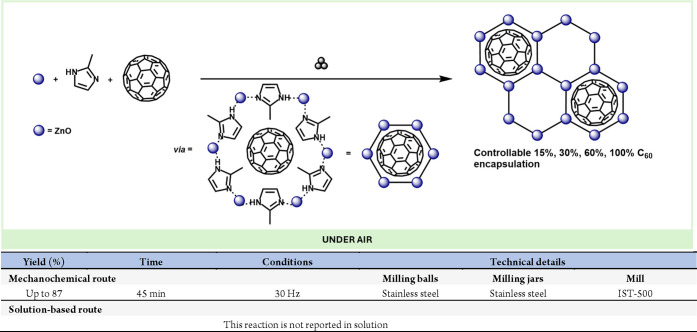
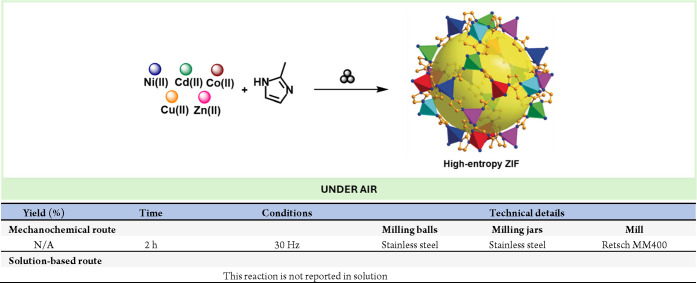
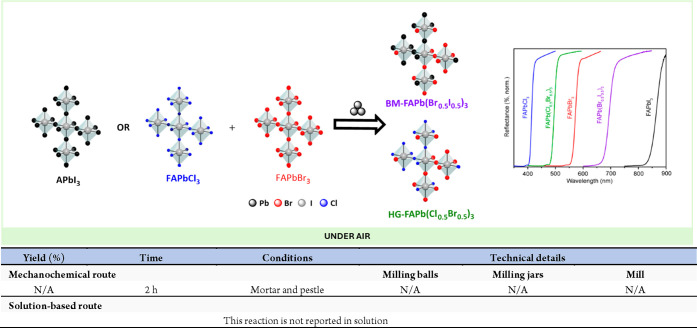
Abstract
In recent years, mechanochemistry has become an innovative and sustainable alternative to traditional solvent-based synthesis. Mechanochemistry rapidly expanded across a wide range of chemistry fields, including diverse organic compounds and active pharmaceutical ingredients, coordination compounds, organometallic complexes, main group frameworks, and technologically relevant materials. This Review aims to highlight recent advancements and accomplishments in mechanochemistry, underscoring its potential as a viable and eco-friendly alternative to conventional solution-based methods in the field of synthetic chemistry.
Keywords: Mechanochemistry, ball milling, green chemistry, solid-state reactions, catalysis, organic chemistry, inorganic chemistry, main group, organocatalysis, organometallic chemistry
Introduction
The need for cleaner, safer chemical processes drives the push for greener synthetic methods.1 Reducing or eliminating solvents is a key strategy with solid-state mechanochemistry leading the way to solvent-free synthesis. Although solvents facilitate reactant interactions, reaction control, and thermal management, their roles in extraction and purification remains critical. Nonetheless, the merits of minimal solvent use are gaining recognition, even among sceptics in synthetic chemistry.2,3
Mechanochemistry involves reactions triggered by mechanical forces like compression and friction, commonly executed through techniques such as ball milling. Traditional methods, such as manual grinding, face variable human and environmental influences. However, modern milling technologies, such as shaker and planetary mills, provide enclosed, controlled environments for more consistent results. These mills are widely used in laboratories for organic and inorganic synthesis due to their efficiency and adaptability. Mechanochemical processes are broadly categorized into batch and continuous methods.3 Batch processing ranges from hand grinding to advanced mills like Simoloyer, which are suitable for laboratory to large-scale operations. Continuous processes utilizing twin-screw or single-screw extrusion offer scalable throughputs with minimal equipment expansion Figure 1.
Figure 1.
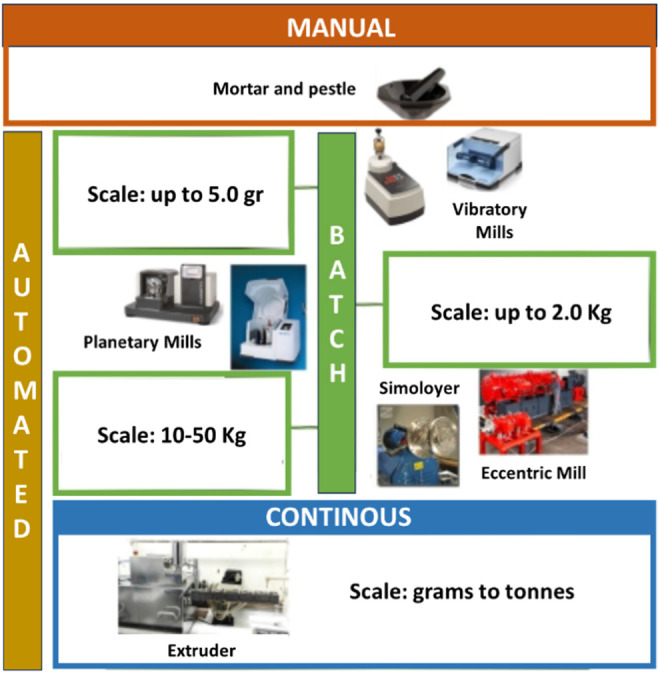
Mechanochemical tools commonly used for synthesis at different scales. Adapted with permission from ref (3). Copyright 2021 Elsevier.
It is worth noting that ultrasound (US) technologies and resonant acoustic mixing (RAM) are will not be discussed in this review. In addition, mechanochemical reactions with gases,4 characterization techniques,2,5−8 and large-scale reactions9 will not be addressed here.
Mechanochemistry for Synthesis
Over the past two decades, mechanochemistry has significantly increased as a field to complement classic synthetic routes carried out in solution. This rise in popularity has resulted in the application of mechanochemistry across various areas of modern chemical and materials synthesis.6,10−16
Due to space constraints, our review will focus on selected examples of mechanochemical syntheses. We illustrate mechanochemistry as a powerful synthetic tool from organic, inorganic, organometallic, and main group molecular compounds through mechanocatalytic reactions to technological materials (i.e., metal–organic frameworks and perovskites).
Synthesis of Inorganic Compounds
In this section, we explore the utilization of mechanochemistry in synthesizing molecular solids. Initially, we will cover its applications in main group chemistry, focusing on s-block elements such as hydrides, alkaline metals, and alkaline-earth metals, as well as p-block molecular compounds. Subsequently, the section reviews significant advancements in applying mechanochemistry for preparing transition metal compounds from d- and f-blocks.
Main Group Mechanochemistry
Main group compounds, frameworks, and materials constitute an important category, encompassing a diverse array of technological materials and widely used chemical reagents. As research on main group elements expands, the development of sustainable methodologies for synthesizing main-group-based compounds and materials still needs to catch up to that of their organic counterparts. However, mechanochemistry has recently emerged as a versatile approach for the development of s- and p-block main group elements. This Review includes some key examples of mechanochemical main group reactions; nevertheless, several comprehensive reviews have recently been published.3,17,18
Group 1
Among s-block molecular compounds, hydrides are widely used in both organic and inorganic synthesis due to their strong reducing power.19 However, these compounds often require handling in an inert atmosphere and nonreactive or nonprotic solvents, which limits the scope and scalability of reactions. Mechanochemical synthesis offers a compelling solution for hydrides, as it circumvents limitations such as solvent compatibility. Notably, the mechanochemical preparation of metal borohydrides has been documented since 1953, exemplified by synthesizing sodium borohydride from boric oxide and sodium hydride.20
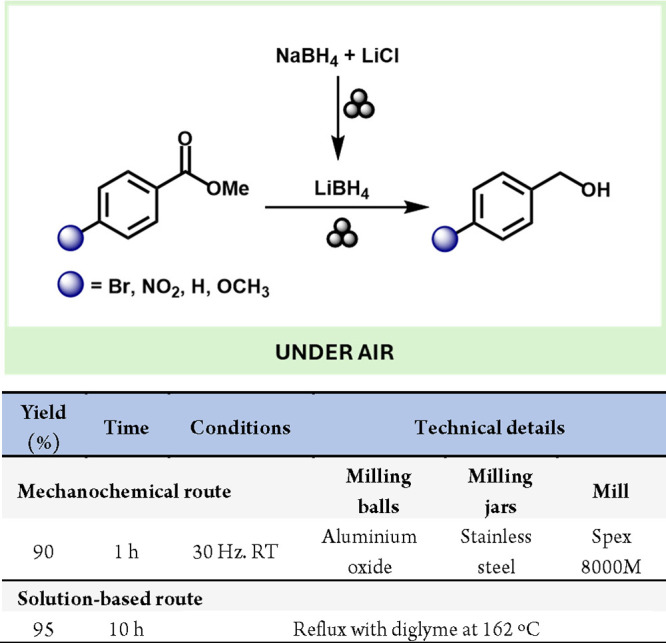
More recently, ball milling has been employed for the in situ preparation of LiBH4, utilizing NaBH4 and LiCl as starting materials for the solvent-free reduction of esters, as shown in Scheme 1.21 Additionally, mechanochemistry has been applied to synthesize other reactive hydride species. For instance, Gupta, Pruskid, Pecharsky and colleagues developed a mechanochemical process to synthesize alane (AlH3), a promising material for hydrogen and energy storage and a reducing agent in alkali batteries and as a hydrogen source for low-temperature fuel cells, using lithium hydride (LiH) and aluminum chloride (AlCl3) as the starting materials.22
Scheme 1. Mechanochemical Generation of LiBH4, Its Use in Reducing Esters, and Comparison with a Representative Solution-Based Method23.
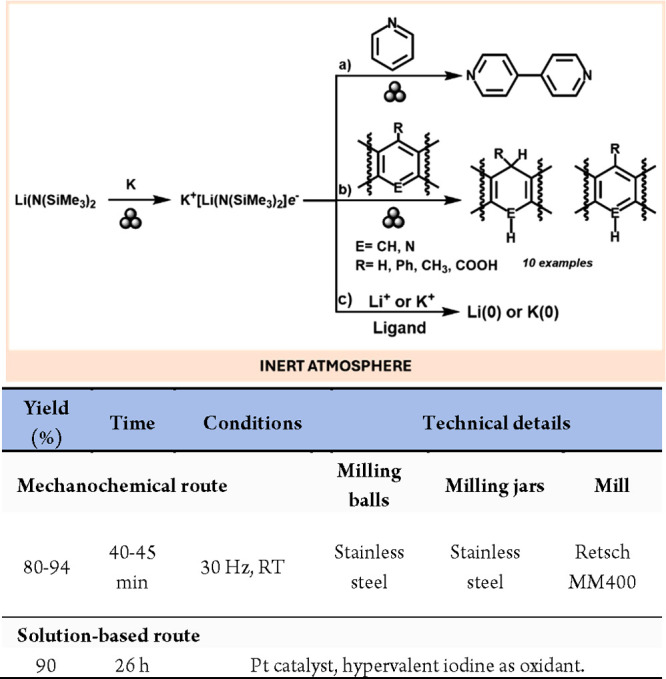
Electride compounds are ionic compounds in which the anion is a free electron; for this reason, electrides are highly reactive (reductive) species that can be difficult to prepare and handle.24 Very recently, Lu and co-workers have reported a mechanochemical, scalable method for the preparation of a novel electride species of formula K+[Li(N(SiMe3)2]e– (Scheme 2). The electride is stable at room temperature, and it has been tested mechanically for reactions such as arene C–H activation (a) and C–C bond formation and Birch reactions (b), with the latter being the first mechanochemical example of this reaction.25 Moreover, this reagent demonstrated the ability to mechanochemically reduce Li+ and K+ to their elemental form, thus demonstrating the versatility of solid-state chemistry (c).26
Scheme 2. Mechanochemical Preparation of K+[Li(N(SiMe3)2]e–, Synthetic Applications, and Comparison with a Representative Solution-Based Method27.
Recently, Ito, Kubota et al. reported a mechanochemical Birch reduction. Traditionally, Birch reductions, which convert arenes into 1,4-cyclohexadiene derivatives, require complex, inert, and cold conditions in liquid ammonia. Their methods are ammonia-free and utilize lithium metal, which is activated by ball milling. Their method covers a broad substrate range and takes less than a minute. Its utility is underscored by the successful reduction of bioactive molecules and its adaptability for gram-scale synthesis.28
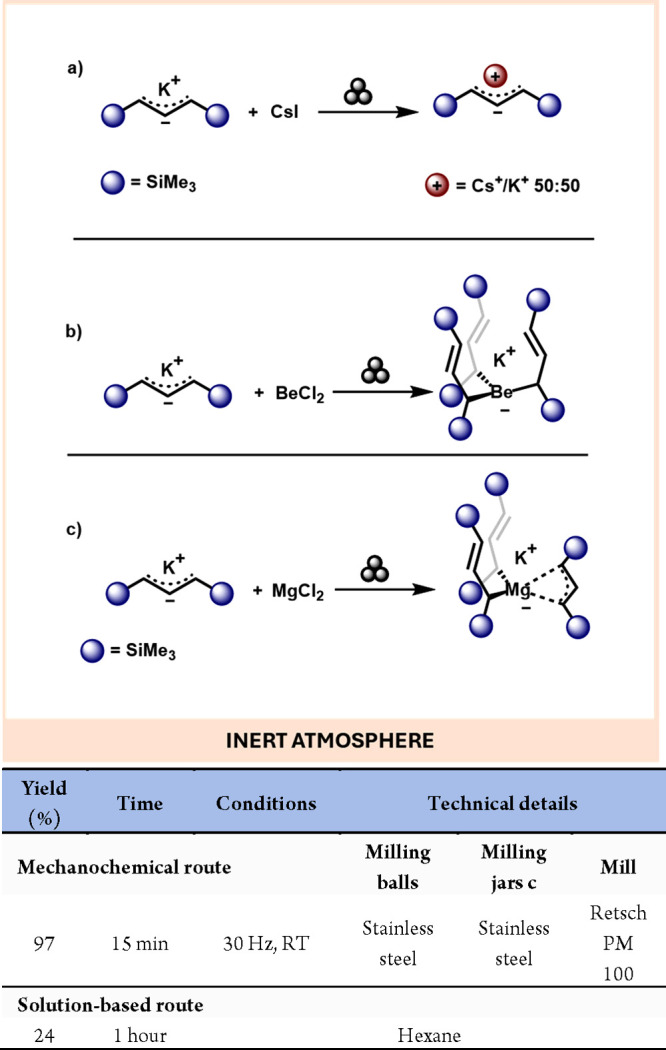
Another example of the distinct reactivity within group 1 elements, achieved through mechanochemistry, was reported by Hanusa.29 This study explored a novel halide metathesis reaction between K[A′] and CsI, resulting in a compound with the simplified formula CsKA’2 (Scheme 3a).
Scheme 3. Mechanochemical Halide Metathesis of K[A’] CsI (a); Mechanochemical Reaction of K[A’] with BeCl2 (b) and MgCl2 (c); Comparison with a Representative Solution-Based Method31.
Group 2
One method to enhance properties such as stability and reactivity of group 2 element compounds involves designing new supporting ligands or modifying their coordination modes.30 In this context, allyl moieties represent a versatile class of ligands for group 2 species. Hanusa et al. successfully synthesized a bis(trimethylsilyl)propylberyllate complex in quantitative yield. This was achieved by milling BeCl2 with the potassium salt of 1,3-bis(trimethylsilyl)propene (K[Me3SiCH2CHCH2SiMe3] or K[A’]) for 15 min (see Scheme 3b). Regarding Mg, the mechanochemical reaction between K[A’] and MgCl2 shows divergent reactivity compared to the same reaction in solution. While these ligands typically exhibit an η1 coordination mode with Mg, an alternative η3 mode was observed when the reaction was conducted via ball milling (see Scheme 3c).31
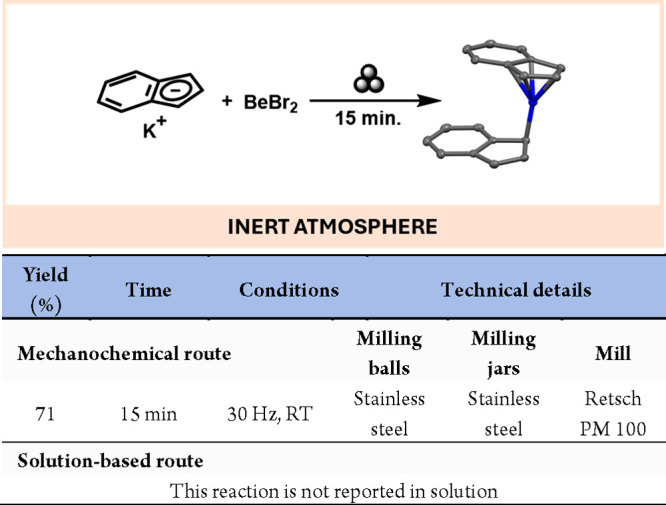
Indenyl main group complexes, larger analogues of the classic cyclopentadienyl counterparts, have been known and studied for a long time. However, despite beryllocene (BeCp2) being known for several decades now,32 its indenyl derivative could not be prepared or isolated using traditional solution-based synthetic routes. Addressing this, Hanusa’s group recently developed a mechanochemical method for the first-ever preparation of Be(Indenyl)2. This was accomplished by milling the indenyl potassium salt with BeBr2 for 15 min (see Scheme 4).30
Scheme 4. Mechanochemical Preparation of Be(Indenyl)2.
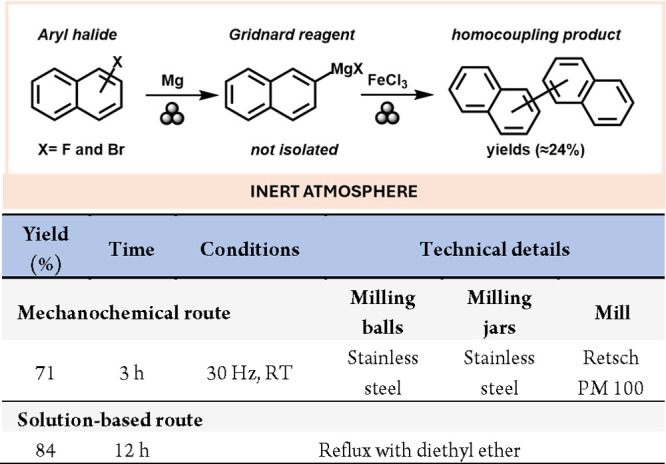
One of the most versatile reagents in organic chemistry is the Grignard reagent, with the general formula RMgX (where X = halogen, and R = alkyl or aryl), playing a crucial role in the formation of C–C bonds in organic synthesis.33,34 However, Grignard reagents are typically highly air- and moisture-sensitive and incompatible with protic solvents, making solvent-free synthetic routes highly desirable. In this context, the mechanochemical reaction of magnesium with naphthalene halides produced Grignard reagents that retained their activity for 10 weeks at room temperature under an inert atmosphere, and even for several months at 4 °C.35 Mechanochemical preparation of Grignard reagents can also yield reactivity that is not observed in solution-based methods. For instance, the strong C–F bond has historically hindered the creation of Grignard species from organic fluorides and magnesium. However, Hanusa’s group described a mechanochemical method for preparing binaphthalenes by milling fluoronaphthalene with an excess of magnesium metal for 2 h, followed by FeCl3 for 1 h (Scheme 5), although the fluoro-based Grignard reagent could not be isolated.36
Scheme 5. Example of the Mechanochemical Preparation and Synthetic Application of Fluoro-Based Grignard Reagent and Comparison with a Representative Solution-Based Method37.
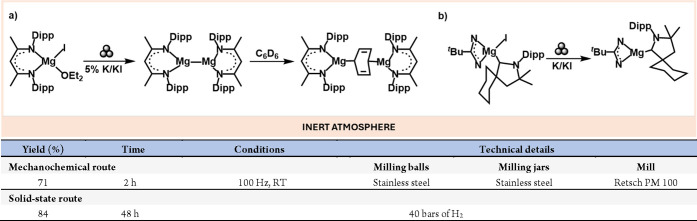
Mg(I) species make up an exciting class of compounds. Monovalent radical Mg species, previously only detected in space or low-temperature matrix experiments, became more accessible after Jones and Stash’s groups prepared the first stable and isolable Mg(I) compound.38 Following this seminal study, mechanochemistry has proven to be an effective tool for the preparation and reactivity of these highly reactive compounds. For example, the Harder group developed a protocol for mechanically preparing dimerized Mg(I) compounds (Scheme 6a).39 These species were so reactive that they activated benzene to yield a bridging dianionic dearomatised derivative. The same group also successfully prepared and isolated a monomeric Mg(I) radical species stabilized by a cyclic(alkyl)aminocarbonyl (CAAC, Scheme 6b).40
Scheme 6. Mechanochemical Preparation of Highly Reactive Mg(I) Dimers (a); Solid State Preparation of CAAC Stabilized Mg(I) Compounds (b).
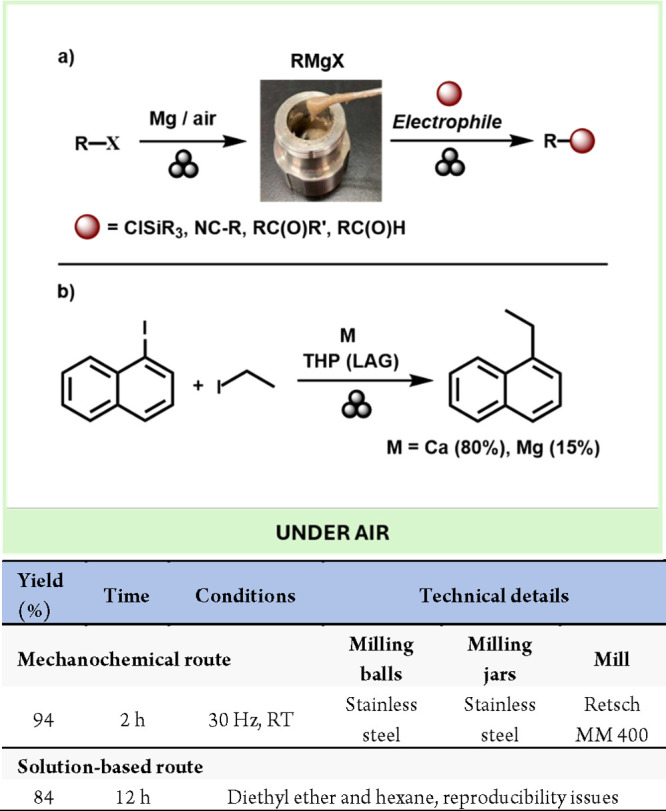
In 2021, Ito et al. described a general synthesis of magnesium-based Grignard reagents (in paste form) in air by mechanochemical means. Moreover, these species can be used directly for the one-pot nucleophilic addition reactions with various electrophiles and nickel-catalyzed cross-coupling reactions under solvent-free conditions (Scheme 7a).41 More recently, the same group made another breakthrough in the mechanochemical preparation of calcium-based heavy Grignard reagents with the formula RCaX. Traditionally, Ca-based Grignard reagents have been poorly explored due to the lack of accessible synthetic routes under mild solvent-based conditions. The developed air-stable process from aryl halides and commercially available calcium metal, without any preactivation steps, enabled the rapid development of novel cross-electrophile-coupling reactions mediated by arylcalcium reagents (b).42 Interestingly, the in situ generated Ca-based Grignard, obtained using tetrahydropyran (THP) as a LAG additive, displayed increased reactivity with some nucleophiles, such as ethyl iodide, compared with the classic Mg-based Grignard, under the same conditions. The increased reactivity is attributed to the elimination of the solvent effects, which stabilize reaction intermediates that reduce Grignard reactivity. In addition, the increased surface area and more efficient mixing under mechanochemical conditions lead to a more uniform reaction environment, which reduces diffusion limitations (Scheme 7b).
