between the rs16969968 genotype and total drinks consumed in the past 90 days [TLFB–total drinks, df = 1146, F = 3.942, P = .049 (uncorrected)], with the GG genotype group showing higher mean alcohol intake than the AA/AG genotype group. There was no significant genotype effect on the SRE and ASQ measures (supplementary Table 3), nor was there a genotype effect on subjective responses of DEQ, AUQ, and BAES, peak and average BrAC, total ethanol consumed across the self-administration period, or during each quarter of the self-administration period (to explore any time-related effects) (supplementary Figure 2). We also conducted a repeated-measures ANOVA within groups, revealing a significant main effect of time, indicating significant changes in BrAC across different time intervals (df = 1.58, F = 42.405, P < .001). However, the interaction between genotype and time was nonsignificant, suggesting that the rate of change in BrAC did not differ between GG and AA/AG groups across various time intervals (supplementary Table 8).
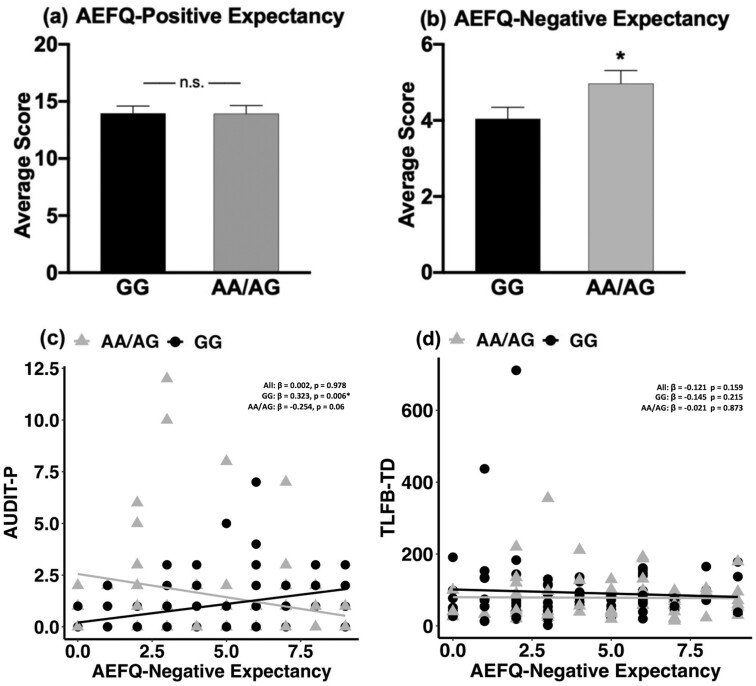
Association Between rs16969968 Genotype and Alcohol Measures Based on AEFQ—
We did not find a significant association of the genotype on AEFQ-positive expectancies (Figure 1A). However, we found an association between the genotype and AEFQ-negative expectancy score (F = 3.913, df = 1133, P = .05), which was significantly higher in the AA/AG genotype group compared with the GG genotype group (Figure 1B). Given this genotype effect, we evaluated the association between alcohol AEFQ-negative expectancy score and AUDIT, TLFB, LDH, and IV-ASA measures by conducting linear regression analyses in the full IV-ASA sample, and each genotype group. There was a significant negative correlation between AEFQ-negative expectancy score and total average BrAC (β = −.197, P = .02), and average and peak BrAC levels at 30-, 60-, and 90-minute time points in the full IV-ASA sample. We also found a positive correlation between AEFQ-negative expectancy and BAES-sedation score (β = .315, P = .001) in the full IV-ASA sample.
Figure 1.
