Abstract
CHD2‐related epilepsy is characterized by early‐onset photosensitive myoclonic epilepsy with developmental delay and a high rate of pharmacoresistance. We sought to evaluate the efficacy of acetazolamide (ACZ) in CHD2‐related epilepsy, due to ACZ's unexpected efficacy in our first patient harboring a pathogenic CHD2 variant. We collected patients from different Eastern European countries with drug‐resistant CHD2‐related epilepsy who were then treated with ACZ. Patients underwent video EEG before and during ACZ treatment. In a zebrafish model of CHD2‐related epilepsy, ictal‐like events were recorded 5 days post‐fertilization after overnight ACZ exposure. Developmental delay preceded the onset of seizures in 10 of the 12 patients. Four had ataxia, and 6 exhibited autistic features. Seizures, primarily myoclonic, began at an average age of 3.4 years and were photosensitive in all 12 patients. Add‐on ACZ treatment controlled photosensitive seizures in all patients: 6 became seizure‐free, and in the remaining 6, seizure frequency decreased by over 75%. Four patients transitioned to ACZ monotherapy. The median follow‐up was 13 months. In the zebrafish model, ACZ exposure reduced ictal‐like events by 72%. ACZ, a well‐tolerated and cost‐effective medication, could be a good option for CHD2‐related epilepsy, predominantly manifesting with myoclonic seizures and photosensitivity.
Plain Language Summary
Epilepsy associated with CHD2 mutations is often pharmacoresistant and associated with developmental delay and eventually ataxia. There are several generalized seizure types, including generalized tonic–clonic seizures, but the most characteristic are jerks triggered by light stimulation. We collected 12 patients who received acetazolamide, a drug usually given as a diuretic and registered as a mild antiseizure medication. All jerks triggered by light disappeared while the frequency of spontaneous seizures decreased by over 75%. Further studies are needed to confirm this promising finding and identify the mechanism by which an old compound seems to have such a specific antiseizure effect.
Keywords: developmental epileptic encephalopathy, myoclonic seizures, photosensitivity, tonic–clonic seizures, zebrafish
Key points.
CHD2‐related epilepsy features predominantly myoclonic seizures with high rate of photosensitivity and is often resistant to standard ASMs.
Add‐on acetazolamide, an underrated drug, could be an effective treatment option for drug‐resistant CHD2‐related epilepsy.
In a zebrafish model, acetazolamide showed superior efficacy to fenfluramine, reducing ictal‐like events by ~72%.
Further large‐scale clinical studies and experimental research are needed to explore acetazolamide's mode of action and effectiveness.
1. INTRODUCTION
The great variability of epilepsy phenotypes and etiology, and the large number of antiseizure medications (ASMs) provide a significant challenge to treatment strategy. Following the initial distinction between focal and generalized epilepsies, the definition of epilepsy syndromes was the next step pointing to specific treatment effects of various ASMs. 1 , 2 Many epilepsy phenotypes, however, do not correspond to any epilepsy syndromes included in the International League Against Epilepsy Classification, 1 and genetics has added opportunities to disclose still unrecognized conditions.
Pathogenic variants in CHD2, encoding the chromodomain helicase DNA‐binding protein 2 (CHD2), are associated with a particular epilepsy phenotype in which myoclonus and photosensitivity are predominant, and over half of patients remain pharmacoresistant. 2 Microcephaly, developmental delay, and cerebellar ataxia are frequent. 3 , 4 In zebrafish, knockdown of chd2 also results in microcephaly and epileptiform activity as well as photosensitive seizures. 5
Acetazolamide (ACZ), an inhibitor of carbonic anhydrase, has been given to patients, including children for the treatment of epilepsy since the 1950s at doses ranging 10–20 mg/kg/d with good tolerability, 6 and is approved by the FDA and European agencies as an adjuvant therapy for epilepsy, although it is currently seldom used, due to poor efficacy. 7 Nevertheless, moderate benefits have been reported for ACZ, and a recent review by Shukralla et al. of 12 observational studies involving 941 patients taking ACZ, found that 20% achieved seizure freedom. 7 , 8
Furthermore, ACZ was recently reported to be effective in patients with a combination of photosensitive myoclonic epilepsy and ataxia, due to monogenic epilepsies, namely mutations in KCNMA1 9 and KCNA2. 10 Since the phenotype of these patients is similar to that of CHD2‐associated epilepsy, we offered parents ACZ as an add‐on treatment, as per usual clinical practice. Following encouraging observations, we advised colleagues of collaborating centers to offer it also to their patients. We then evaluated the effect of ACZ in 12 patients with CHD2‐related epilepsy and found remarkable efficacy.
