Abstract
Proteolytic activation of protease-activated receptor 2 (PAR2) may represent a major mechanism of regulating the transient receptor potential vanilloid 4 (TRPV4) non-selective cation channel in pathophysiological conditions associated with protease activation (e.g. during inflammation). To provide electrophysiological evidence for PAR2-mediated TRPV4 regulation, we characterised the properties of human TRPV4 heterologously expressed in Xenopus laevis oocytes in the presence and absence of co-expressed human PAR2. In outside-out patches from TRPV4 expressing oocytes, we detected single-channel activity typical for TRPV4 with a single-channel conductance of about 100 pS for outward and 55 pS for inward currents. The synthetic TRPV4 activator GSK1016790A stimulated TRPV4 mainly by converting previously silent channels into active channels with an open probability of nearly one. In oocytes co-expressing TRPV4 and PAR2, PAR2 activation by trypsin or by specific PAR2 agonist SLIGRL-NH2 potentiated the GSK1016790A-stimulated TRPV4 whole-cell currents several fold, indicative of channel sensitisation. Pre-incubation of oocytes with the calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM did not reduce the stimulatory effect of PAR2 activation on TRPV4, which indicates that the effect is independent of intracellular calcium signalling. Neutrophil elastase, a biased agonist of PAR2 that does not induce intracellular calcium signalling, also caused a PAR2-dependent sensitisation of TRPV4. The Rho-kinase inhibitor Y27362 abolished elastase-stimulated sensitisation of TRPV4, which indicates that Rho-kinase signalling plays a critical role in PAR2-mediated TRPV4 sensitisation by the biased agonist neutrophil elastase. During acute inflammation, neutrophil elastase may sensitise TRPV4 by a mechanism involving biased agonism of PAR2 and activation of Rho-kinase.
Keywords: TRPV4, PAR2, Proteases, Proteolytic activation, Elastase, Rho-kinase, Two-electrode voltage clamp, Xenopus laevis oocytes
Introduction
Transient receptor potential vanilloid 4 (TRPV4), a member of the TRP channel superfamily, has a broad spectrum of pathophysiological functions and is expressed in a wide range of tissues [20, 31, 48]. As TRPV4 can be activated by hypotonicity, it may serve as a mechanosensor or as an osmosensor [20, 39, 40]. TRPV4 also plays an important role in inflammation [7, 69, 79], where its activation can induce formation of proinflammatory mediators during osmotic stress [53]. Moreover, TRPV4 may mediate mechanical nociception and hyperalgesia [4, 8, 24, 62]. The importance of TRPV4 is reinforced by the identification of TRPV4 mutations that are associated with neurological disorders [14, 36] or with abnormalities in bone and joint function and growth [35, 37, 61].
Multiple stimuli activate TRPV4, including moderate heat (>25 °C), ultraviolet radiation [46], cell swelling, endogenous chemicals such as arachidonic acid and its metabolite 5,6-epoxyeicosatrienoic acid, and a growing number of exogenous chemical ligands [20, 48], including many which are plant-derived, e.g. phorbol esters such as 4α-phorbol 12,13-didecanoate (4α-PDD). Moreover, there are several synthetic agonists of TRPV4, including RN-1747 and GSK1016790A [30, 71]. The selective agonist GSK1016790A is 300-fold more potent than 4α-PDD [67]; however, the mechanism of TRPV4 activation by GSK1016790A is not yet understood. Antagonists of TRPV4 include ruthenium red, which is a non-selective pore blocker of several TRPV channels [48], and the selective antagonists GSK205 [53] and HC067047 [21].
TRP channels are major downstream targets of G protein-coupled receptors (GPCRs), which can activate signalling pathways that lead to altered channel gating or to the generation of channel agonists. In particular, there is good evidence that activation of protease-activated receptor-2 (PAR2) sensitises TRPV4 [68]. For example, in human respiratory epithelial cells, diesel exhaust particles have been reported to initiate signal transduction from PAR2 to TRPV4 [38]. Mechanisms of PAR2-mediated sensitisation of TRPV4 include the activation of protein kinase C and the generation of endogenous agonists [24, 54, 62]. PAR2 belongs to the group of four protease-activated GPCRs that mediate the effects of diverse proteases on haemostasis, inflammation, pain and healing [52, 56]. Trypsin cleaves human PAR2 at R36↓S37 to expose the tethered ligand S37LIGKV, which binds to and activates the cleaved receptor. Synthetic peptides that mimic this domain directly activate the receptor [3, 50]. Other proteases that cleave at this canonical site include trypsin IV [11, 32], tryptase [10, 45], coagulation factors VIIa and Xa [5], acrosin [63], granzyme A [27], membrane-type serine protease 1 or matriptase [66], TMPRSS2 [78] and kallikrein 2, 4, 5, 6 and 14 [44, 51, 58, 59]. Other proteases can cleave PAR2 at distinct sites, which may destroy or remove the tethered ligand domain, and thereby disarm the receptor. However, recent observations suggest that cleavage at alternative sites may cause biased agonism by stabilizing distinct active conformations of the receptor and thereby activate alternative signalling pathways. Neutrophil elastase cleaves PAR2 at S68↓V69, which removes the tethered ligand, and thereby prevents trypsin-stimulated PAR2 signalling [18, 56]. Elastase cleavage of PAR2 also induces PAR2-dependent activation of extracellular-signal-regulated kinases 1/2 (ERK1/2) by a Rho-kinase-dependent pathway [56] that is distinct from trypsin-induced mitogen-activated protein kinase (MAPK) activation mediated by β-arrestins [13]. Importantly, neutrophil elastase cleavage of PAR2 does not involve intracellular calcium signalling [29, 56]. These observations suggest that neutrophil elastase is a biased agonist of PAR2, stimulating the receptor to signal by pathways that differ from those activated by trypsin. However, the functional relevance of elastase activation of PAR2 is unknown.
To define the molecular mechanisms by which trypsin- and elastase-activated PAR2 can regulate TRPV4, we studied TRPV4 activation by channel agonists and proteases using the Xenopus laevis oocyte expression system, a powerful tool to study the function and regulation of ion channels and PARs.
Material and methods
Chemicals
GSK1016790A, HC067047, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM, trypsin I from bovine pancreas and soybean trypsin inhibitor (SBTI) were from Sigma. Human neutrophil elastase was from SERVA electrophoresis (81 U/mg); elastase inhibitor II and the selective Rho-kinase inhibitor Y27632 were from Merck. N-methyl-D-glucamine (NMDG) was from Fluka. GB88, 2-furoyl LIGRL-NH2, and SLIGRL-NH2 were from American Peptide Company, Inc. (Sunnyvale, CA, USA).
Plasmids
Complementary DNA (cDNA) for human wild-type (wt) TRPV4 was subcloned into pTLN vector. Full-length cDNA for human PAR2 (N-terminal FLAG and C-terminal HA11 epitopes) was subcloned into pcDNA3.1. Linearised plasmids were used as templates for complementary RNA (cRNA) synthesis (mMESSAGE mMACHINE) using T7 promoter.
Isolation of oocytes and injection of cRNA
Oocytes were obtained from adult X. laevis in accordance with the principles of German legislation, with approval by the animal welfare officer for the Friedrich-Alexander-University Erlangen-Nürnberg and under the governance of the state veterinary health inspectorate (permit no. 621–2531.32–05/02). The animals were anaesthetised in 0.2 % MS222. Ovarian follicles were surgically removed through a small abdominal incision. After suture, the animals were allowed to recover fully in a separate tank before they were returned to the frog colony 1 day later. Oocytes were treated with 600–700 U/ml collagenase type 2 from Clostridium histolyticum (CLS 2, Worthington) dissolved in a solution containing (in mM: NaCl 82.5, KCl 2, MgCl2 1 and HEPES 1, pH 7.4, with NaOH) at 19 °C for 3–4 h. The enzymatic digestion permits the isolation of the oocytes from the ovarian lobe. Defolliculated stage V–VI oocytes were injected (Nanoject II automatic injector, Drummond) with 0.5 ng of TRPV4 cRNA and 10 ng of PAR2 cRNA. Injected oocytes were stored at 19 °C in ND96 solution (in mM: NaCl 96, KCl 2, CaCl2 1.8, MgCl2 1, HEPES 5, pH 7.4, with Tris) supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin.
Two-electrode voltage clamp
The oocytes were studied 2 days after injection using the two-electrode voltage clamp technique as described previously [25, 60]. Oocytes were placed in a small experimental chamber and constantly superfused at room temperature with Ca2+-free solution (in mM: NaCl 96, KCl 2, MgCl2 1, HEPES 5, EGTA 1, pH 7.4, with NaOH) at a flow rate of approximately 2–3 ml/min. We used a Ca2+-free solution to prevent an activation of endogenous calcium-activated chloride channels by TRPV4-mediated calcium influx and to delay a calcium-induced decay of TRPV4 currents [75]. The simplistic approach of using a Ca2+-free solution has certain advantages in our experimental system but ignores aspects of TRPV4 channel function and regulation that are dependent on the presence of extracellular Ca2+. To prevent sodium influx, we used a Ca2+-free solution in which NaCl was replaced by NMDG-Cl (in mM: NMDG-Cl 96, KCl 2, MgCl2 1, HEPES 5, EGTA 1, pH 7.4, with Tris). Oocytes were voltage-clamped at −60 mV. Bath solution and drug exchanges were controlled by a magnetic valve system (ALA BPS-8) in combination with a TIB14 interface (HEKA). Voltage-clamp experiments were performed using an OC-725C amplifier (Warner Instruments Corp.) interfaced via LIH-1600 (HEKA) to a PC with PULSE 8.67 software (HEKA) for data acquisition and analysis. To test the effect of trypsin and neutrophil elastase, individual oocytes were pre-incubated in 130 μl of a protease-containing or a protease-free solution (Ca2+-free). Afterwards, the oocytes were transferred to the experimental chamber, and GSK1016790A was used to activate TRPV4.
Single-channel recordings in outside-out patches
Single-channel recordings in outside-out membrane patches of TRPV4 expressing oocytes were performed 2 days after cRNA injection essentially as described previously [15, 16, 26, 60], using conventional patch clamp technique. Patch pipettes were pulled from borosilicate glass capillaries and had a tip diameter of about 1–1.5 μm after fire polishing. Pipettes were filled with K gluconate pipette solution (in mM: K gluconate 90, NaCl 5, Mg-ATP 2, EGTA 2, HEPES 10, pH 7.28, with Tris). Seals were routinely formed in a low-sodium NMDG-Cl bath solution (in mM: NMDG-Cl 95, NaCl 1, KCl 4, MgCl2 1, CaCl2 1, HEPES 10, pH 7.4, with Tris). In this bath solution, the pipette resistance averaged ~7.5 MΩ. After seal formation, the NMDG-Cl solution was switched to a NaCl Ca2+-free bath solution in which NMDG-Cl (95 mM) was replaced by NaCl (95 mM), CaCl2 was removed, and EGTA (1 mM) was added. For continuous current recordings, membrane patches were routinely voltage-clamped at −82 mV, close to the calculated equilibrium potential of Cl− (ECl=−77.4 mV) and K+ (EK=−79.4 mV) under our experimental conditions. Experiments were performed at room temperature. Single-channel current data were initially filtered at 2.5 kHz and sampled at 10 kHz. The current traces were re-filtered at 250 Hz to resolve the single-channel current amplitude (i) and channel activity. The latter was derived from binned amplitude histograms as the product NPO, where N is the number of channels and PO is open probability [15, 16, 33, 34]. Single-channel data were analysed using the program “Patch for Windows” written by Dr. Bernd Letz (HEKA Elektronik) and the program “Nest-o-Patch” written by Dr. V. Nesterov (Institut für Zelluläre und Molekulare Physiologie, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany). Using a 3 M KCl flowing boundary electrode, the liquid junction potential occurring at the pipette/NaCl bath junction was measured to be ~12 mV (bath positive) and was not significantly affected by the removal of divalent cations. Vhold values are corrected for the liquid junction potential.