Association between CHRNA5 rs16969968 SNP genotype and alcohol expectancies. Mean + SE of (A) Alcohol Effects Questionnaire (AEFQ)-positive and (B) AEFQ-negative expectancy scores between the GG and AA/AG rs16969968 SNP genotype groups. (C) Correlation between Alcohol Use Disorder Identification Test (AUDIT-P) and AEFQ- negative expectancy score in the full i.v. alcohol self-administration (IV-ASA) study sample, GG genotype, and AA/AG genotype group. (D) Correlation between Timeline Followback–Total drinks (TLFB-TD) and AEFQ-negative expectancy score in the full IV-ASA sample, GG genotype, and AA/AG genotype group. Mean AEFQ-negative expectancy score was significantly higher in the AA/AG genotype group compared with the GG genotype group (F = 3.913, df = 1133, P = .05). Significant positive association was observed between AUDIT-P and AEFQ-negative expectancy score only in the GG genotype group (β = .323, P = .006), but not in the full IV-ASA sample, and in the AA/AG genotype group. Age, sex, and ancestry scores were used as covariates in the analyses. Abbreviations: *, significant P ≤.05; n.s., nonsignificant.
In the GG genotype group, AEFQ-negative expectancy was significantly positively correlated with AUDIT-P (β = .323, P = .006; Figure 1C) and BAES-sedation scores (β = .459, P = .0002) and negatively correlated with average (β = −.291, P = .016) and peak BrAC (β = −.243, P = .043) at the 30-minute time point. There was no significant association of AEFQ-negative expectancy with AUDIT, TLFB, LDH, and IV-ASA measures in the AA/AG genotype group (Figure 1D).
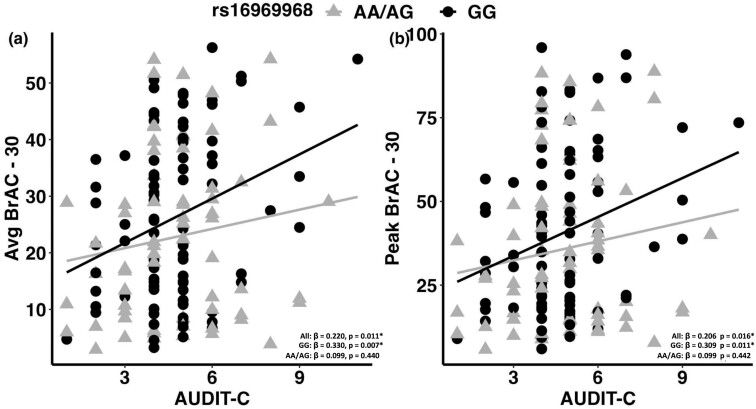
Association Between AUDIT-C and IV-ASA Measures across rs16969968 Genotype Groups
We conducted regression analyses in the full IV-ASA sample and in the GG and AA/AG genotype groups. In the full IV-ASA sample, there was a significant positive correlation between AUDIT-C and total average BrAC (β = .179, P = .038), average and peak BrAC levels at 30- and 60-minute time points (avg BrAC 30 minutes: β = .220, P = .011; peak BrAC 30 minutes: β = .206, P = .016; avg BrAC 60 minutes: β = .175, P = .042; peak BrAC 60 minutes: β = .171, P = .046), and subjective alcohol “like” effect (β = .181, P = .038). In the GG genotype, AUDIT-C was significantly positively correlated with average and peak BrAC levels at the 30-minute time point (avg BrAC 30 minutes: β = .330, P = .007; peak BrAC 30 minutes: β = .309, P = .011) and subjective alcohol “like” effect (β = .254, P = .037). In contrast, there was no significant association of AUDIT-C and IV-ASA measures in the AA/AG genotype group (Figure 2). We also explored the association between TLFB and IV-ASA measures and did not find any significant relationships (data not shown).
Figure 2.
Association between AUDIT-C and IV-ASA measures. Correlation between Alcohol Use Disorder Identification Test (AUDIT-C) and i.v. alcohol self-administration (IV-ASA) measures in the full IV-ASA samples, GG genotype and AA/AG rs16969968 SNP genotype groups after controlling for age, sex, and ancestry scores. Significant positive associations have been observed between AUDIT-C and IV-ASA measures (average and peak breath alcohol concentration (BrAC) at 30-minute time point) in the full IV-ASA sample and in the GG genotype group but not in the AA/AA genotype group. *P ≤.05.