2. PATIENTS AND METHODS
At MediClubGeorgia in Tbilisi, ACZ was offered to all 4 patients with CHD2 mutations, after the failure of the usual ASMs, as per normal clinical practice, in an attempt to improve their condition since encouraging observations had been reported in cases with a similar epilepsy phenotype. In the absence of dysmorphia, neither karyotype nor CGHarray was indicated. Findings of Epilepsy Gene Panel or whole‐exome sequencing, including CNV analysis, were interpreted according to the ACMG guidelines (Table 1A). Clinical data were analyzed based on files and parent interviews. As a usual practice, seizure diaries were given to the parents. Data were drawn before and upon ACZ treatment. Following encouraging observations, we informed colleagues from Eastern Europe with whom we usually interact. In 6 Eastern European epilepsy centers, 13 pediatric patients were identified with CHD2 mutations, who had not received ACZ, but whose epilepsy was resistant to at least 2 ASMs, and whose parents gave informed consent for the administration of ACZ as add‐on therapy.
TABLE 1A.
Clinical features and previous treatments.
| Patient No/current age/sex | CHD2 Gene alteration | Age of onset/type of first seizure | Seizure types | Cognitive development before seizures | Currently intellectual disability/autism spectrum disorder | Age ataxia | ASM used |
|---|---|---|---|---|---|---|---|
| 1/14/F |
c.1934C > A pathogenic missense |
6/Eyelid fluttering | Upward deviation of eyes/eyelid fluttering, evaluated as myoclonic | Borderline at 3 | Severe/Yes | 5 | ESX, LZP |
| 2/8/M |
c.236 T > C missense VUS |
2/Myoclonic jerks | Myoclonic jerks, eyelid myoclonus | Normal | No/No | No | CZP, ESX |
| 3/8/F |
c.2647_2649 Del in‐frame VUS |
6/Staring, then loss of consciousness | Absence with hypersalivation and oral automatisms, with postictal confusion and inability to communicate, myoclonic jerks, eyelid myoclonus | Mild speech disability | Mild/No | No | LEV, CBZ |
| 4/14/F |
c.3774Dup (p.Tyr1246IllfsTer13) likely pathogenic frameshift |
2/Febrile convulsive seizure | Myoclonic seizures, GTCS | Mild disability | Mild/No | No | CBZ, LEV, VPA |
| 5/17/M |
CHD2‐c.4176_4177Del pathogenic frameshift |
2y/Febrile convulsive seizure | GTCS, eyelid myoclonus | Normal | Moderate/Yes | 3 | VPA. LEV, CBZ |
| 6/13/F |
c.1717dupA likely pathogenic frameshift |
4/GTCS |
Myoclonic‐atonic seizures, oro‐alimentary automatisms, GTCS Comment: Spontaneous seizures stopped on LEV, Before ACZ treatment, only myoclonic seizures on IPS |
Mild disability from birth | Mild disability/No | No | LTG, VPA, LEV |
| 7/10/F |
c.1453C > T pathogenic nonsense |
3/Eyelid myoclonus | Eyelid myoclonus, GTCS | Disability from first year | Mild/Yes | No | VPA, ESX, CBD, LEV |
| 8/6/M |
c.294 + 1G > A noncoding likely pathogenic |
1/Myoclonic jerks | Myoclonic jerks, eyelid myoclonus | Disability from first year | Mild/No | No | LEV, VPA |
| 9/15/F |
c.1143_1146delAAGA p.Glu381fs likely pathogenic nonsense |
1/Eyelid myoclonus | Eyelid myoclonus, GTCS | Disability from first year | Mild/No | No | VPA, LEV |
| 10/4/F |
c.2686C > T pathogenic nonsense |
1.5/Absences with eyelid myoclonus | Absences with eyelid myoclonus | Moderate disability | Moderate/Yes | 1.5 | LEV, VPA, ESX |
| 11/9/F | Microdeletion chromosome 15 including exons 6–12 of the CHD2 gene | 7/Myoclonic jerks | Myoclonic jerks | Moderate disability | Moderate/Yes | 2 | VPA, LEV |