Statistical methods
Data are presented as mean ± SEM. Statistical significance was assessed by appropriate version of Student’s t test with GraphPad Prism 4.03 (GraphPad Software) for Windows.
Results
Functional evidence for TRPV4 expression in the oocyte expression system
To confirm functional TRPV4 expression in oocytes injected with TRPV4 cRNA, we made continuous whole-cell current recordings at a holding potential of −60 mV as illustrated in Fig. 1. The known TRPV4 agonist GSK1016790A [67] and the selective TRPV4 inhibitor HC067047 (100 nM) [21] were used as tools to activate and inhibit TRPV4 currents, respectively. The representative whole-cell current recording shown in Fig. 1a demonstrates that 50 nM GSK1016790A stimulated a substantial inward current, whereas 1 and 10 nM GSK1016790A had no effect. Washout of GSK1016790A caused a delayed and incomplete return towards the baseline current level. A subsequent application of GSK1016790A in a concentration of 100 nM elicited an even larger stimulatory response, which was partially reversible after washout of the agonist. The sustained stimulated inward current component that remained after the washout of the agonist was inhibited by HC067047, confirming that the inward current elicited by GSK1016790A is mediated by TRPV4. As expected, the non-specific TRPV4 blocker ruthenium red also inhibited the TRPV4-mediated inward current activated by GSK1016790A (data not shown). Importantly, in control experiments using non-injected oocytes, GSK1016790A did not stimulate any inward current (data not shown). We then applied GSK1016790A (50 nM) for different periods of time (30 s, 1 min and 2 min; Fig. 1b). These experiments indicate that the stimulatory effect of GSK1016790A increased with longer exposure time. Furthermore, the stimulatory response to GSK1016790A increased with repeated applications of the agonist (Fig. 1c). A similar phenomenon of increased current amplitude with repeated agonist application has been described for TRPA1 [55]. Under our experimental conditions with a holding potential of −60 mV and in the absence of extracellular calcium ions, the TRPV4 currents activated by GSK1016790A should be carried by an influx of sodium ions. To confirm this, we studied oocytes in which extracellular NaCl was replaced by NMDG-Cl, which is too large to permeate the TRPV4 pore (Fig. 1d). After an initial inward current activation by GSK1016790A, changing the bath solution to NMDG-Cl resulted in a small reduction of the inward current back to baseline level. This is consistent with the interpretation that the inward current component that persists after application of GSK1016790A is carried by sodium ions. Importantly, in the absence of extracellular sodium ions, a second application of GSK1016790A failed to elicit an inward current. The responsiveness to GSK1016790A was restored after changing back to a sodium ion containing bath solution (Fig. 1d). These findings confirm that the inward currents observed upon GSK1016790A application are carried by the influx of sodium ions through activated TRPV4 channels. These experiments demonstrate that X. laevis oocytes are a suitable system to express functional TRPV4 channels and that the expressed channels can be activated reliably with GSK1016790A. To achieve comparable levels of TRPV4 activation, we applied GSK1016790A in a concentration of 50 nM for 45 s in all subsequent two-electrode voltage-clamp experiments.
Fig. 1.
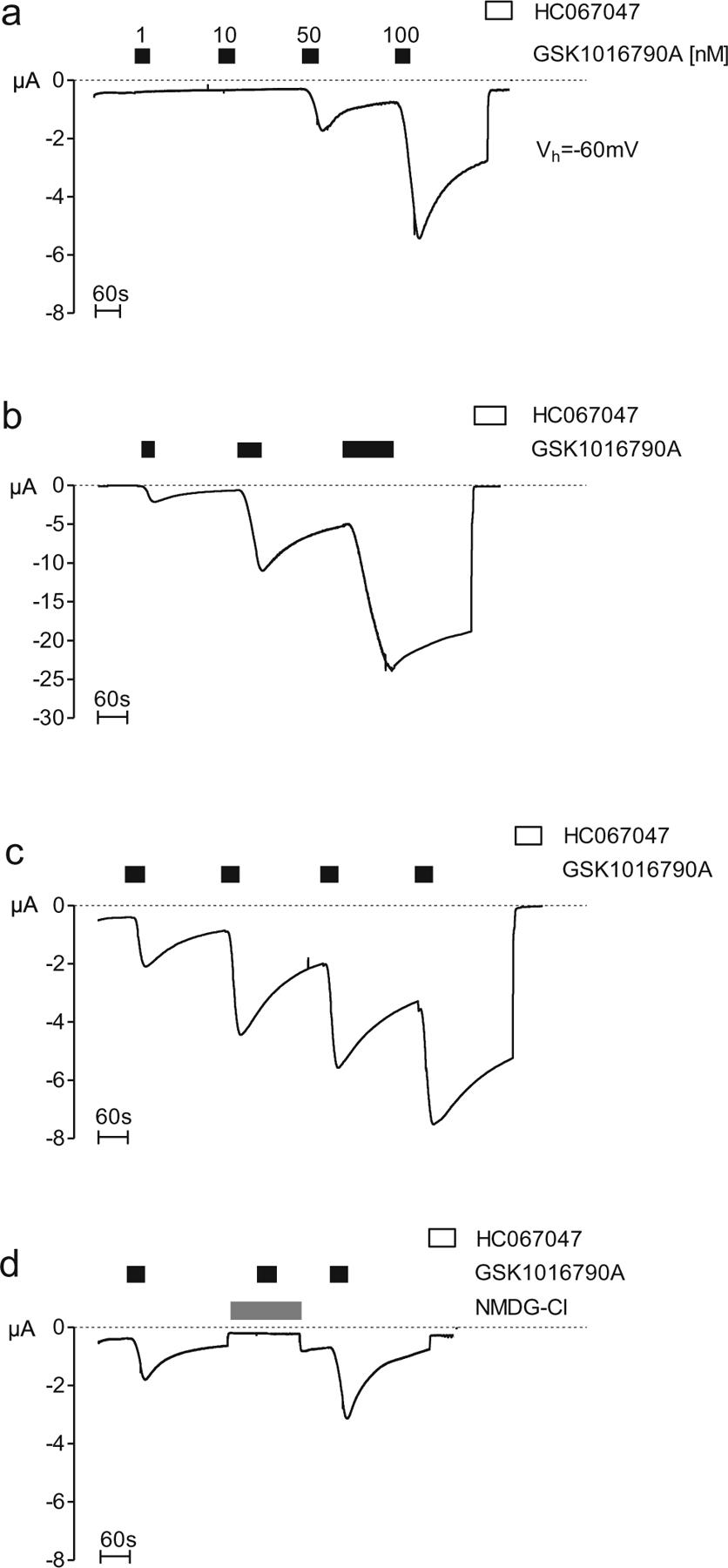
Functional evidence for TRPV4 expression in the oocyte expression system. Oocytes were injected with 0.5 ng of cRNA coding for TRPV4 and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. GSK1016790A and the TRPV4 inhibitor HC067047 were present in the bath solution, as indicated by closed and open bars, respectively. a–d Representative whole-cell current traces of oocytes expressing TRPV4 recorded in Ca2+-free solution. a Concentration-dependent effect of GSK1016790A on TRPV4. Concentrations ranging from 1 to 100 nM were used. b Time-dependent effect of GSK1016790A on TRPV4. Application times ranging from 30 s to 2 min. c Repeated application (four times) of GSK1016790A (50 nM). d The period of NMDG-Cl application is indicated by the grey bar
Single-channel properties of TRPV4 and its activation by GSK1016790A
Single-channel properties of human TRPV4 expressed in oocytes have not yet been studied in detail. To resolve TRPV4 single-channel currents, we made patch clamp recordings in the outside-out configuration using oocytes expressing TRPV4. Typical single-channel current traces recorded at different holding potentials are shown in Fig. 2a. Interestingly, this type of spontaneous single-channel activity was observed without prior application of GSK1016790A in five out of ten successful outside-out membrane patches obtained from TRPV4 expressing oocytes but not in non-injected control oocytes (Fig. 2d). The closed channel level (−C) was determined by visual inspection of the traces. At this level, the electrical noise was significantly lower than at the channel open level (−1). The average single-channel I-V plot shown in Fig. 2b summarises data from similar experiments as shown in Fig. 2a. A polynomial fit of these data revealed a reversal potential of −0.5 mV (n=11) indicative of a non-selective cation channel that does not discriminate between the monovalent cations Na+ and K+. Under our experimental conditions, TRPV4-mediated inward currents are carried by Na+ at hyperpolarising holding potentials, and outward currents are carried by K+ at depolarising holding potentials. From the polynomial fit shown in Fig. 2b, we estimated the following single-channel slope conductance values: 55 pS at Vhold=−80 mV, 8 pS at Vhold=−40 mV, 30 pS at Vhold=+40 mV and 99 pS at Vhold=+80 mV (n=11). Thus, the channel displays inward as well as outward rectification with a slightly larger outward than inward conductance. These are typical features of TRPV4 [20]. The mechanism by which GSK1016790A activates TRPV4 is not known. To investigate the activation of TRPV4 by GSK1016790A at the single-channel level, we made outside-out patch clamp recordings from oocytes expressing TRPV4 (Vhold=−82 mV). As shown in Fig. 2c, spontaneous TRPV4 channel activity was observed with one active channel in the patch even before application of the TRPV4 activator GSK1016790A. Long bursts of channel activity (>10 s) with an open probability of nearly one alternated with periods of complete channel closure. Application of extracellular GSK1016790A caused a stepwise appearance of several additional channel levels resulting in a significant increase in the overall activity (NPO) of TRPV4, as summarised in Fig. 2e. This significant increase in the number of active channels in the patch (Nactive) after application of GSK1016790A was confirmed in nine additional recordings (Fig. 2f). These single-channel data indicate that recruitment of near-silent channels largely contributes to the stimulatory effect of GSK1016790A on TRPV4. Application of GSK1016790A did not alter the single-channel current amplitude of TRPV4 (Fig. 2c). Before and after application of GSK1016790A at Vhold=−82 mV, the single-channel current amplitude averaged at −4.66±0.32 pA (n=5) and −4.84±0.34 pA (n=5, n.s.), respectively. Washout of GSK1016790A for ~30 s did not significantly change the single-channel activity. However, extracellular application of the TRPV4 inhibitor HC067047 decreased NPO and Nactive towards baseline values, confirming that the channel activity elicited by application of GSK1016790A can be attributed to TRPV4 (Fig. 2c, summary in Fig. 2e, f). In control experiments using non-injected oocytes, no comparable spontaneous single-channel activity was detected and application of both GSK1016790A and HC067047 had no detectable effect (Fig. 2d). In summary, these data demonstrate that TRPV4 expressed in oocytes functions as a non-selective cation channel with a similar single-channel conductance for Na+ and K+ and with an inwardly and outwardly rectifying I-V relationship typical for this channel [20]. GSK1016790A activates TRPV4 by converting previously silent channels in the excised membrane patch into active channels without changing the single-channel current amplitude.
Fig. 2.