DISCUSSION
Our findings confirmed the strong association of smoking with various alcohol-related phenotypic traits and showed a preliminary indication of the rs16969968 genotype effect on quantity of alcohol consumption. Smoking status predicted greater history of alcohol consumption, problematic drinking, the risk for AUD diagnosis, and increased overall quantity, frequency, and binge drinking episodes in the past 90 days. The total lifetime quantity of drinking and binge drinking years was also higher in smokers than nonsmokers. These results extend previous study findings showing an increased level of alcohol consumption and hazardous drinking in smokers compared with nonsmokers (McKee et al., 2007). Human laboratory studies have also demonstrated the effect of nicotine use on enhanced alcohol consumption and subjective alcohol effects, as well as increased motivation for alcohol administration (Kouri et al., 2004). The effect of smoking was consistent across the CHRNA5 genotype groups; smokers with the GG or AA/AG genotype both showed increased alcohol measures compared with nonsmokers, further indicating the strong influence of smoking on alcohol-related phenotypes, independent from the rs16969968 genotype. Beyond smoking group differences, we also found significant positive correlations between several alcohol and smoking measures. Mainly, TLFB–total drinks, drinking days, number of binge drinking days, and LDH–binge drinking years were significantly positively correlated with nicotine use and dependence measures, confirming the quantitative relationships between nicotine and alcohol consumption. The increased alcohol consumption in smokers could be due to the action of either alcohol or nicotine or both on the mesolimbic (DA) system, which comprises DAergic neurons within the VTA and their projection targets in the NAcc and the olfactory tubercle within the ventral striatum (Morel et al., 2019; Oettl et al., 2020).
The CHRNA5 rs16969968 ‘A’ allele impacts the VTA-DA neurons by causing a partial loss of function effect that has been associated with decreased reward properties of nicotine and increased dose of self-administered nicotine (Bierut et al., 2008; Morel et al., 2014). Alcohol may also impact drug reinforcement by influencing the mesolimbic pathway via nAChRs or by altering synaptic plasticity in the mesolimbic system through the DAergic mechanism (Adams, 2017). Neuroimaging studies have linked the CHRNA5 rs16969968 minor allele ‘A’ with decreased intrinsic resting functional connectivity strength in corticostriatal circuits that are associated with nicotine addiction severity (Hong et al., 2010). Alcohol exposure is also known to cause alterations in behavioral flexibility that are mediated by the dysregulation of corticostriatal circuits (Barker et al., 2015). Considering the strong functional role of the rs16969968 SNP in nicotine addiction through the corticostriatal and mesolimbic system and the known effects of alcohol on these systems, we investigated the association of the smoking and CHRNA5 rs16969968 SNP on alcohol consumption and related phenotypes. A previous study reported a higher frequency of the rs16969968 ‘A’ allele in smokers than nonsmokers, whereas we observed a higher ‘A’ allele frequency in nonsmokers than smokers (Ayesh et al., 2018). We detected an interactive association between the rs16969968 genotype and smoking on recent total drinks. There was a significant association of smoking with increased alcohol consumption and a preliminary indication of association of the AA/AG genotype with decreased alcohol consumption. Among groups with African American ancestry, we observed a similar trend, with the AA/AG genotype linked to decreased alcohol intake compared with the GG genotype. Conversely, in individuals of White ancestry, no such association was observed. The AA/AG genotype showed an association with increased drinking frequency compared with the GG group. These differences are likely attributed to variations in allele frequencies. The ‘A’ allele was less common among African Americans, who are more frequently smokers, while it was more prevalent among Whites, who are predominantly nonsmokers. A novel finding of our exploratory analyses in the IV-ASA sample is the greater AEFQ-negative expectancies scores in the minor allele, AA/AG genotype group than in the GG genotype group. Negative expectancies of alcohol have been previously associated with decreased frequency of alcohol consumption in social drinkers (Lee et al., 1999). In problematic drinkers and patients undergoing treatment for AUD, alcohol negative expectancies were found to motivate them for quitting alcohol consumption and continuing to abstain after completing the treatment program (Mahon and Jones, 1993; Jones and McMahon, 1994; Jones et al., 2001).