| 12/17/F |
c.5035C > T pathogenic nonsense |
6/Absences with eyelid myoclonus |
Absences with eyelid myoclonus, GTCS. Comment: Spontaneous seizures stopped on VPA, eyelid myoclonus remained on IPS |
Moderate disability | Moderate/Yes | No | VPA, LEV, TPM |
Abbreviations: ASM, antiseizure medication; CBD, cannabidiol; CLB, clobazam; CZP, carbamazepine; ESX, ethosuximide; F, female; GTCS, generalized tonic–clonic seizure; IPS, intermittent photic stimulation; LEV, levetiracetam; LTG, lamotrigine; LZP, lorazepam; M, male (Age is given in years); TPM, topiramate; VPA, valproate; ZNS, zonisamide.
In these centers, parents of all patients with pharmacoresistant CHD2‐associated epilepsy were offered it as add‐on therapy. Therefore, the 13 included patients represent the exhaustive population of patients with pharmacoresistant CHD2‐related epilepsy, who were given ACZ and were followed in the various contributing centers. One child was lost to follow‐up for familial reasons, soon after the inclusion, and the studied series comprises therefore 12 patients, 9 females, and 3 males. Parents gave their written permission to publish the data.
Video‐EEG monitoring employed a standard 10–20 electrode placement system. Intermittent photic stimulation and hyperventilation were performed according to American Clinical Neurophysiology Society guidelines. 11
A zebrafish model of CHD2‐related epilepsy was generated by antisense knockdown using a morpholino oligomer (MO) targeting the zebrafish chd2 gene, as described. 5 Electrophysiological analysis of ictal‐like events was performed by local field potential (LFP) recordings of zebrafish larvae at 5 days post‐fertilization (dpf). 12 Drug treatment was carried out by overnight exposure of zebrafish larvae to the maximum tolerated concentration (MTC) of ACZ (1 mM) in embryo medium. Zebrafish experiments were performed under ethical approval by the Norwegian Food Safety Authority (Mattilsynet), approval number 23935 dated 1 July 2020.
3. RESULTS
Two patients had a history of febrile seizures (Table 1A). Developmental delay preceded seizure onset in 10 out of 12 patients. In 1 patient, delay was noticed only after the seizure onset at the age of 3 years. Six patients showed autistic features, and 4 had ataxia.
The age of onset of non‐febrile seizures ranged from 1 to 7 years (average 3.4 years; SD = 2.05). Before ACZ therapy, seizure types included eyelid myoclonus in 9 patients, generalized tonic–clonic (GTCSs) in 6, myoclonic seizures (other than eyelid myoclonus) in 6, absences with oral automatisms in 2, myoclonic absences in 2, and myoclonic‐atonic seizures in 1. None of the patients exhibited self‐induced seizures. All patients had multiple seizure types and a variable myoclonic component: myoclonic jerks of both upper limbs, eyelid myoclonus, myoclonic absences, and/or myoclonic‐atonic seizures. Although seizures were occasionally asymmetric, no focal seizure was reported.
Interictal EEG showed normal background activity, including for patients with developmental delay. Bilateral spike–wave complexes predominated in posterior areas, while there were very few focal spikes. Photic stimulation triggered in all patients either eyelid myoclonus or myoclonic jerks of the upper limbs with polyspike‐slow wave discharges (Figure 1).