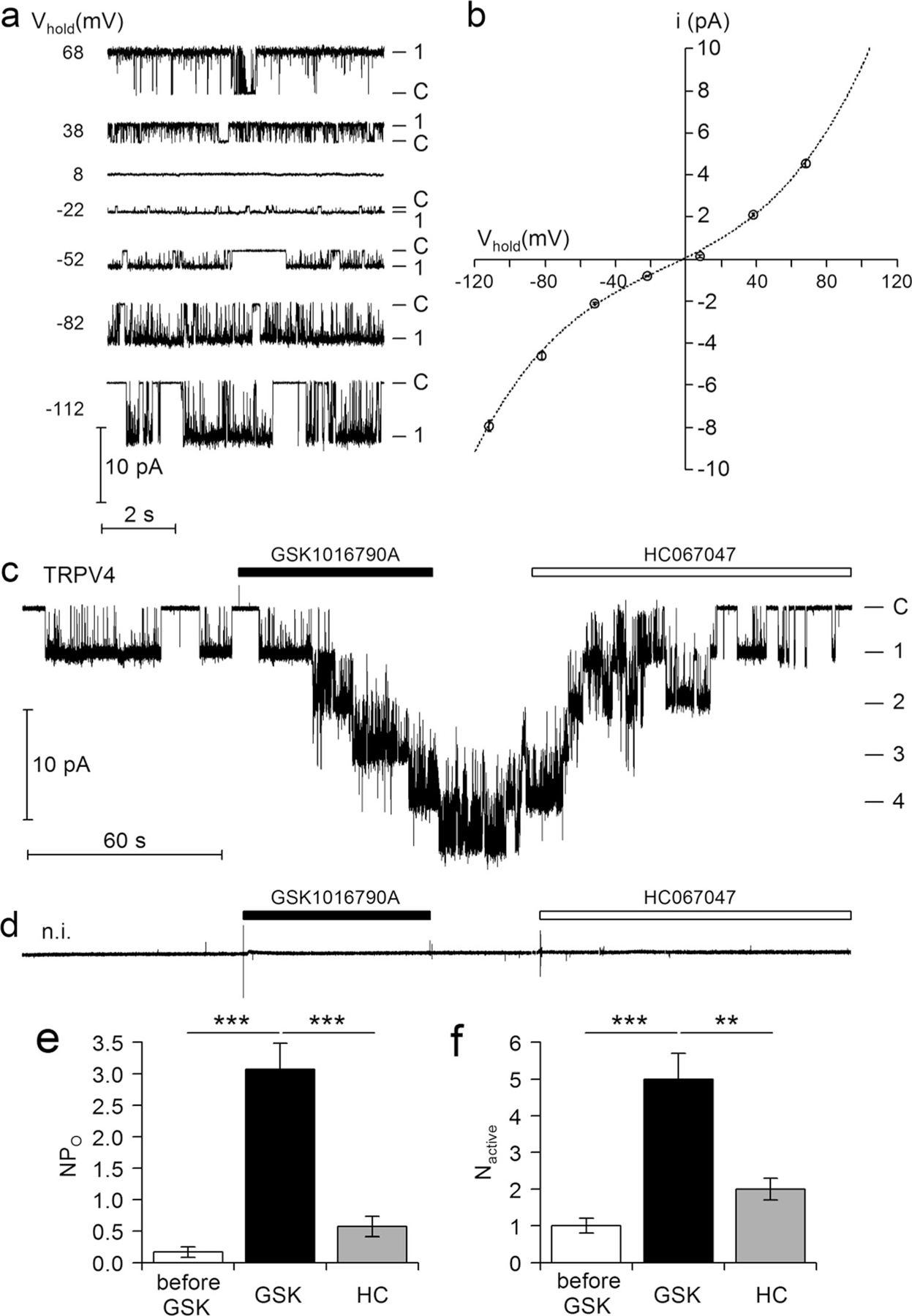
Single-channel properties of TRPV4 and its activation by GSK1016790A a Representative single-channel current traces at different holding potentials (Vhold) from an outside-out patch of an oocyte expressing TRPV4. b Average single-channel I-V plot calculated from recordings (11 patches from three batches of oocytes) similar to those shown in a. Binned current amplitude histograms (not shown for clarity) were used to determine the single-channel current amplitude (i) at each holding potential. The dashed line represents a polynomial fit of the data. c, d Representative single-channel current recordings obtained at Vhold=−82 mV from outside-out patches of an oocyte expressing TRPV4 and of a non-injected control oocyte (n.i.), respectively. GSK1016790A (50 nM) and HC067047 (100 nM) were present in the bath solution as indicated by the bars above the traces. Single-channel traces from experiments as shown in c were further analysed to determine NPO (e) and the number of active channel channels in a patch (Nactive; f) before application of GSK1016790A (before GSK, n= 10), after application GSK1016790A (GSK, n=10) and in the presence of HC067047 (HC, n=8). **p<0.01; ***p<0.001 unpaired t test
Functional evidence for PAR2 expression in the oocyte expression system
X. laevis oocytes are known to express endogenous calcium-activated chloride channels [2, 22]. Moreover, they may express endogenous PAR2-like receptors since application of high concentrations of trypsin (e.g. 2 μg/ml=85 nM) to non-injected oocytes has been shown to elicit a transient inward current, probably caused by an activation of endogenous calcium-activated chloride channels as a result of receptor-mediated calcium signalling [9, 15, 19]. In the present study, we demonstrated that trypsin applied in a low concentration (8 nM) had a negligible effect on baseline currents of non-injected control oocytes (Fig. 3a). In contrast, oocytes injected with cRNA for PAR2 consistently responded to the same concentration of trypsin (8 nM) with a large transient inward current peak. This stimulatory effect of trypsin was prevented by application of trypsin with SBTI, which indicates that the response requires proteolytic trypsin activity (Fig. 3b). Application of the PAR2 agonists 2-furoyl-LIGRL-NH2 or SLIGRL-NH2, which mimic the effect of the tethered ligand released by trypsin, elicited a current response similar to that caused by trypsin (Fig. 3c). Moreover, pre-incubating PAR2 expressing oocytes for 15 min with the non-competitive non-peptide PAR2 antagonist GB88 [41, 64] reduced the current response to trypsin by ~60 % (Fig. 3d). This inhibition is in good agreement with the effect of GB88 on PAR2 in HEK293 cells (data not shown). Finally, we demonstrated that pre-incubation of PAR2 expressing oocytes with BAPTA-AM to buffer intracellular calcium abolished the current response to trypsin (Fig. 3e) and also to the PAR2 agonists 2-furoyl-LIGRL-NH2 or SLIGRL-NH2 (Fig. 3f) in these oocytes. This finding is consistent with the interpretation that trypsin and the PAR2 agonists stimulate a PAR2-dependent current that is mediated by stimulation of calcium-activated chloride channels. Taken together, our findings indicate functional expression of PAR2 in oocytes injected with PAR2 cRNA.
Fig. 3.
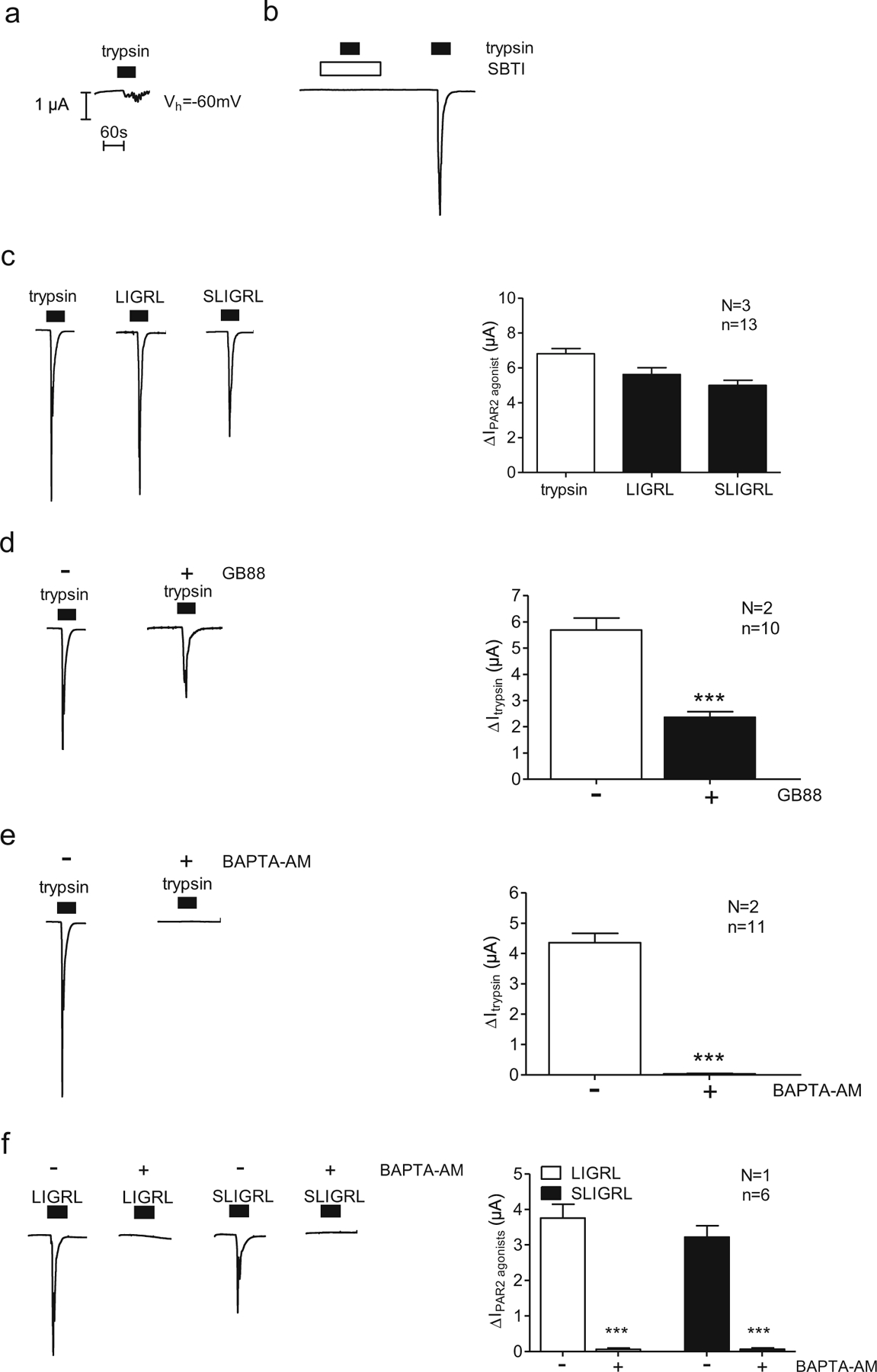
Functional evidence for PAR2 expression in the oocyte expression„ system. Oocytes were injected with 10 ng of cRNA coding for PAR2 and incubated for 2 days in ND96 solution, or non-injected oocytes were used. The whole-cell currents caused by trypsin or the synthetic activating peptides of PAR2 (2-furoyl-LIGRL-NH2 or SLIGRL-NH2) were measured using the two-electrode voltage-clamp technique. a–e Representative whole-cell current traces of a non-injected oocyte (a) or oocytes expressing PAR2 (b, e) recorded in Ca2+-free solution. Trypsin (8 nM), LIGRL (10 μM), SLIGRL (100 μM) or the trypsin inhibitor SBTI (0.08 μM) was present in the bath solution, as indicated by closed and open bars, respectively. Columns represent mean ΔIPAR2 agonist values from similar experiments. d Representative whole-cell current traces of oocytes expressing PAR2 incubated for 15 min in Ca2+-free solution in the absence (−) or presence (+) of GB88 (10 μM). Columns represent mean ΔItrypsin values from similar experiments. e, f Representative whole-cell current traces of oocytes expressing PAR2 incubated for 3 h in Ca2+-free solution in the absence (−) or presence (+) of BAPTA-AM (100 μM). Columns represent mean ΔItrypsin or ΔIPAR2 agonist values from similar experiments. n indicates number of individual oocytes measured. N indicates the number of batches of oocytes. ***p<0.001, unpaired t test
Proteolytic activation of PAR2 by trypsin sensitises TRPV4
To investigate a possible role of PAR2 in TRPV4 regulation, we co-expressed TRPV4 with PAR2. Co-expression of PAR2 per se did not significantly alter TRPV4-mediated currents elicited by the application of GSK1016790A in a concentration of 50 nM for 45 s (TRPV4 currents averaged 1.73±0.13 μA or 1.93±0.10 μA (N=2, n=14; data not shown) in oocytes expressing TRPV4 or TRPV4/PAR2, respectively). We pre-incubated oocytes expressing TRPV4 alone or co-expressing TRPV4 and PAR2 for 1, 5 or 25 min with 8 nM trypsin. Subsequently, we assessed the GSK1016790A-induced TRPV4 whole-cell currents using two-electrode voltage-clamp measurements (Fig. 4). Our results show that TRPV4 currents were significantly larger in trypsin pre-treated oocytes co-expressing TRPV4 and PAR2 compared to those in oocytes expressing TRPV4 alone. The stimulatory effect of trypsin was maximal in TRPV4/PAR2 co-expressing oocytes treated with trypsin for 5 min (Fig. 4a, b). Importantly, we demonstrated in control experiments that the trypsin inhibitor SBTI prevented the stimulatory effect of trypsin on TRPV4 currents in TRPV4/PAR2 co-expressing oocytes (Fig. 4c). In summary, our results indicate that sensitisation of TRPV4 currents by trypsin pre-incubation is dependent on PAR2 co-expression and is likely to be mediated by proteolytic PAR2 activation.