In line with previous findings, we observed a decreased quantity of alcohol consumption in the past 90 days in the AA/AG genotype group compared with the GG genotype group, and there was a lack of positive association of the AEFQ-negative expectancy scores with AUDIT-P in the AA/AG genotype group, which was instead observed in the GG genotype group. These results suggest that increased alcohol negative expectancies may decrease the risk for greater alcohol consumption and problematic drinking in non-AUD drinkers carrying the rs16969968 AA/AG genotype. The AA/AG genotype may be associated with a protective effect on alcohol consumption quantity and problematic drinking in non-AUD drinkers. Our finding that lower expectancies of the negative effect of alcohol are related to increased problematic drinking and quantity of alcohol consumption in the GG genotype group supports a previous study in individuals with European ancestry that reported a significant association between the rs16969968 SNP ‘G’ allele and increased risk of AUD symptoms (Chen et al., 2009). Interestingly, similar associations have been reported in cocaine users, showing a protective effect of the rs16969968 ‘A’ allele on cocaine dependence (Grucza et al., 2008; Aroche et al., 2020).
Finally, it is important to note that AUDIT-C scores were positively correlated with average and peak BrAC during the early phase (first 30 minutes) as well as with subjective “like” effects during the IV-ASA session in the full IV-ASA sample and in the GG genotype group. These results indicate that BrAC levels achieved during the laboratory session are highly correlated with real-world alcohol consumption, consistent with our previous human laboratory studies (Zimmermann et al., 2013; Stangl et al., 2017; Sloan et al., 2020). On the other hand, the absence of this correlation in the AA/AG genotype group suggests that increased AEFQ-negative expectancies, including cognitive and physical impairments and carelessness, potentially may have contributed to the reduced liking effect for alcohol and reduced the overall alcohol consumption quantity.
Evidence suggests that lone drinkers have higher negative alcohol expectancy scores than individuals who drink in groups (Jones and McMahon, 1992). Alcohol negative expectancies may be attenuated by altering the perception of negative experiences of alcohol when drinking in a group rather than drinking alone (Jones and McMahon, 1992). Our IV-ASA laboratory settings allow the participants to be more sensitive to the negative expectancies of alcohol than in natural settings, and administering alcohol through the IV method enables a more rapid and reliable time course of effects than oral alcohol consumption (Ramchandani et al., 2009). This negative expectancy, which was higher in the AA/AG group, may have influenced participants to administer a smaller quantity of alcohol than their regular drinking quantity, potentially leading to the lack of association between AUDIT-C and SA measures in the AA/AG genotype group. Although the mechanism of how rs16969968 SNP influences alcohol negative expectancies is not known, we may speculate that behaviorally, the rs16969968 SNP may reduce the reward salience for alcohol by increasing the negative expectancies of alcohol. These results further support the notion of a protective effect of the rs16969968 ‘AA/AG’ genotype on alcohol self-administration; however, additional studies will need to be conducted to examine this further.
Although early studies have associated the rs16969968 ‘A’ allele/CHRNA5 398N protein with nicotinic agonist–mediated lower calcium permeability, increased short-term desensitization, and partial loss of function in the VTA region, the mutation may affect the response to alcohol differently (Bierut et al., 2008; Morel et al., 2014). A previous preclinical alcohol study by Dawson et al., (2018) demonstrated attenuated ethanol reward in conditioned place preference and reduced ethanol intake following restraint stress in α5-lacking mice relative to the wild type. Although we did not consider the effect of stress in our study, supporting Dawson’s study findings we observed a trend of decreased alcohol consumption in the AA/AG genotype group compared with the GG group, possibly due to a less rewarding effect of alcohol. However, there is a lack of power to test the robustness of this translation due to the smaller number of IV-ASA study participants. In the future, we will consider a larger sample to validate this translational effect. Our findings contradict studies in rodents that have shown an effect of the rs16969968 SNP minor allele ‘A’ on increased alcohol consumption (Besson et al., 2019; Quijano Cardé et al., 2022). In our recent publication, we also addressed the impact of the α5 knockout gene effect on alcohol-related behavior in mice and found increased alcohol consumption in adolescent female mice carrying the α5 knockout gene compared with the wild types, but this effect was not translated in our human samples (Quijano Cardé et al., 2022).