Scheme 7. Preparation of Grignard Reagents under Air and Broad Scope Reactivity (a); Preparation and Application of Heavier Ca-Based Grignard Reagent (b); Representative Solution-Based Method for Comparison43.
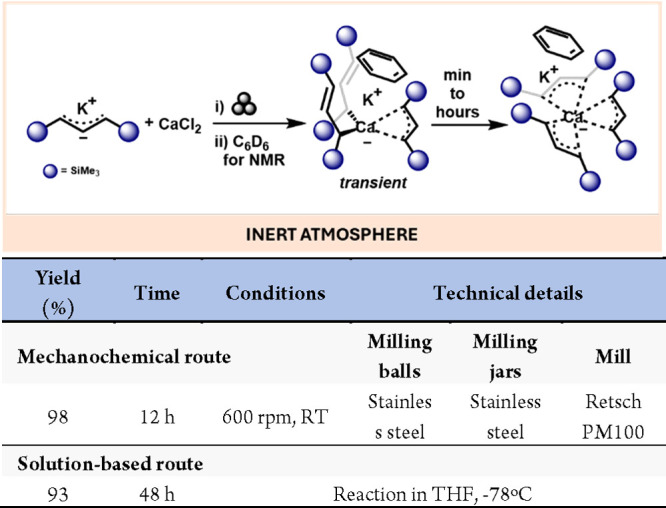
The use of mechanochemistry to prepare nonsolvated main group compounds has led to the observation of transient, previously unknown species. For example, the milling of K[A′] with CaI2 led to the formation of a K[CaA′3] intermediate. This species is proposed to have a mixture of σ and π bound allyl ligands as it can be clearly distinguished in 1H NMR (benzene-d6) from the isolable fully η3-bound allyl structure (Scheme 8).44
Scheme 8. Mechanochemical Reaction of K[A’] with CaI2 and Representative Solution-Based Method for Comparison46.
Regarding heavier group 2 compounds, it is worth mentioning that LAG techniques have been used to prepare strontium-based semiconductor precursors of formula Sr(Cp*)2. This compound is generally obtained via a salt metathesis reaction between alkali cyclopentadienides (i.e., KCp’) and metal halides (e.g., SrI2) in solution. However, this reaction suffers from both the low solubility of strontium iodide and the formation of stable adducts with SrCp2 with polar solvents, such as dimethyl ether and tetrahydrofuran (THF), which ultimately compromise the semiconducting properties.45
Group 13
These elements, characterized by their p-block electron configuration, play significant roles in various fields, ranging from semiconductor technology and aerospace engineering to medicinal applications, reflecting their diverse chemical reactivities and physical characteristics.
Functional layers featuring aluminum tris(8-hydroxyquinoline) (AlQ3) constitute a commonly used electron-transport and emitting layers in the field of electroluminescent materials.47,48 To this end, a simple and scalable synthesis of AlQ3 AcOH can be achieved by milling the aluminum(III) complex [Al(OAc)2(OH)] and 8-hydroxyquinoline (Q) in a 1:3 M ratio. Remarkably, this complex can be transformed into AlQ3 by heating the mixture to 200 °C for 2 h.49,50
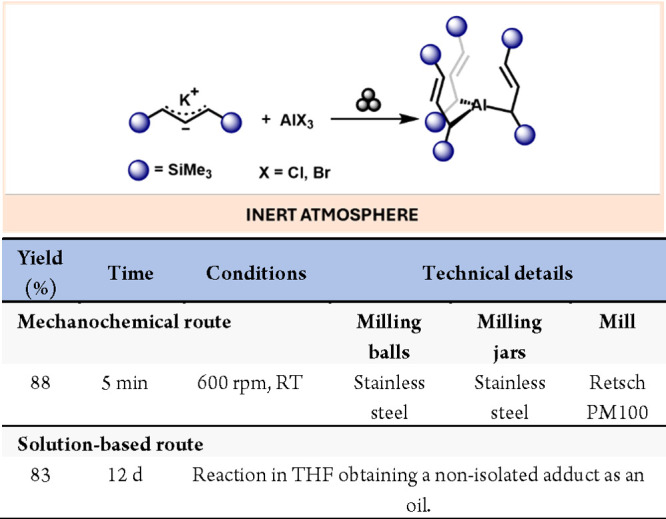
Attempts to prepare and isolate nonsolvated tris(allyl)aluminum species have been unsuccessful, with only THF, OPPh3, and pyridine adducts successfully characterized using traditional solvent-based methods. However, milling AlX3 (X = Cl, Br) with potassium 1,3-bis(trimethylsilyl)allyl (A’) anion species yielded Al(A’)3 efficiently.51 Employing a tube disperser apparatus, up to 150 mg of the complex can be generated with an 85% yield, and using a planetary mill, the reaction can be scaled up to 1.3 g with an 88% yield (Scheme 9).
Scheme 9. Preparation of Nonsolvated Al(A’)3 by Mechanochemical Means and Comparison with Solution-Based Methods54.
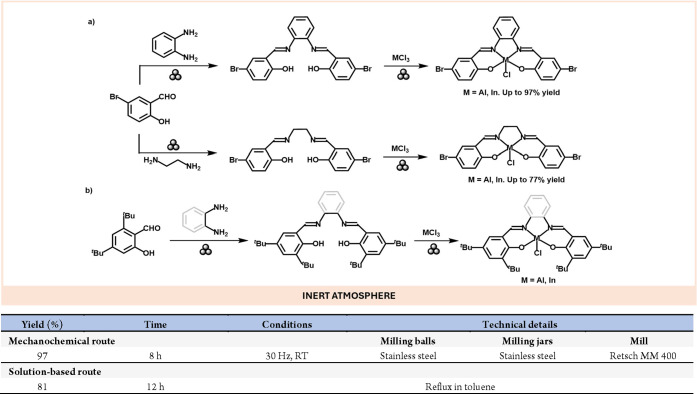
Besides the possibility of preparing species that traditional solution-based methods cannot prepare, mechanochemistry also enables the scale-up reactions to levels difficult to achieve otherwise.52 For instance, the preparation of Al(III) and In(III) complexes bearing salen and salophen ligands at kilogram scales has been demonstrated.53 Using a planetary mill, the salen and salophen ligands could be prepared by mechanochemical condensation of o-phenylenediamine with the corresponding aromatic hydroxy aldehyde (Scheme 10a). Noteworthy, these high-scale syntheses displayed lower green metrics (E-Factor, PMI and RME) in all the cases compared with the solution-based methods. The same group recently reported a mechanochemical method for preparing structurally related tBu salen and salophen Al and In complexes with tunable emissive properties with up to 85% yield (Scheme 10b).55
Scheme 10. (a) Large-Scale Mechanochemical Preparation of Al and In Complexes Bearing Salen and Salophen Ligands; (b) Mechanochemical Preparation of Al and in Luminophores Based on Bulky Salen and Salophen Ligands; Representative Solution-Based Method53.
Continuing with indium(III) complexes, they have attracted significant attention due to their low toxicity, water stability, and catalytic activity in various organic reactions.56 When combined with suitable ligands, these indium species can undergo metal-to-ligand charge transfer and potentially serve as efficient photosensitizers with long-lived excited states.57
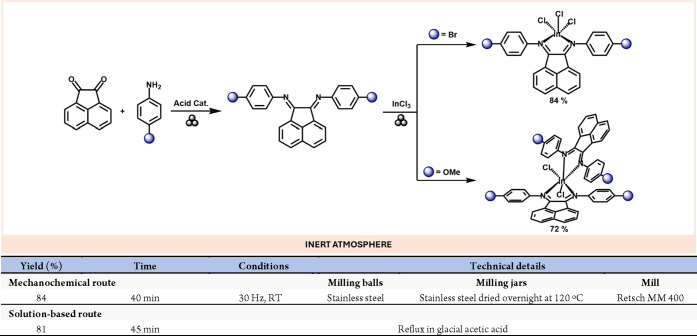
Bis(arylamino)acenaphthene ligands (Ar-BIAN) are essential ligands in the main-group complexes arena.58 These ligands are typically obtained through condensation reactions between acenaphthoquinone and the corresponding aniline derivative under acidic conditions, although a transition metal templating agent is commonly required.59 However, the acid-catalyzed ball-milling of acenaphthoquinone with aniline derivatives gives the desired Ar-BIAN ligands in good yields. Moreover, In(III) complexes In(BIAN)Cl3 and [In(BIAN)2Cl2][InCl4], can then be obtained by further milling equimolar quantities of the respective BIAN ligand with indium trichloride (the starting materials we loaded into the milling jars using a glovebox).60 Remarkably, these compounds can also be obtained by milling the ligand starting materials and InCl3 in a one-pot fashion, although the yield using this route is lower (Scheme 11).
Scheme 11. Ar-BIAN Ligands and Indium(III) Complex Synthesis by Mechanochemistry; Representative Solution-Based Method58.
Group 14
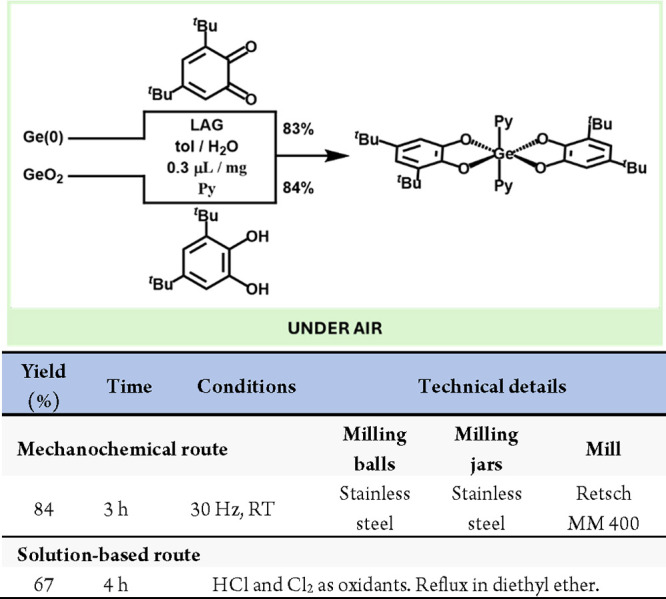
Regarding group 14, besides C and Si compounds, there are a few examples of mechanochemically prepared group 14 molecular compounds. Germanium compounds are critical due to their increasing demand and low concentration in the earth’s crust. The purification of crude germanium ores usually involves using strong oxidants (and highly toxic) such as HCl or Cl2 to obtain the corresponding metal chlorides.61 Additionally, the traditionally generated GeCl4 can be challenging to handle due to its air and moisture sensitivity, and it is a poor reagent for substitution reactions.62 To this end, Friščić et al. developed a novel protocol for the mechanochemical preparation of highly pure and bench-stable organogermanium compounds from metallic germanium or germanium oxide using benzoquinones or catecholates, respectively (Scheme 12).63 Interestingly, both starting materials led to similar yields after grinding the reagents using the LAG technique (LAG) in the presence of pyridine as a coordinating ligand.
Scheme 12. Mechanochemical Purification of Ge(0) and GeO2 via Catecholates Preparation and Comparison with a Representative Solution-Based Method62.
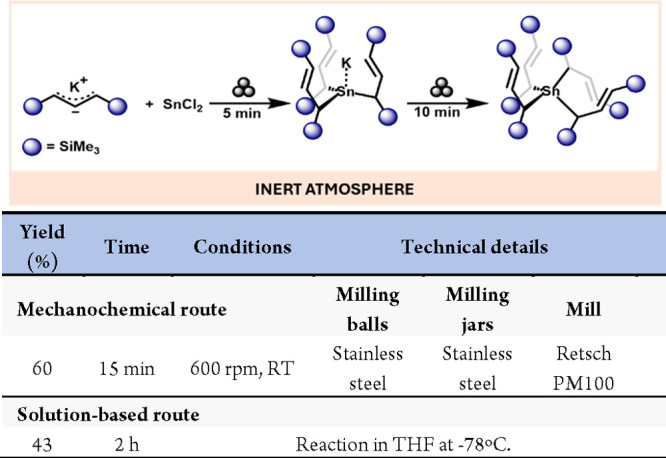
Descending in the group, allylstannane compounds of formula SnA’3K-THF have been previously prepared using solution-based methods.64 However, the nonsolvated counterparts were unknown until the group of Hanusa described the mechanochemical preparation of SnA’3K. To this end, the milling of K[A’] with SnCl2 for only 5 min produced SnA’3K (Scheme 13).65 Interestingly, a longer milling time from the starting materials led to homoleptic Sn(IV)A’4, constituting the first example of mechanochemically produced organometallic disproportionation without external oxidant.
Scheme 13. Preparation of Nonsolvated SnA’3K and SnA’4 Mechanochemically and Comparison with a Representative Solution-Based Method66.
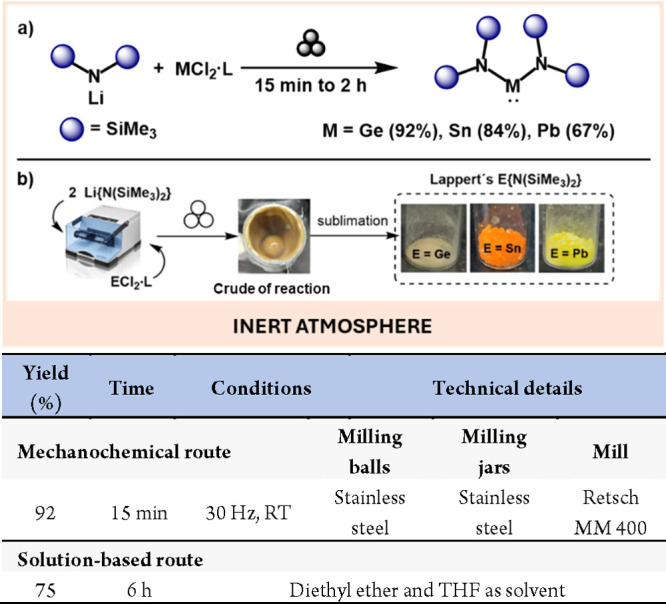
Another versatile class of group 14-based compounds is heavier tetrylenes (HTs). These carbene analogues have attracted much attention due not only to their activating and catalytic activity67,68 but also to their use as ligands.69 Although the synthesis of tetrylenes of general formula E{N(SiMe3)2}2 (E = Ge, Sn, Pb) was described as early as 1974 by Lappert,70 and has been optimized over time,71 it would be highly desirable to develop a greener and more efficient methodology.
To this end, García-Alvarez et al. very recently described the preparation of Lappert’s HTs mechanochemically.72 Interestingly, not only were the reaction times shorter than those described for solution-based methods but the yields were higher in all the cases (Scheme 14).
Scheme 14. Mechanochemical Preparation of Lappert’s Heavier Tetrylenes (a); Rheology of the Crude Reaction and Sublimed Products (Reproduced with Permission from ref (72). Copyright 2023 Royal Society of Chemistry) (b); and Comparison with Representative Solution-Based Reaction71.
Group 15
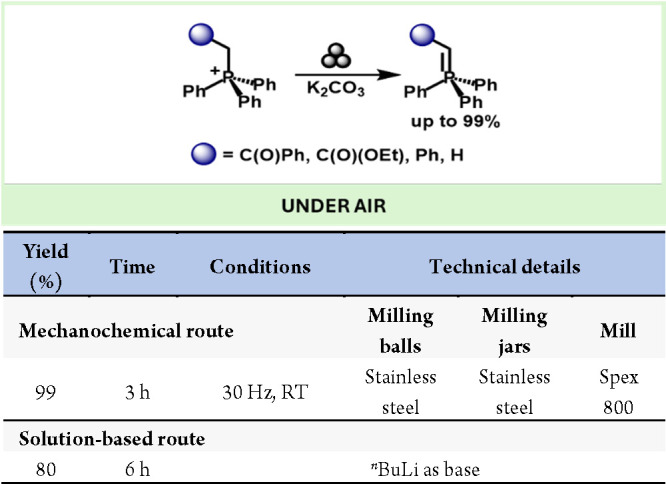
Wittig reagents constitute a broadly used tool for preparing olefins via metathesis. These reagents are commonly prepared by deprotonating alkylphosphonium salts with strong bases such as tBuONa, tBuOK or KHDMS.73 Pecharsky described a simple mechanochemical preparation of Wittig reagents by milling the corresponding alkylphosphonium salts with K2CO3 with yields up to 99% (Scheme 15).74
Scheme 15. Mechanochemical Preparation of Wittig Reagents and Comparison with Representative Solution-Based Method73.
Developing orthogonal mechanochemical reactions in which several reactants are ground together can be a powerful and efficient strategy to obtain a desired product selectively. Such orthogonal syntheses are rare, especially those involving main group elements. Cyclophoph(V)azanes constitute an exciting class of compounds that have displayed applications in many areas such as coordination chemistry,75 supramolecular chemistry,76 polymers,77 medicinal chemistry,78 and catalysis.80 Traditional solution-based preparation of these species often requires two steps: (i) nucleophilic addition to the starting dichlorocyclophosphazane and (ii) oxidation of the substituted derivative (Scheme 16, left a).79 Notably, the direct formation of air- and moisture-stable cyclophoph(V)azanes enabled by an orthogonal one-pot mechanochemical process revealed the long-term stability of these compounds (Scheme 16, left b).81
Scheme 16. (a) Comparison between Solution-Based and Mechanochemical Preparation of Cyclophoph(V/V)azanes; (b) Comparison between Previously Reported Approach (a) and Mechanochemical Preparation of Adamantoid Phosphazanes (b)79.
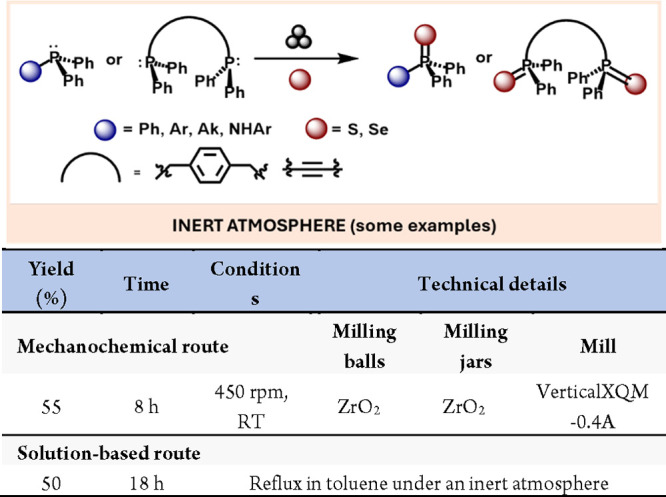
Also related to mechanoxidations from P(III) to P(V), Balakrishna’sroup has also reported the mechanochemical oxidation of a wide variety of phosphines containing different functional groups with good to quantitative yields.82 The methodology proved to be compatible with amines, heterocycles, or alkynes, which highlights the versatility of this mechanochemical approach (Scheme 17).
Scheme 17. Solid State Oxidation of Several Substituted Phosphines (Selected Examples) And Comparison with Representative Solution-Based Methods83.
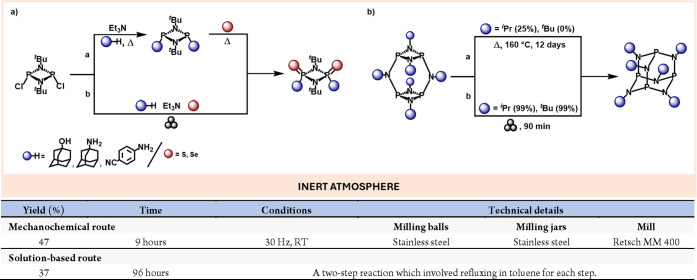
Mechanochemistry has also unlocked the synthesis of main group compounds previously described as being unattainable. Thus, back in the ’80s, the Scherer’s group reported the preparation of an adamantoid phosphazane of formula P4(NiPr)6 by heating the isomeric cyclic dimeric cyclophosphazane for 12 days at 160 °C (Scheme 16, right a).84 Since then, the iPr substituted adamantoid derivative was considered the bulkiest achievable structure of this type.
However, more recently, the first synthesis of the adamatoid phosphazane P4(NtBu)6 has been demonstrated by a solvent-free mechanochemical approach based on ball milling, highlighting the importance of mechanochemical reaction environments in (re)evaluating the chemical reactivity. Furthermore, they achieved by the same technique the reduction of the reaction time and temperature conditions for the synthesis of the previously described P4(NiPr)6 from 12 days at 160 °C to 90 min at ambient temperature (Scheme 16, right b).85
Transition Metal Mechanochemistry
Transition elements (termed transition metals) are metallic elements with incomplete d or f shells. These elements are classified into d-block metals, consisting of 3d elements from Sc to Cu, 4d elements from Y to Ag, and 5d elements from Hf to Au, and f-block metals, consisting of lanthanoid elements from La to Lu and actinoid elements from Ac to Lr.86 Although the use of mechanochemistry for synthesizing transition metals has already been covered in previous reviews,2,87,88 we will highlight some key mechanochemical transformations involving d-block and f-block transition metals.
d-block Metals
d-Block metals, in their different forms, display excellent thermal, electrical, chemical and catalytic properties, and many are considered critical elements due to their low natural abundance in the Earth’s crust.89
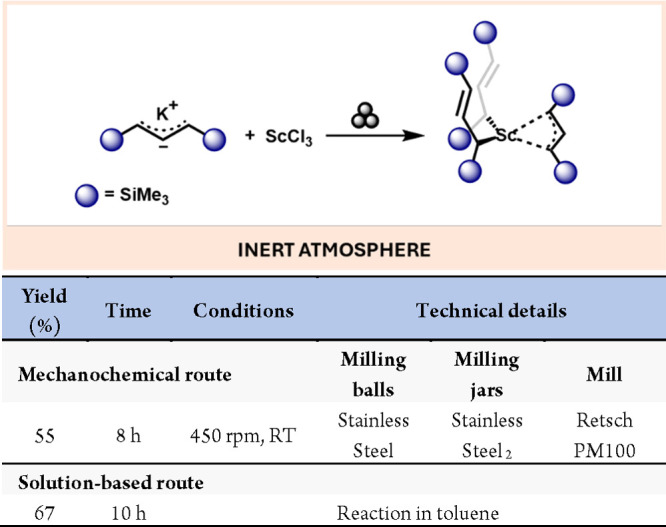
The mechanochemical synthesis of scandium and yttrium (group 3) has been much less explored than most of their transition metal counterparts. The lack of investigation of these metal complexes is mainly due to several crucial limitations, such as the limited number of accessible oxidation states, which makes group 3 metal complexes often tedious to synthesize and isolate, and the high cost of starting materials due to their low natural abundance. Nevertheless, Rightmire, Hanusa and Rheingold developed a tris(allyl)scandium complex using a planetary ball mill. In this case, milling potassium 1,3-bis-TMS-allyl anion species 24 with ScCl3 for 10 min at 600 rpm afforded the target complex with 48% yield and isolating a product that could not be achieved in solution (Scheme 18).51
Scheme 18. Mechanochemical Synthesis of Tris(allyl)scandium Complex and Comparison with a Representative Solution-Based Method90.