Fig. 4.
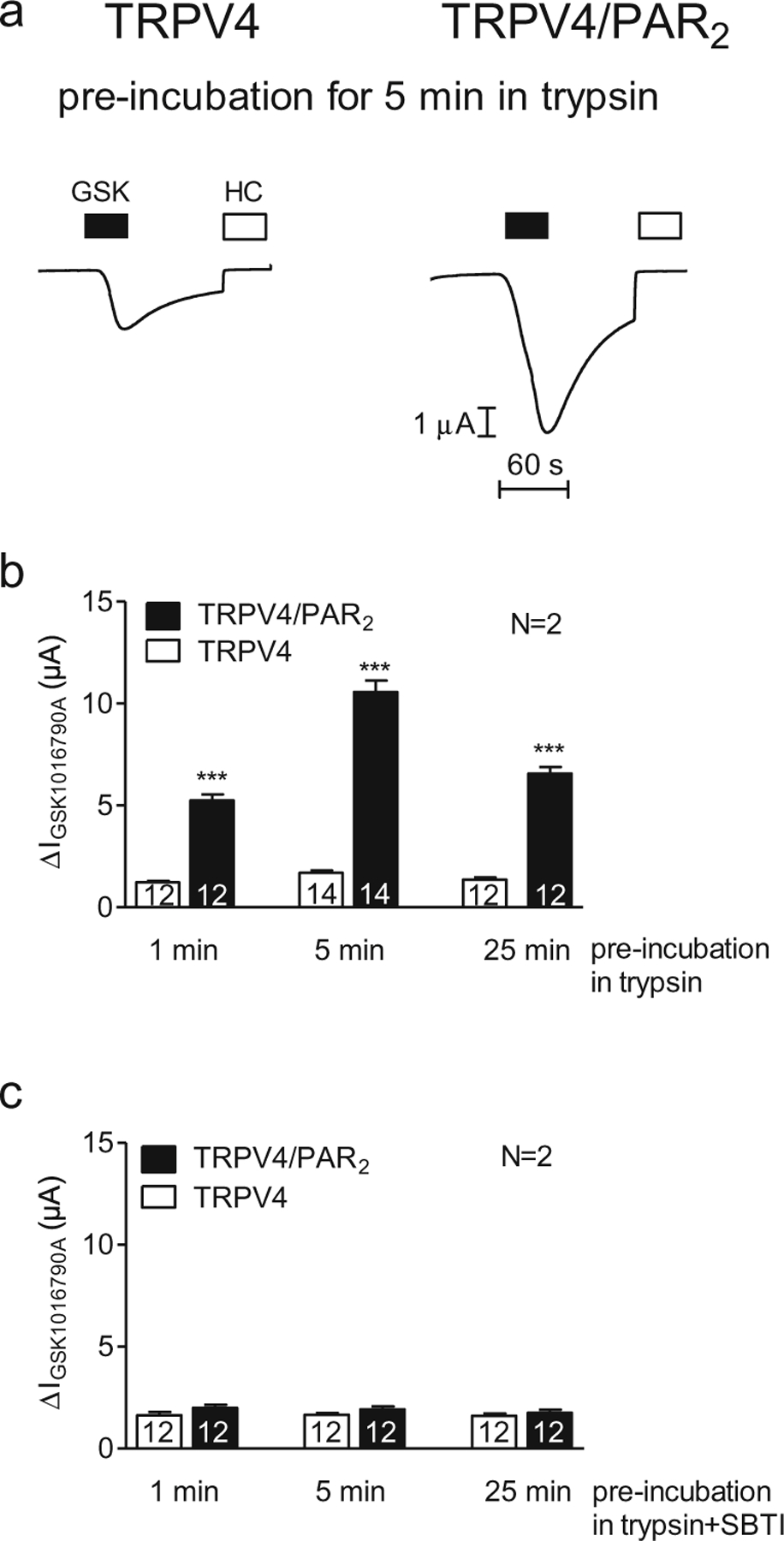
Proteolytic activation of PAR2 by trypsin sensitises TRPV4. Oocytes were injected with cRNA coding for TRPV4 0.5 ng or TRPV4 0.5 ng/PAR2 10 ng and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. Activation of TRPV4 by GSK1016790A (50 nM) after incubation in Ca2+-free solution in the presence of trypsin (8 nM) for different time points (1, 5 and 25 min). a Representative whole-cell current traces of oocytes expressing TRPV4 (left side) or TRPV4/PAR2 (right side) recorded in Ca2+-free solution. Activation and inhibition of TRPV4 by GSK1016790A (GSK, closed bars; 50 nM) and HC067047 (HC, open bars; 100 nM), respectively, after pre-incubation for 5 min in Ca2+-free solution in the presence of trypsin (8 nM). b, c Columns represent mean ΔIGSK1016790A values of oocytes pre-incubated in trypsin (b) or trypsin in the presence of the trypsin inhibitor SBTI (0.08 μM; c). N indicates the number of batches of oocytes. Numbers inside the columns indicate the number of individual oocytes measured. ***p<0.001, unpaired t test
Activation of PAR2 by PAR2 agonists (2-furoyl-LIGRL-NH2 or SLIGRL-NH2) also sensitises TRPV4
In additional experiments, we investigated the effect of two known PAR2-activating peptides (2-furoyl-LIGRL-NH2 and SLIGRL-NH2) on TRPV4 currents in oocytes expressing TRPV4 alone or co-expressing TRPV4/PAR2 (Fig. 5). As illustrated in Fig. 3c, the two peptides elicited a similar transient inward current response as activation of PAR2 by trypsin. TRPV4 and TRPV4/PAR2 expressing oocytes were pre-incubated for 5 min in 2-furoyl-LIGRL-NH2 (10 μM) or SLIGRL-NH2 (100 μM). Subsequently, GSK1016790A-induced TRPV4 currents were measured (Fig. 5a). TRPV4 currents were significantly larger in TRPV4/PAR2 expressing oocytes compared to those measured in oocytes expressing TRPV4 alone (Fig. 5b). These results indicate PAR2-dependent sensitisation of TRPV4 by PAR2 agonists.
Fig. 5.
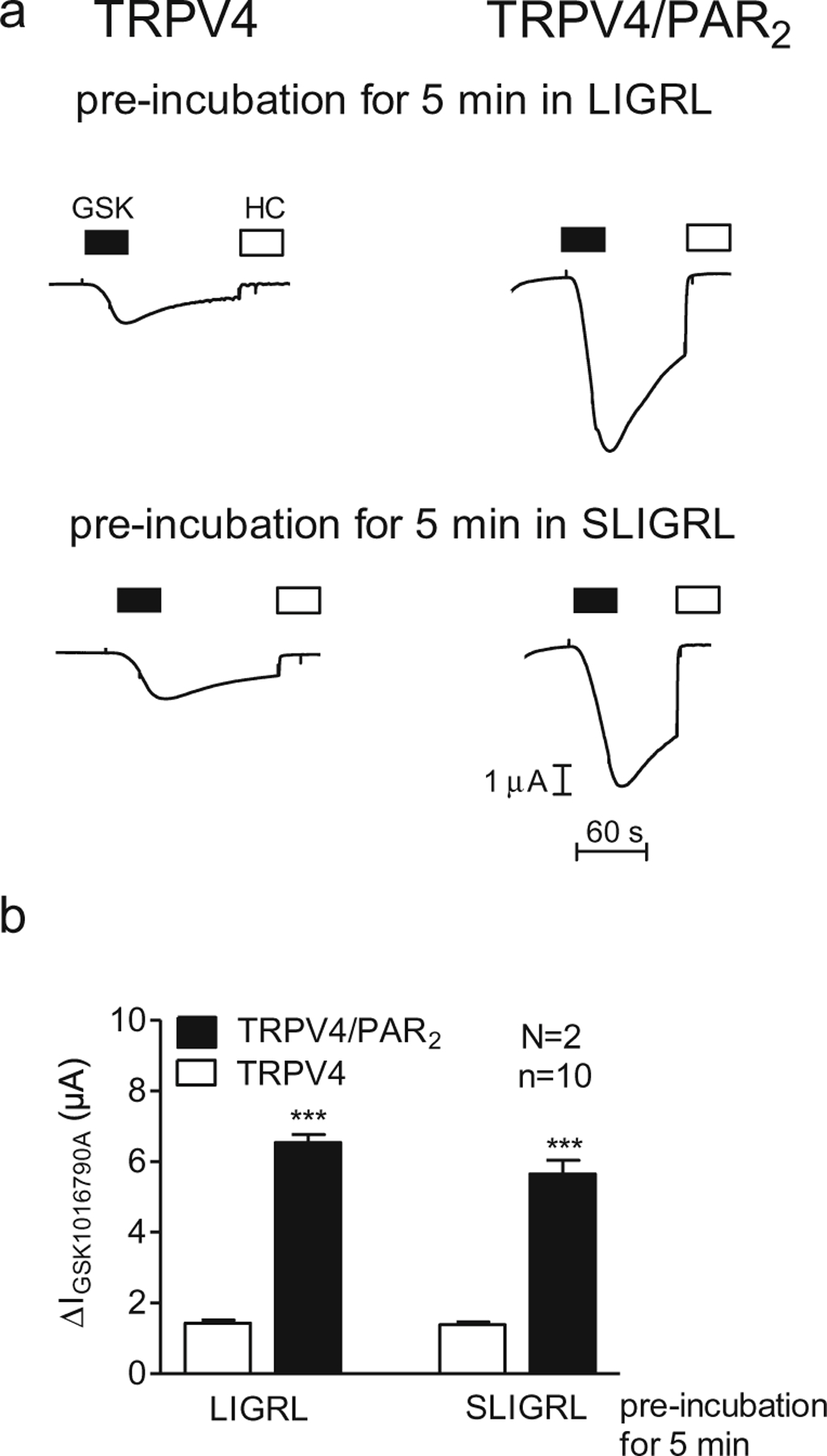
Activation of PAR2 by PAR2 agonists (2-furoyl-LIGRL-NH2 or SLIGRL-NH2) also sensitises TRPV4. Oocytes were injected with cRNA coding for TRPV4 0.5 ng or TRPV4 0.5 ng/PAR2 10 ng and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. Activation of TRPV4 by GSK1016790A (50 nM) after incubation for 5 min in Ca2+-free solution in the presence of LIGRL (10 μM) or SLIGRL (100 μM). a Representative whole-cell current traces of oocytes expressing TRPV4 (left side) or TRPV4/PAR2 (right side) recorded in Ca2+-free solution. Activation or inhibition of TRPV4 by GSK1016790A (GSK, closed bars; 50 nM) or HC067047 (HC, open bars; 100 nM), respectively. b Columns represent mean ΔIGSK1016790A values. n indicates number of individual oocytes measured. N indicates the number of batches of oocytes. ***p<0.001, unpaired t test
Inhibition of PAR2 by GB88 causes a reduction of TRPV4 sensitisation
To further confirm the role of PAR2 in trypsin-induced sensitisation of TRPV4 currents in TRPV4/PAR2 co-expressing oocytes, we studied the effect of the PAR2 antagonist GB88. Oocytes co-expressing TRPV4/PAR2 were pre-incubated for 15 min with GB88 (10 μM) or vehicle (control). Subsequently, oocytes were exposed to trypsin for 5 min, and the GSK1016790A-induced TRPV4 currents were measured. Inhibition of PAR2 by GB88 reduced trypsin-induced activation of TRPV4 currents by ~70 % compared to control (Fig. 6).