Our study is not without limitations. A major limitation is that the IV-ASA analysis was conducted in nonsmokers and therefore only examined association of the CHRNA5 genotype, leaving any potential interactive effects of the 2 drugs unstudied. We are currently conducting a prospective study in smokers and nonsmokers, stratified by genotype, to directly compare the IV-ASA and subjective measures to better understand the interactive effect of smoking and the CHRNA5 variant on alcohol response phenotypes. Another limitation of our study is that we did not analyze any sex differences in the effect of the rs16969968 SNP on alcohol consumption due to the lack of power to examine sex-by-genotype interactions. Previous work conducted in mice by members of our group (Gangitano et al., 2009) has suggested progesterone-modulated alteration in α5 mRNA expression in the brain. The variation in progesterone levels among females may alter α5 expression in humans and could affect the reinforcing properties of alcohol, although additional studies are needed to evaluate whether these findings translate to humans. We aim to examine this in our ongoing work with larger samples to help characterize any sex-dependent effect of the CHRNA5 genotype on alcohol-related measures.
In conclusion, we found what appears to be a protective effect of the AA/AG genotype on alcohol consumption and problematic drinking in humans. These effects may be related to increased negative expectancies of alcohol in the AA/AG genotype group and decreased negative expectancies of alcohol in the GG genotype group. Our findings also extend previous research showing a significant association of smoking with increased alcohol consumption and problematic drinking to individuals who did not have an AUD diagnosis, suggesting a potential source of vulnerability or risk before development of alcohol problems. Given the relationship between this pattern of expectancies to increase the risk for higher alcohol consumption, further research is needed to address the potential biobehavioral mechanisms underlying the effect of the CHRNA5 genotype on alcohol response phenotypes and how this may drive the risk for AUD.
Supplementary Material
Acknowledgments
The authors thank Dr Mary Lee and Dr David T. George and nurse practitioners LaToya Sewell, Tonette Vinson, and Yvonne Horneffer for their medical supervision of the alcohol self-administration sessions. The authors also thank the staff of the 5SWS day hospital and 1-SE alcohol clinic at the NIH Clinical Center. The Computer-Assisted Infusion Software (CAIS) used for the i.v. alcohol self-administration session was developed with support from Dr Sean O’Connor, Dr Martin Plawecki, and Mr Victor Vitvitskiy from the Indiana Alcohol Research Center, Indiana University School of Medicine (NIH P60 AA007611).
Contributor Information
Shyamala K Venkatesh, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA; Human Psychopharmacology Laboratory, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Bethany L Stangl, Human Psychopharmacology Laboratory, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Jia Yan, Human Psychopharmacology Laboratory, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Natalia A Quijano Cardé, Pharmacology Graduate Group, Biomedical Graduate Studies, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Elliot A Stein, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, Maryland, USA.
Nancy Diazgranados, Office of the Clinical Director, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Melanie L Schwandt, Office of the Clinical Director, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Hui Sun, Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Reza Momenan, Clinical Neuroimaging Research Core, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
David Goldman, Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA; Office of the Clinical Director, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Mariella De Biasi, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Vijay A Ramchandani, Human Psychopharmacology Laboratory, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
This work was supported by the National Institutes of Health grant U01 AA025931 to M.D.B. and V.A.R. and Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health Z1A AA000466 to V.A.R. and Z1A AA000130 to N.D.
Interest Statement
None declared.
Data Availability
The data supporting the analyses and findings of this study are available on request from the corresponding author.
Author Contributions
Shyamala Venkatesh (Conceptualization [Equal], Formal analysis [Equal], Investigation [Equal], Methodology [Equal], Project administration [Equal], Visualization [Equal], Writing—original draft [Equal], Writing—review and editing [Equal]), Bethany Stangl (Investigation, Writing—review and editing), Jia Yan (Investigation, Writing—review and editing), Natalia Quijano Cardé (Writing—review and editing), Elliot Stein (Conceptualization, Writing—review and editing), Nancy Diazgranados (Writing—review and editing), Melanie Schwandt (Methodology, Writing—review and editing), Hui Sun (Writing—review and editing), Reza Momenan (Writing—review and editing), David Goldman (Writing—review and editing), Mariella De Biasi (Conceptualization [Equal], Funding acquisition [Equal], Supervision [Equal], Writing—review and editing [Equal]), and Vijay Ramchandani (Conceptualization [Equal], Funding acquisition [Equal], Methodology [Equal], Supervision [Equal], Writing—review and editing [Equal]).