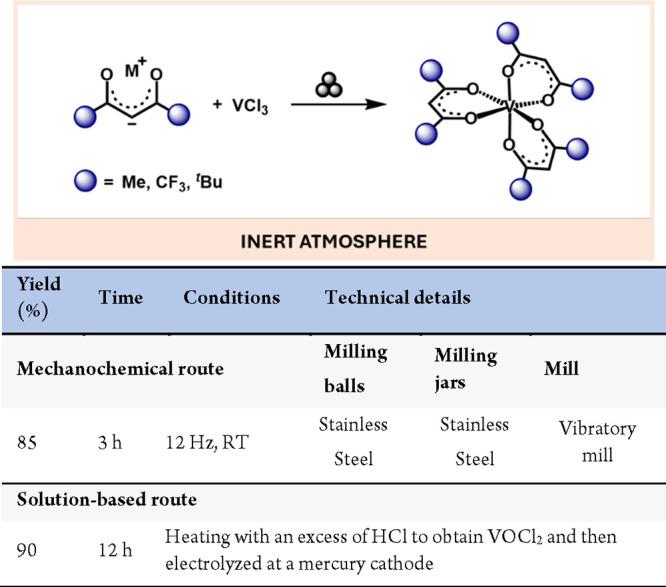
Another family of versatile ligands includes β-diketones, which exhibit distinctive electronic properties. Being outstanding weak-field ligands, they provide pronounced π-acid character. Their potential to form stable complexes with a wide range of metals has allowed them to play an exceptional role in different fields, such as catalysts91 and photoluminescence materials.92 The mechanosynthesis of vanadium(III) β-diketonates from the reaction of vanadium chloride with a slight excess of a series of β-diketonates has been reported by Makhaev and Petrova (Scheme 19).93 They managed to synthesize a family of vanadium complexes obtaining yields between 43 and 85% without using any solvent, even in the purification step, which was performed by sublimation.
Scheme 19. Mechanochemical Synthesis of Vanadium Diketonates and Comparison with a Representative Solution-Based Method94.
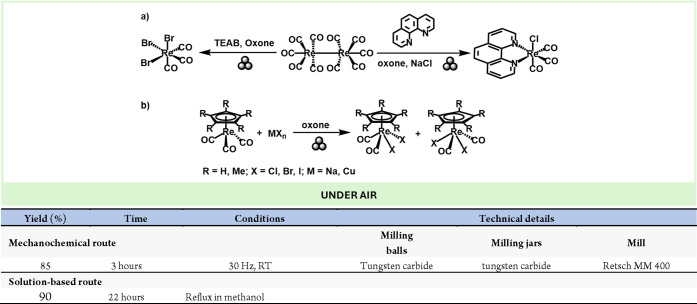
Mechanochemistry has also been successfully applied to the synthesis of rhenium complexes with the general formula fac- Re(CO)3(L)3, where (L)3 = Br3 or (Cl)(N–N) (N–N = 1,10-phenanthroline). These complexes are promising for the development of new model radiopharmaceuticals due to the Re-186 and Re-188 radiochemical properties, the stable coordination chemistry and the versatile ligand framework such as the fac-Re(CO)3(Cl)(phen) that can enhance the complex’s ability to target specific biological molecules or structures (e.g., tumors).95
These complexes can be easily obtained in high yields by grinding the precursor [Re2(CO)10] with tetraethylammonium bromide (TEAB) and Oxone for 3 h, as proposed by Hernandez and co-workers (Scheme 20a).96 Additionally, they demonstrated that the fac-Re(CO)3(Cl)(N–N) complex can be obtained by reacting NaCl, Oxone, and phenanthroline from the same precursor.
Scheme 20. Mechanochemical Synthesis of Rhenium Complexes and Comparison with a Representative Solution-Based Method101.
Re(I) complexes have also been studied by Hernandez, Friščić et al., who developed a simple solvent-free mechanochemical oxidative halogenation of model organometallic Re(I) compounds with excellent yields and tunable stereoselectivity, showing that mechanochemistry can advance and simplify fundamental organometallic transformations (Scheme 20b).97
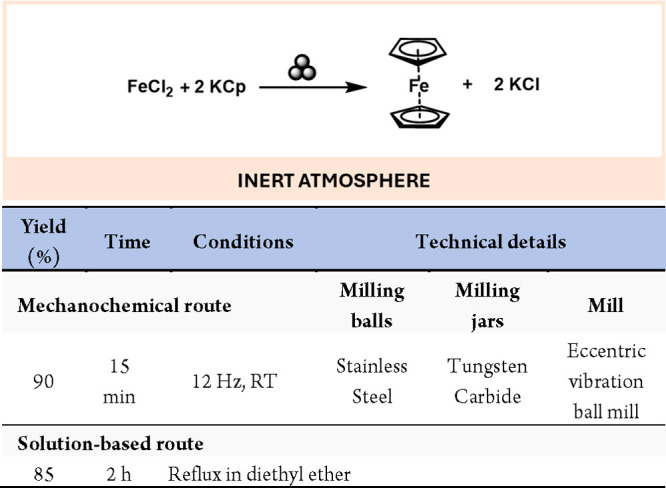
The mechanochemical synthesis of group 9 elements was mainly focused on iron and ruthenium. Iron complexes have a wide range of applications in analysis, pigments, pharmacology, and catalysis, inter alia.98 Moreover, iron is the third most abundant metal on the earth’s crust, making it especially important for large-scale industrial applications.2,99 Ferrocene species were first prepared mechanically by milling potassium cyclopentadienide (KCp) and anhydrous FeCl2 for 15 min, followed by sublimation, achieving conversions to up to 90% (Scheme 21).100
Scheme 21. Mechanochemical Synthesis of Ferrocene and Comparison with a Representative Solution-Based Method102.
Other authors used [C2B9H11] ligand instead of cyclopentadienyl to synthesize iron complexes, allowing good reactivity and selectivity.103 Ferra(III)bis(dicarbollide) species are versatile agents for the incorporation of iron(III) into organic molecules, such as DNA-dinucleotides. The complex [Fe(C2B9H11)2][Me4N] containing bisdicarbollyl ligands, a versatile agent in organic molecules such as DNA-nucleotides, can be prepared by milling FeCl3 and Tl2C2B9H11 for 30 min, followed by treatment with tetramethylammonium hydrogen sulfate.104
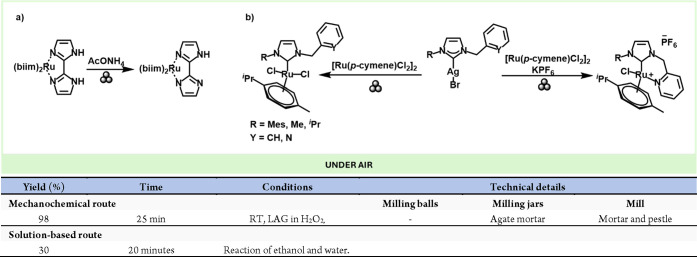
Moving down to the group, only a handful of reported mechanochemical examples of Ruthenium complexes exist. For instance, Tan and co-workers synthesized the [Ru(Hbiim)3] (H2biim = 2,2′-biimidazole) complex quantitatively and rapidly via a LAG mechanochemical approach in just few minutes of reaction mixing [Ru(H2biim)3](PF6)2 and NH4OAc with a few drops of H2O2 as an oxidant and solvent (Scheme 22a), concomitant with color changes.105
Scheme 22. (a) Mechanochemical Synthesis of Ru(biim)3 complex; (b) Mechanochemical Preparation of Ru Complexes by Transmetallation and Comparison with a Representative Solution-Based Method105.
A different type of ruthenium complexes are the Noels-type NHC species, which are used in ring-opening metathesis (ROM) as a catalyst.106 In 2020, Lamaty, Bantreil and co-workers performed a transmetalation with ruthenium via mechanochemistry that allowed rapid access (1.5 min to 1 h) to the corresponding complexes having a structure similar to Noels-type precatalysts (Scheme 22b).107 Evaluation of the complexes in the ring-opening metathesis polymerization of norbornene in different solvents, including nontoxic ones, showed high catalytic activity for one of them, comparable to that of the Noels catalyst.
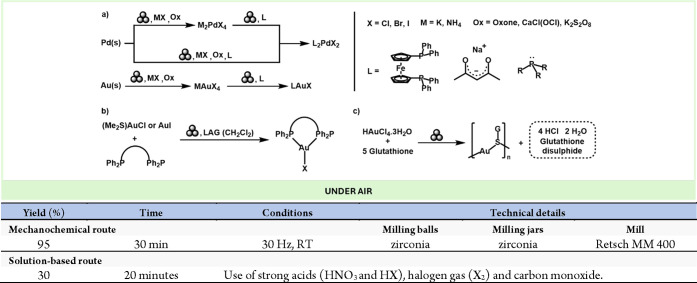
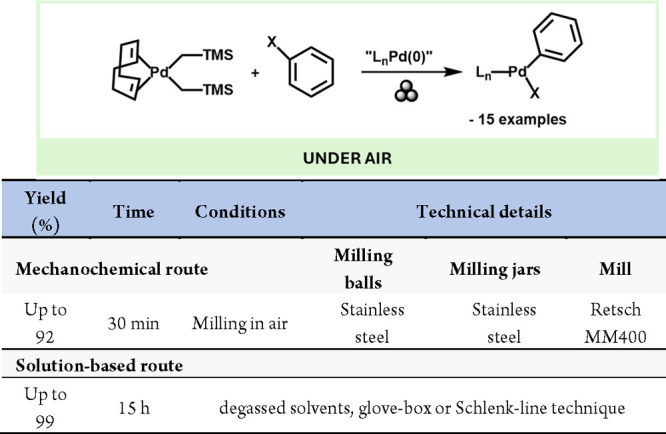
Other noble metals, such as Pd and Au, are of critical interest due to their chemical, thermal, catalytic, and other properties.108−110 However, due to their inert nature the preparation of reactive precursors from the zerovalent reserves found on the earth crust is a highly challenging issue which often requires the use of very strong acids, toxic gases, and heat.111
To this end, a highly relevant study by Friščić’s group described the preparation of Pd and Au precursors from elementary metals by mild, mechanochemical conditions.112 Moreover, the obtained metal precursors could be further functionalized by reaction with appropriate ligands to generate highly valuable catalytic species (Scheme 23a). Another study by Deák, Colacino, and co-workers reported a mechanochemical method to Au(diphos)X complexes comprising diphosphine ligands and halide ions. Their work showcased the effectiveness of mechanochemistry in a fast (4 min reaction time), efficient (up to 98% yield), and eco-friendly route to luminescent and stimuli-responsive gold(I) complexes (Scheme 23b).113 Finally, the same team also introduced an innovative and eco-friendly mechanochemical approach to oligomeric glutathione-based gold nanocluster species, which are already being utilized in the biomedical field as effective radiosensitizers for cancer radiotherapy (Scheme 23c).114
Scheme 23. Mechanochemical Preparation of Au and Pd Salts and Complexes from Their Elemental Metallic Form and Comparison with a Representative Solution-Based Method112.
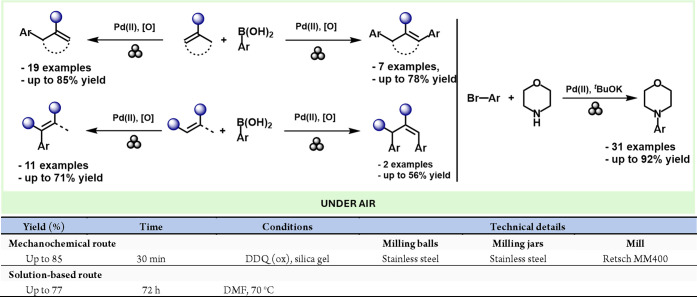
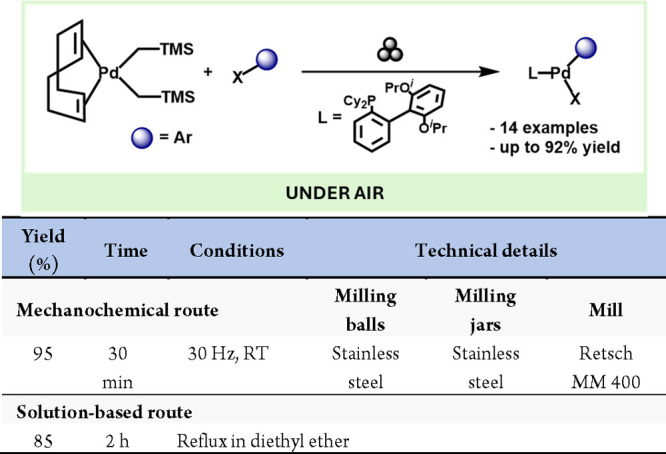
Continuing with Pd complexes, a recent work by the Ito group describes the mechanochemical synthesis of Pd complexes.115 In this work, the oxidative addition of aryl halides to Pd(0) species, which usually requires glovebox or Schlenk techniques, could be carried out under air with high yields (Scheme 24).
Scheme 24. Mechanochemical Preparation of Pd Complexes via Oxidative Additions Of Aryl Halides And Comparison With A Comparative Solution-Based Method116.
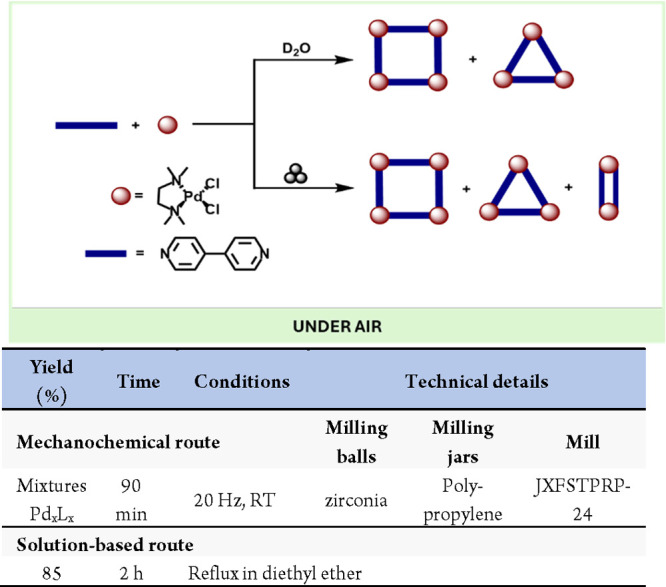
Another remarkable application of mechanochemistry is preparing species that are not thermally achievable in solution. For instance, Yan and co-workers have recently described the preparation of previously unreported “rectangular” Pd2L2 dimers.117 In this work, the milling of the Pd precursor (tmeda)Pd(X2) with bipyridine as a linker yielded the known Pd4L4 and Pd3L3 cycles118 together with the previously unknown Pd2L2, which was detected by NMR and mass spectrometry techniques (Scheme 25). Using a similar approach, Yan and co-workers used the same self-assembly mechanochemical approach to prepare and isolate several unstable Pd cages and their intermediates.119
Scheme 25. Mechanochemical Preparation of Short-Lived Cyclic Pd Dimer.
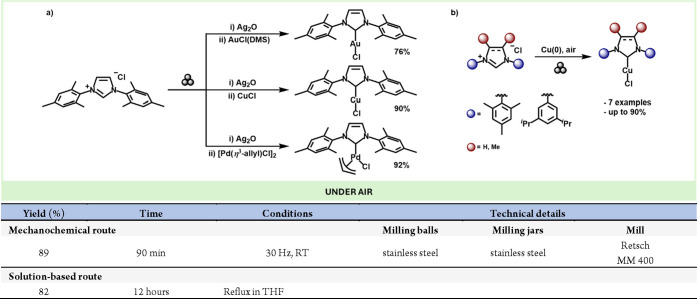
Regarding N,N-diaryl NHC metal complexes, mechanochemistry has proven to be a handy tool for preparing these types of compounds, which usually require prolonged reactions in reflux conditions to achieve metalation.120 To this end, Lamaty’s group described a very convenient preparation of Cu, Au, and Pd NHC complexes under mechanochemical conditions. In this work, milling the imidazolium precursor with Ag2O led to the corresponding Ag-NHC complexes in high yields (Scheme 26a). Moreover, the obtained Ag complexes could undergo further transmetalation with Cu, Au and Pd precursors in a one-pot two-step fashion to yield the NHC complexes with excellent yields.121
Scheme 26. (a) Mechanochemical Preparation of Au, Cu, and Pd NHC Complexes; (b) Synthesis of Cu NHC Complexes Using Air As the Oxidising Agent; Comparison with a Representative Solution-Based Method.
The same group also developed a mechanochemical route for preparing Cu-NHC complexes by directly reacting the imidazolium salts with Cu (0) (Scheme 26b). Interestingly, both the yields and the reaction times were significantly improved with respect to the corresponding solution-based method.122
A family of N-Oxy-Heterocyclic Carbenes (NOHC) complexes has been prepared with high yields following a similar approach.123 Moreover, the starting N-alkoxy imidazolium was also prepared using mechanochemistry from the corresponding diketones and alkoxyamines
Regarding salen-type ligands, in a comparable manner to what was described for main group salen and salophen complexes, James et al. described the quantitative mechanochemical preparation of salen-type ligands and their corresponding Zn, Ni, and Cu complexes.124 Interestingly, the complexes could be prepared both by one-pot two-step procedure and by all in one-pot way in quantitative yields.
Finally, regarding other late transition metals as well as main group metals, it is relevant to mention the large-scale preparation of Ni, Zn, and Al metal–organic frameworks (MOFs) by James et al., where the preparation of these MOFs by twin and single screw extrusion was accomplished at rates up to kg h–1.125
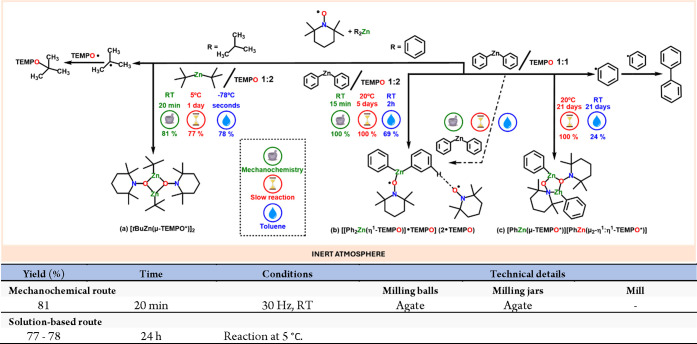
In terms of zinc126 complexes, Lewinski et al. have performed several studies in the area.127 For instance, they conducted a comparative study on the reactions of TEMPO with organozinc compounds,128 specifically ditest-duty zinc and diphenylzinc, using mechanochemical, slow-chemistry, and solution methods. They found that the tBu2Zn/TEMPO reaction yields a dimeric diamagnetic complex [tBuZn(μ-TEMPO*)]2 with varying results based on the chosen method. In contrast, mixing TEMPO with diphenylzinc in a 2:1 molar ratio results in a high-yield, novel paramagnetic Lewis acid–base adduct [[Ph2Zn(η1-TEMPO)]TEMPO], irrespective of the method (Scheme 27). This adduct is also produced in the slow-chemistry process when TEMPO and Ph2Zn are mixed in a 1:1 ratio and left at ambient temperature for 2 weeks, eventually yielding a diamagnetic dinuclear compound [PhZn(μ-TEMPO*)][PhZn(μ2-η1:η1-TEMPO*)] and biphenyl. The same reaction in toluene showed a lower conversion rate. The group also explored reactions involving bis(pentafluorophenyl)zinc ((C6F5)2Zn) and TEMPO to study the kinetics and thermodynamics of wet and solvent-free solid-state processes.129
Scheme 27. Comparative Study (i.e., Mechanochemistry, Slow Reaction and Solution) of Zinc Organometallic Species with TEMPO and Representative Solution-Based Method for Comparison128.
Inner Transition Metals (f-Block): Lanthanides and Actinides d-Block Metals
f-Block element chemistry, including scandium and yttrium, lanthanides, and actinides, has been a thriving area of research for many years. The primarily ionic and Lewis acidic character of lanthanide metals allows for a wide range of structural features supported by numerous ligands.130,131
The lanthanides are generally more like one another than any ordinary transition metal series members. They typically exhibit only one stable oxidation state, and their chemistry provides an excellent opportunity to examine the effects of small changes in size and nuclear charge along a series of otherwise similar elements. In contrast, the chemistry of actinides is more complex due to both the existence of a wide range of oxidation states and their radioactive nature.132
The complexity of working with f-block elements has resulted in a limited number of investigations compared with those involving d-block elements. However, mechanochemistry has enabled the synthesis of lanthanide and actinide complexes. In 2001, Lee and co-workers synthesized lanthanum oxychloride (LaOCl), oxybromide (LaOBr), and their solid solutions, LaOCl1-xBrx (0 ≤ x ≤ 1, Δx = 0.25), by reacting a mixture of lanthanum oxide (La2O3), chloride (LaCl3), and bromide (LaBr3) in a planetary ball mill.133
A few years later, Fetrow and co-workers developed a series of borohydride ligands, particularly aminodiboranates (H3BNR2BH3–) and phosphinodiboranates (H3BPR2BH3–), for synthesizing trivalent f-element borohydride complexes of uranium, cerium, neodymium, lanthanum, and praseodymium using ball milling.134,134,135
In 2018, Salazar-Zertuche and colleagues successfully synthesized and characterized Ln4Zr3O12 (Ln = yttrium, holmium, erbium, and ytterbium) zirconates by mechanochemistry.154 Their electrical properties were studied for potential applications as solid electrolytes in solid oxide fuel cells (SOFCs).136
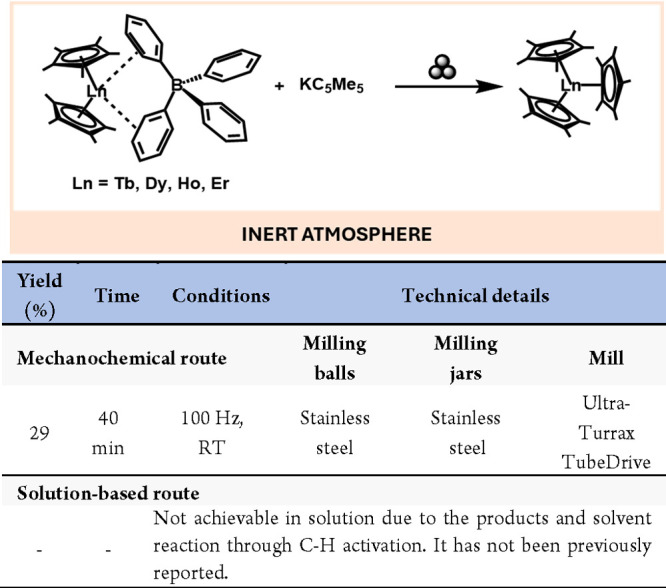
Mechanochemistry has also facilitated the synthesis of f-metal complexes that were not accessible via solution-based methodologies. For instance, Woen and colleagues reported the synthesis of various tris(pentamethylcyclopentadienyl) complexes of late lanthanides ((C5Me5)3Ln), such as terbium, dysprosium, holmium, and erbium (Scheme 28). These complexes and the yttrium analogue could be synthesized by a solvent-free mechanochemical method, avoiding the reaction of the products with solvents through C–H activation.137
Scheme 28. Mechanochemical Preparation Lanthanides Triscyclopentadienyl Complexes and Comparison with a Representative Solution-Based Method138.