Fig. 6.
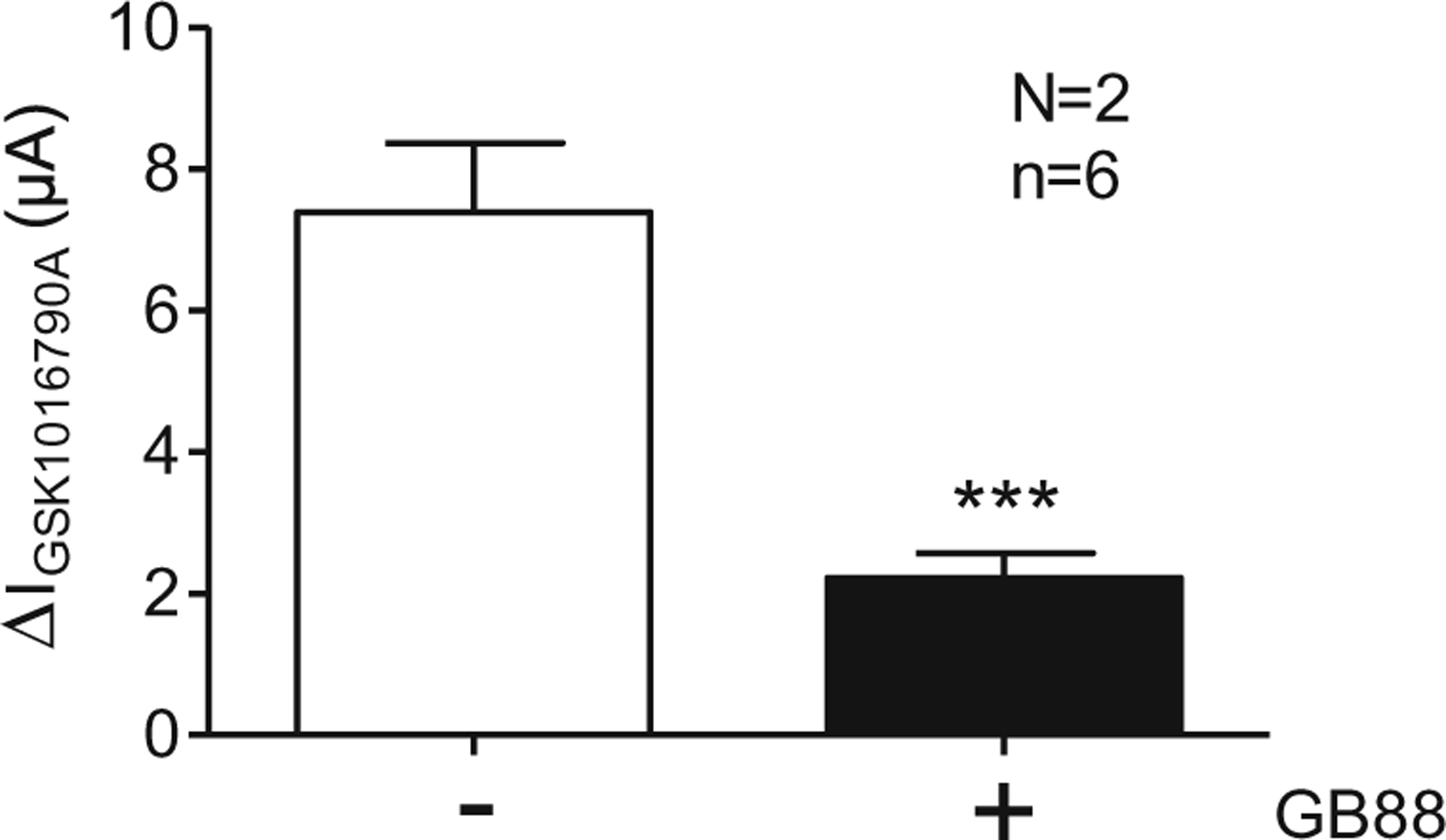
Inhibition of PAR2 by GB88 causes a reduction of TRPV4 sensitisation. Oocytes were injected with cRNA coding for TRPV4 0.5 ng/PAR2 10 ng and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. Oocytes were pre-incubated for 15 min in Ca2+-free solution in the absence (−) or in the presence (+) of GB88 (10 μM). After a subsequent pre-incubation for 5 min in trypsin, TRPV4 was activated by GSK1016790A (50 nM). Columns represent mean ΔIGSK1016790A values. n indicates number of individual oocytes measured. N indicates the number of batches of oocytes. ***p<0.001, unpaired t test
PAR2-mediated sensitisation of TRPV4 is not affected by BAPTA-AM
In control experiments, similar to those shown in Fig. 3e, we confirmed that pre-incubation in BAPTA-AM abolished the trypsin-induced activation of calcium-activated chloride currents in TRPV4/PAR2 co-expressing oocytes (data not shown). This finding indicates that pre-incubation in BAPTA-AM prevents a PAR2-mediated rise in intracellular calcium. In matched control oocytes co-expressing TRPV4/PAR2, without BAPTA-AM pre-treatment, we confirmed the PAR2-mediated sensitisation of TRPV4 by trypsin that is not observed in oocytes expressing TRPV4 alone (Fig. 7; also see Fig. 4). Importantly, a similar sensitisation of TRPV4 currents was observed in TRPV4/PAR2 co-expressing oocytes pretreated with BAPTA-AM. This indicates that PAR2-mediated intracellular calcium signalling is not required for sensitisation of TRPV4 by PAR2 activation.
Fig. 7.
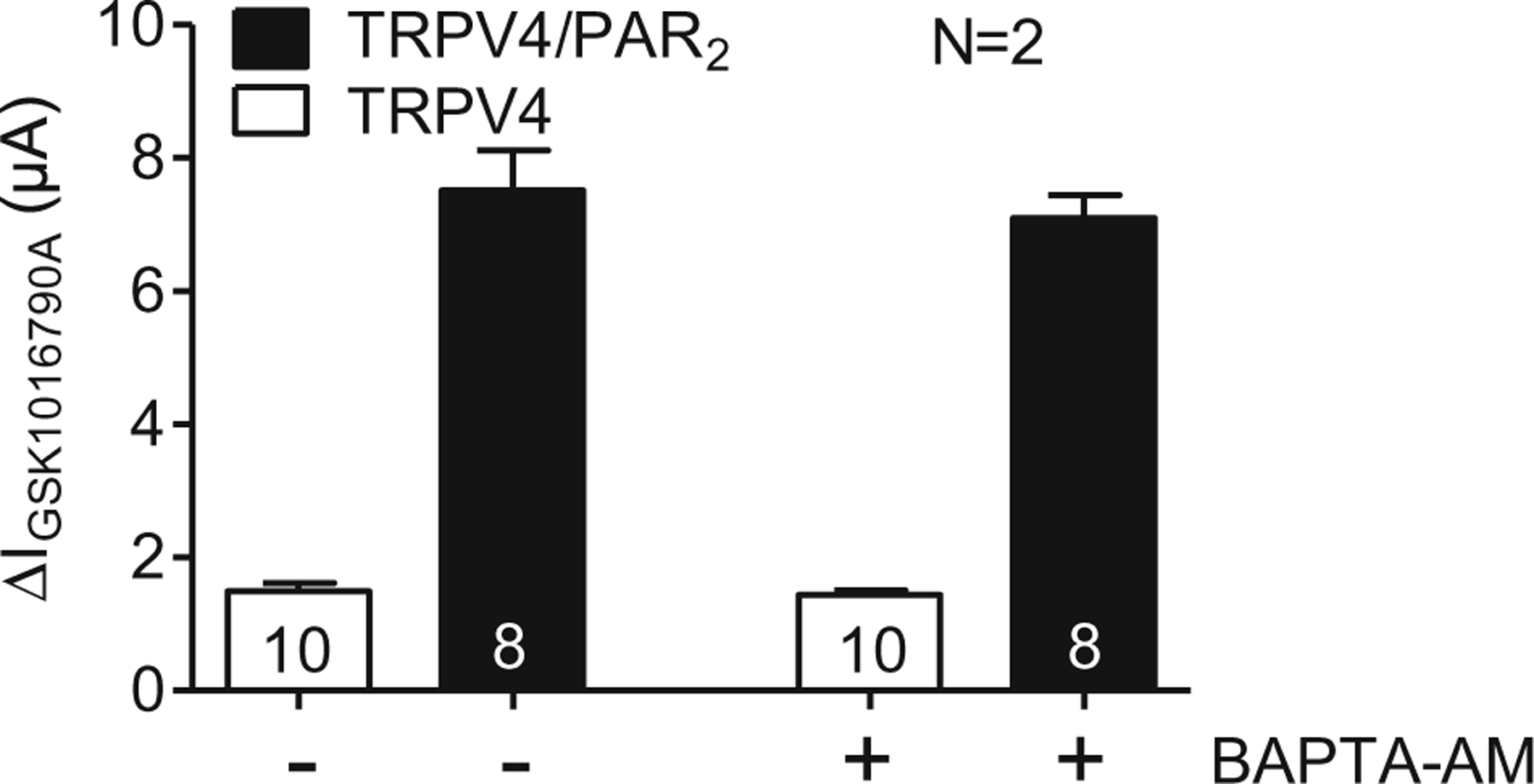
PAR2-mediated sensitisation of TRPV4 is not affected by BAPTA-AM. Oocytes were injected with cRNA coding for TRPV4 0.5 ng or TRPV4 0.5 ng/PAR2 10 ng and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. Oocytes were pre-incubated for 3h in Ca2+-free solution in the absence (−) or in the presence (+) of BAPTA-AM (100 μM). After a subsequent pre-incubation for 5 min in trypsin (8 nM), TRPV4 was activated by GSK1016790A (50 nM). Columns represent mean ΔIGSK1016790A values. N indicates the number of batches of oocytes. Numbers inside the columns indicate the number of individual oocytes measured
Proteolytic activation of PAR2 by the biased agonist human neutrophil elastase sensitises TRPV4
Our experiments using BAPTA-AM (see Fig. 7) suggest that calcium signalling is not involved in PAR2-mediated sensitisation of TRPV4. In additional experiments, we tested the effect of the biased agonist neutrophil elastase (Fig. 8), which activates PAR2 without eliciting an intracellular calcium signal [29, 56]. The experimental protocol was similar to that used to investigate the effect of trypsin (Fig. 4). We exposed oocytes to vehicle (control; a) or human neutrophil elastase (3 μM; b) for 5 min and then activated TRPV4 with GSK1016790A (50 nM). We compared the magnitude of the response to the TRPV4 agonist in oocytes expressing TRPV4 alone to that in oocytes expressing TRPV4/PAR2. GSK1016790A-activated currents were similar in vehicle-treated control oocytes expressing TRPV4 alone or TRPV4/PAR2 (Fig. 8a, c). Pre-treatment of oocytes expressing TRPV4 alone with neutrophil elastase did not alter the response to GSK1016790A (Fig. 8b, left; Fig. 8c). In contrast, pre-incubation of oocytes expressing TRPV4/PAR2 with elastase for 5 min amplified the response to GSK1016790A (Fig. 8b, right; Fig. 8c). To confirm that the observed increase in ΔIGSK1016790A is caused by the elastase activity of the protease preparation used, we examined the effect of an elastase inhibitor on PAR2-mediated sensitisation of TRPV4 by neutrophil elastase. The elastase inhibitor prevents PAR2-mediated sensitisation of TRPV4 by elastase but not by trypsin (Fig. 8d). In control experiments, pre-incubation of oocytes in elastase inhibitor alone had a negligible effect on GSK1016790A-mediated TRPV4 currents (Fig. 8d). Thus, elastase activates PAR2, which can sensitise TRPV4. Since elastase does not evoke PAR2-dependent intracellular calcium signals, our findings further support the conclusion that intracellular calcium signalling is not involved in PAR2-mediated sensitisation of TRPV4.