FIGURE 1.
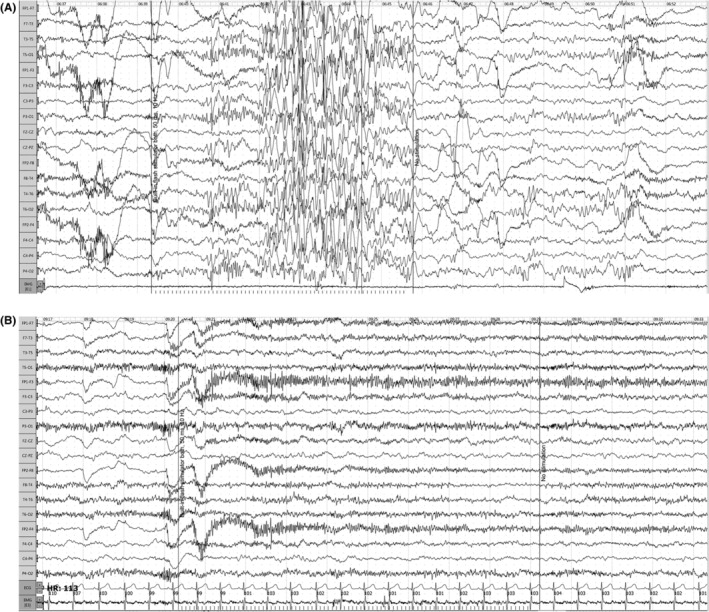
Patient 3: EEG on intermittent photic stimulation (10 Hz), before (A) and on (B) acetazolamide. Amplitude 10 mv/mm, sweep 30 mm/s, low‐pass filter −0.5 Hz, high‐pass filter −70 Hz.
Brain MRI was normal for all patients except for Patient #1 who displayed a right temporal lobe dysplasia although neither seizure types nor EEG revealed focal epileptic activity.
Gene sequencing uncovered 6 patients with missense and truncating point mutations, 2 intragenic duplications, and 4 deletions. Point mutations and intragenic duplications result in premature termination.
Epilepsy was resistant to various ASMs, including levetiracetam (10 patients), valproic acid (9 patients), ethosuximide (4 patients), carbamazepine (2 patients), and lamotrigine, topiramate, lorazepam, clobazam, and cannabidiol in various combinations. Until ACZ was started, all patients were photosensitive. Ten had intractable spontaneous seizures, while 2 had seizures only during photic stimulation.
According to seizure diary and EEG recording, ACZ stopped the remaining photosensitive seizures for all 12 patients (Supporting Information) and reduced or stopped the spontaneous seizures (Table 1B). Four seizure‐free patients were gradually transitioned to monotherapy, yielding seizure freedom in 2 patients, and reducing seizure frequency in the remaining 2 patients by ≥90% and ≥75%, respectively. For the 8 others, ACZ was given in combination with levetiracetam (5 patients), valproic acid (1 patient), levetiracetam plus valproic acid (1 patient), or valproic acid plus ethosuximide (1 patient): 4 became seizure‐free, and seizures were reduced by >75% in the other 4. The average effective dose of ACZ was 9.2 mg/kg ranging from 3.5 to 15. The follow‐up period ranged from 6 months to 3.5 years (median 13 months). None of the patients withdrew from the study, and ACZ was generally well‐tolerated with no significant adverse effects (Table 1B).
TABLE 1B.