Organic Synthesis
Mechanochemistry is revolutionizing the way chemists approach molecular construction. This technique has shown remarkable efficacy in synthesizing a variety of organic compounds. Its environmentally friendly nature, stemming from reduced solvent use, aligns with the principles of green chemistry. Its ability to yield products with high purity and its scalability–as well as the ability to facilitate reactions that are challenging in traditional solution-based methods - presents a promising avenue for developing sustainable synthetic methodologies in organic chemistry.139−141
Mechanochemical Rearrangement Reactions
In chemistry, a rearrangement reaction occurs when the carbon skeleton of a molecule is reorganized to yield a structural isomer of the original molecule. Alongside substitution and addition reactions, rearrangements are crucial in both organic and inorganic synthesis since they enable the transformation of molecules into more stable, functional, or desirable structures with diverse applications. For instance, the pinacol rearrangement is used to prepare the antiepileptic phenytoin (Scheme 29a). Molecular rearrangements serve as a powerful tool for creating complex structures in an atom- and step-economic manner, transforming multistep processes into more viable and sustainable alternatives.143
Scheme 29. (a) First Reported Mechanochemical Pinacol Rearrangement Finalised to the Preparation Phenytoin (API); Mechanochemical Beckmann Rearrangement for the Synthesis of (b) ε-Caprolactame and (c) Paracetamol; Comparison with a Representative Solution-Based Method142.
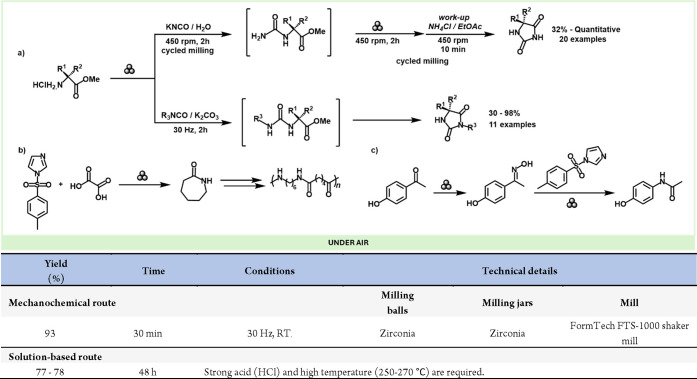
Mechanochemical molecular rearrangements, which have already been reached in previous reviews,143 are becoming an increasingly attractive green synthetic approach, particularly in preparing active pharmaceutical ingredients (APIs) and natural products.99,144 Despite being a relatively new method, mechanochemical rearrangements offer promising avenues for scientists to merge molecular diversity with green chemistry principles, achieving greater efficiency and higher selectivity in environmentally friendly reactions.143 Mechanochemical rearrangements have proven to be a powerful approach to value-added compounds. For example, the Beckmann mechano-rearrangement reported by Mocci and co-workers for the synthesis of ε-caprolactam, use in the industry of nylon-6,6 or the obtention of an API and World Health Organization (WHO) essential medicines such as paracetamol.145 They reported on a sustainable mechanochemical process that allows the design of new amide frameworks via an eco-efficient “cut-and-paste” process of C–C and C–N bonds on the oxime backbone using cheap and sustainable reagents such as p-tosyl imidazole (p-Ts-Im) and oxalic acid. To obtain ε-caprolactam, they performed the mechanochemical Beckmann rearrangement of cyclohexanone with p-Ts-Im and oxalic acid using a zirconia jar and balls of 15 mL and 8 mm, respectively, at 30 Hz for 30 min (Scheme 29b), obtaining excellent yields (93%) and opening promising perspectives for its industrialization as a precursor for the production of nylon-6,6. Using the same reaction conditions, they achieved the preparation of paracetamol in a two-step procedure via the rearrangement of the oxime, by using 4′-hydroxyacethophenone, which is a crucial intermediate in its synthesis, obtaining an excellent yield of 84% (Scheme 29c), displaying the great potential of this approach for industrial applications.
The pinacol rearrangement, a pioneering solid-state rearrangement, was first demonstrated by Toda et al., who achieved high yields and selectivity through the reaction of powdered 1,1,2-ethan-1,2-diol with dry HCl gas or p-toluenesulfonic acid under specific conditions. This method presented faster kinetics compared to solution-based processes and yielded a ketone product with 90% efficiency.146 Later, in 1995, Kaupp et al. furthered this approach by synthesizing triphenylacetophenone via a proton-catalyzed pinacol rearrangement of benzopinacol, marking an advancement in solvent-free synthesis.147 In 2000, Sekiya et al. expanded the scope of pinacol rearrangements by inducing a 1,4-migration in thienothienyl-substituted-9,10-dihydroxy-9,10-dihydroanthracenes, leading to significant crystal structure changes.148 These studies laid the foundation for various promising mechanochemical pinacol rearrangements.143
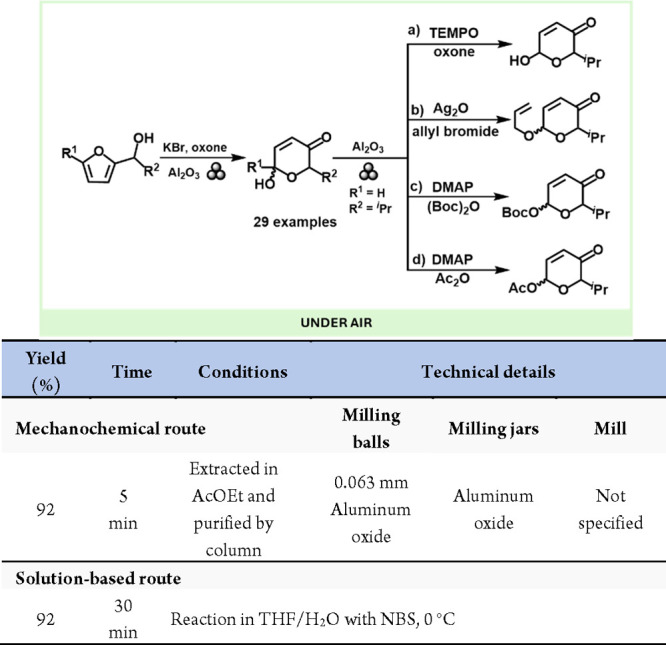
The Achmatowicz rearrangement, involving the conversion of 2-furyl carbinols to pyranoses, has become a valuable tool in synthesizing nitrogen and oxygen heterocycles including a wide range of natural products. This reaction’s versatility in stereodifferentiation and its application in organic synthesis has given it a unique position in recent decades.149,150 Mechanochemical methods have been developed to enhance the environmental friendliness and stereochemical control of the Achmatowicz rearrangement. Notably, in 2015, Falenczyk et al. reported the first solvent-free mechanochemical Achmatowicz rearrangements, converting furfurals to furfuryl alcohols.151 Zhao and Tong introduced an innovative approach using magnetically stirred chromatographic alumina (Al2O3), which allowed for the solvent-free rearrangement and easy scalability from milligrams to grams, along with the integration of multiple reactions in a single process.152 These advancements underscore the evolving landscape of mechanochemical synthesis in organic chemistry (Scheme 30).
Scheme 30. Solvent-Free Protocol for the Achmatowicz Rearrangement Using Al2O3 and Representative Solution-Based Method (a) for Comparison152.
In addition to the molecular organic rearrangements described above and despite not being as extensively studied, the main group compound rearrangement has also been studied via mechanochemistry. For example, as already mentioned, Shi and co-workers performed the synthesis of Phosphazane-Based frameworks through mechanochemical rearrangement.153
In 2020, Ardila-Fierro and colleagues implemented in situ monitoring of a mechanochemical benzil–benzilic acid molecular rearrangement using synchrotron powder X-ray diffraction, Raman spectroscopy, and real-time temperature sensing. This approach aimed to understand the mechanisms of mechanochemical reactions facilitated by ball milling.154 This study marked a pioneering use of in situ monitoring techniques, providing a real-time visualization of molecular rearrangements as they occur.
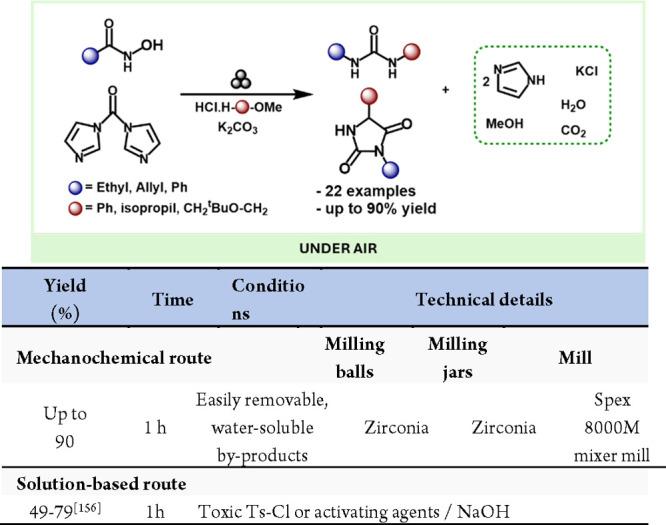
Additionally, the field has seen other mechanochemical rearrangements, such as the Loosen rearrangement. Porcheddu, Colacino and co-workers developed a novel and environmentally friendly mechanochemical method for synthesizing unsymmetrical ureas and 3,5-disubstituted hydantoins. (Scheme 31).155 This approach utilizes safer starting materials instead of hazardous and toxic isocyanates, and for the first time, the Lossen rearrangement has been successfully employed to create a variety of medicinally relevant structures through a one-pot mechanochemical process, eliminating the use of organic solvents even during the workup. This procedure proved effective for producing API ethotoin.
Scheme 31. Mechanochemical Protocol for the Loosen Rearrangement and Comparison with Representative Derivatives in Solution-Based Methods155,156.
Mechanochemical Catalysis
Catalysis plays a pivotal role in both chemistry and society. A significant portion of chemicals used in academia and industry are produced with the aid of catalysts at some point in their manufacturing processes.10,157 A large portion of the world’s gross chemical production depends on catalytic processes, a cornerstone of ’green chemistry.’ This is due to their ability to reduce energy requirements and minimize waste generation in synthetic processes.158 Catalytic reactions are typically classified based on the method of overcoming the activation barrier, such as using photons in photocatalysis,159 electrical potential in electrocatalysis,160 or thermal energy in conventional thermal catalysis.161 However, mechanical energy, an often overlooked source, can also initiate chemical and catalytic reactions.162 During the last years, mechanochemistry has extensively studied different catalytic reactions, and some critical reviews have been written involving metal-mediated and metal-catalyzed reactions,163 organometallics,164 catalytic materials,165 and zerovalent metals in synthesis.166
Recent years have seen extensive exploration of catalytic reactions in ball mills,167 especially in organic synthesis. These studies range from C–H bond functionalization,168 C–C and C–N coupling,169−171 and cross-coupling,172,173 to aromatic substitution,174 Lewis acid and base chemistry,175,176 and even the synthesis of porous carbon-based catalysts under solvent-free conditions.177
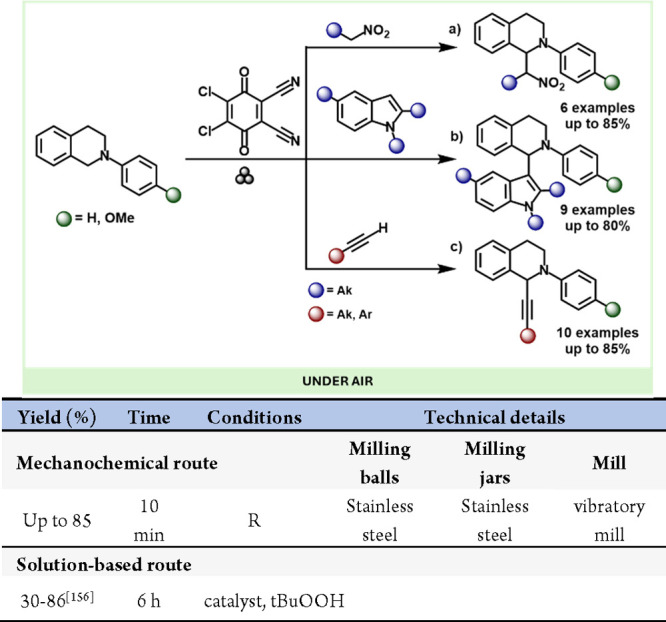
In 2009, Fulmer and colleagues successfully executed the archetypical Sonogashira coupling reaction using high-speed ball milling. They synthesized a variety of para-substituted aryl halides with trimethylsilylacetylene or phenylacetylene, using both iodo- and bromo-substituted substrates.178 Later, in 2011, Su and co-workers utilized this technique for cross-dehydrogenative coupling reactions between tetrahydroisoquinolines and various nucleophiles, including nitroalkanes, alkynes, and indoles (Scheme 32).179
Scheme 32. Coupling Reaction of Tetrahydroisoquinolines with Nitroalkanes, Indoles, and Alkynes by Ball Milling Mechanochemistry and Comparison with Representative Example (a) in a Solution-Based Method156,181.
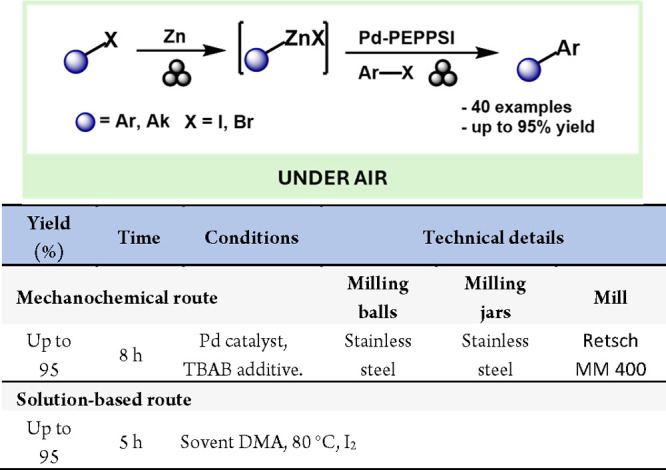
The Negishi coupling is another highly versatile reaction for forming C–C bonds. Cao and colleagues reported a Negishi cross-coupling reaction facilitated by mechanochemistry, demonstrating a broad substance scope for both C(sp3)–C(sp2) and C(sp2)–C(sp2) bond formation (Scheme 33). Notably, the required organozinc reagent was also prepared by using mechanochemistry. This approach may open up significant opportunities for the in situ generation of organometallic compounds from base metals and their subsequent involvement in synthetic reactions via mechanochemical methods.180
Scheme 33. Mechanochemical Preparation of Organozinc Reagents and Their Use in Negishi Reactions and Comparison with Representative Examples in the Literature184.
A mechanochemical Pd-catalyzed cross-coupling reaction involving aryl halides and organozinc pivalates, which can be conducted at ambient temperature and atmosphere, has been recently reported. This straightforward procedure yields a diverse array of biaryl and aryl-heteroaryl derivatives in high yields and within short timeframes.182
Asymmetric organocatalytic reactions have become of great interest due to their ability to activate inert substrates and their success in photocatalysis and electrocatalytic reactions. However, their practical application is still in its early stages, often hindered by extreme reaction conditions and the need for large amounts of solvents.183 For instance, the alkaloid-mediated asymmetric opening of cyclic meso anhydrides typically requires low temperatures (−60 °C) and organic solvents like toluene or tetrachloride mixtures.185 Addressing this challenge, Rodriguez and colleagues conducted this reaction in a solvent-free mechanochemical manner, achieving very high yields.186 Furthermore, this catalysis process is tolerant to air and moisture, does not require purification of the reagents, and thus reduces experimental setup and costs.187
An asymmetric organocatalytic domino Mannich addition was also performed via diastereoselective fluorination. The Mannich reactions involving pyrazolones and, to a lesser degree, isoxazolones demonstrated effectiveness under solvent-free ball-milling conditions. This method, coupled with a chiral squaramide catalyst, yielded products with high yields and enantiomeric purities reaching up to 99:1 e.r. and as a singular diastereomer.188
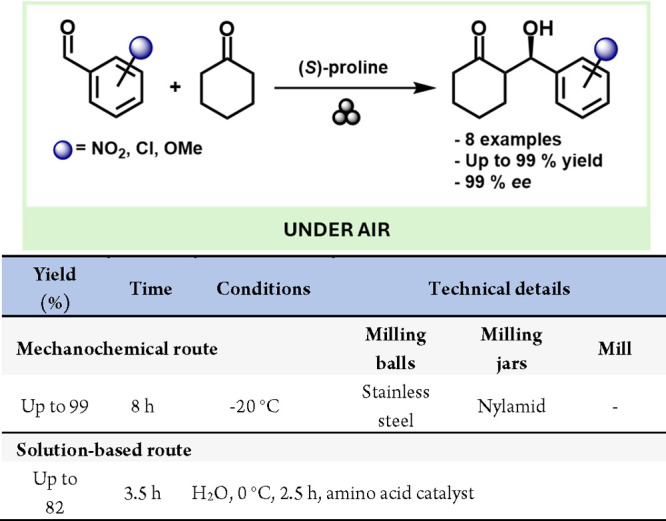
In 2011, Hernandez and Juaristi expanded upon previous studies by achieving asymmetric aldol reactions via ball milling. They combined cyclohexanone and acetone with various aromatic aldehydes, successfully forming aldol products (Scheme 34). Notably, these reactions exhibited higher diastero- and enantioselectivity compared to their solution-based counterparts.190
Scheme 34. Solid State Enantioselective Aldol Reaction Catalyzed by (S)-Proline and Comparison with Solution Based-Methods189.
Mechanochemistry has also become vital for enhancing the reactivity of insoluble or poorly soluble substances, opening new avenues for catalysis. For instance, Friščić’s group performed a screening for cross metathesis (Scheme 35a) and ring-closing metathesis (Scheme 35b) using a second generation Hoveyda-Grubbs catalyst. They achieved excellent yields with minimal or no solvent.191
Scheme 35. Mechanochemical Catalytic Reactions for Cross Metathesis (a) and Ring-Closing Metathesis (b) and Comparison with Solution-Based Method for (a)194.
In 2008, Bolm et al. developed a mechanocatalytic process for alkynylation reactions using Rh(III) and Au(I) catalysts. These reactions demonstrated excellent functional group tolerance and better yields than their solution-based analogues (Scheme 36).192,193
Scheme 36. Mechanochemical Alkynylations of Indoles Using Rh and Au Catalysts and Comparison with Solution-Based Method195.
Palladium complexes, important in catalytic transformations like the Heck reaction197,198 and the Buchwald-Hartwig amination reactions199,200 have seen further advancements due to mechanochemistry.201 For instance, Tullberg and co-workers performed the Heck reaction using Palladium(II) acetate in a mechanochemical, solvent-free method, efficiently synthesizing substituted dehydroalanines (Scheme 37).202
Scheme 37. Mechanochemical Pd-Catalyzed Heck Reaction and Comparison with Solution-Based Methods196.
Continuing with the Heck reaction, in 2019, Yu and colleagues presented an efficient mechanochemical method for chemo-, regio-, and stereoselective Heck coupling. They used Palladium(II) acetate to catalyze reactions between arylboron/heteroaromatics and cyclic or acyclic olefins, achieving excellent yields and selectivity even on a gram scale (Scheme 38, left).203
Scheme 38. Mechanochemical Pd-Catalyzed Oxidative Heck Reaction (Left), Buchwald-Hartwig Reaction (Right), and Representative Solution-Based Reaction205.
On a related note, Browne et al. devised a mechanochemical method for the Pd-catalyzed Buchwald-Hartwig amination of arylhalides with secondary amines. They utilized a Palladium pyridine-enhanced precatalyst preparation stabilization and initiation (Pd-PEPPSI) catalyst system. This method, applied to over 30 solid and liquid substrates, showed higher reaction rates and slower catalyst deactivation compared with solution-based methods (Scheme 38, right).
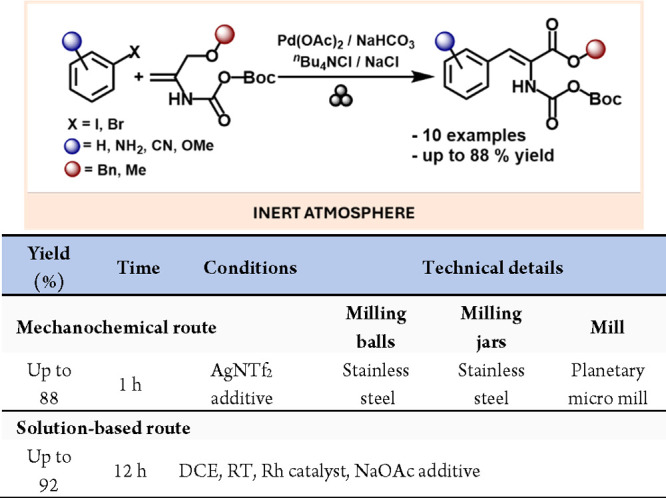
Continuing with C–N bond formation reactions, a significant development was reported by Friščić and co-workers. They achieved the mechanochemical synthesis of N-sulfonylguanidines by coupling sulfonamides and carbodiimides (Scheme 39).170 These compounds are highly relevant in the pharmaceutical and agrochemical industries. Still, their solution-based reactions often failed or yielded very low conversions, underscoring the importance of mechanochemistry for this synthetic approach.
Scheme 39. Mechanochemical Cu Catalyzed Preparation of N-Sulfonylguanidines and Comparative Solution-Based Method204.
More recently, Friščić and co-workers developed a copper-catalyzed C–N coupling of amides with cyclohexyl isocyanate (CyNCO) into carbamoyl isatins and benzamides using mechanochemistry (Scheme 40). These conditions resulted in higher yields than solution-based methods, which either did not occur or required high temperatures and energetic conditions.171
Scheme 40. Mechanochemical C–N Coupling of Amides and Isocyanates and Comparison with Solution-Based Method206.