Fig. 8.
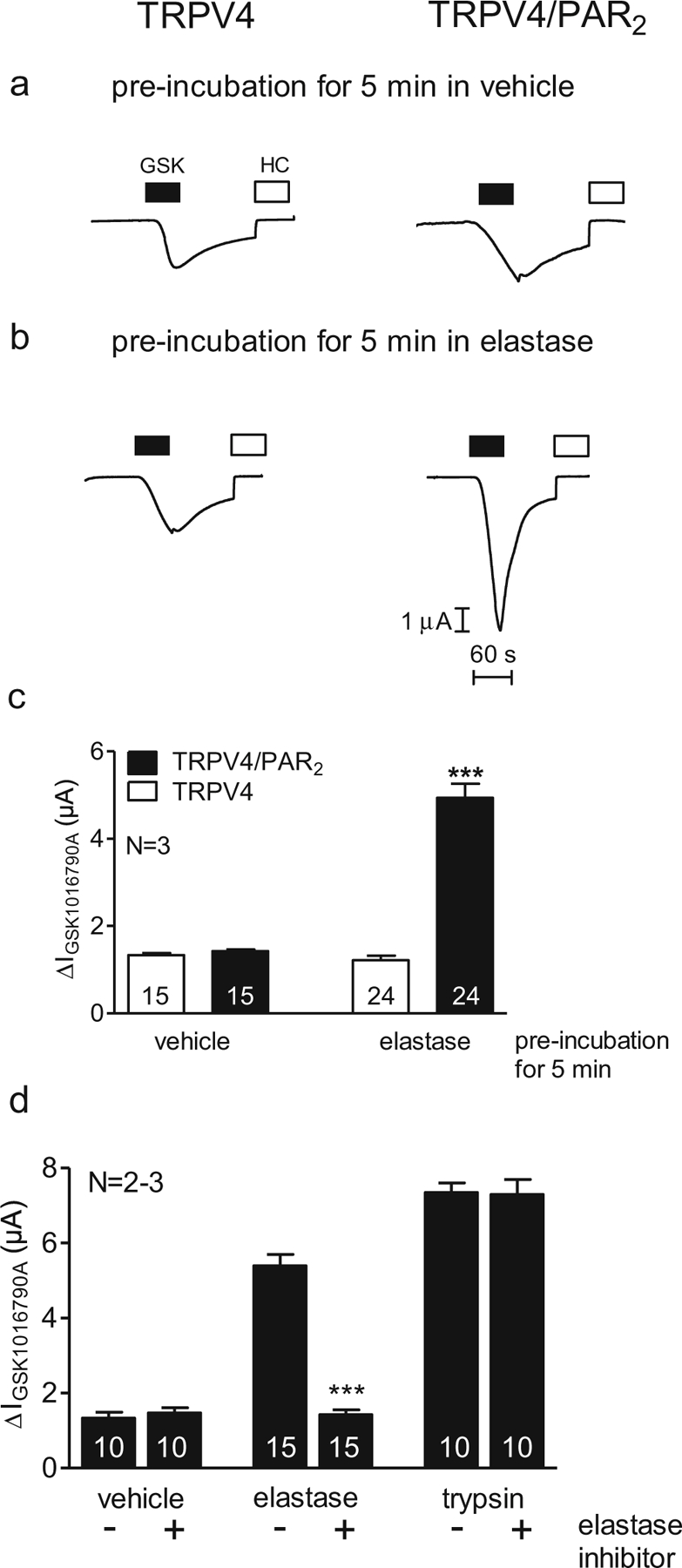
Proteolytic activation of PAR2 by the biased agonist human neutrophil elastase sensitises TRPV4. Oocytes were injected with cRNA coding for TRPV4 0.5 ng or TRPV4 0.5 ng/PAR2 10 ng and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. a, b Representative whole-cell current traces of oocytes expressing TRPV4 (left side) or TRPV4/PAR2 (right side) recorded in Ca2+-free solution. Activation and inhibition of TRPV4 by GSK1016790A (GSK, closed bars; 50 nM) and HC067047 (HC, open bars; 100 nM), respectively, after pre-incubation for 5 min in only Ca2+-free solution (vehicle; a) or in Ca2+-free solution in the presence of elastase (3 μM; b). c Columns represent mean ΔIGSK1016790A values. d Columns represent mean ΔIGSK1016790A of oocytes expressing TRPV4/PAR2. N indicates the number of batches of oocytes. Numbers inside the columns indicate the number of individual oocytes measured. ***p<0.001, unpaired t test
Rho-kinase inhibitor Y27362 inhibits PAR2-mediated sensitisation of TRPV4 by neutrophil elastase
Rho-kinase inhibitors block elastase and PAR2-dependent p44/42 MAPK signalling [56]. Therefore, we investigated the effect of the Rho-kinase inhibitor Y27362 on PAR2-mediated sensitisation of TRPV4. Oocytes expressing TRPV4/PAR2 were pre-incubated in neutrophil elastase (3 μM) or trypsin (8 nM) in the absence or presence of Rho-kinase inhibitor Y27362 (10 μM). Y27362 almost completely prevented PAR2-mediated sensitisation of TRPV4 by neutrophil elastase (Fig. 9). In contrast, Y27362 did not affect PAR2-mediated TRPV4 sensitisation by trypsin. In control experiments, Y27362 had no effect on the GSK1016790A-mediated TRPV4 currents in TRPV4-expressing oocytes. Furthermore, to rule out the possibility that the Rho-kinase inhibitor prevents PAR2 activation by non-specifically inhibiting the proteolytic activity of elastase, we tested the channel-activating effect of elastase in the presence or absence of Rho-kinase inhibitor on oocytes expressing the human epithelial sodium channel (ENaC). Elastase is known to proteolytically activate ENaC [1, 28]. We demonstrated that proteolytic ENaC activation by elastase was preserved in the presence of the Rho-kinase inhibitor (data not shown). Thus, the inhibitory effect of the Rho-kinase inhibitor on the PAR2-mediated sensitisation of TRPV4 by elastase cannot be explained by an inhibition of the proteolytic activity of elastase. Taken together, these findings indicate that the signalling pathway for PAR2-mediated sensitisation of TRPV4 by elastase critically involves Rho-kinase.
Fig. 9.
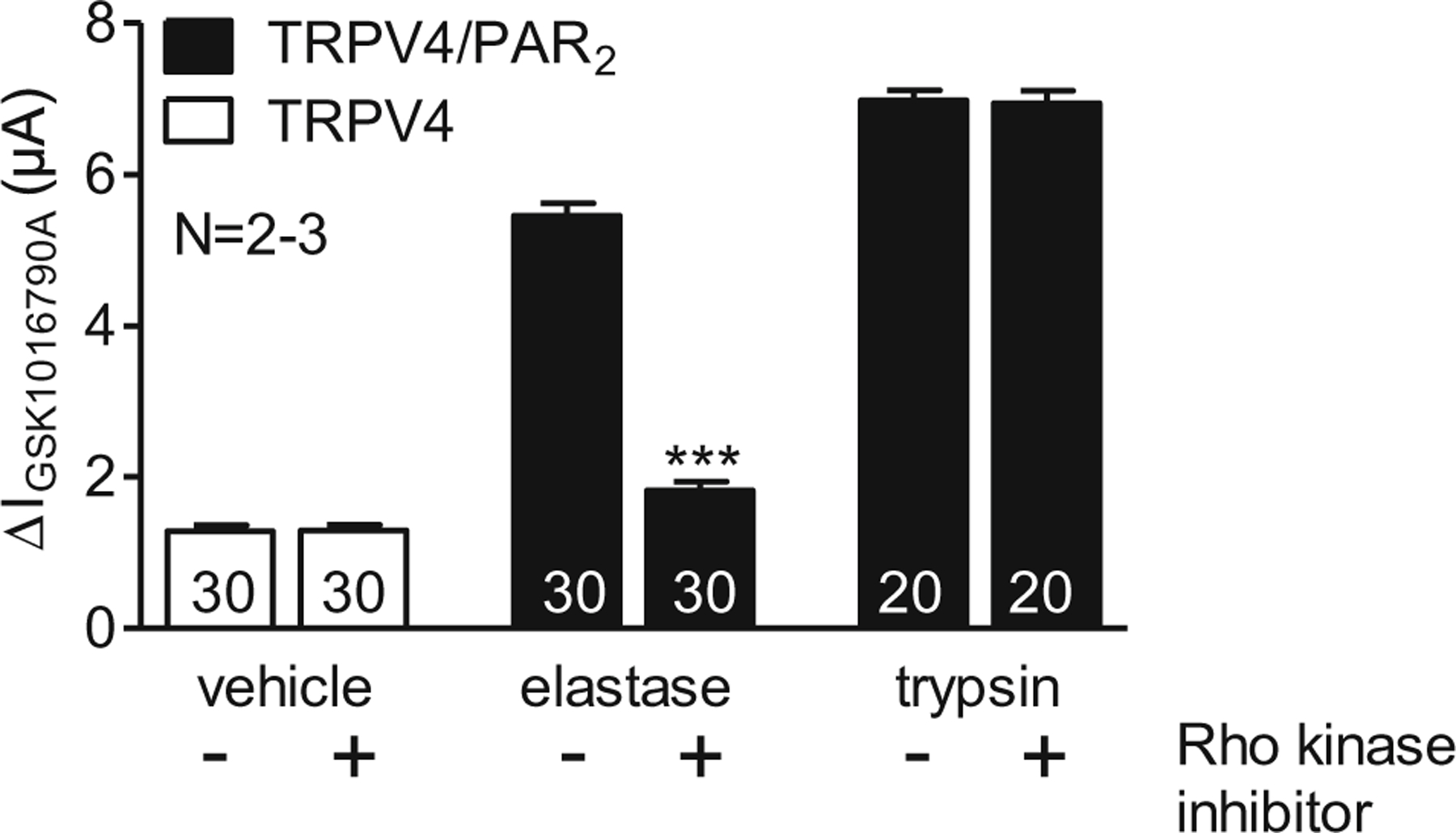
Rho-kinase inhibitor Y27362 inhibits PAR2-mediated sensitisation of TRPV4 by neutrophil elastase Oocytes were injected with cRNA coding for TRPV4 0.5 ng or TRPV4 0.5 ng/PAR2 10 ng and incubated for 2 days in ND96 solution. GSK1016790A-mediated whole-cell currents were measured using the two-electrode voltage-clamp technique. Oocytes expressing TRPV4 (white bars) were pre-incubated for 15 min in Ca2+-free solution in the absence (−) or in the presence (+) of Rho-kinase inhibitor Y27362 (10 μM). Oocytes expressing TRPV4/PAR2 (black bars) were pre-incubated for 15 min in elastase (3 μM) or trypsin (8 nM) in the absence (−) or in the presence (+) of Rho-kinase inhibitor (10 μM). Columns represent mean ΔIGSK1016790A values. N indicates the number of batches of oocytes. Numbers inside the columns indicate the number of individual oocytes measured. ***p<0.001, unpaired t test
Discussion
To our knowledge, this is the first study to demonstrate that, in addition to trypsin, the biased PAR2 agonist elastase also sensitises TRPV4 via PAR2 signalling. This suggests that PAR2-mediated sensitisation of TRPV4 is independent of intracellular calcium signalling, which is further supported by our finding that buffering intracellular calcium does not prevent PAR2-mediated TRPV4 sensitisation. Moreover, we identified Rho-kinase as one of the principal signalling pathways involved in PAR2-dependent sensitisation of TRPV4 by neutrophil elastase. In addition, we report that the stimulatory effect of the known TRPV4 channel activator GSK1016790A results from the recruitment of near-silent channels in the plasma membrane. Upon exposure to GSK1016790A, these near-silent channels are converted to channels with an open probability of nearly one.