References
- Adams S (2017) Psychopharmacology of tobacco and alcohol comorbidity: a review of current evidence. Curr Addict Rep 4:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroche AP, Rovaris DL, Grevet EH, Stolf AR, Sanvicente-Vieira B, Kessler FHP, von Diemen L, Grassi-Oliveira R, Bau CHD, Schuch JB (2020) Association of CHRNA5 gene variants with crack cocaine addiction. Neuromolecular Med 22:384–390. [DOI] [PubMed] [Google Scholar]
- Ayesh BM, Al-Masri R, Abed AA (2018) CHRNA5 and CHRNA3 polymorphism and lung cancer susceptibility in Palestinian population. BMC Res Notes 11:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders, JB, Monteiro, MG (2001) The Alcohol Use Disorders Identification Test (AUDIT) manual: Guidelines for use in primary care. 2nd ed., Department of Mental Health and Substance Dependence. Geneva: World Health Organization, WHO/MSD/MSB/016a, 4-32 [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ (2015) Corticostriatal circuitry and habitual ethanol seeking. Alcohol 49:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maître C, Rueff B (1995) Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction 90:977–980. [DOI] [PubMed] [Google Scholar]
- Bernaards CM, Twisk JW, Snel J, Van Mechelen W, Kemper HC (2001) Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction 96:1653–1661. [DOI] [PubMed] [Google Scholar]
- Besson M, Forget B, Correia C, Blanco R, Maskos U (2019) Profound alteration in reward processing due to a human polymorphism in CHRNA5: a role in alcohol dependence and feeding behavior. Neuropsychopharmacology 44:1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606. [DOI] [PubMed] [Google Scholar]
- Brown-Rice KA, Scholl JL, Fercho KA, Pearson K, Kallsen NA, Davies GE, Ehli EA, Olson S, Schweinle A, Baugh LA, Forster GL (2018) Neural and psychological characteristics of college students with alcoholic parents differ depending on current alcohol use. Prog Neuropsychopharmacol Biol Psychiatry 81:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargiulo T (2007) Understanding the health impact of alcohol dependence. Am J Health Syst Pharm 64:S5–11. [DOI] [PubMed] [Google Scholar]
- Chen LS, et al. (2015) CHRNA5 risk variant predicts delayed smoking cessation and earlier lung cancer diagnosis--a meta-analysis. J Natl Cancer Inst 107:djv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS (2009) Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet 150b:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Lotfipour S, Leslie FM (2017) Mechanisms and genetic factors underlying co-use of nicotine and alcohol or other drugs of abuse. Am J Drug Alcohol Abuse 43:171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Wolstenholme JT, Roni MA, Campbell VC, Jackson A, Slater C, Bagdas D, Perez EE, Bettinger JC, De Biasi M, Miles MF, Damaj MI (2018) Knockout of alpha 5 nicotinic acetylcholine receptors subunit alters ethanol-mediated behavioral effects and reward in mice. Neuropharmacology 138:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M (2002) Nicotinic mechanisms in the autonomic control of organ systems. J Neurobiol 53:568–579. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Söderpalm B (2009) The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther 329:225–230. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ; Comparative Risk Assessment Collaborating Group (2002) Selected major risk factors and global and regional burden of disease. Lancet 360:1347–1360. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhöfel S (2006) An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health 29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Stein EA (2015) Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci 1349:64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming KA, Bartholow BD, Hilgard J, McCarthy DM, O’Neill SE, Steinley D, Sher KJ (2016) The alcohol sensitivity questionnaire: evidence for construct validity. Alcohol Clin Exp Res 40:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M (2009) Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav 8:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MG, et al. (2018) Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, et al. (2008) A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry 64:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin KA, Gu F, Wacholder S, Freedman ND, Panagiotou OA, Reyes-Guzman C, Caporaso NE (2015) Time to first morning cigarette and risk of chronic obstructive pulmonary disease: smokers in the PLCO cancer screening trial. PLoS One 10:e0125973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hällfors J, Loukola A, Pitkäniemi J, Broms U, Männistö S, Salomaa V, Heliövaara M, Lehtimäki T, Raitakari O, Madden PA, Heath AC, Montgomery GW, Martin NG, Korhonen T, Kaprio J (2013) Scrutiny of the CHRNA5-CHRNA3-CHRNB4 smoking behavior locus reveals a