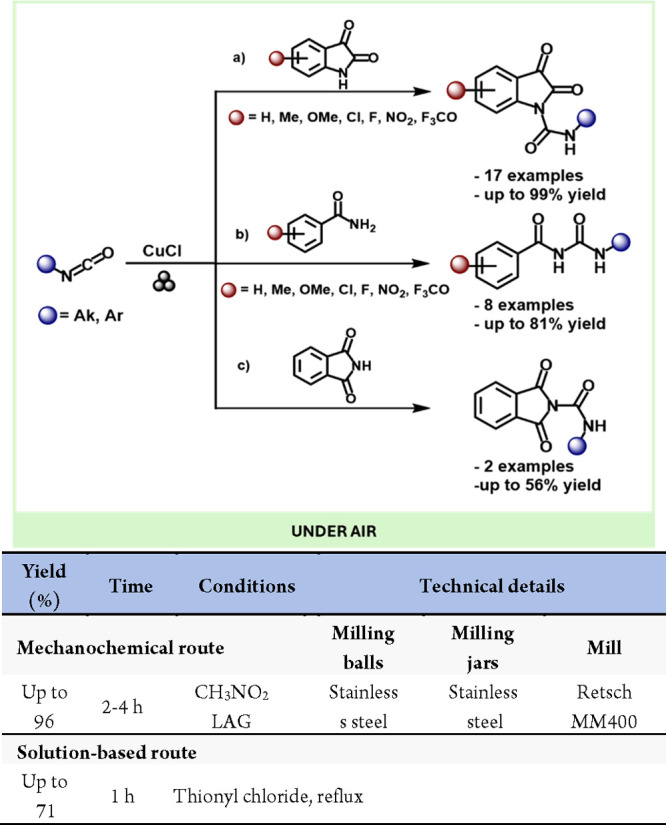
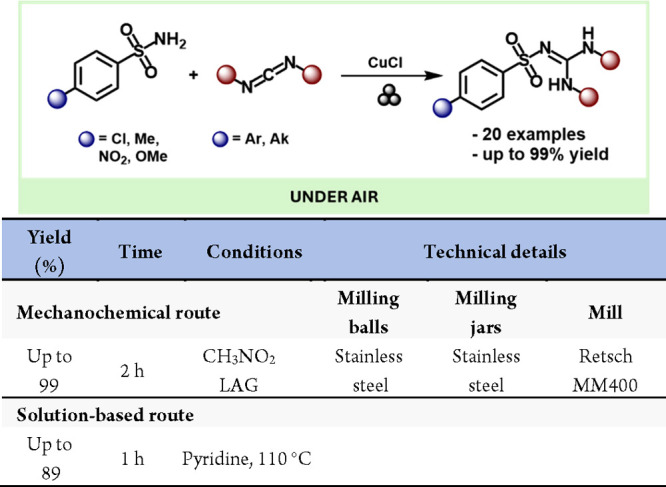
A notable example of “click” chemistry is the copper-catalyzed azide/alkyne cycloaddition reaction (CuAAC).209 In 2013, Mack et al. reported the first CuAAC reaction under solvent-free mechanochemical conditions, achieving the isolated triazole product in just 15 min without further purification (Scheme 41).210 Furthermore, this is the first example of a mechanocatalysis reaction induced by grinding media with a copper vial.
Scheme 41. Mechanochemical CuAAC Reaction and Comparison with a Representative Solution-Based Method156,207.
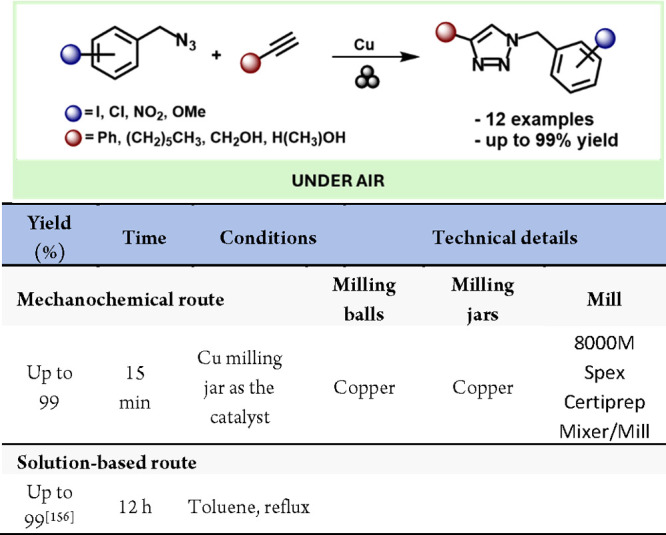
Continuing with C–N bond formation reactions, Bolm’s study on the mechanochemical C–H bond amidation of arenes is also noteworthy.211 This work involved the direct mechanochemical Rh(III)-catalyzed amidation of benzamides using dioxazolones as the nitrogen source. Remarkably, the reaction was compatible with arenes bearing both electron-donating and withdrawing groups, as well as with various substituents on both the arene and the dioxazolones (Scheme 42)
Scheme 42. Mechanochemical C–H Amidation of Arenes and Comparison with Solution-Based Methods156,208.
Regarding cycloaddition reactions, in 2016, Mack and co-workers developed an innovative solvent-free, nickel-catalyzed [2 + 2+2 + 2] cycloaddition of alkynes to synthesize substituted cyclooctatetraene (COT) derivatives via high-speed ball milling (Scheme 43). This mechanochemical method leverages the frictional energy created by reusable nickel pellets, which also act as the catalyst. Notably, it predominantly yields cyclooctatetraene isomers rather than substituted benzenes typically obtained in solution.212
Scheme 43. Mechanochemical CuAAC Reaction and Comparison with a Solution-Based Representative Method213.
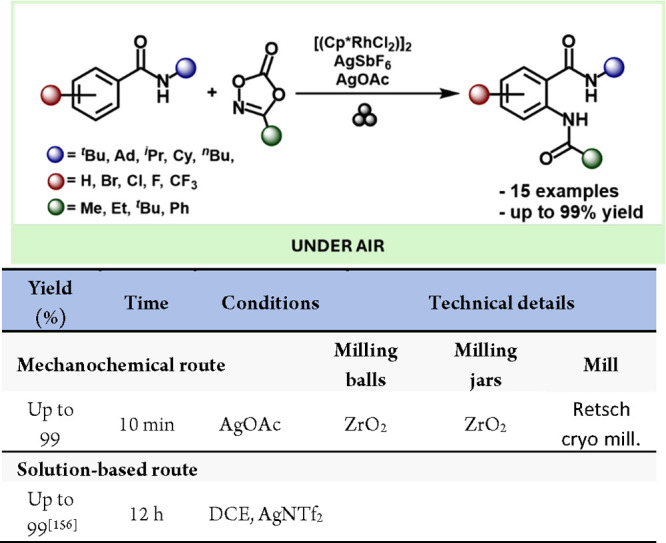
Mechanochemical methods have also enabled the direct observation and the occasional isolation of reactive intermediates. The Ito group showcased this by reporting the straightforward synthesis and solid-state isolation of organopalladium halides, which are known intermediates in Suzuki-Miyaura and Heck-type coupling reactions (Scheme 44).115
Scheme 44. Mechanochemical Palladium-Based Oxidative Addition Complexes Air-Sensitive Pd(0) Intermediates and Comparison with a Solution-Based Method116.
Mechanochemical techniques can modify the chemical reactivity and selectivity compared to analogous solution-based procedures. As a result, solvent-free milling can lead to product mixtures or equilibrium compositions different from those obtained in solution.214 In 2010, Lamaty and co-workers conducted a Horner–Wadsworth–Emmons reaction using a mild carbonate base under mechanochemical conditions. Starting from a phosphonate-substituted glycine, this method yielded tert-butoxycarbonyl-protected unsaturated amino esters with outstanding yield and selectivity.215 Parallel to these efforts, Zhang and colleagues developed a Diels–Alder cycloaddition of cyclopentadiene with maleic anhydride and maleimide derivatives via mechanochemistry.216 They successfully obtained endonorbornenes in quantitative yield at ambient temperature without using any organic solvent or catalyst, simplifying the purification process. In this context, selective carbon–hydrogen (C–H) arylation of arenes via mechanochemistry has been extensively studied in recent years.217
For example, Lou and co-workers achieved rapid and selective biaryl synthesis through dehydrogenative C–H/C-H arylation in a ball mill, generating C–C bonds between various arenes and both electron-rich and electron-poor oximes in good to excellent yields (Scheme 45a). They also successfully applied this approach to the arylation of anilides (Scheme 45b).218
Scheme 45. Mechanochemical C–C Bond Formation with Oximes and Anilides and Solution-Based Method for Comparison for (a)219.
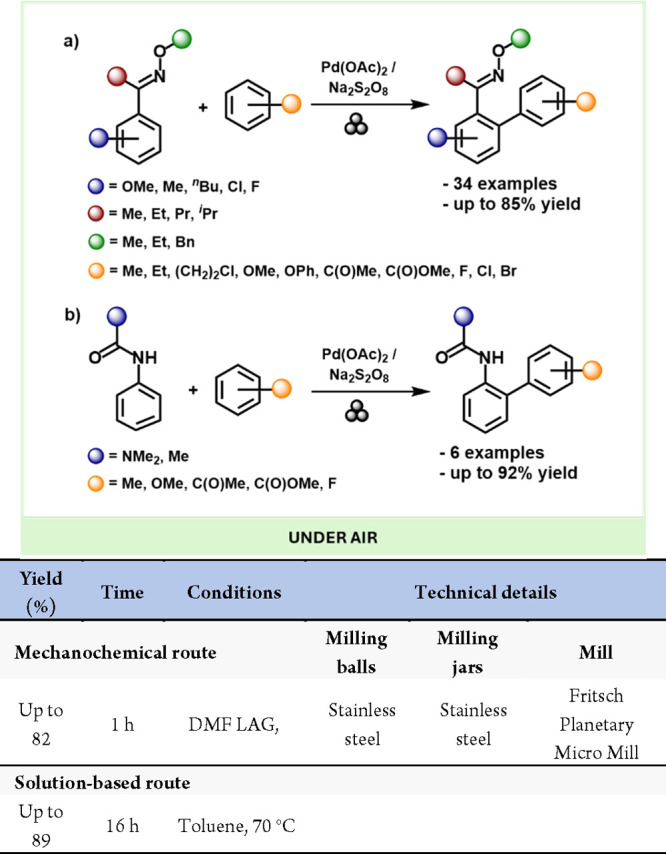
Colacino and co-workers recently investigated the Kabachnik-Fields domino reaction by mechanochemistry for the first time, preparing α-aminophosphonate derivatives with very high yields and total selectivity compared to solution methods.220 α-Aminophosphonates are biologically active compounds garnering significant interest in medicinal chemistry due to their potential to inhibit enzymes in amino acid metabolism.
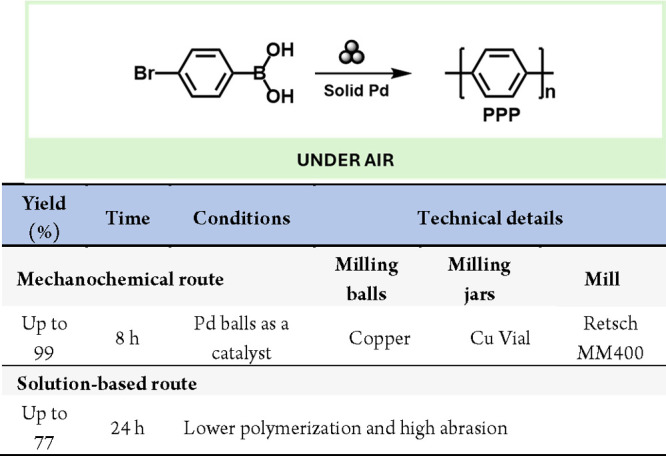
Another intriguing aspect of mechanochemistry is using milling media (i.e., jars and/or balls) as catalysts.221 The grinding surfaces and leaching metallic particles can exhibit catalytic activity, especially when M(0) species act as catalysts or precatalysts. For example, Borchardt and colleagues conducted a Suzuki polymerization reaction using Pd solid balls as both milling media and catalyst, achieving the highest degree of polymerization reported to date (199) (Scheme 46).222 Subsequently, they enhanced the reaction by using copper alloys as milling tools, increasing yield and reducing abrasion while improving catalyst stability and reusability.223
Scheme 46. Mechanochemical Suzuki Polymerization of 4-Bromophenylboronic Acid224.
Similarly, Jiang’s group described the cross-dehydrogenative coupling of 2-phenyltetrahydroisoquinoline with nitromethane, alkynes, and indoles.179 Here, copper milling balls were used directly as catalysts, with 2,3-dichloro-5,6-dicyanoquinone (DDQ) as the oxidant (Scheme 47).
Scheme 47. Copper Ball Catalyzed Cross Dehydrogenative Coupling Reactions and Representative Solution-Based Method for Comparison226.
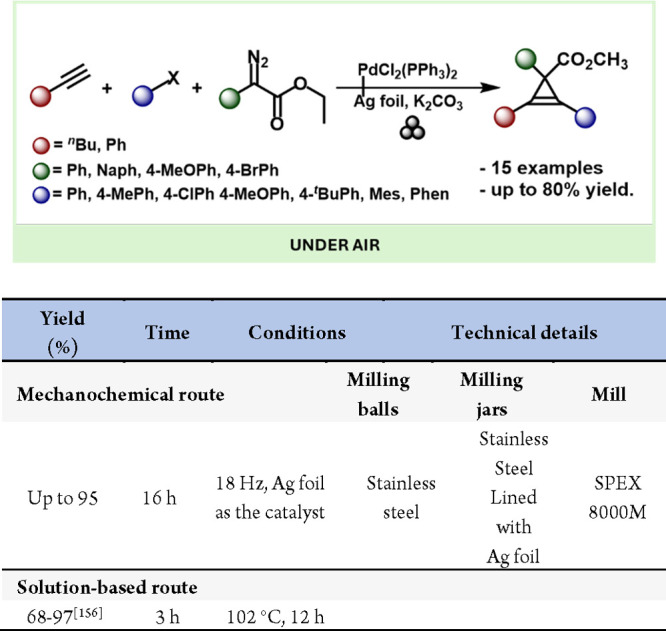
In addition, Mack and co-workers also discovered that silver and copper foil are effective, practical, versatile, and selective heterogeneous catalysts for cyclopropenation of terminal and internal alkynes under mild mechanochemical conditions.225 They also pioneered one-pot palladium(II)/silver foil-catalyzed Sonogashira-cyclopropenation reactions for complex cyclopropene formation by mechanochemistry (Scheme 48).225
Scheme 48. One-Pot Mechanochemical Sonogashira Coupling/Cyclopropenation and Comparison with a Solution-Based Method156,227.
Lastly, the Borchard group made a notable contribution by introducing the term “mechanocatalysis”, which is defined as solvent-free catalytic reactions initiated by mechanical forces in mechanochemical reactors like ball mills. A distinctive feature is that the milling materials, such as the milling balls themselves, act as the catalyst of the reaction.228
Organocatalysis
Asymmetric catalytic synthesis, using nature-inspired methods instead of transition metals, has transformed organic chemistry by enabling access to diverse chiral compounds.229−232
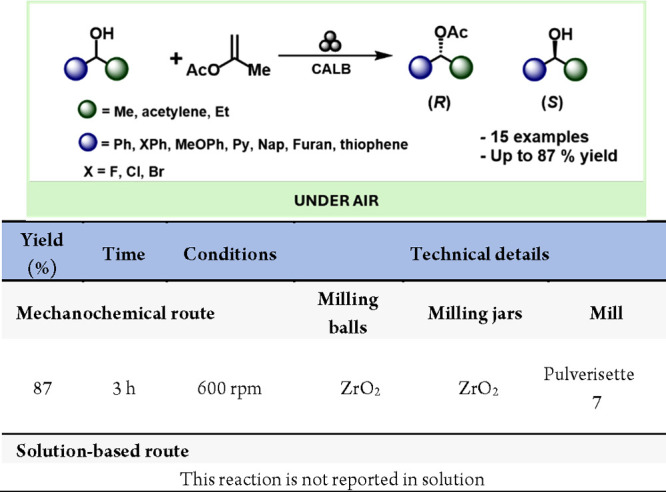
Enzymes serve as highly effective and adaptable catalysts extensively utilized in both industry and academia.233 Over the past three decades, significant research has focused on their application in stereoselective chemical transformations. This journey began with early achievements like the synthesis of antibiotic analogues234 {ref} and has evolved to include sophisticated protein engineering techniques.235 Biocatalysts possess unique attributes such as resilience to extreme temperatures,236 pH ranges,237 and nonaqueous solvents,238,239 making them increasingly valuable in organic synthesis.240,241 They are particularly instrumental in conducting asymmetric reactions that yield biologically active compounds with exceptional enantiopurity.242−244 In a groundbreaking advance, Hernández et al. introduced a novel approach that integrates biocatalysis with mechanical force (Scheme 49). They devised a mechanochemical method using immobilized lipase B from Candida antarctica (CALB, Novozym 435) to resolve a racemic mixture of secondary alcohols through an enantioselective acylation reaction.245 Since then, several articles have been published, but it is still a field in development.246−252
Scheme 49. Mechanochemical Enzymatic Resolution of Secondary Alcohols.
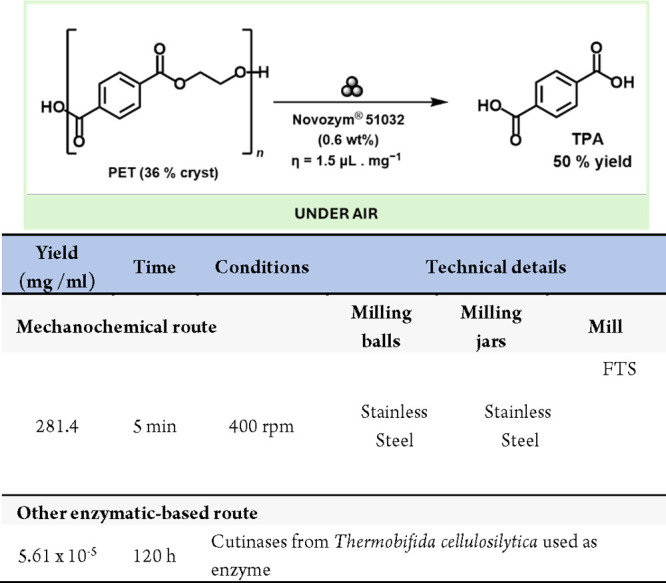
Polyethylene terephthalate (PET) is the most recycled plastic, but traditional recycling reduces the quality of the recycled material. Breaking PET into its building blocks can create high-quality PET, yet this chemical depolymerization needs hazardous conditions. Enzymes are safer and work under mild conditions. While some enzymes can depolymerize low-crystallinity PET, they struggle with highly crystalline PET found in consumer products. Friščić, Auclair et al. reveals that the cutinase enzyme from Humicola insolens (HIC, Novozym 51032) can efficiently break down highly crystalline PET to terephthalic acid (TPA) with a 50% yield, without pretreatment (Scheme 50).253
Scheme 50. General Reaction Scheme of the Mechanoenzymatic Hydrolysis of PET using HiC (Novozym 51032) by Ball-Milling and Comparison with Other Enzymatic-Based Method254.
Organocatalysis focused on stereoselectivity is now gaining a lot of strength, being at the same level as transition-metal and enzymatic methods in this field. The Nobel Prize in Chemistry 2021 recognized Benjamin List and David MacMillan for their pioneering work on enamine255 and iminium activation,{ref} advancing from covalent to noncovalent catalytic approaches such as carbene,256,257 phosphine,258,259 hydrogen bonding (HB),260,261 ion-pairing,262,263 phase-transfer,263−265 and other reactions that lead to valuable transformations.
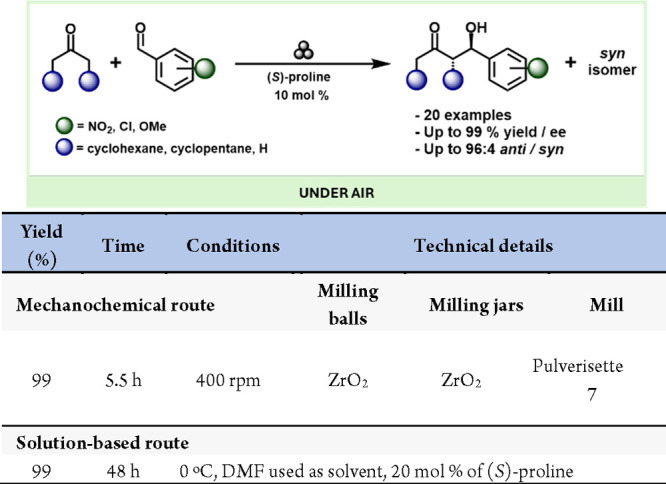
In recent years, mixing organocatalysis with mechanochemistry has attracted the interest of organic seeking more efficient and sustainable processes to obtain biologically active compounds and APIs.248,266−269 For example, one of the first uses of mechanochemistry to synthesize cocrystals of biologically significant molecules was reported by the Etter group. They manually ground 1-methylthymine and 9-methyladenine to create a cocrystal, where the complementary bases were connected by Hoogsteen-type hydrogen bonds.270 In 2006, Trask et al. conducted the first systematic study using neat grinding and liquid-assisted grinding (LAG) to screen for salt forms of APIs. They examined the reactions of two structurally similar APIs, trimethoprim and pyrimethamine, with pharmaceutically acceptable carboxylic acids.271 In the Bolm’s group successfully combined mechanochemistry with asymmetric organocatalysis, performing an enantioselective (S)-proline-catalyzed aldol reaction under ball-milling conditions.186,187 This landmark report demonstrated that solvent-free organocatalytic reactions can be carried out in a ball mill while maintaining both yield and enantioselectivity with up to 99% of enantiomeric excess (ee) (Scheme 51)
Scheme 51. Enantioselective (S)-Proline-Catalyzed Aldol Reaction under Ball-Milling Conditions and Representative Solution-Based Method for Comparison272.
Inspired by this work, researchers have since developed various asymmetric organocatalytic transformations using both covalent and noncovalent activation strategies under mechanochemical conditions.229
For ease of discussion, we will divide the organocatalytic reactions into two groups: (i) transformations that require covalent bond formation between catalysts and substrates and (ii) transformations where catalysts interact with reagents through weak noncovalent interactions (NCI).
Covalent organocatalysis involves covalently driven transformations, such as enamine and iminium activation of carbonyl compounds with primary and secondary amines. The concept later expanded to include carbene and phosphine catalysts, forming covalently bonded intermediates.
Following this initial report by Bolm and co-workers, Juaristi et al. published several reports on dipeptide-catalyzed aldol reactions under mechanochemical conditions.273−276 Notably three of these reports utilize a dental amalgamator (used in dentistry to prepare amalgams for cavity treatment) to perform their experiments. The first of these used an (S)-proline-(S)-phenylalanine dipeptide catalyst, achieving high yields and stereoselectivities in just 4 h.190
Michael/1,4-addition reactions, well-established in solution chemistry, can also be effectively mediated by organocatalysts. For instance, enolizable aldehydes can be readily transformed into nitroalkenes using ball milling techniques using the Jørgensen-Hayashi secondary amine as a catalyst.267
Other examples of this type of reactions include, α-aminoxylation and α-hydrazination of aldehydes with nitrosobenzene and dibenzyl azodicarboxylate, respectively, catalyzed by an O-silylated-(S)-proline was reported,274 In 2014, Goldfuss and co-workers developed ten new hydrogen-bonding catalysts derived from open-chain PV-amides of BINOL and cinchona alkaloids were tested in the asymmetric Michael addition of 2-hydroxynaphthoquinone to β-nitrostyrene. The open-chain 9-epi-amino-cinchona-based phosphorus amides demonstrated high catalytic activity, achieving nearly quantitative yields of up to 98% and ee of up to 51%.277 In 2019, Kowalczyk et al. performed the stereoselective addition of nitrometane to conjugated en-ynones by ball milling achieving 1,4-addition products with 91% conversion and 88% of ee.278
In terms of organocatalytic processes occurring via weak NCI, over the past decade, this type of reaction has significantly advanced beyond HB and other concepts such as ion-pairing, halogen bonding, and phase-transfer catalysis have been described in a wide range of valuable transformations.279 Mechanochemistry has been primarily used for hydrogen-bonding catalysis, mainly involving thioureas and squaramides, with mechanochemical conditions yielding notable improvements in these organocatalytic transformations.280
These types of reactions posed interesting questions under ball milling conditions since it might be expected that their performance would suffer in solvent-free or ball-milling setups. However, Xu et al. demonstrated that the Michael addition of diverse 2,4-dicarbonyls to nitroalkenes can be efficiently achieved in a ball mill using cinchona alkaloid-derived squaramide catalysts, with reaction times as short as 5 min and a catalyst loading of just 0.5 mol % (Scheme 52).218
Scheme 52. Squaramide Catalyzed Michael Addition of Aldehydes to Nitroalkenes by Ball-Milling and Representative Solution-Based Method for Comparison.