In single-channel current recordings from outside-out patches, we confirmed that human TRPV4 functions as a non-selective cation channel permeable for sodium and potassium ions. The single-channel conductance of about 100 pS for outward currents and 55 pS for inward currents and the small degree of inward and outward rectification in our experiments are in good agreement with previously reported data. Indeed, in recent years, several groups have made TRPV4 single-channel recordings showing inward and outward rectification mainly using mouse and rat TRPV4. Watanabe et al. have reported a single-channel conductance of about 100 pS for outward currents and 60 pS for inward currents in HEK293 cells transfected with mouse TRPV4 [74, 76, 77]. In the oocyte expression system, single-channel recordings have been described for rat TRPV4 (100 pS for outward currents and 45 pS for inward currents) [42]. A higher single-channel conductance of 310 pS has been reported for chicken VR-OAC (TRPV4) expressed in CHO cells [40]. However, this value was estimated under different experimental conditions from one inside-out patch clamp recording at a holding potential of +80 mV without analysis of the current voltage relationship. For the human heteromeric TRPV4/TRPC1 channel, a single-channel conductance of about 90 pS for outward currents and 60 pS for inward currents was reported in HEK293 cells using cell-attached patches [43].
In 50 % of outside-out membrane patches from TRPV4 expressing oocytes, we observed a sizeable spontaneous single-channel activity without prior application of GSK1016790A (Fig. 2d). In contrast, spontaneous TRPV4 activity prior to the application of GSK1016790A was minimal in whole-cell current measurements in TRPV4 expressing oocytes (e.g. Fig. 1). The latter finding is expected since our experiments were performed at room temperature and TRPV4 is known to be spontaneously active only at temperatures >24 to 27 °C [20]. Thus, TRPV4 is probably inactive in oocytes maintained at room temperature but may have some degree of spontaneous activity in mammalian cells at 37 °C. At present, the molecular mechanism of heat activation remains unclear but may involve additional heat-sensitive proteins associated with the channel in the plasma membrane [72, 73]. Therefore, the sensitivity of TRPV4 to heat may be altered by patch excision [49], which may explain our finding of spontaneous TRPV4 activity in outside-out patches from oocytes expressing TRPV4.
The selective TRPV4 agonist GSK1016790A has been widely used to study the role of TRPV4 channels [23, 67]. However, the mechanism by which GSK1016790A activates TRPV4 is not well understood. In the present study, we used the outside-out configuration of the patch clamp technique to observe the effect of GSK1016790A at the single-channel level. Analysis of our data obtained in the outside-out configuration of the patch clamp technique revealed that the activation of TRPV4 by GSK1016790A was mainly mediated by recruitment of previously silent channels that are already present in the plasma membrane and not by an increase in single-channel current amplitude. As our recordings were performed in outside-out patches under calcium-free conditions on the extracellular and intracellular sides of the plasma membrane, it is unlikely that the recruitment of additional channels was mediated by insertion of vesicles containing TRPV4, because vesicle fusion with the plasma membrane usually requires a calcium signal. Our findings provide direct electrophysiological evidence to support the conclusion of a recent study using total internal reflection fluorescence microscopy to record TRPV4 channel activity in primary human endothelial cells. The authors report that TRPV4 protein is evenly distributed throughout the plasma membrane, but that most channels are silent. Moreover, their optical recordings indicate that GSK1016790A acts by recruiting previously inactive TRPV4 channels rather than by increasing elevation of basal activity [65]. GSK1016790A may activate the silent TRPV4 channels by changing their conformation possibly by interacting with a specific binding site. However, further studies are needed to elucidate the precise molecular mechanism(s) of TRPV4 activation by GSK1016790A and the physiological role of an apparently large pool of near silent channels in the plasma membrane.
It is well known that TRPV4 mediates neurogenic inflammation and pain [47]. TRPV4 is also discussed as a potential therapeutic target to treat intestinal inflammation and inflammatory bowel disease [69]. One major downstream target of PAR2 is TRPV4 [6, 24, 54, 62]. After activation, PAR2 can regulate multiple pathophysiological processes, including inflammation, pain, haemostasis and wound healing. For instance, it has been reported that PAR2 activation by trypsin causes widespread inflammation [70]. PAR2 is also believed to play a role in allergic lung inflammation, and PAR2 antagonists may be useful drugs to treat inflammatory airway disease [12]. Furthermore, PARs may be involved in neurogenic inflammation, in neurodegenerative processes and also in nociception [70]. Here, we demonstrated for the first time that proteolytic activation of PAR2 by neutrophil elastase potentiates the response to a TRPV4 agonist leading to a sensitisation, which may play an important role in the context of nociception. The “coupling” of TRPV4/PAR2 may be relevant in inflammatory diseases such as arthritis or inflammatory bowel disease, where multiple PAR2-activating proteases are produced [24]. The observed PAR2-mediated TRPV4 sensitisation by neutrophil elastase suggests a potential pathophysiological link between PAR2 and TRPV4 in the context of inflammation. Elastase released from neutrophils has been correlated to the pathologic processes of a variety of inflammatory diseases, including idiopathic pulmonary fibrosis, rheumatoid arthritis, adult respiratory distress syndrome and cystic fibrosis [17]. Activation of PAR2 by elastase may explain the recently reported activation of TRPV4 by ultraviolet radiation, which depends on an up-regulation of neutrophil elastase in response to ultraviolet exposure of the skin [46].
In summary, our study demonstrates that the oocyte expression system is suitable to study the mechanisms of TRPV4 activation by GSK1016790A at the single-channel level and to investigate the regulation of TRPV4 by PAR2 in co-expression experiments using different PAR2 agonists. In whole-cell recordings, we confirmed previous findings that trypsin-mediated activation of PAR2 markedly enhanced TRPV4 currents. Moreover, we demonstrated for the first time that the biased PAR2 agonist neutrophil elastase could also sensitise TRPV4 in a PAR2-dependent manner. PAR2 signalling elicited by neutrophil elastase does not involve intracellular calcium signalling [29, 57]. This is in good agreement with our finding that PAR2-mediated sensitisation of TRPV4 was unaffected by buffering intracellular calcium by BAPTA. Thus, our BAPTA experiments and the fact that neutrophil elastase is a biased PAR2 agonist without calcium signalling indicate that PAR2-mediated TRPV4 activation is independent of intracellular calcium. Our finding that neutrophil elastase causes a PAR2-dependent sensitisation of TRPV4 reveals, for the first time, a potential physiological consequence of biased agonism of PAR2 by elastase. Moreover, we identified Rho-kinase as one of the principal signalling pathways involved in PAR2-mediated TRPV4 sensitisation by neutrophil elastase. Interestingly, PAR2-mediated TRPV4 sensitisation by trypsin was not affected by inhibition of Rho-kinase. This is in good agreement with the concept that the biased PAR2 agonist elastase activates a more limited number of signalling pathways than trypsin. Our results indicate that PAR2-mediated TRPV4 sensitisation by trypsin does neither depend on calcium signalling nor on Rho-kinase signalling but involves another signalling pathway that remains to be elucidated. Further studies are required to determine whether elastase-induced activation of PAR2 and sensitisation of TRPV4 could contribute to the proinflammatory and nociceptive effects of neutrophil elastase. In this case, inhibition of Rho-kinases may become a therapeutic strategy to prevent PAR2-mediated TRPV4 sensitisation by elastase in inflammatory diseases.
Acknowledgments
The expert technical assistance of Ralf Rinke is gratefully acknowledged. This study was supported by a PhD fellowship from the Bayerische Forschungsstiftung (S.S.) and by NHMRC grants 63303, 1031886, 1046860 and 1049682 and Monash University (N.W.B., P.M.).
Abbreviations
- TRPV4
Transient receptor potential vanilloid 4
- PAR2
Protease-activated receptor 2
- GPCR
G protein-coupled receptor
Contributor Information
Silvia Sostegni, Institut für Zelluläre und Molekulare Physiologie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Waldstr. 6, 91054 Erlangen, Germany.
Alexei Diakov, Institut für Zelluläre und Molekulare Physiologie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Waldstr. 6, 91054 Erlangen, Germany.
Peter McIntyre, Health Innovations Research Institute, School of Medical Sciences, RMIT University, Bundoora, VIC 3083, Australia.
Nigel Bunnett, Monash Institute of Pharmaceutical Sciences, 381 Royal Parade, Parkville, VIC 3052, Australia, Department of Pharmacology and Therapeutics, University of Melbourne, 1-100 Grattan Street, Parkville, VIC 3010, Australia.
Christoph Korbmacher, Institut für Zelluläre und Molekulare Physiologie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Waldstr. 6, 91054 Erlangen, Germany.
Silke Haerteis, Institut für Zelluläre und Molekulare Physiologie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Waldstr. 6, 91054 Erlangen, Germany.