novel association with alcohol use in a Finnish population based study. Int J Mol Epidemiol Genet 4:109–119. [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D (2008) Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol 43:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D (2003) Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1–46. [DOI] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA (2010) A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A 107:13509–13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icick R, Forget B, Cloëz-Tayarani I, Pons S, Maskos U, Besson M (2020) Genetic susceptibility to nicotine addiction: advances and shortcomings in our understanding of the CHRNA5/A3/B4 gene cluster contribution. Neuropharmacology 177:108234. [DOI] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M (2015) A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology 40:2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BT, Corbin W, Fromme K (2001) A review of expectancy theory and alcohol consumption. Addiction 96:57–72. [DOI] [PubMed] [Google Scholar]
- Jones BT, McMahon J (1992) Negative and positive expectancies in lone and group problem drinkers. Br J Addict 87:929–930. [DOI] [PubMed] [Google Scholar]
- Jones BT, McMahon J (1994) Negative and positive alcohol expectancies as predictors of abstinence after discharge from a residential treatment program: a one-month and three-month follow-up study in men. J Stud Alcohol 55:543–548. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE (2004) Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend 75:55–65. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J (2011) Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)₂α5 AChR function. Mol Pharmacol 79:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Parikh V, Gould TJ (2015) Nicotine addiction and psychiatric disorders. Int Rev Neurobiol 124:171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res 24:155–163. [DOI] [PubMed] [Google Scholar]
- Lee NK, Greely J, Oei TP (1999) The relationship of positive and negative alcohol expectancies to patterns of consumption of alcohol in social drinkers. Addict Behav 24:359–369. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Gorwood P (2010) In alcohol-dependent drinkers, what does the presence of nicotine dependence tell us about psychiatric and addictive disorders comorbidity? Alcohol Alcohol 45:167–172. [DOI] [PubMed] [Google Scholar]
- MacLean RR, Sofuoglu M, Rosenheck R (2018) Tobacco and alcohol use disorders: evaluating multimorbidity. Addict Behav 78:59–66. [DOI] [PubMed] [Google Scholar]
- Mahon JM, Jones BT (1993) Negative expectancy in motivation. Addiction Research 1:145–155. [Google Scholar]
- Marees AT, et al. (2018) Exploring the role of low-frequency and rare exonic variants in alcohol and tobacco use. Drug Alcohol Depend 188:94–101. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993) Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17:140–146. [DOI] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG (2007) Smoking status as a clinical indicator for alcohol misuse in US adults. Arch Intern Med 167:716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL (2012) Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 223:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS (2013) The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl) 227:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, De Biasi M, Lathrop M, Fratta W, Maskos U, Faure P (2014) Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry 19:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C, Montgomery S, Han MH (2019) Nicotine and alcohol: the role of midbrain dopaminergic neurons in drug reinforcement. Eur J Neurosci 50:2180–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettl LL, Scheller M, Filosa C, Wieland S, Haag F, Loeb C, Durstewitz D, Shusterman R, Russo E, Kelsch W (2020) Phasic dopamine reinforces distinct striatal stimulus encoding in the olfactory tubercle driving dopaminergic reward prediction. Nat Commun 11:3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Reboussin BA, Green KM, LaFlair LN, Storr CL, Alvanzo AAH, Mojtabai R, Cullen B, Young AS, Tormohen K, Riehm K, Crum RM (2019) Current tobacco use, nicotine dependence, and transitions across stages of alcohol involvement: a latent transition analysis approach. Int J Methods Psychiatr Res 28:e1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano Cardé NA, Shaw J, Carter C, Kim S, Stitzel JA, Venkatesh SK, Ramchandani VA, De Biasi M (2022) Mutation of the α5 nicotinic acetylcholine receptor subunit increases ethanol and nicotine consumption in adolescence and impacts adult drug consumption. Neuropharmacology 216:109170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Engleman EA, Bell RL (2014) Nicotinic receptor modulation to treat alcohol and drug dependence. Front Neurosci 8:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O’Connor S (2009) Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res 33:938–944. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ (1983) Drinking habits and expectancies about alcohol’s effects for self versus others. J Consult Clin Psychol 51:752–756. [DOI] [PubMed] [Google Scholar]