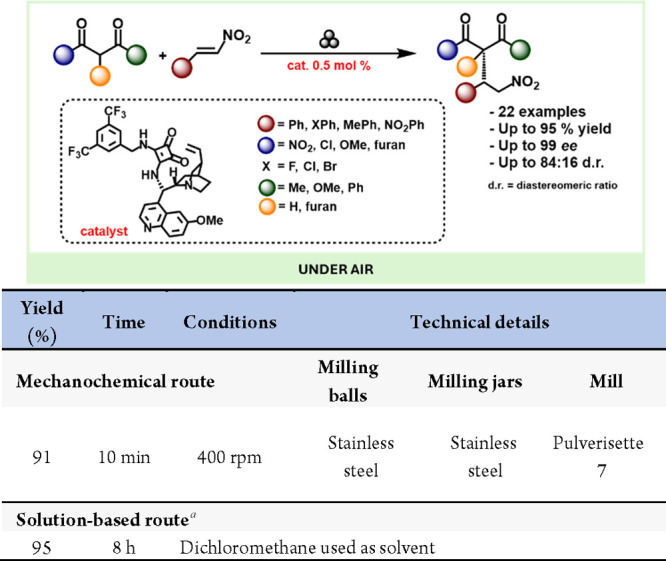
Conducted by authors of mechanochemical study.
Bolm and co-workers subsequently described thiourea-catalyzed Michael addition of α-nitrocyclohexanone to different nitroalkenes under ball-milling conditions. Using optimized parameters, they achieved Michael addition products with yields up to 95%, an enantiomeric excess (ee) of 98% and an anti/syn ratio of 98:2, completing the reaction in 30 min.281
More recently, Šebesta et al. reported a squaramide catalyzed asymmetric domino Mannich-fluorination process under ball-milling conditions. They discovered that mechanical activation significantly accelerated the organocatalyzed domino Mannich reaction/fluorination with just 50 μL of CH2Cl2 as a liquid-assisted grinding (LAG) agent A bifunctional squaramide catalyst facilitated the initial asymmetric Mannich reaction of enolizable pyrazolones with oxindole imines. Complete conversion of starting materials was achieved within five minutes of milling, and the intermediate was used directly in the next step without further purification. The second step, diastereoselective fluorination, typically took 20–25 min under ball-milling conditions, yielding a variety of fluorinated oxindole derivatives.188
Mack and Shumba reported a ball-milling-enabled Morita-Baylis-Hillman (MBH) reaction between aryl aldehydes and methyl acrylate, catalyzed by diazabicyclo[2.2.2]octane (DABCO).282 They achieved product yields of up to 98% in just 30 min when using p-nitrobenzaldehyde (Scheme 53).
Scheme 53. Tertiary Amine (DABCO)-Catalyzed MBH Reaction by Mechanochemistry and Representative Solution-Based Method for Comparison283.
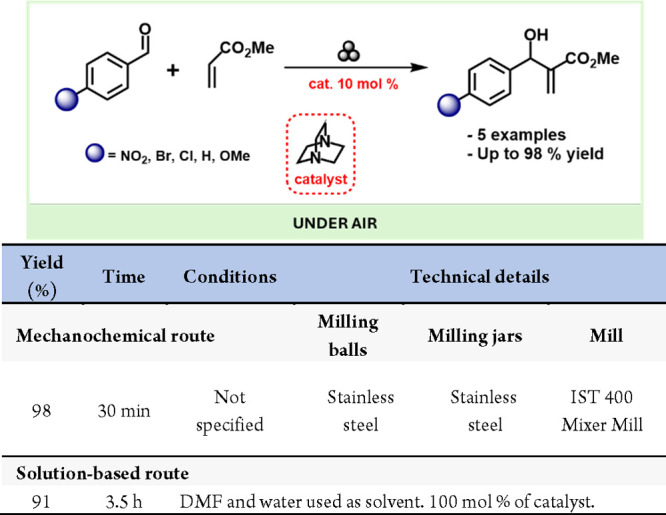
Inspired by the work of Mack and Shumba, Browne and colleagues recently reported an aza-Morita-Baylis-Hillman (aza-MBH) reaction under ball-milling conditions, involving imines and α,β-unsaturated compounds.176 They demonstrated that 3-hydroxyquinuclidine could effectively catalyze the reaction in just 99 min, using toluene in LAG quantities and sodium chloride as a grinding auxiliary, achieving the desired products in moderate to excellent yields.
Acyl anions, activated carbonyls with umpolung reactivity, enable previously inaccessible functionalizations. This reactivity, accessed using N-heterocyclic carbenes (NHCs), was pioneered by Breslow.284 Notable transformations using acyl anion chemistry include the benzoin and Stetter reactions, where benzaldehyde derivatives react with carbonyls or α,β-unsaturated carbonyls, respectively. Browne and co-workers recently reported the first acyl anion NHC organocatalysis under ball-milling conditions.241 They demonstrated inter- and intramolecular benzoin and Stetter reactions with a notable rate enhancement compared with solution-phase methods. Their approach utilized sand as a grinding auxiliary and a LAG agent, employing triazolium or thiazolium pre-NHC catalysts and cesium carbonate as a base.
Finally, Lamaty and co-workers reported the asymmetric α-alkylation of imines with alkyl bromides, catalyzed by a cinchonidine-derived ammonium salt and potassium hydroxide as a base, under ball-milling conditions.285
Mechanoredox Reactions
Photoredox chemistry has emerged as a significant development in synthetic chemistry. In these reactions, a photoexcited catalyst can either be a reductant by donating an excited electron or an oxidant by filling the generated hole.286 Regarding mechanochemistry, executing photomechanochemical reactions remains a considerable challenge. First, the required light sources must be attached to machines with high-speed moving parts. Second, the milling jars must be made from materials transparent to the needed wavelength (such as plastic, glass, or quartz) and robust enough to withstand the high-speed impacts essential for mechanochemical processes. While there have been successful reports of photomechanochemical reactors,287 numerous limitations still need to be addressed.288
In this context, Strukil described the first example of photomechanochemical catalysis in 2017.289 This proof-of-concept study successfully demonstrates the first transition-metal-free photocatalysis in the solid state, achieved through the combination of visible light irradiation and mechanochemical ball milling (Scheme 54). In the same year, Hernandez and co-workers also reported a photomechanochemical borylation of aryldiazonium salts, studying the role of eosin Y.290
Scheme 54. First Example of Photomechanochemical Catalysis and Representative Solution-Based Method for Comparison291.
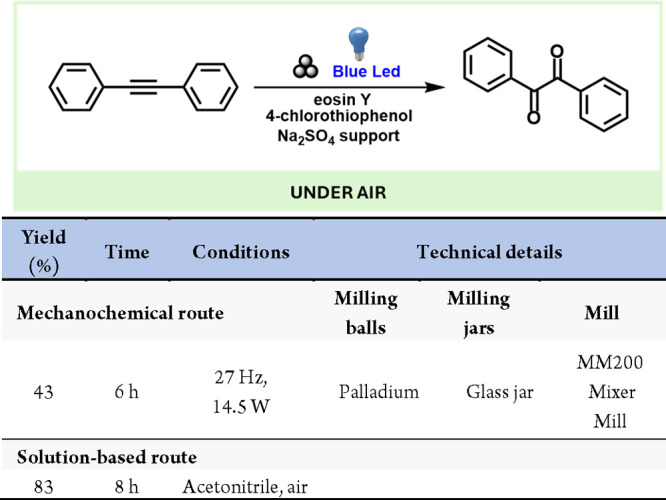
In this context, MacGillivray’s group has described the solid-state photodimerization of a hydrogen-bonded adduct between 4,6-dichlororesorcinol and trans-1,2-bis(4- pyridyl)ethylene.292 The initial hydrogen-bonded adduct was obtained through manual grinding and then exposed to UV light in the solid state to induce dimerization (Scheme 55). Interestingly, this dimer could be reverted to the original adduct upon further grinding. In a similar vein, the same group reported the [2 + 2] solid-state photodimerization of p-di[2-(4-pyridyl)ethenyl]benzene to produce [2.2]paracyclophane.293
Scheme 55. Supramolecular Solid-State Photodimerization and Mechanochemical Depolymerization Steps.
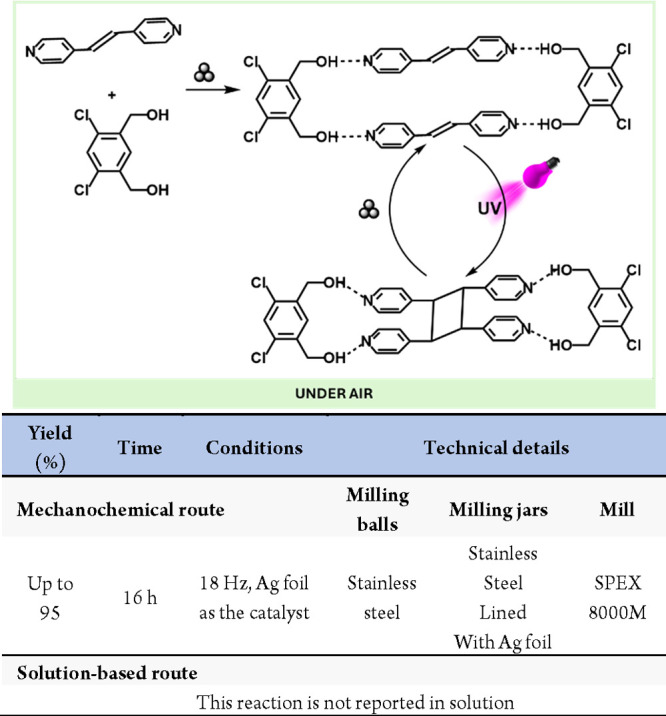
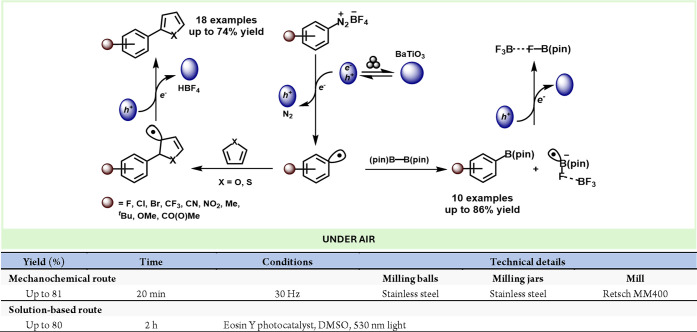
Kubota, Ito and co-workers developed an alternative mechanoredox system based on piezoelectric materials. In this seminal work, they exploited the ability of piezoelectric materials to generate charge separation and transient species under mechanical stress. These transient polarized species could then donate an excited electron to a suitable acceptor, functioning analogously to classic catalysts. Utilizing BaTiO3 as a piezocatalyst, they successfully reduced aryl diazonium salts, generating radical aryl groups that underwent radical borylation and arylation reactions (Scheme 56).295 This breakthrough opens new avenues for solid-state redox chemistry.
Scheme 56. Mechanoredox Reduction of Azonium Salts Mediated by Piezoelectric Materials and Comparison with a Representative Solution-Based Method298.
Expanding upon these applications, the same group carried out the mechanoredox reduction of trifluoromethyl sulfonium salts (Umemoto’s reagent), achieving the trifluoromethylation of various arenes (Scheme 57).296
Scheme 57. Solid State Reduction of Umemoto′s Reagent for Radical Trifluoromethylation Reactions and Comparison with a Solution-Based Method294.
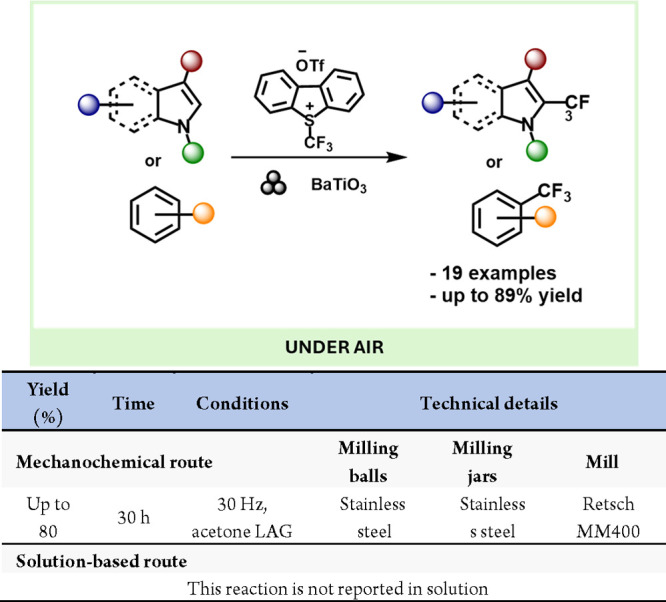
The formation and regeneration of active copper(I) are crucial mechanistic steps in copper-catalyzed atom transfer radical cyclizations (ATRC). Conventionally, ensuring the presence of catalytically active Cu(I) species involves high copper(I) catalyst loadings or the addition of complementary reducing agents. In 2020, Bolm and colleagues demonstrated how the piezoelectric properties of BaTiO3 enabled the mechanoredox reduction of a copper(II) precatalyst into the active copper(I) species for copper-catalyzed, mechanochemical, solvent-free ATRC reactions (Scheme 58).297
Scheme 58. Mechanoredox Generation of Catalytically Active Cu(I) Species.
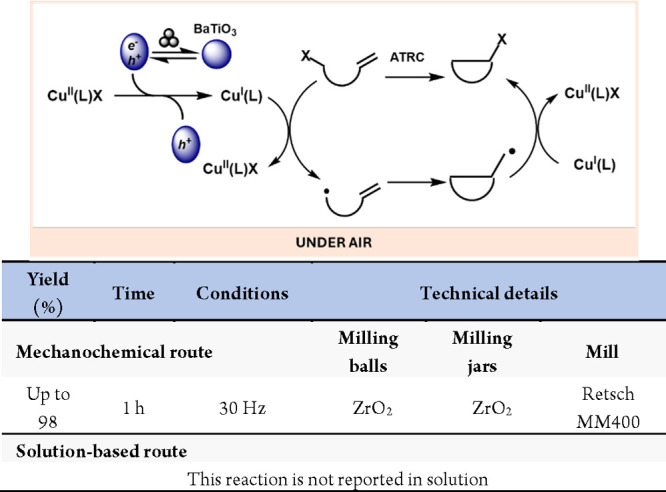
More recently, the same group has described the mechanoredox radical addition of sulfoximidoyl chlorides to allenes.299 In this work, the piezoelectric material was also used to activate the Cu(II) precatalyst to yield the Cu(I) active species (Scheme 59).
Scheme 59. Mechanoredox Addition of Sulfoximidoyl Chlorides to Allenes.
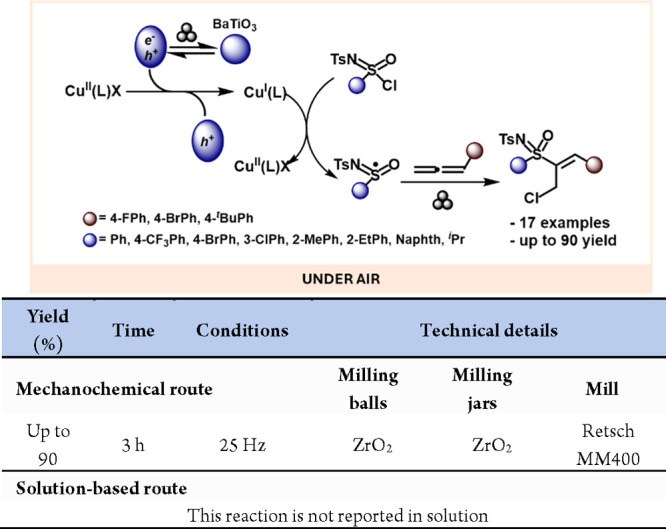
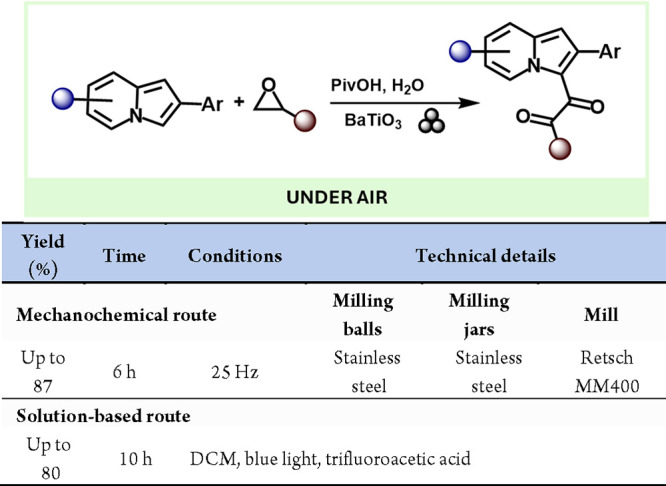
Finally, in 2021, Wang and colleagues employed BaTiO3 as the piezoelectric material in the mechanochemical-induced synthesis of 1,2-diketoindolizine derivatives from indolizines and epoxides.300 This method provided a simple and efficient alternative to transition-metal-catalyzed or visible-light-induced methods. It offers a novel approach to synthesizing these products using solvent-free processes with scalable potential and high conversion efficiency (Scheme 60).
Scheme 60. Mechanoredox Preparation of 1,2-Diketoindolizine and Comparison with a Representative Solution-Based Method301.
Mechanochemistry of Materials
In materials science, mechanochemistry has emerged as a powerful tool for synthesizing a wide range of materials, enabling the creation of novel materials with unique properties that may not be attainable through conventional synthetic routes.
Mechanochemistry offers a unique approach to chemical synthesis and material processing, which differs from traditional methods that often rely on solvents or high temperatures. The appeal of mechanochemistry lies in its simplicity, energy efficiency, and environmentally friendly nature.
Mechanochemistry is an increasingly represented methodology in the design of new energy-efficient and low-cost routes to new and existing materials with applications in energy storage,10,302 OLED materials,50 textiles,303 nanocrystals,127,304,305 piezoelectric materials,297,306 metal hydrides,307−310 nanomaterials,311 hydrogen storage materials,312 MOFS, and perovskites.313 Also, several authors have published review articles focused on mechanochemistry and materials.10,314,315
The ability of mechanochemistry to produce unique, technologically relevant materials is illustrated in the synthesis of MOFs and perovskite material, which have been extensively studied over the past few years. Some selected examples are discussed in this section. In addition, further examples can be obtained in reviews focused on MOFs316−318 and Perovskite materials.319−321
Metal–Organic Frameworks (MOFs)
A versatile class of materials are metal–organic frameworks (MOFs).322−324 Highly microporous and tunable functional materials are used in gas storage, catalytic separation, etc.325−327 For example, the encapsulation and confinement of fullerene hosts in metal–organic frameworks (MOFs) leads to a new class of fullerene crystalline materials with unique physicochemical properties and a plethora of potential applications.328 However, the main limitation is their low solubility and the competition between the solvents and the guest to occupy framework voids - which can be prevented using mechanochemistry.329 In 2020, Užarević et al. developed a fast, green, efficient, and stoichiometry-controlled ion- and liquid-assisted grinding330 (ILAG) route to four C60-zeolitic imidazolate frameworks 8 (ZIF-8) containing different mol % of C60 (Scheme 61).331
Scheme 61. ILAG Procedure for the Encapsulation of C60 Fullerene into Cavities of ZIF-8.
Current routes to MOFs are based on nonscalable solvothermal synthesis, which generally display high energy consumption, low yields, and use of toxic solvents.332,333 In this regard, mechanochemical approaches have been shown to be capable of addressing the above-mentioned drawbacks of solvothermal routes.334 In 2010, Emmerling et al. used a mechanochemical synthesis to obtain metal–organic frameworks (MOFs) with high surface areas for two model systems.335 The compounds HKUST-1 (Cu3(BTC)2, BTC = 1,3,5-benzenetricarboxylate), and MOF-14 (Cu3(BTB)2, BTB = 4,4,4-benzenetribenzoate) were synthesized by ball milling and characterized by powder X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscopy (SEM), and thermal analysis (DTA/DTG/MS).
Also, in 2010, James and co-workers studied the mechanochemical formation and resulting properties of the Cu3(BTC)2 (BTC = 1,3,5-benzenetricarboxylate) MOF. They reported that appreciable surface areas (>1300 m2 g–1) can be obtained if the product is washed prior to activation, yielding a material with the same essential functionality as the one prepared using solution-based preparations.336
Thanks to their physical and chemical properties, such as porosity, crystallinity, rich structural diversity, and exceptional chemical and thermal stability, zeolitic imidazolate frameworks (ZIFs) show significant potential applications for gas storage, catalysis, etc.337 However, the transition metals that can form ZIFs are limited (mainly Zn(II) and Co(II)), limiting their applications. However, the development of high-entropy ZIFs (HE-ZIFs) allows obtaining ZIFs that contain different transition metals.
High-entropy materials are compounds with five or more metal species incorporated into a single lattice with a random occupancy. Although the concept of high-entropy is well-known in inorganic hard materials such as alloys (HEAs).338 However, the temperature needed to provide the energy required for the synthesis is challenging because it is crucial to choose a temperature that makes the reaction possible without destroying the HE-ZIF. To solve this issue problem, Xu et al. developed a mechanochemical synthesis of HE-ZIFs at ambient temperature in which five ions were dispersed in the ZIF lattices, employing 2-methylimidazole (MIM) linkers (Scheme 62).This is in stark contrast with solvothermal methods, where only Zn(II) and Co(II) HE-ZIFs ions could be obtained.337
Scheme 62. Mechanochemical Synthesis of HE-ZIF (Adapted with Permission from Ref (337). Copyright 2019 Wiley).
Užarević et al. designed a series of zirconium (Zr) MOFs composed of Zr6 cluster nodes, UiO-66, UiO-66-NH2, MOF-801, and MOF-804, using a water-assisted mechanochemical approach.339 The authors achieved high-quality MOFs using nonconventional zirconium dodecanuclear acetate cluster, and minute amounts of water, in less than 60 min of milling, avoiding solvents such as dimethylformamide. Furthermore, the synthesis was also scaled up using twin single-screw extrusion to produce more than 100 g.
