Abstract
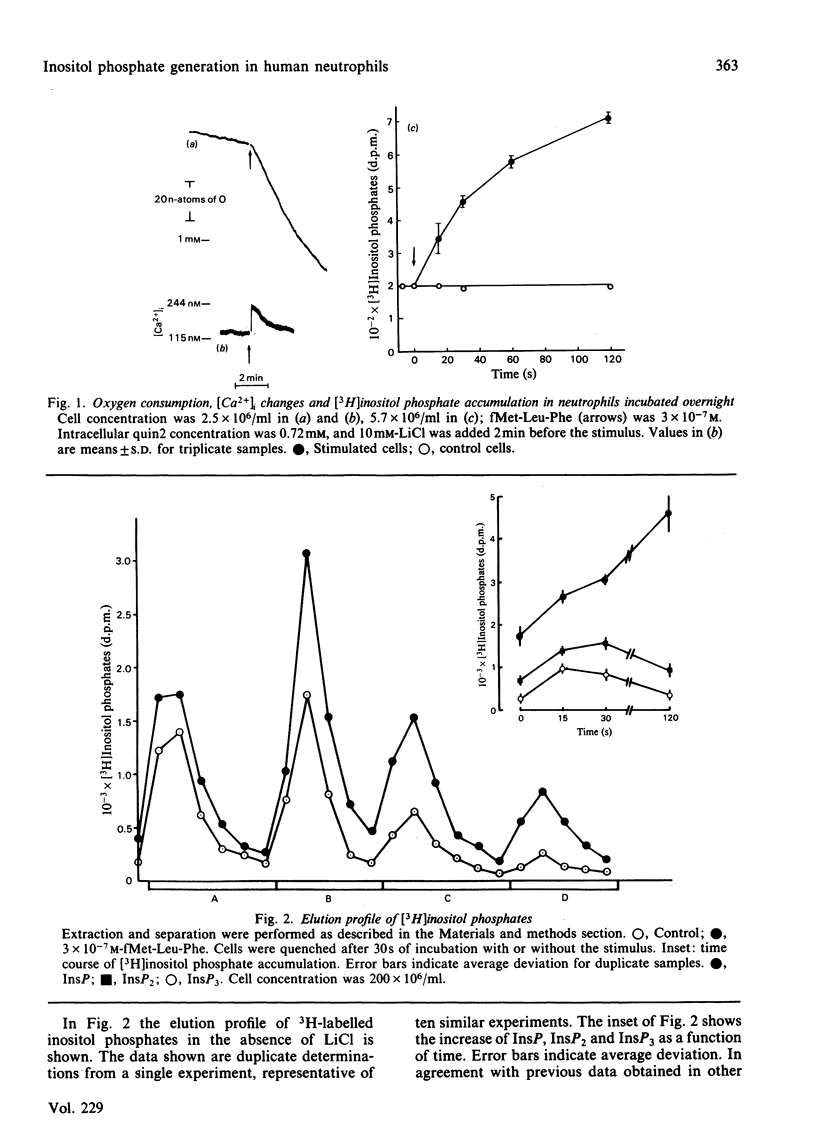
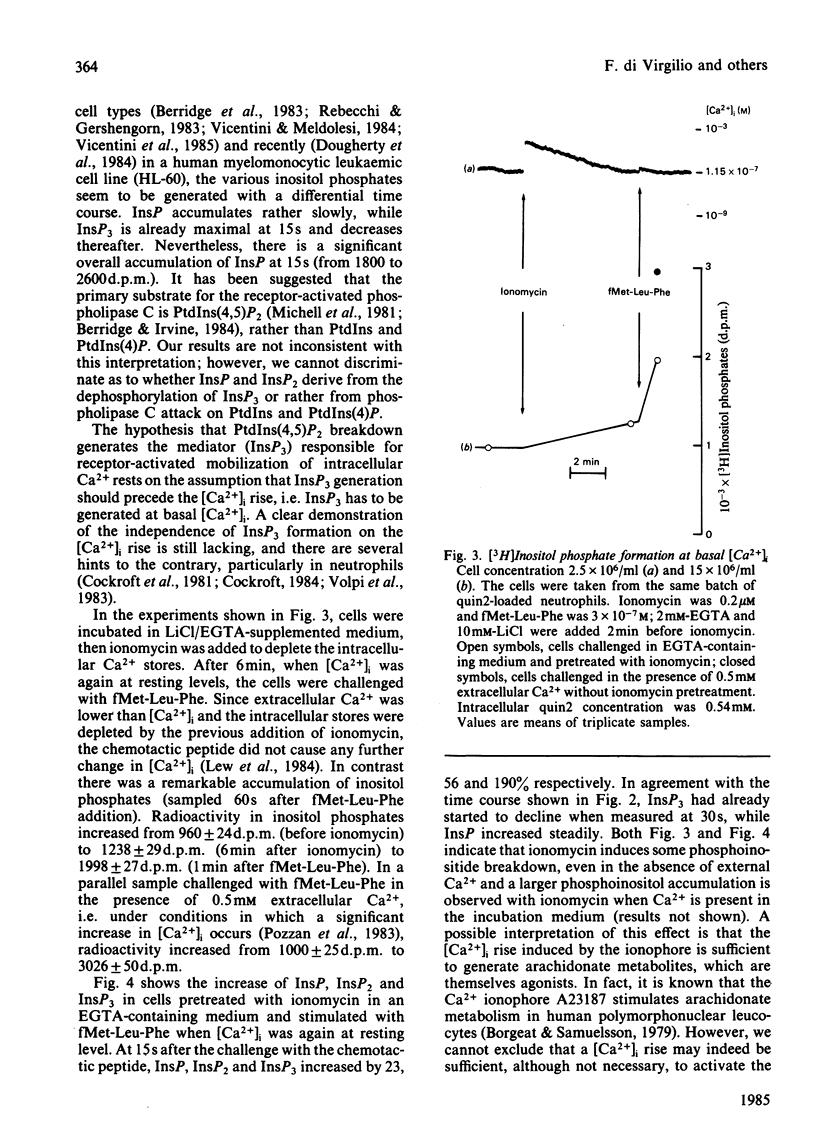
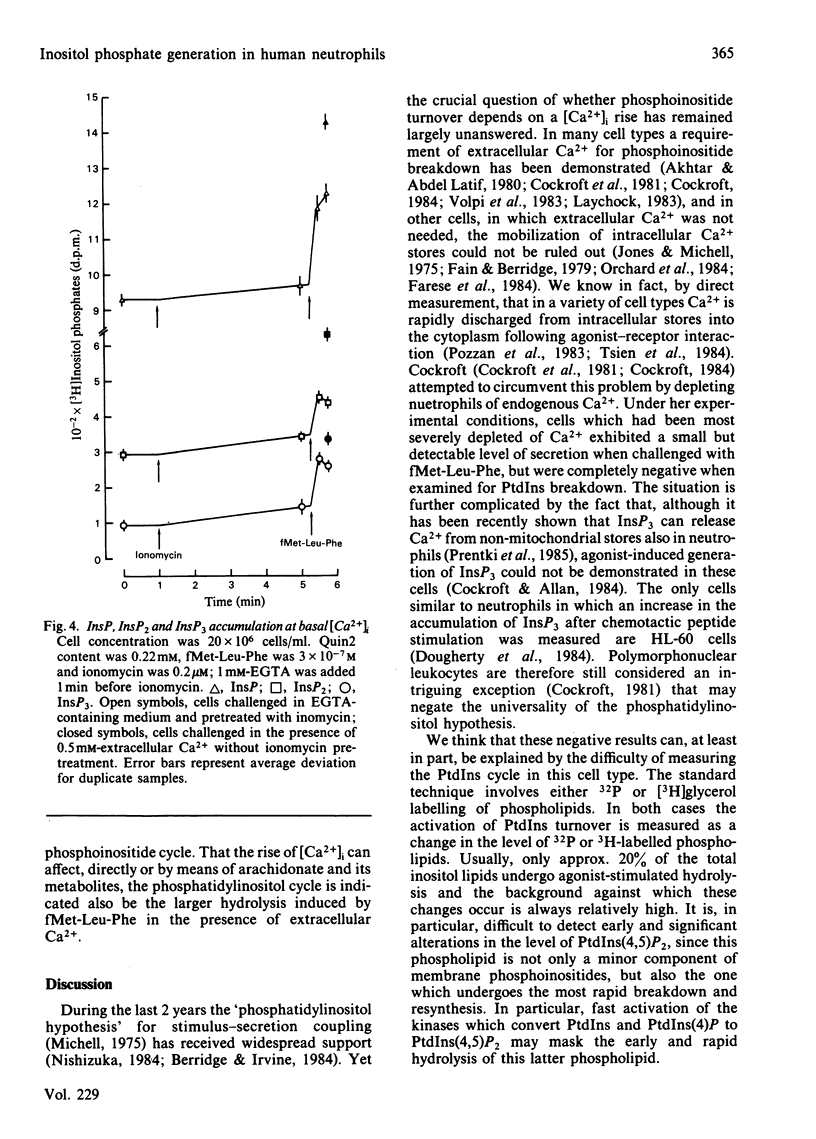
The accumulation of inositol phosphates in myo-[3H]inositol-labelled human neutrophils stimulated with the chemotactic peptide fMet-Leu-Phe was measured. The challenge with the chemotactic peptide caused the generation of inositol monophosphate (InsP), inositol bisphosphate (InsP2) and inositol trisphosphate (InsP3). The formation of the three inositol phosphates followed a differential time course: InsP3 accumulated very rapidly and transiently, whereas InsP increased steadily for more than 2 min. Inositol phosphate formation was only partially decreased by procedures which prevented the fMet-Leu-Phe-dependent increase of cytosolic free Ca2+ concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar R. A., Abdel-Latif A. A. Requirement for calcium ions in acetylcholine-stimulated phosphodiesteratic cleavage of phosphatidyl-myo-inositol 4,5-bisphosphate in rabbit iris smooth muscle. Biochem J. 1980 Dec 15;192(3):783–791. doi: 10.1042/bj1920783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cockcroft S., Baldwin J. M., Allan D. The Ca2+-activated polyphosphoinositide phosphodiesterase of human and rabbit neutrophil membranes. Biochem J. 1984 Jul 15;221(2):477–482. doi: 10.1042/bj2210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. The dependence