Acetazolamide treatment and its effect.
| Patient number/Age (y) acetazolamide (ACZ) onset/Treatment lag from seizure onset to acetazolamide (ACZ) treatment(y) | Current dose (mg/kg/d) | Duration years/months | Current ASM | Effect of acetazolamide (ACZ) | Comment |
|---|---|---|---|---|---|
| 1/13/7 | 12 | 3/9 | ACZ | Reduction of myoclonic seizures >90% | Seizures controlled and behavior normalized |
| 2/8/6 | 10 | 1/3 | ACZ | Reduction of myoclonic seizures >75% | Seizures and photosensitivity were fully controlled for 6 months, then seizure reduction >75% remains |
| 3/8/2 | 9 | 0/9 | ACZ | Seizure‐free | Seizures controlled |
| 4/7/5 | 11 | 2/9 | ACZ | Seizure‐free | Seizures controlled |
| 5/17/4 | 15 | 0/9 | ACZ + LEV | Seizure‐free | Seizures controlled |
| 6/13/9 | 6 | 0/6 | ACZ + LEV | Seizure‐free | Photosensitivity controlled and behavior normalized |
| 7/10/7 | 9 | 0/6 | ACZ + LEV | Reduction of myoclonic seizures >75% | Nearly all seizures controlled |
| 8/6/5 | 15 | 0/6 | ACZ + LEV+VPA | Reduction of myoclonic seizures >90% | Nearly all seizures controlled |
| 9/15/14 | 3.5 | 0/6 | ACZ + LEV | Seizure‐free | Photosensitivity controlled |
| 10/4/3.5 | 7.8 | 0/6 | ACZ + LEV + ETX | Reduction of absences with eyelid myoclonus >75% | Seizures and photosensitivity reduced |
| 11/9/2 | 8 | 0/6 | ACZ + LEV | Reduction of myoclonic jerks >75% | Nearly all seizures controlled and photosensitivity reduced |
| 12/17/11 | 5 | 0/6 | ACZ + VPA | Seizure‐free | Photosensitivity controlled |
Abbreviations: ACZ, acetazolamide; m, months; y, years.
Partial knockdown of chd2 in zebrafish larvae, via microinjection of an antisense morpholino oligomer targeting the zebrafish chd2 gene, caused developmental delays and dysmorphology including microcephaly, body curvature, lack of swim bladder, twisted tail, pericardial edema, delayed morphogenesis of the pectoral fin as well as delays in craniofacial development (Figure 2a), as previously reported. 5 LFP recordings of chd2 knockdown larvae revealed ictal‐like events, as previously reported, 13 which were reduced by treatment with ACZ (Figure 2b). Treatment with ACZ reduced ictal‐like events by ca. 72%, from an average of 3.29 (n = 14) in chd2 MO larvae, to an average of 0.92 (n = 13) in ACZ‐treated chd2 knockdown larvae, while treatment with FEN did not result in a statistically significant reduction of ictal‐like events, with an average of 2.0 (n = 17) (Figure 2c).
FIGURE 2.
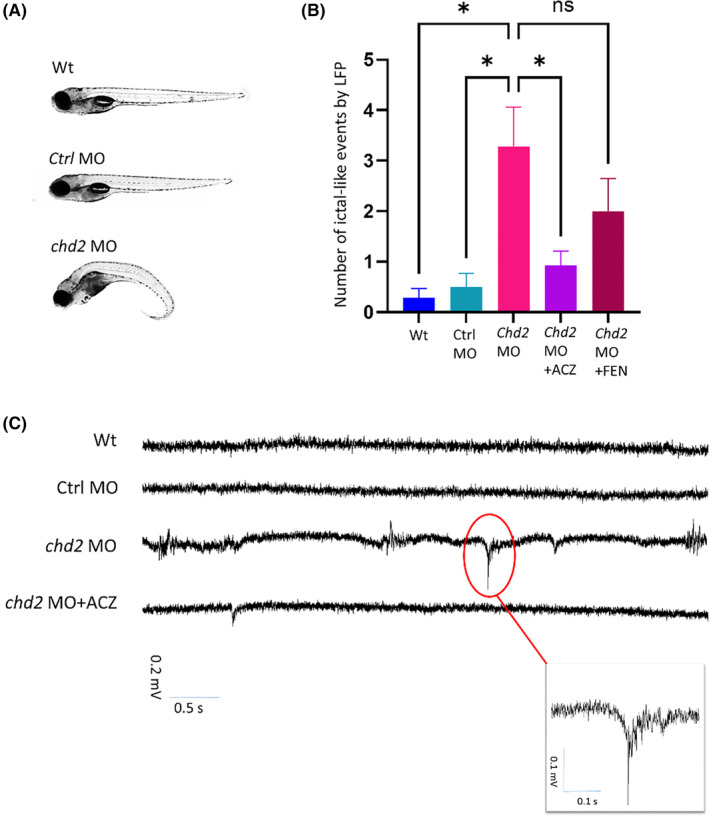
Antisense knockdown of chd2 in zebrafish larvae—morphological and electrophysiological analysis (A) chd2 knockdown and non‐injected control zebrafish larvae at 5 dpf. (B‐C) Local field potential (LFP) recording revealed epileptiform events in 5‐dpf chd2 knockdown larvae; chd2 knockdown larvae exhibited an increase in ictal‐like events compared to non‐injected control larvae at 5 dpf, non‐injected control larvae with a mean of 0.29 ± 0.18 (SEM), chd2 knockdown