Previous to this work, James, Crawford and co-workers employed continuous twin- and single-screw extrusion for the synthesis of different Ni(salen) complexes and commercial MOFs such as Cu3(BTC)2 (HKUST-1), Zn(2-methylimidazolate)2 (ZIF-8), and Al(fumarate)(OH), achieving a maximum rate of 4 kg per hour of ZIF-8.125
The growing demand and public interest in nanomaterials are hindered by the risks associated with the use of hazardous chemicals during their synthesis and stabilization.340 Most high-purity metal nanoparticles reported to date have been obtained by using hazardous chemicals and toxic surfactants. During the last years, different mechanochemical techniques have been used to produce nanomaterials comprising cooper,341 nickel,342 iron,343,344 and silver.345 For instance, Rak and co-workers reported the solvent-free synthesis of silver nanoparticles from a simple silver salt (i.e., AgNO3) using lignin as a biodegradable reducer and polyacrylamide polymer as support, obtaining a very effective antimicrobial filter for both Gram-positive and Gram-negative bacteria.346
Perovskite Materials
Organic–inorganic halide perovskite materials have been extensively explored due to their unique optoelectronic properties and wide range of applications (photovoltaic solar cells,347,348 lighting,349,350 photodetectors,351,352 and lasers353,354). These materials display tunable bandgaps and can be deposited quickly and inexpensively from low-cost precursors, making them ideal candidate materials for solar cells, either by themselves as the wide-bandgap top cell material paired with low-bandgap silicon or copper indium diselenide bottom cells or by using both wide- and small-bandgap perovskite semiconductors to produce all-perovskite solar cells.355,356 Among the top six certified best energy conversion efficiencies reported by the National Renewable Energy Laboratory on perovskite-based solar cells, five are based on mixed perovskites such as MAPbI1–xBrx, FA0.85MA0.15PbI2.55Br0.45, and Cs0.1FA0.75MA0.15PbI2.49Br0.51 (MA = methylammonium, FA = formamidinium).357
Over the past decade, mechanochemical approaches have become versatile routes for preparing a range of hybrid perovskites.321,358−364 They provide a high degree of stoichiometric control and allow for the growth of relatively large crystalline grains, making the mechanosynthesised perovskites exhibit lower hysteretic behavior, slow charge recombination and low trap density in comparison to the conventional solvothermal synthesis.321,365
In 2016, Jodlowski and co-workers developed an efficient, simple, and reproducible method for preparing four types of hybrid perovskites. These were obtained in large quantities of high-purity polycrystalline powders, which displayed excellent optoelectronic properties.319 Later, in 2018, Karmakar and co-workers prepared seven mixed-halide lead perovskites (MHPs) using a solvent-free mechanochemical method. The obtained materials displayed solid-solution behavior identical to that of traditional solvent-based synthesis. However, mechano-perovskites showed superior stoichiometry control and higher reproducibility, stability, and material phase purity, which is essential for device engineering. These results were later supported by Michaelis and co-workers, who demonstrated that mechanochemistry efficiently allows the formation of various phase pure hybrid lead and lead-free halide perovskite compositions that show the same benefits.366
Moreover, as demonstrated by Michaelis and co-workers, mechanochemistry has even enabled the formation of some perovskite materials with compositions never achieved in solution, such as certain formamidinium-based mixed halide perovskites (FA-MHPs). The authors reported the one-pot mechanochemical synthesis of FAPBX3 (X = Cl, Br, I, respectively) by both hand grinding and ball milling, comparing their normalized reflectance spectra (Scheme 63).366
Scheme 63. Mechanochemical Preparation of FA-MHPs Together with Their Crystal Structure Diagram, Underlining the [PbXxX′6–x]4– Octahedral Configuration and the Normalized Reflectance Spectra for FAPbX3 and MHPs Performed by Hand Grinding (HG-FAPb(Cl0.5Br0.5)3) and Ball Milling (BM-FAPb(Br0.5I0.5)3.
In 2019, Chen and co-workers demonstrated that a mechanosynthesized family of APbX3 (A = MA, FA AND Cs; X = Cl, Br, and I) perovskites comprising halogen-rich surfaces yield visible full-spectral emissions with maximal photoluminescence quantum yield up to 92% (Figure 2). The authors also synthesized Mn2+-doped CsPbCl3 nanocrystals showing dual-modal emissions of both dopants and excitons, demonstrating their application as blue/green/red color converters in UV-excitable white-light-emitting diodes.367,368
Figure 2.
Representative luminescence photographs of MAPbX3 perovskite solutions under a 365 nm UV lamp irradiation. Reproduced with permission from ref (368). Copyright 2019 ACS.
Finally, and with a view to their future industrialization, Leupold and co-workers demonstrated that mechanochemically synthesized lead halide perovskites such as MAPbI3 display higher thermal stability than the ones obtained by conventional thin-films methods with no degradation after more than two years and only a meaningless degradation after heat treatment at 220 °C for 14 h. This work established the potential of mechanochemically synthesized halide perovskite powders for long-time storage and upscaling, leading the way to commercializing perovskite-based optoelectronic devices.369
Conclusions and Outlook
We hope this Review illustrates some relevant mechanochemical reactions in both organic and inorganic chemistry by covering main group and transition metal molecular compounds, organic and catalytic reactions, and the preparation of materials.
The wide variety of applications in virtually all areas of modern chemistry showcases the increasing importance of mechanochemistry. Beyond the initial perception of “yet another technique”, mechanochemistry has demonstrated that it constitutes an all-new branch of chemistry—since it has displayed divergent reactivity with respect to solution-based methods
Another advantage of mechanochemistry is its environmentally benign nature. The absence of solvent (or minute amounts of it) constitutes a drastic decrease in chemical waste generation. Moreover, shorter reaction times or lower temperatures are often required when using mechanochemistry methods, which implies a reduction in energy consumption. These advantages are especially appealing for industrial applications where any decrease in waste, time or energy can constitute drastically cheaper processes.9,370
Despite all of the highlighted advantages, the study of mechanochemical processes remains outnumbered by its solution-based counterparts. However, the increasing number of scientific studies, together with new technical advances (e.g., newly developed mechanochemical milling and grinding apparatus, control temperature devices, and monitoring setups), strongly supports an even faster expansion of the field of mechanochemistry in the following years.
Acknowledgments
We acknowledge the support from the Agencia Estatal de Investigación (PID2021-127407NB-I00, and RED2022-134074-T) and Fundación para el Fomento en Asturias de la Investigación Científica Aplicada y la Tecnología (FICYT) through the Margarita Salas Senior Program (AYUD/2021/59709). F.G. acknowledges Monash University for financial support. F.L. acknowledges the support of the MSCA Project “PhotoFLPs”, ID101109138.
Data Availability Statement
The data underlying this study are available in the published article.
Author Contributions
# J.F.R. and F.L. contributed equally. CRediT: Javier F. Reynes conceptualization, writing-original draft, writing-review & editing; Felix Leon conceptualization, writing-original draft, writing-review & editing; Felipe García conceptualization, funding acquisition, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
References
- Anastas P. T.; Warner J. C.; Warner J. C.. Green Chem.: Theory and Practice; Oxford University Press: Oxford, 2000. [Google Scholar]
- James S. L.; Adams C. J.; Bolm C.; Braga D.; Collier P.; Friščić T.; Grepioni F.; Harris K. D. M.; Hyett G.; Jones W.; Krebs A.; Mack J.; Maini L.; Orpen A. G.; Parkin I. P.; Shearouse W. C.; Steed J. W.; Waddell D. C. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. 10.1039/C1CS15171A. [DOI] [PubMed] [Google Scholar]
- Colacino E.; Isoni V.; Crawford D.; García F. Upscaling Mechanochemistry: Challenges and Opportunities for Sustainable Industry. Trends Chem. 2021, 3, 335–339. 10.1016/j.trechm.2021.02.008. [DOI] [Google Scholar]
- Bolm C.; Hernández J. G. Mechanochemistry of Gaseous Reactants. Angew. Chem., Int. Ed. 2019, 58, 3285–3299. 10.1002/anie.201810902. [DOI] [PubMed] [Google Scholar]
- Michalchuk A. A. L.; Emmerling F. Time-Resolved In Situ Monitoring of Mechanochemical Reactions. Angew. Chem., Int. Ed. 2022, 61, e202117270 10.1002/anie.202117270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukin S.; Germann L. S.; Friščić T.; Halasz I. Toward Mechanistic Understanding of Mechanochemical Reactions Using Real-Time In Situ Monitoring. Acc. Chem. Res. 2022, 55, 1262–1277. 10.1021/acs.accounts.2c00062. [DOI] [PubMed] [Google Scholar]
- Michalchuk A. A. L.; Kabelitz A.; Emmerling F.. Monitoring Mechanochemical Processes in Situ and in Real Time. In Nontraditional Activation Methods in Green and Sustainable Applications; Elsevier, 2021; pp 369–419. [Google Scholar]
- Julien P. A.; Friščić T. Methods for Monitoring Milling Reactions and Mechanistic Studies of Mechanochemistry: A Primer. Cryst. Growth Des. 2022, 22, 5726–5754. 10.1021/acs.cgd.2c00587. [DOI] [Google Scholar]
- Reynes J. F.; Isoni V.; García F. Tinkering with Mechanochemical Tools for Scale Up. Angew. Chem., Int. Ed. 2023, 62, e202300819 10.1002/anie.202300819. [DOI] [PubMed] [Google Scholar]
- Friščić T.; Mottillo C.; Titi H. M. Mechanochemistry for Synthesis. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. 10.1002/anie.201906755. [DOI] [PubMed] [Google Scholar]
- Martinez V.; Stolar T.; Karadeniz B.; Brekalo I.; Užarević K. Advancing Mechanochemical Synthesis by Combining Milling with Different Energy Sources. Nat. Rev. Chem. 2023, 7, 51–65. 10.1038/s41570-022-00442-1. [DOI] [PubMed] [Google Scholar]
- Mateti S.; Mathesh M.; Liu Z.; Tao T.; Ramireddy T.; Glushenkov A. M.; Yang W.; Chen Y. I. Mechanochemistry: A Force in Disguise and Conditional Effects towards Chemical Reactions. Chem. Commun. 2021, 57, 1080–1092. 10.1039/D0CC06581A. [DOI] [PubMed] [Google Scholar]
- O’Neill R. T.; Boulatov R. The Many Flavours of Mechanochemistry and Its Plausible Conceptual Underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. 10.1038/s41570-020-00249-y. [DOI] [PubMed] [Google Scholar]
- Ozer D.Mechanochemistry: A Power Tool for Green Synthesis. In Advances in Green Synthesis; Inamuddin, Boddula R., Ahamed M. I., Khan A., Eds.; Advances in Science, Technology & Innovation; Springer International Publishing: Cham, 2021; pp 23–39. [Google Scholar]
- Reichle S.; Felderhoff M.; Schüth F. Mechanocatalytic Room-Temperature Synthesis of Ammonia from Its Elements Down to Atmospheric Pressure. Angew. Chem., Int. Ed. 2021, 133, 26589–26593. 10.1002/ange.202112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrute A. P.; De Bellis J.; Felderhoff M.; Schüth F. Frontispiece: Mechanochemical Synthesis of Catalytic Materials. Chem. – Eur. J. 2021, 27, chem.202182361 10.1002/chem.202182361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanese C.; Jensen T. R.; Hauback B. C.; Pistidda C.; Dornheim M.; Yang H.; Lombardo L.; Zuettel A.; Filinchuk Y.; Ngene P.; De Jongh P. E.; Buckley C. E.; Dematteis E. M.; Baricco M. Complex Hydrides for Energy Storage. Int. J. Hydrog. Energy 2019, 44, 7860–7874. 10.1016/j.ijhydene.2018.11.208. [DOI] [Google Scholar]
- Gečiauskaitė A. A.; García F. Main Group Mechanochemistry. Beilstein J. Org. Chem. 2017, 13, 2068–2077. 10.3762/bjoc.13.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goralski C. T.; Singaram B. Special feature section: hydride reductions. Org. Process Res. Dev. 2006, 10, 947–948. 10.1021/op0601363. [DOI] [Google Scholar]
- Schlesinger H.; Brown H. C.; Finholt A. The Preparation of Sodium Borohydride by the High Temperature Reaction of Sodium Hydride with Borate Esters1. J. Am. Chem. Soc. 1953, 75, 205–209. 10.1021/ja01097a054. [DOI] [Google Scholar]
- Mack J.; Fulmer D.; Stofel S.; Santos N. The First Solvent-Free Method for the Reduction of Esters. Green Chem. 2007, 9, 1041. 10.1039/b706167f. [DOI] [Google Scholar]
- Gupta S.; Kobayashi T.; Hlova I.; Goldston J.; Pruski M.; Pecharsky V. Solvent-Free Mechanochemical Synthesis of Alane, AlH 3: Effect of Pressure on the Reaction Pathway. Green Chem. 2014, 16, 4378–4388. 10.1039/C4GC00998C. [DOI] [Google Scholar]
- Zhu H.-J.; Pittman C. U. Reductions of Carboxylic Acids and Esters with NaBH 4 in Diglyme at 162°C. Synth. Commun. 2003, 33, 1733–1750. 10.1081/SCC-120018935. [DOI] [Google Scholar]
- Liu C.; Nikolaev S. A.; Ren W.; Burton L. A. Electrides: A Review. J. Mater. Chem. C 2020, 8, 10551–10567. 10.1039/D0TC01165G. [DOI] [Google Scholar]
- Davison N.; Quirk J. A.; Tuna F.; Collison D.; McMullin C. L.; Michaels H.; Morritt G. H.; Waddell P. G.; Gould J. A.; Freitag M.; Dawson J. A.; Lu E. A Room-Temperature-Stable Electride and Its Reactivity: Reductive Benzene/Pyridine Couplings and Solvent-Free Birch Reductions. Chem. 2023, 9, 576–591. 10.1016/j.chempr.2022.11.006. [DOI] [Google Scholar]
- Davison N.; Waddell P. G.; Lu E. Reduction of K + or Li + in the Heterobimetallic Electride K + [LiN(SiMe 3) 2 ]e –. J. Am. Chem. Soc. 2023, 145, 17007 10.1021/jacs.3c06066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konze W. V.; Scott B. L.; Kubas G. J. C–H Activation and C–C Coupling of Arenes by Cationic Pt(II) Complexes. J. Am. Chem. Soc. 2002, 124, 12550–12556. 10.1021/ja020798h. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Kubota K.; Ito H. Mechanochemical Approach for Air-Tolerant and Extremely Fast Lithium-Based Birch Reductions in Minutes. Angew. Chem., Int. Ed. 2023, 62, e202217723 10.1002/anie.202217723. [DOI] [PubMed] [Google Scholar]
- Koby R. F.; Rightmire N. R.; Schley N. D.; Hanusa T. P.; Brennessel W. W. Halide Metathesis in Overdrive: Mechanochemical Synthesis of a Heterometallic Group 1 Allyl Complex. Beilstein J. Org. Chem. 2019, 15, 1856–1863. 10.3762/bjoc.15.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koby R. F.; Schley N. D.; Hanusa T. P. C. Di (Indenyl) Beryllium. Angew. Chem., Int. Ed. 2021, 133, 21344–21348. 10.1002/ange.202107980. [DOI] [PubMed] [Google Scholar]
- Boyde N.; Rightmire N.; Hanusa T.; Brennessel W. Symmetric Assembly of a Sterically Encumbered Allyl Complex: Mechanochemical and Solution Synthesis of the Tris(Allyl)Beryllate, K[BeA′3] (A′ = 1,3-(SiMe3)2C3H3). Inorganics 2017, 5, 36. 10.3390/inorganics5020036. [DOI] [Google Scholar]
- Fischer E. O.; Hofmann H. P. Über Aromatenkomplexe von Metallen, XXV. Di-cyclopentadienyl-beryllium. Chem. Ber. 1959, 92, 482–486. 10.1002/cber.19590920233. [DOI] [Google Scholar]
- Seyferth D. The Grignard Reagents. Organometallics 2009, 28, 1598–1605. 10.1021/om900088z. [DOI] [Google Scholar]
- Samineni R.; Eda V.; Rao P.; Sen S.; Oruganti S. Grignard Reagents as Niche Bases in the Synthesis of Pharmaceutically Relevant Molecules. ChemistrySelect 2022, 7, e202102853. 10.1002/slct.202102853. [DOI] [Google Scholar]
- Harrowfield J. M.; Hart R. J.; Whitaker C. R. Magnesium and Aromatics: Mechanically-Induced Grignard and McMurry Reactions. Aust. J. Chem. 2001, 54, 423–425. 10.1071/CH01166. [DOI] [Google Scholar]
- Speight I. R.; Hanusa T. P. Exploration of Mechanochemical Activation in Solid-State Fluoro-Grignard Reactions. Molecules 2020, 25, 570. 10.3390/molecules25030570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T.; Hayashi T. Iron-Catalyzed Oxidative Homo-Coupling of Aryl Grignard Reagents. Org. Lett. 2005, 7, 491–493. 10.1021/ol047509+. [DOI] [PubMed] [Google Scholar]
- Green S. P.; Jones C.; Stasch A. Stable Magnesium(I) Compounds with Mg-Mg Bonds. Science 2007, 318, 1754–1757. 10.1126/science.1150856. [DOI] [PubMed] [Google Scholar]
- Jędrzkiewicz D.; Langer J.; Harder S. Low-valent Mg(I) Complexes by Ball-milling. Z. Für Anorg. Allg. Chem. 2022, 648, e202200138 10.1002/zaac.202200138. [DOI] [Google Scholar]
- Jędrzkiewicz D.; Mai J.; Langer J.; Mathe Z.; Patel N.; DeBeer S.; Harder S. Access to a Labile Monomeric Magnesium Radical by Ball-Milling. Angew. Chem., Int. Ed. 2022, 61, e202200511 10.1002/anie.202200511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R.; Hu A.; Gao P.; Gao Y.; Pang Y.; Seo T.; Jiang J.; Maeda S.; Takaya H.; Kubota K.; et al. Mechanochemical Synthesis of Magnesium-Based Carbon Nucleophiles in Air and Their Use in Organic Synthesis. Nat. Commun. 2021, 12, 1–10. 10.1038/s41467-021-26962-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P.; Jiang J.; Maeda S.; Kubota K.; Ito H. Mechanochemically Generated Calcium-based Heavy Grignard Reagents and Their Application to Carbon–Carbon Bond-forming Reactions. Angew. Chem., Int. Ed. 2022, 61, e202207118 10.1002/anie.202207118. [DOI] [PubMed] [Google Scholar]
- Trost B. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- Koby R. F.; Doerr A. M.; Rightmire N. R.; Schley N. D.; Brennessel W. W.; Long B. K.; Hanusa T. P. Mechanochemical Formation, Solution Rearrangements, and Catalytic Behavior of a Polymorphic Ca/K Allyl Complex. Chem. – Eur. J. 2021, 27, 8195–8202. 10.1002/chem.202100589. [DOI] [PubMed] [Google Scholar]
- Peters D. W.; Blair R. G. Mechanochemical Synthesis of an Organometallic Compound: A High Volume Manufacturing Method. Faraday Discuss. 2014, 170, 83–91. 10.1039/C3FD00157A. [DOI] [PubMed] [Google Scholar]
- Harvey M. J.; Hanusa T. P.; Young V. G. Jr. Synthesis and Crystal Structure of the Bis(Allyl)Calcium Complex [Ca{C3(SiMe3)2H3}2·(Thf)2]. Angew. Chem., Int. Ed. 1999, 38, 217–219. . [DOI] [Google Scholar]
- Tang C. W.; VanSlyke S. A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. 10.1063/1.98799. [DOI] [Google Scholar]
- Tokito S.; Tanaka H.; Noda K.; Okada A.; Taga Y. Temperature Dependences of Electroluminescent Characteristics in the Devices Fabricated with Novel Triphenylamine Derivatives. IEEE Trans. Electron Devices 1997, 44, 1239–1244. 10.1109/16.605461. [DOI] [Google Scholar]
- Crawford D. E.; James S. L.; McNally T. Use of Batch Mixing to Investigate the Continuous Solvent-Free Mechanical Synthesis of OLED Materials by Twin-Screw Extrusion (TSE). ACS Sustain Chem. Eng. 2018, 6, 193–201. 10.1021/acssuschemeng.7b02202. [DOI] [Google Scholar]
- Ma X.; Lim G. K.; Harris K. D.; Apperley D. C.; Horton P. N.; Hursthouse M. B.; James S. L. Efficient, Scalable, and Solvent-Free Mechanochemical Synthesis of the OLED Material Alq3 (Q= 8-Hydroxyquinolinate). Cryst. Growth Des. 2012, 12, 5869–5872. 10.1021/cg301291w. [DOI] [Google Scholar]
- Rightmire N. R.; Hanusa T. P.; Rheingold A. L. Mechanochemical Synthesis of [1, 3-(SiMe3) 2C3H3] 3 (Al, Sc), a Base-Free Tris (Allyl) Aluminum Complex and Its Scandium Analogue. Organometallics 2014, 33, 5952–5955. 10.1021/om5009204. [DOI] [Google Scholar]