References
- 1.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ (2007) A segment of γENaC mediates elastase activation of Na+ transport. J Gen Physiol 130:611–629. doi: 10.1085/jgp.200709781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barish ME (1983) A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol 342:309–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW (1996) Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J Biol Chem 271:22003–22016. doi: 10.1074/jbc.271.36.22003 [DOI] [PubMed] [Google Scholar]
- 4.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA (2008) Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134:2059–2069. doi: 10.1053/j.gastro.2008.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camerer E, Huang W, Coughlin SR (2000) Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A 97:5255–5260. doi: 10.1073/pnas.97.10.5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N (2008) Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135:937–946. doi: 10.1053/j.gastro.2008.05.024, 946 e1–2 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RW, Taylor AB, Wang F, Guilak F, Liedtke W (2013) Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain 154:1295–1304. doi: 10.1016/j.pain.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Yang C, Wang ZJ (2011) Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 193:440–451. doi: 10.1016/j.neuroscience.2011.06.085 [DOI] [PubMed] [Google Scholar]
- 9.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD (1998) Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol 111:127–138. doi: 10.1085/jgp.111.1.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW (1997) Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest 100:1383–1393. doi: 10.1172/JCI119658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottrell GS, Amadesi S, Grady EF, Bunnett NW (2004) Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem 279:13532–13539. doi: 10.1074/jbc.M312090200 [DOI] [PubMed] [Google Scholar]
- 12.de Boer JD, Van’t Veer C, Stroo I, van der Meer AJ, de Vos AF, van der Zee JS, Roelofs JJ, van der Poll T (2013) Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun doi: 10.1177/1753425913503387 [DOI] [PubMed]
- 13.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW (2000) beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148:1267–1281. doi: 10.1083/jcb.148.6.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng HX, Klein CJ, Yan J, Shi Y, Wu Y, Fecto F, Yau HJ, Yang Y, Zhai H, Siddique N, Hedley-Whyte ET, Delong R, Martina M, Dyck PJ, Siddique T (2010) Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet 42:165–169. doi: 10.1038/ng.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C (2008) Cleavage in the γ-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol 586:4587–4608. doi: 10.1113/jphysiol.2008.154435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diakov A, Korbmacher C (2004) A novel pathway of ENaC activation involves an SGK1 consensus motif in the C-terminus of the channel’s a-subunit. J Biol Chem 279:38134–38142. doi: 10.1074/jbc.M403260200 [DOI] [PubMed] [Google Scholar]
- 17.Doring G (1994) The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med 150:S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114 [DOI] [PubMed] [Google Scholar]
- 18.Dulon S, Cande C, Bunnett NW, Hollenberg MD, Chignard M, Pidard D (2003) Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Am J Respir Cell Mol Biol 28:339–346. doi: 10.1165/rcmb.4908 [DOI] [PubMed] [Google Scholar]
- 19.Durieux ME, Salafranca MN, Lynch KR (1994) Trypsin induces Ca(2+)-activated Cl− currents in X. laevis oocytes. FEBS Lett 337: 235–238. doi: 10.1016/0014-5793(94)80198-3 [DOI] [PubMed] [Google Scholar]
- 20.Everaerts W, Nilius B, Owsianik G (2010) The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T (2010) Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A 107:19084–19089. doi: 10.1073/pnas.1005333107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson JE, Hanley MR (1992) Phosphatidic acid and lysophosphatidic acid stimulate receptor-regulated membrane currents in the Xenopus laevis oocyte. Arch Biochem Biophys 297:388–392 [DOI] [PubMed] [Google Scholar]
- 23.Gradilone SA, Masyuk TV, Huang BQ, Banales JM, Lehmann GL, Radtke BN, Stroope A, Masyuk AI, Splinter PL, LaRusso NF (2010) Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology 139(304–14):e2. doi: 10.1053/j.gastro.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578:715–733. doi: 10.1113/jphysiol.2006.121111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haerteis S, Krappitz M, Bertog M, Krappitz A, Baraznenok V, Henderson I, Lindstrom E, Murphy JE, Bunnett NW, Korbmacher C (2012) Proteolytic activation of the epithelial sodium channel (ENaC) by the cysteine protease cathepsin-S. Pflugers Arch 464: 353–365. doi: 10.1007/s00424-012-1138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haerteis S, Krappitz M, Diakov A, Krappitz A, Rauh R, Korbmacher C (2012) Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the γ-subunit of the human epithelial sodium channel. J Gen Physiol 140:375–389. doi: 10.1085/jgp.201110763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen KK, Sherman PM, Cellars L, Andrade-Gordon P, Pan Z, Baruch A, Wallace JL, Hollenberg MD, Vergnolle N (2005) A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci U S A 102: 8363–8368. doi: 10.1073/pnas.0409535102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC (2007) A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 282:58–64. doi: 10.1074/jbc.M605125200 [DOI] [PubMed] [Google Scholar]
- 29.Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, Ramachandran R (2014) Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br J Pharmacol 171:1180–1194. doi: 10.1111/bph.12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin M, Wu Z, Chen L, Jaimes J, Collins D, Walters ET, O’Neil RG (2011) Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PLoS ONE 6: e16713. doi: 10.1371/journal.pone.0016713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassmann M, Harteneck C, Zhu Z, Nurnberg B, Tepel M, Gollasch M (2013) Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol (Oxf) 207:546–564. doi: 10.1111/apha.12051 [DOI] [PubMed] [Google Scholar]
- 32.Knecht W, Cottrell GS, Amadesi S, Mohlin J, Skaregarde A, Gedda K, Peterson A, Chapman K, Hollenberg MD, Vergnolle N, Bunnett NW (2007) Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J Biol Chem 282:26089–26100. doi: 10.1074/jbc.M703840200 [DOI] [PubMed] [Google Scholar]
- 33.Korbmacher C, Volk T, Segal AS, Boulpaep EL, Frömter E (1995) A calcium-activated and nucleotide-sensitive nonselective cation channel in M-1 mouse cortical collecting duct cells. J Membr Biol 146:29–45 [DOI] [PubMed] [Google Scholar]
- 34.Krueger B, Haerteis S, Yang L, Hartner A, Rauh R, Korbmacher C, Diakov A (2009) Cholesterol depletion of the plasma membrane prevents activation of the epithelial sodium channel (ENaC) by SGK1. Cell Physiol Biochem 24:605–618. doi: 10.1159/000257516 [DOI] [PubMed] [Google Scholar]
- 35.Lamande SR, Yuan Y, Gresshoff IL, Rowley L, Belluoccio D, Kaluarachchi K, Little CB, Botzenhart E, Zerres K, Amor DJ, Cole WG, Savarirayan R, McIntyre P, Bateman JF (2011) Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat Genet 43:1142–1146. doi: 10.1038/ng.945 [DOI] [PubMed] [Google Scholar]
- 36.Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, Choo SS, Phelps CB, Paudel R, Houlden H, Ludlow CL, Caterina MJ, Gaudet R, Kleta R, Fischbeck KH, Sumner CJ (2010) Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet 42:170–174. doi: 10.1038/ng.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leddy HA, McNulty AL, Lee SH, Rothfusz NE, Gloss B, Kirby ML, Hutson MR, Cohn DH, Guilak F, Liedtke W (2014) Follistatin in chondrocytes: the link between TRPV4 channelopathies and skeletal malformations. FASEB J doi: 10.1096/fj.13-245936 [DOI] [PMC free article] [PubMed]
- 38.Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, Oliver T, Yasuda R, Ghio A, Simon SA, Liedtke W (2011) TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect 119:784–793. doi: 10.1289/ehp.1002807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liedtke W (2005) TRPV4 as osmosensor: a transgenic approach. Pflugers Arch 451:176–180. doi: 10.1007/s00424-005-1449-8 [DOI] [PubMed] [Google Scholar]
- 40.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535. doi: 10.1016/S0092-8674(00)00143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohman RJ, Cotterell AJ, Suen J, Liu L, Do AT, Vesey DA, Fairlie DP (2012) Antagonism of protease-activated receptor 2 protects against experimental colitis. J Pharmacol Exp Ther 340:256–265. doi: 10.1124/jpet.111.187062 [DOI] [PubMed] [Google Scholar]
- 42.Loukin S, Su Z, Zhou X, Kung C (2010) Forward genetic analysis reveals multiple gating mechanisms of TRPV4. J Biol Chem 285: 19884–19890. doi: 10.1074/jbc.M110.113936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma X, Nilius B, Wong JW, Huang Y, Yao X ( 2011) Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochim Biophys Acta 1808:2789–2797. doi: 10.1016/j.bbamem.2011.07.049 [DOI] [PubMed] [Google Scholar]
- 44.Mize GJ, Wang W, Takayama TK (2008) Prostate-specific kallikreins-2 and −4 enhance the proliferation of DU-145 prostate cancer cells through protease-activated receptors-1 and −2. Mol Cancer Res 6:1043–1051. doi: 10.1158/1541-7786.MCR-08-0096 [DOI] [PubMed] [Google Scholar]
- 45.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF (1997) Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem 272:4043–4049 [DOI] [PubMed] [Google Scholar]
- 46.Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, Parekh P, Lee SH, Kontchou NA, Yeh I, Jokerst NM, Fuchs E, Steinhoff M, Liedtke WB (2013) UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A 110: E3225–E3234. doi: 10.1073/pnas.1312933110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilius B (2007) TRP channels in disease. Biochim Biophys Acta 1772:805–812. doi: 10.1016/j.bbadis.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 48.Nilius B, Voets T (2013) The puzzle of TRPV4 channelopathies. EMBO Rep 14:152–163. doi: 10.1038/embor.2012.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilius B, Voets T (2005) TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch 451:1–10. doi: 10.1007/s00424-005-1462-y [DOI] [PubMed] [Google Scholar]
- 50.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J (1994) Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A 91:9208–9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, Scarisbrick I, Andrade-Gordon P, Cottrell GS, Bunnett NW, Diamandis EP, Hollenberg MD (2006) Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem 281:32095–32112. doi: 10.1074/jbc.M513138200 [DOI] [PubMed] [Google Scholar]
- 52.Ossovskaya VS, Bunnett NW (2004) Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84:579–621. doi: 10.1152/physrev.00028.2003 [DOI] [PubMed] [Google Scholar]
- 53.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F (2009) Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum 60:3028–3037. doi: 10.1002/art.24799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu T, Darby W, Liedtke W, Lew MJ, McIntyre P, Bunnett NW (2013) Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem 288:5790–5802. doi: 10.1074/jbc.M112.438184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raisinghani M, Zhong L, Jeffry JA, Bishnoi M, Pabbidi RM, Pimentel F, Cao DS, Evans MS, Premkumar LS (2011) Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception. Am J Physiol Cell Physiol 301:C587–C600. doi: 10.1152/ajpcell.00465.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD (2011) Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J Biol Chem 286:24638–24648. doi: 10.1074/jbc.M110.201988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, Defea K, Hollenberg MD (2009) Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol 76: 791–801. doi: 10.1124/mol.109.055509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramsay AJ, Dong Y, Hunt ML, Linn M, Samaratunga H, Clements JA, Hooper JD (2008) Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J Biol Chem 283:12293–12304. doi: 10.1074/jbc.M709493200 [DOI] [PubMed] [Google Scholar]
- 59.Ramsay AJ, Reid JC, Adams MN, Samaratunga H, Dong Y, Clements JA, Hooper JD (2008) Prostatic trypsin-like kallikrein-related peptidases (KLKs) and other prostate-expressed tryptic proteinases as regulators of signalling via proteinase-activated receptors (PARs). Biol Chem 389:653–668. doi: 10.1515/BC.2008.078 [DOI] [PubMed] [Google Scholar]
- 60.Rauh R, Diakov A, Tzschoppe A, Korbmacher J, Azad AK, Cuppens H, Cassiman JJ, Dotsch J, Sticht H, Korbmacher C (2010) A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition. J Physiol 588:1211–1225. doi: 10.1113/jphysiol.2009.180224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, Lachman RS, Wilcox WR, Reyno S, Quadrelli R, Vaglio A, Owsianik G, Janssens A, Voets T, Ikegawa S, Nagai T, Rimoin DL, Nilius B, Cohn DH (2008) Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet 40:999–1003. doi: 10.1038/ng.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW (2008) Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008 [DOI] [PubMed] [Google Scholar]
- 63.Smith R, Jenkins A, Lourbakos A, Thompson P, Ramakrishnan V, Tomlinson J, Deshpande U, Johnson DA, Jones R, Mackie EJ, Pike RN (2000) Evidence for the activation of PAR-2 by the sperm protease, acrosin: expression of the receptor on oocytes. FEBS Lett 484:285–290. doi: 10.1016/S0014-5793(00)02146-3 [DOI] [PubMed] [Google Scholar]
- 64.Suen JY, Barry GD, Lohman RJ, Halili MA, Cotterell AJ, Le GT, Fairlie DP (2012) Modulating human proteinase activated receptor 2 with a novel antagonist (GB88) and agonist (GB110). Br J Pharmacol 165:1413–1423. doi: 10.1111/j.1476-5381.2011.01610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S (2012) Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol Pharmacol 82:464–472. doi: 10.1124/mol.112.078584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS (2000) Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 275:26333–26342. doi: 10.1074/jbc.M002941200 [DOI] [PubMed] [Google Scholar]
- 67.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD (2008) N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}−3-hydroxypropanoyl)-1-piperazinyl]carbonyl}−3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther 326:432–442. doi: 10.1124/jpet.108.139295 [DOI] [PubMed] [Google Scholar]
- 68.Veldhuis NA, Bunnett NW (2013) Proteolytic regulation of TRP channels: implications for pain and neurogenic inflammation. Proc Aust Physiol Soc 44:101–108 [Google Scholar]
- 69.Vergnolle N (2014) TRPV4: new therapeutic target for inflammatory bowel diseases. Biochem Pharmacol 89:157–161. doi: 10.1016/j.bcp.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 70.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD (2001) Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 22:146–152. doi: 10.1016/S0165-6147(00)01634-5 [DOI] [PubMed] [Google Scholar]
- 71.Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA (2009) Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389:490–494. doi: 10.1016/j.bbrc.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 72.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754. doi: 10.1038/nature02732 [DOI] [PubMed] [Google Scholar]
- 73.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A 101:396–401. doi: 10.1073/pnas.0303329101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577. doi: 10.1074/jbc.M200062200 [DOI] [PubMed] [Google Scholar]
- 75.Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, Nilius B (2003) Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium 33:489–495. doi: 10.1016/S0143-4160(03)00064-2 [DOI] [PubMed] [Google Scholar]
- 76.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424:434–438. doi: 10.1038/nature01807 [DOI] [PubMed] [Google Scholar]
- 77.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B (2002) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277:47044–47051. doi: 10.1074/jbc.M208277200 [DOI] [PubMed] [Google Scholar]
- 78.Wilson S, Greer B, Hooper J, Zijlstra A, Walker B, Quigley J, Hawthorne S (2005) The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem J 388:967–972. doi: 10.1042/BJ20041066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ, Laznik D, Kamenecka TM, Song X, Liedtke W, Mootha VK, Puigserver P, Griffin PR, Clapham DE, Spiegelman BM (2012) TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 151: 96–110. doi: 10.1016/j.cell.2012.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]