larvae with a mean of 3.29 ± 0.77, chd2 knockdown treated with 1 mM acetazolamide with a mean of 0.92 ± 0.29, chd2 knockdown treated with 100 μM fenfluramine overnight with a mean of 2.0 ± 0.64 ictal‐like events. Statistical analysis by one‐way ANOVA showed that treatment with 1 mM acetazolamide significantly reduced ictal‐like events (p < 0.05), but that for 100 μM fenfluramine, the result was not significant (p > 0.29), with all groups passing the D'Agostino‐Pearson normality test.
4. DISCUSSION
This series highlights the effect of ACZ on CHD2‐related epilepsy which, beyond its various types of generalized seizures, is mainly characterized by photosensitive myoclonus. Although epilepsy had lasted for several years, after ACZ initiation half of the patients reached total seizure freedom, while the other half had over 75% seizure reduction.
The mean age of seizure onset in our series is consistent with that of the largest series. 14 Most striking is the occurrence of photosensitive myoclonic seizures. CHD2 was found to be the main causative gene associated with photosensitive eyelid myoclonus, provided it was in the context of developmental delay. 15 GTCSs affected only half of our patients, while all patients of two other series involving older patients exhibited this type of seizures. 3
The phenotype of CHD2‐related epilepsy has been variously labeled, including (1) as juvenile myoclonic epilepsy (JME), 3 however the age of onset is too young for classical JME; (2) as Jeavons' syndrome of eyelid myoclonia with or without absences but there are developmental delay and frequent GTCSs 16 ; (3) as Dravet‐like syndrome, 5 but there was selection bias; (4) as epilepsy with myoclonic‐atonic seizures 17 ; (5) epileptic spasms with hypsarrhythmia, 4 (6) and Lennox–Gastaut syndrome, 18 but myoclonus and photosensitivity as prominent features is unusual in these conditions. Therefore, the phenotype of CHD2‐related epilepsy may deserve being considered as a clinically recognizable epilepsy syndrome and added to the classification of epilepsies. 1 Ataxia was present in four of our patients, but milder than reported by Thomas et al., 3 possibly because of the limited follow‐up period.
We could find no reports of patients who had received ACZ, while in our hands, this compound appeared to be very effective. If our results are confirmed by further studies, a new avenue to the specific treatment of CHD2‐related epilepsy could be opened.
We extended the findings in the human population in a zebrafish model of CHD2‐related epilepsy, where administration of ACZ to chd2 knockdown larvae resulted in a reduction of ictal events by approximately 72% (Figure 2c). In contrast, while chd2 knockdown larvae treated with fenfluramine also exhibited a reduction in ictal events, this effect was less pronounced and not statistically significant, suggesting that the effect of ACZ may be specific to the particular phenotype of CHD2‐related epilepsy.