- Russell M, Marshall JR, Trevisan M, Freudenheim JL, Chan AW, Markovic N, Vána JE, Priore RL (1997) Test-retest reliability of the cognitive lifetime drinking history. Am J Epidemiol 146:975–981. [DOI] [PubMed] [Google Scholar]
- Saccone SF, et al. (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, et al. (2010) Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet 6:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos N, Chatterjee S, Henry A, Holgate J, Bartlett SE (2013) The α5 neuronal nicotinic acetylcholine receptor subunit plays an important role in the sedative effects of ethanol but does not modulate consumption in mice. Alcohol Clin Exp Res 37:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Ehringer MA (2008) The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev 1:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE (1997) The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction 92:979–988. [PubMed] [Google Scholar]
- Selya AS, Oancea SC, Thapa S (2016) Time to first cigarette, a proxy of nicotine dependence, increases the risk of pulmonary impairment, independently of current and lifetime smoking behavior. Nicotine Tob Res 18:1431–1439. [DOI] [PubMed] [Google Scholar]
- Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, Anton RF, Oslin D, Farrer LA, Gelernter J (2010) Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology 35:1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Janakiraman R, Ester CD, Stoddard J, Stangl B, Ramchandani VA (2020) High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addict Biol 25:e12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LCSaMB (1992) Timeline follow-back: a technique for assessing self-reported alcohol consumption. Measuring alcohol consumption: psychosocial and biological methods. Totowa, NJ: Humana Press, pp 41–72. [Google Scholar]
- Stangl BL, Vatsalya V, Zametkin MR, Cooke ME, Plawecki MH, O’Connor S, Ramchandani VA (2017) Exposure-response relationships during free-access intravenous alcohol self-administration in nondependent drinkers: influence of alcohol expectancies and impulsivity. Int J Neuropsychopharmacol 20:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE (2008) Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev 17:3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA, Laird AR (2016) Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Carroll JC, Brown MC, Chen Z, Mirshams M, Patel D, Boyd K, Pierre A, Goldstein DP, Giuliani ME, Xu W, Eng L, Khodayari Moez E, Liu G, Hung RJ (2020) Nicotine dependence as a risk factor for upper aerodigestive tract (UADT) cancers: a mediation analysis. PLoS One 15:e0237723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, et al. (2008) A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452:638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, McKee SA (2017) An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am J Drug Alcohol Abuse 43:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pilver CE, Hoff RA, Mazure CM, McKee SA (2013) Changes in smoking for adults with and without alcohol and drug use disorders: longitudinal evaluation in the US population. Am J Drug Alcohol Abuse 39:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Platt J, Jiang B, Goodwin RD (2015) Cigarette smoking and risk of alcohol use relapse among adults in recovery from alcohol use disorders. Alcohol Clin Exp Res 39:1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Towb PC, Hodgkinson CA, Shen PH, Freeman C, Miller G, Lindgren E, Shokri-Kojori E, Demiral Ş B, Kim SW, Tomasi D, Sun H, Wang GJ, Goldman D, Volkow ND (2018) Association of genetic ancestry with striatal dopamine D2/D3 receptor availability. Mol Psychiatry 23:1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg RE, Wolfman SL, De Biasi M, Dani JA (2020) Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology 177:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, McLaughlin I, Shaw JK, Quijano-Cardé N, Dani JA, De Biasi M (2023) CHRNA5 gene variation affects the response of VTA dopaminergic neurons during chronic nicotine exposure and withdrawal. Neuropharmacology 235:109547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nelson K, Toth J, Muscat JE (2019) Nicotine dependence as an independent risk factor for atherosclerosis in the National Lung Screening Trial. BMC Public Health 19:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the analyses and findings of this study are available on request from the corresponding author.