on Ca2+ of phosphatidylinositol breakdown and enzyme secretion in rabbit neutrophils stimulated by formylmethionyl-leucylphenylalanine or ionomycin. Biochem J. 1981 Dec 15;200(3):501–508. doi: 10.1042/bj2000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- De Togni P., Cabrini G., Di Virgilio F. Cyclic AMP inhibition of fMet-Leu-Phe-dependent metabolic responses in human neutrophils is not due to its effects on cytosolic Ca2+. Biochem J. 1984 Dec 1;224(2):629–635. doi: 10.1042/bj2240629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Togni P., Della Bianca V., Grzeskowiak M., Di Virgilio F., Rossi F. Mechanism of desensitization of neutrophil response to N-formylmethionylleucylphenylalanine by slow rate of receptor occupancy. Studies on changes in Ca2+ concentration and phosphatidylinositol turnover. Biochim Biophys Acta. 1985 Jan 28;838(1):23–31. doi: 10.1016/0304-4165(85)90245-4. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Dougherty R. W., Godfrey P. P., Hoyle P. C., Putney J. W., Jr, Freer R. J. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem J. 1984 Sep 1;222(2):307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Davis J. S. Rapid effects of angiotensin-II on polyphosphoinositide metabolism in the rat adrenal glomerulosa. Endocrinology. 1984 Jan;114(1):302–304. doi: 10.1210/endo-114-1-302. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. The relationship of calcium to receptor-controlled stimulation of phosphatidylinositol turnover. Effects of acetylcholine, adrenaline, calcium ions, cinchocaine and a bivalent cation ionophore on rat parotid-gland fragments. Biochem J. 1975 Jun;148(3):479–485. doi: 10.1042/bj1480479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laychock S. G. Identification and metabolism of polyphosphoinositides in isolated islets of Langerhans. Biochem J. 1983 Oct 15;216(1):101–106. doi: 10.1042/bj2160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orchard J. L., Davis J. S., Larson R. E., Farese R. V. Effects of carbachol and pancreozymin (cholecystokinin-octapeptide) on polyphosphoinositide metabolism in the rat pancreas in vitro. Biochem J. 1984 Jan 1;217(1):281–287. doi: 10.1042/bj2170281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Ambrosini A., Di Virgilio F., Pozzan T., Meldolesi J. Muscarinic receptor-induced phosphoinositide hydrolysis at resting cytosolic Ca2+ concentration in PC12 cells. J Cell Biol. 1985 Apr;100(4):1330–1333. doi: 10.1083/jcb.100.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Meldolesi J. alpha Latrotoxin of black widow spider venom binds to a specific receptor coupled to phosphoinositide breakdown in PC12 cells. Biochem Biophys Res Commun. 1984 Jun 15;121(2):538–544. doi: 10.1016/0006-291x(84)90215-8. [DOI] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]