Apart from its canonical and well‐known mode of action via the inhibition of carbonic anhydrase, thus causing acidosis, a variety of alternative explanations of ACZ's possible mode of action has emerged during the last two decades. ACZ has been reported to act on various membrane channels, including acid‐sensing ion channels, R‐type calcium channels, the water channel aquaporin‐4 (AQP4), and large conductance calcium and voltage‐activated potassium channel (BK channel), 19 which has recently been identified as a target of cannabidiol. 20 It is widely expressed in CNS, with notable prevalence in Purkinje Cells of cerebellum. 21 Each of the above‐mentioned pathways may contribute to epilepsy when altered. 22 Further experimental studies are needed to elucidate the mechanism of action of ACZ in CHD2‐related epilepsy.
4.1. Limitations
We gathered a small sample size with no control group, experienced difficulties in quantification of very brief seizures, and had limited follow‐up. However, CHD2‐related epilepsy is a rare condition representing 1% of unexplained pediatric epilepsies 23 ; ACZ is widely used and marketed as an ASM. Parents are unlikely to accept inclusion into a structured trial with a placebo group, repeat visits, blood samplings, and EEGs. For vigabatrin, inclusions into a prospective trial as first‐line drug for infantile spasms suddenly stopped when the drug was marketed and parents had access to it, and the patient sample remained too small to reach statistical significance. 24
Lack of experimental data involving electrophysiological studies on potential targets of ACZ requires in‐depth analysis in animal models to explore the molecular mechanisms behind this drug's effectiveness for CHD2‐related epilepsy.
4.2. Clinical Relevance or Future Directions
A prospective structured clinical study should improve seizure quantification, optimize ACZ dose ranging, and evaluate comedication strategies to set the basis of a randomized study.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Data S1
ACKNOWLEDGMENTS
We are grateful to Ana Mingorance, Domenico Tircarico, and Rusudan Karseladze for their valuable suggestions to the manuscript.
Melikishvili G, Striano P, Shojeinia E, Gachechiladze T, Kurua E, Tabatadze N, et al. Effectiveness of add‐on acetazolamide in children with drug‐resistant CHD2‐related epilepsy and in a zebrafish CHD2 model. Epilepsia Open. 2024;9:1972–1980. 10.1002/epi4.13034
Elham Shojeinia, Tamar Gachechiladze and Ekaterine Kurua are equally contributing authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bureau M, Genton P, Dravet C, Delgado‐Escueta A. Epileptic syndromes in infancy, childhood and adolescence. Paris: John Libbey Eurotext; 2019. [Google Scholar]
- 3. Thomas RH, Zhang LM, Carvill GL, Archer JS, Heavin SB, Mandelstam SA, et al. CHD2 myoclonic encephalopathy is frequently associated with self‐induced seizures. Neurology. 2015;84:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Zhang J, Liu A, Zhang L, Li H, Zeng Q, et al. CHD2‐related epilepsy: novel mutations and new phenotypes. Dev Med Child Neurol. 2020;62:647–653. [DOI] [PubMed] [Google Scholar]
- 5. Suls A, Jaehn JA, Kecskés A, Weber Y, Weckhuysen S, Craiu DC, et al. De novo loss‐of‐function mutations in CHD2 cause a fever‐sensitive myoclonic epileptic encephalopathy sharing features with Dravet syndrome. Am J Hum Genet. 2013;93:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Millichap JG. Anticonvulsant action of diamox in children. Neurology. 1956;6:552–559. [DOI] [PubMed] [Google Scholar]
- 7. Shukralla AA, Dolan E, Delanty N. Acetazolamide: old drug, new evidence? Epilepsia Open. 2022;7:378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katayama F, Miura H, Takanashi S. Long‐term effectiveness and side effects of acetazolamide as an adjunct to other anticonvulsants in the treatment of refractory epilepsies. Brain Dev. 2002;24:150–154. [DOI] [PubMed] [Google Scholar]
- 9. Bailey CS, Moldenhauer HJ, Park SM, Keros S, Meredith AL. KCNMA1‐linked channelopathy. J Gen Physiol. 2019;151:1173–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balaram N, Jose J, Gafoor A, Balachandran S, Saritha F, Dileep KV, et al. Acetazolamide responsive early‐onset absence epilepsy and ataxia in a toddler with a KCNA2 genetic variant; a case report. Seizure. 2023;110:157–159. [DOI] [PubMed] [Google Scholar]
- 11. Kuratani J, Pearl PL, Sullivan LR, Riel‐Romero RMS, Cheek J, Stecker MM, et al. American clinical neurophysiology society guideline 5: minimum technical standards for pediatric electroencephalography. Neurodiagn J. 2016;56:266–275. [DOI] [PubMed] [Google Scholar]
- 12. Turrini L, Sorelli M, de Vito G, et al. Multimodal characterization of seizures in zebrafish larvae. Biomedicine. 2022;10:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galizia EC, Myers CT, Leu C, de Kovel CGF, Afrikanova T, Cordero‐Maldonado ML, et al. CHD2 variants are a risk factor for photosensitivity in epilepsy. Brain. 2015;138:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Maria B, Balestrini S, Mei D, et al. Expanding the genetic and phenotypic spectrum of CHD2‐related disease: from early neurodevelopmental disorders to adult‐onset epilepsy. Am J Med Genet A. 2022;188:522–533. [DOI] [PubMed] [Google Scholar]
- 15. Coppola A, Krithika S, Iacomino M, Bobbili D, Balestrini S, Bagnasco I, et al. Dissecting genetics of spectrum of epilepsies with eyelid myoclonia by exome sequencing. Epilepsia. 2023;65:779–791. [DOI] [PubMed] [Google Scholar]
- 16. Striano S, Capovilla G, Sofia V, Romeo A, Rubboli G, Striano P, et al. Eyelid myoclonia with absences (Jeavons syndrome): a well‐defined idiopathic generalized epilepsy syndrome or a spectrum of photosensitive conditions? Epilepsia. 2009;50(Suppl 5):15–19. [DOI] [PubMed] [Google Scholar]
- 17. Trivisano M, Striano P, Sartorelli J, Giordano L, Traverso M, Accorsi P, et al. CHD2 mutations are a rare cause of generalized epilepsy with myoclonic‐atonic seizures. Epilepsy Behav. 2015;51:53–56. [DOI] [PubMed] [Google Scholar]
- 18. Lund C, Brodtkorb E, Øye AM, Røsby O, Selmer KK. CHD2 mutations in Lennox‐Gastaut syndrome. Epilepsy Behav. 2014;33:18–21. [DOI] [PubMed] [Google Scholar]
- 19. Tricarico D, Barbieri M, Mele A, Carbonara G, Conte Camerino D. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+‐deficient rats. FASEB J. 2004;18:760–761. [DOI] [PubMed] [Google Scholar]
- 20. Monat J, Altieri LG, Enrique N, Sedán D, Andrinolo D, Milesi V, et al. Direct inhibition of BK channels by cannabidiol, one of the principal therapeutic cannabinoids derived from Cannabis sativa . J Nat Prod. 2024;87:1368–1375. [DOI] [PubMed] [Google Scholar]
- 21. Ancatén‐González C, Segura I, Alvarado‐Sánchez R, Chávez AE, Latorre R. Ca2+− and voltage‐activated K+ (BK) channels in the nervous system: one gene, a myriad of physiological functions. Int J Mol Sci. 2023;24:3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teppema LJ. Multifaceted clinical effects of acetazolamide: will the underlying mechanisms please stand up? J Appl Physiol. 1985;2014(116):713–714. [DOI] [PubMed] [Google Scholar]
- 23. Kim SH, Seo J, Kwon SS, Teng LY, Won DJ, Shin S, et al. Common genes and recurrent causative variants in 957 Asian patients with pediatric epilepsy. Epilepsia. 2024;65:766–778. [DOI] [PubMed] [Google Scholar]
- 24. Appleton RE, Peters AC, Mumford JP, Shaw DE. Randomised, placebo‐controlled study of vigabatrin as first‐line treatment of infantile spasms. Epilepsia. 1999;40:1627–1633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