- Lee S. H.; Shin N.; Kwak S. W.; Hyun K.; Woo W. H.; Lee J. H.; Hwang H.; Kim M.; Lee J.; Kim Y.; Lee K. M.; Park M. H. Intriguing Indium-Salen Complexes as Multicolor Luminophores. Inorg. Chem. 2017, 56, 2621–2626. 10.1021/acs.inorgchem.6b02797. [DOI] [PubMed] [Google Scholar]
- Singh V. K.; Chamberlain-Clay A.; Ong H. C.; León F.; Hum G.; Par M. Y.; Daley-Dee P.; García F. Multigram Mechanochemical Synthesis of a Salophen Complex: A Comparative Analysis. ACS Sustain. Chem. Eng. 2021, 9, 1152–1160. 10.1021/acssuschemeng.0c06374. [DOI] [Google Scholar]
- Lichtenberg C.; Robert D.; Spaniol T. P.; Okuda J. Bis(Allyl)Aluminum Cation, Tris(Allyl)Aluminum, and Tetrakis(Allyl)Aluminate: Synthesis, Characterization, and Reactivity. Organometallics 2010, 29, 5714–5721. 10.1021/om100809h. [DOI] [Google Scholar]
- Leon F.; Li C.; Reynes J. F.; Singh V. K.; Lian X.; Ong H. C.; Hum G.; Sun H.; García F. Mechanosynthesis and Photophysics of Colour-Tunable Photoluminescent Group 13 Metal Complexes with Sterically Demanding Salen and Salophen Ligands. Faraday Discuss. 2023, 241, 63–78. 10.1039/D2FD00117A. [DOI] [PubMed] [Google Scholar]
- Li C.-J.; Chen L. Organic Chemistry in Water. Chem. Soc. Rev. 2006, 35, 68–82. 10.1039/B507207G. [DOI] [PubMed] [Google Scholar]
- Kee J. W.; Ng Y. Y.; Kulkarni S. A.; Muduli S. K.; Xu K.; Ganguly R.; Lu Y.; Hirao H.; Soo H. S. Development of Bis(Arylimino)Acenaphthene (BIAN) Copper Complexes as Visible Light Harvesters for Potential Photovoltaic Applications. Inorg. Chem. Front. 2016, 3, 651–662. 10.1039/C5QI00221D. [DOI] [Google Scholar]
- Wang J.; Soo H. S.; Garcia F. Synthesis, Properties, and Catalysis of p-Block Complexes Supported by Bis(Arylimino)Acenaphthene Ligands. Commun. Chem. 2020, 3, 113. 10.1038/s42004-020-00359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini M.; Ragaini F.; Cenini S. Synthesis of Ar-BIAN Ligands (Ar-BIAN = Bis(Aryl)Acenaphthenequinonediimine) Having Strong Electron-Withdrawing Substituents on the Aryl Rings and Their Relative Coordination Strength toward Palladium(0) and -(II) Complexes. Organometallics 2002, 21, 2950–2957. 10.1021/om020147u. [DOI] [Google Scholar]
- Wang J.; Ganguly R.; Yongxin L.; Díaz J.; Soo H. S.; García F. A Multi-Step Solvent-Free Mechanochemical Route to Indium (III) Complexes. Dalton Trans. 2016, 45, 7941–7946. 10.1039/C6DT00978F. [DOI] [PubMed] [Google Scholar]
- Moore J. J.Metal Extraction Processes. In Chemical Metallurgy; Elsevier, 1990; pp 243–309. [Google Scholar]
- Cerveau G.; Chuit C.; Corriu R. J. P.; Reye C. Reactivity of Dianionic Hexacoordinate Germanium Complexes toward Organometallic Reagents. A New Route to Organogermanes. Organometallics 1991, 10, 1510–1515. 10.1021/om00051a049. [DOI] [Google Scholar]
- Glavinović M.; Krause M.; Yang L.; McLeod J. A.; Liu L.; Baines K. M.; Friščić T.; Lumb J. A Chlorine-Free Protocol for Processing Germanium. Science Advances 2017, 3, e1700149 10.1126/sciadv.1700149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S. A.; Layfield R. A. The Coordination Chemistry of Silyl-Substituted Allyl Ligands. Dalton Trans 2010, 39, 2469–2483. 10.1039/B918619K. [DOI] [PubMed] [Google Scholar]
- Koby R. F.; Hanusa T. P.; Schley N. D. Mechanochemically Driven Transformations in Organotin Chemistry: Stereochemical Rearrangement, Redox Behavior, and Dispersion-Stabilized Complexes. J. Am. Chem. Soc. 2018, 140, 15934–15942. 10.1021/jacs.8b09862. [DOI] [PubMed] [Google Scholar]
- Layfield R. A.; García F.; Hannauer J.; Humphrey S. M. Ansa-Tris(Allyl) Complexes of Alkali Metals: Tripodal Analogues of Cyclopentadienyl and Ansa-Metallocene Ligands. Chem. Commun. 2007, 47, 5081. 10.1039/b712285c. [DOI] [PubMed] [Google Scholar]
- Yao S.; Saddington A.; Xiong Y.; Driess M. Chelating Bis-Silylenes As Powerful Ligands To Enable Unusual Low-Valent Main-Group Element Functions. Acc. Chem. Res. 2023, 56, 475–488. 10.1021/acs.accounts.2c00763. [DOI] [PubMed] [Google Scholar]
- Rivard E. Group 14 Inorganic Hydrocarbon Analogues. Chem. Soc. Rev. 2016, 45, 989–1003. 10.1039/C5CS00365B. [DOI] [PubMed] [Google Scholar]
- Cabeza J. A.; García-Álvarez P. Tetrelanes versus Tetrylenes as Precursors to Transition Metal Complexes Featuring Tridentate PEP Tetryl Ligands (E = Si, Ge, Sn). Chem. – Eur. J. 2023, 29, e202203096 10.1002/chem.202203096. [DOI] [PubMed] [Google Scholar]
- Harris D. H.; Lappert M. F. Monomeric, Volatile Bivalent Amides of Group IV B Elements, M(NR 12) 2 and M(NR 1 R 2) 2 (M=Ge, Sn, or Pb; R 1 =Me 3 Si, R 2 =Me 3 C). J. Chem. Soc. Chem. Commun. 1974, 21, 895–896. 10.1039/C39740000895. [DOI] [Google Scholar]
- Glock C.; Krieck S.; Westerhausen M.; Lavin C. M.; Gillett-Kunnath M. M.; Ruhlandt K.; Hill M. S.; Anker M. D.; Wilson A. S. S.; Weetman C.; Arnold P. L.; Veinot A. J.; Stack D. L.; Clyburne J. A. C.; Masuda J. D.; Dickie D. A.; Chadha U.; Kemp R. A.. Calcium, strontium, germanium, tin, and lead bis(trimethylsilyl)amido derivatives and 2,2,6,6-tetramethylpiperidido and n-isopropylphenylamido dervatives of potassium and calcium. In Inorganic Syntheses; Power P. P., Ed.; Wiley, 2018; Vol. 37, pp 15–31. [Google Scholar]
- Cabeza J. A.; Reynes J. F.; García F.; García-Álvarez P.; García-Soriano R. Fast and Scalable Solvent-Free Access to Lappert’s Heavier Tetrylenes E{N(SiMe 3)2}2(E = Ge, Sn, Pb) and ECl{N(SiMe 3)2 } (E = Ge, Sn). Chem. Sci. 2023, 14, 12477–12483. 10.1039/D3SC02709K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette R. J.; Rawn J. D.. Aldehydes and Ketones: Nucleophilic Addition Reactions. In Organic Chemistry; Elsevier, 2018; pp 595–623. [Google Scholar]
- Balema V. P.; Wiench J. W.; Pruski M.; Pecharsky V. K. Mechanically Induced Solid-State Generation of Phosphorus Ylides and the Solvent-Free Wittig Reaction. J. Am. Chem. Soc. 2002, 124, 6244–6245. 10.1021/ja017908p. [DOI] [PubMed] [Google Scholar]
- Stahl L. Bicyclic and Tricyclic Bis(Amido)Cyclodiphosph(III)Azane Compounds of Main Group Elements. Coord. Chem. Rev. 2000, 210, 203–250. 10.1016/S0010-8545(00)00312-X. [DOI] [Google Scholar]
- Calera S. G.; Wright D. S. Macrocyclic Phosphazane Ligands. Dalton Trans. 2010, 39, 5055. 10.1039/b926428k. [DOI] [PubMed] [Google Scholar]
- Nordheider A.; Hüll K.; Athukorala Arachchige K. S.; Slawin A. M. Z.; Woollins J. D.; Thirumoorthi R.; Chivers T. Spirocyclic, Macrocyclic and Ladder Complexes of Coinage Metals and Mercury with Dichalcogeno P 2 N 2 -Supported Anions. Dalton Trans. 2015, 44, 5338–5346. 10.1039/C5DT00159E. [DOI] [PubMed] [Google Scholar]
- Rashid A.; Ananthnag G. S.; Naik S.; Mague J. T.; Panda D.; Balakrishna M. S. Dinuclear Cu I Complexes of Pyridyl-Diazadiphosphetidines and Aminobis(Phosphonite) Ligands: Synthesis, Structural Studies and Antiproliferative Activity towards Human Cervical, Colon Carcinoma and Breast Cancer Cells. Dalton Trans 2014, 43, 11339–11351. 10.1039/C4DT00832D. [DOI] [PubMed] [Google Scholar]
- Shi Y. X.; Liang R. Z.; Martin K. A.; Weston N.; Gonzalez-Calera S.; Ganguly R.; Li Y.; Lu Y.; Ribeiro A. J. M.; Ramos M. J.; Fernandes P. A.; García F. Synthesis and Hydrolytic Studies on the Air-Stable [(4-CN-PhO)(E)P(μ-N t Bu)] 2 (E = O, S, and Se) Cyclodiphosphazanes. Inorg. Chem. 2015, 54, 6423–6432. 10.1021/acs.inorgchem.5b00735. [DOI] [PubMed] [Google Scholar]
- Otang M. E.; Lief G. R.; Stahl L. Alkoxido-, Amido-, and Chlorido Derivatives of Zirconium- and Hafnium Bis(Amido)Cyclodiphosph(V)Azanes: Ligand Ambidenticity and Catalytic Productivity. J. Organomet. Chem. 2016, 820, 98–110. 10.1016/j.jorganchem.2016.08.008. [DOI] [Google Scholar]
- Sim Y.; Tan D.; Ganguly R.; Li Y.; García F. Orthogonality in Main Group Compounds: A Direct One-Step Synthesis of Air- and Moisture-Stable Cyclophosphazanes by Mechanochemistry. Chem. Commun. 2018, 54, 6800–6803. 10.1039/C8CC01043A. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kumar S.; Pandey M. K.; Kashid V. S.; Radhakrishna L.; Balakrishna M. S. Synthesis of Phosphine Chalcogenides Under Solvent-Free Conditions Using a Rotary Ball Mill. Eur. J. Inorg. Chem. 2018, 2018, 1028–1037. 10.1002/ejic.201701414. [DOI] [Google Scholar]
- Raj J. G. J. Metal-Organophosphine Complexes: Structure, Bonding, and Applications. Rev. Inorg. Chem. 2015, 35, 25–56. 10.1515/revic-2014-0006. [DOI] [Google Scholar]
- Scherer O. J.; Andres K.; Krüger C.; Tsay Y.-H.; Wolmerhäser G. P4(N-i-C3H7)6, a P4 × 6 Molecule with and without Adamantane Structure. Angew. Chem., Int. Ed. 1980, 19, 571–572. 10.1002/anie.198005711. [DOI] [Google Scholar]
- Shi Y. X.; Xu K.; Clegg J. K.; Ganguly R.; Hirao H.; Friščić T.; García F. The First Synthesis of the Sterically Encumbered Adamantoid Phosphazane P4 (NtBu) 6: Enabled by Mechanochemistry. Angew. Chem., Int. Ed. 2016, 55, 12736–12740. 10.1002/anie.201605936. [DOI] [PubMed] [Google Scholar]
- Crabtree R. H.The Organometallic Chemistry of the Transition Metals; Wiley, 2009. [Google Scholar]
- Effaty F.; Ottenwaelder X.; Friščić T. Mechanochemistry in Transition Metal-Catalyzed Reactions. Curr. Opin. Green Sustain. Chem. 2021, 32, 100524 10.1016/j.cogsc.2021.100524. [DOI] [Google Scholar]
- Boldyreva E. Mechanochemistry of Inorganic and Organic Systems: What Is Similar, What Is Different?. Chem. Soc. Rev. 2013, 42, 7719. 10.1039/c3cs60052a. [DOI] [PubMed] [Google Scholar]
- Cracknell A. P. The Fermi Surface. II. d-Block and f-Block Metals. Adv. Phys. 1971, 20, 1–141. 10.1080/00018737100101211. [DOI] [Google Scholar]
- Kuciński K.; Pawluć P.; Marciniec B.; Hreczycho G. Highly Selective Hydrothiolation of Unsaturated Organosilicon Compounds Catalyzed by Scandium(III) Triflate. Chem. – Eur. J. 2015, 21, 4940–4943. 10.1002/chem.201406412. [DOI] [PubMed] [Google Scholar]
- Krajewski S. M.; Crossman A. S.; Akturk E. S.; Suhrbier T.; Scappaticci S. J.; Staab M. W.; Marshak M. P. Sterically Encumbered β-Diketonates and Base Metal Catalysis. Dalton Trans. 2019, 48, 10714–10722. 10.1039/C9DT02293G. [DOI] [PubMed] [Google Scholar]
- Andreiadis E. S.; Gauthier N.; Imbert D.; Demadrille R.; Pecaut J.; Mazzanti M. Lanthanide Complexes Based on β-Diketonates and a Tetradentate Chromophore Highly Luminescent as Powders and in Polymers. Inor. Chem. 2013, 52, 14382–14390. 10.1021/ic402523v. [DOI] [PubMed] [Google Scholar]
- Makhaev V.; Petrova L. Mechanochemical Synthesis of Vanadium (III) β-Diketonates. Russian Journal of General Chemistry 2017, 87, 1105–1109. 10.1134/S1070363217060019. [DOI] [Google Scholar]
- Schaefer W. P. Acetylacetone Complexes of Vanadium(II). Inorg. Chem. 1965, 4, 642–648. 10.1021/ic50027a009. [DOI] [Google Scholar]
- Alberto R.; Braband H.; Nadeem Q. Bioorganometallic Technetium and Rhenium Chemistry: Fundamentals for Applications. Chimia 2022, 74, 953. 10.2533/chimia.2020.953. [DOI] [PubMed] [Google Scholar]
- Hernández J. G.; Butler I. S.; Friščić T. Multi-Step and Multi-Component Organometallic Synthesis in One Pot Using Orthogonal Mechanochemical Reactions. Chem. Sci. 2014, 5, 3576–3582. 10.1039/C4SC01252F. [DOI] [Google Scholar]
- Hernández J. G.; Macdonald N. A.; Mottillo C.; Butler I. S.; Friščić T. A Mechanochemical Strategy for Oxidative Addition: Remarkable Yields and Stereoselectivity in the Halogenation of Organometallic Re (I) Complexes. Green Chem. 2014, 16, 1087–1092. 10.1039/C3GC42104J. [DOI] [Google Scholar]
- Riener K.; Haslinger S.; Raba A.; Högerl M. P.; Cokoja M.; Herrmann W. A.; Kühn F. E. Chemistry of Iron N -Heterocyclic Carbene Complexes: Syntheses, Structures, Reactivities, and Catalytic Applications. Chem. Rev. 2014, 114, 5215–5272. 10.1021/cr4006439. [DOI] [PubMed] [Google Scholar]
- Tan D.; Loots L.; Friščić T. Towards Medicinal Mechanochemistry: Evolution of Milling from Pharmaceutical Solid Form Screening to the Synthesis of Active Pharmaceutical Ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. 10.1039/C6CC02015A. [DOI] [PubMed] [Google Scholar]
- Makhaev V.; Borisov A.; Petrova L. Solid-State Mechanochemical Synthesis of Ferrocene. J. Organomet. Chem. 1999, 590, 222–226. 10.1016/S0022-328X(99)00460-X. [DOI] [Google Scholar]
- Christoforou A. M.; Fronczek F. R.; Marzilli P. A.; Marzilli L. G. Fac -Re(CO) 3L Complexes Containing Tridentate Monoanionic Ligands (L–) with a Seldom-Studied Sulfonamido Group As One Terminal Ligating Group. Inorg. Chem. 2007, 46, 6942–6949. 10.1021/ic700594a. [DOI] [PubMed] [Google Scholar]
- Kealy T. J.; Pauson P. L. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. 10.1038/1681039b0. [DOI] [Google Scholar]
- Hawthorne M. F.; Young D. C.; Wegner P. A. Carbametallic Boron Hydride Derivatives. I. Apparent Analogs of Ferrocene and Ferricinium Ion. J. Am. Chem. Soc. 1965, 87, 1818–1819. 10.1021/ja01086a053. [DOI] [Google Scholar]
- Wojtczak B. A.; Andrysiak A.; Grüner B.; Lesnikowski Z. J. Chemical Ligation”: A Versatile Method for Nucleoside Modification with Boron Clusters. Chem.—Eur. J. 2008, 14, 10675–10682. 10.1002/chem.200801053. [DOI] [PubMed] [Google Scholar]
- Tan Y.-H.; Yang L.-F.; Cao M.-L.; Wu J.-J.; Ye B.-H. Liquid-Assisted Solid-State Reaction: Assembly of (6,3) and (10,3) Hydrogen-Bonded Networks Based on [M(Hbiim)3] by Oxidation of [M(H2biim)3]2+ Complexes in the Presence of Acetate Anions. CrystEngComm 2011, 13, 4512. 10.1039/c1ce00009h. [DOI] [Google Scholar]
- Delaude L.; Demonceau A. Retracing the Evolution of Monometallic Ruthenium–Arene Catalysts for C–C Bond Formation. Dalton Trans. 2012, 41, 9257–9268. 10.1039/c2dt30293d. [DOI] [PubMed] [Google Scholar]
- Quintin F.; Pinaud J.; Lamaty F.; Bantreil X. Mechanosynthesis of Noels-Type NHC–Ruthenium Complexes and Applications in Ring-Opening Metathesis Polymerization. Organometallics 2020, 39, 636–639. 10.1021/acs.organomet.0c00013. [DOI] [Google Scholar]
- Renner H.; Schlamp G.; Hollmann D.; Lüschow H. M.; Tews P.; Rothaut J.; Dermann K.; Knödler A.; Hecht C.; Schlott M.; Drieselmann R.; Peter C.; Schiele R.. Gold, Gold Alloys, and Gold Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, 2000. [Google Scholar]
- Pflästerer D.; Hashmi A. S. K. Gold Catalysis in Total Synthesis – Recent Achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. 10.1039/C5CS00721F. [DOI] [PubMed] [Google Scholar]
- Izarova N. V.; Pope M. T.; Kortz U. Noble Metals in Polyoxometalates. Angew. Chem., Int. Ed. 2012, 51, 9492–9510. 10.1002/anie.201202750. [DOI] [PubMed] [Google Scholar]
- Froehlich P.; Lorenz T.; Martin G.; Brett B.; Bertau M. Valuable Metals—Recovery Processes, Current Trends, and Recycling Strategies. Angew. Chem., Int. Ed. 2017, 56, 2544–2580. 10.1002/anie.201605417. [DOI] [PubMed] [Google Scholar]
- Do J.; Tan D.; Friščić T. Oxidative Mechanochemistry: Direct, Room-Temperature, Solvent-Free Conversion of Palladium and Gold Metals into Soluble Salts and Coordination Complexes. Angew. Chem., Int. Ed. 2018, 57, 2667–2671. 10.1002/anie.201712602. [DOI] [PubMed] [Google Scholar]
- Deák A.; Jobbágy C.; Demeter A.; Čelko L.; Cihlář J.; Szabó P. T.; Ábrányi-Balogh P.; Crawford D. E.; Virieux D.; Colacino E. Mechanochemical Synthesis of Mononuclear Gold(i) Halide Complexes of Diphosphine Ligands with Tuneable Luminescent Properties. Dalton Trans. 2021, 50, 13337–13344. 10.1039/D1DT01751A. [DOI] [PubMed] [Google Scholar]
- Deák A.; Szabó P. T.; Bednaříková V.; Cihlář J.; Demeter A.; Remešová M.; Colacino E.; Čelko L. The First Solid-State Route to Luminescent Au(I)—Glutathionate and Its pH-Controlled Transformation into Ultrasmall Oligomeric Au10–12(SG)10–12 Nanoclusters for Application in Cancer Radiotheraphy. Front. Chem. 2023, 11, 1178225 10.3389/fchem.2023.1178225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K.; Takahashi R.; Ito H. Mechanochemistry Allows Carrying out Sensitive Organometallic Reactions in Air: Glove-Box-and-Schlenk-Line-Free Synthesis of Oxidative Addition Complexes from Aryl Halides and Palladium(0). Chem. Sci. 2019, 10, 5837–5842. 10.1039/C9SC01711A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingoglia B. T.; Buchwald S. L. Oxidative Addition Complexes as Precatalysts for Cross-Coupling Reactions Requiring Extremely Bulky Biarylphosphine Ligands. Org. Lett. 2017, 19, 2853–2856. 10.1021/acs.orglett.7b01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Liu F.; Yan K. Mechanochemical Access to a Short-Lived Cyclic Dimer Pd 2 L 2 : An Elusive Kinetic Species En Route to Molecular Triangle Pd 3 L 3 and Molecular Square Pd 4 L 4. Angew. Chem., Int. Ed. 2022, 61, e202116980 10.1002/anie.202116980. [DOI] [PubMed] [Google Scholar]
- Holló-Sitkei E.; Tárkányi G.; Párkányi L.; Megyes T.; Besenyei G. Steric Effects in the Self-Assembly of Palladium Complexes with Chelating Diamine Ligands. Eur. J. Inorg. Chem. 2008, 2008, 1573–1583. 10.1002/ejic.200701189. [DOI] [Google Scholar]
- Liu Y.; Liu F.; Li S.; Liu H.; Yan K. Biasing the Formation of Solution-Unstable Intermediates in Coordination Self-Assembly by Mechanochemistry. Chem. – Eur. J. 2023, 29, e202302563 10.1002/chem.202302563. [DOI] [PubMed] [Google Scholar]
- Lin J. C. Y.; Huang R. T. W.; Lee C. S.; Bhattacharyya A.; Hwang W. S.; Lin I. J. B. Coinage Metal– N -Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. 10.1021/cr8005153. [DOI] [PubMed] [Google Scholar]
- Beillard A.; Bantreil X.; Métro T.-X.; Martinez J.; Lamaty F. Mechanochemistry for Facilitated Access to N,N-Diaryl NHC Metal Complexes. New J. Chem. 2017, 41, 1057–1063. 10.1039/C6NJ02895K. [DOI] [Google Scholar]
- Beillard A.; Métro T.-X.; Bantreil X.; Martinez J.; Lamaty F. Cu(0), O 2 and Mechanical Forces: A Saving Combination for Efficient Production of Cu–NHC Complexes. Chem. Sci. 2017, 8, 1086–1089. 10.1039/C6SC03182J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróblewska A.; Lauriol G.; Mlostoń G.; Bantreil X.; Lamaty F. Expedient Synthesis of NOxy-Heterocyclic Carbenes (NOHC) Ligands and Metal Complexes Using Mechanochemistry. J. Organomet. Chem. 2021, 949, 121914 10.1016/j.jorganchem.2021.121914. [DOI] [Google Scholar]
- Ferguson M.; Giri N.; Huang X.; Apperley D.; James S. L. One-Pot Two-Step Mechanochemical Synthesis: Ligand and Complex Preparation without Isolating Intermediates. Green Chem. 2014, 16, 1374–1382. 10.1039/C3GC42141D. [DOI] [Google Scholar]
- Crawford D.; Casaban J.; Haydon R.; Giri N.; McNally T.; James S. L. Synthesis by Extrusion: Continuous, Large-Scale Preparation of MOFs Using Little or No Solvent. Chem. Sci. 2015, 6, 1645–1649. 10.1039/C4SC03217A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochowicz D.; Nawrocki J.; Terlecki M.; Marynowski W.; Lewiński J. Facile Mechanosynthesis of the Archetypal Zn-Based Metal–Organic Frameworks. Inorg. Chem. 2018, 57, 13437–13442. 10.1021/acs.inorgchem.8b02026. [DOI] [PubMed] [Google Scholar]
- Krupiński P.; Grala A.; Wolska-Pietkiewicz M.; Danowski W.; Justyniak I.; Lewiński J. From Uncommon Ethylzinc Complexes Supported by Ureate Ligands to Water-Soluble ZnO Nanocrystals: A Mechanochemical Approach. ACS Sustain. Chem. Eng. 2021, 9, 1540–1549. 10.1021/acssuschemeng.0c06081. [DOI] [Google Scholar]
- Budny-Godlewski K.; Justyniak I.; Leszczyński M. K.; Lewiński J. Mechanochemical and Slow-Chemistry Radical Transformations: A Case of Diorganozinc Compounds and TEMPO. Chem. Sci. 2019, 10, 7149–7155. 10.1039/C9SC01396B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budny-Godlewski K.; Leszczyński M. K.; Tulewicz A.; Justyniak I.; Pinkowicz D.; Sieklucka B.; Kruczała K.; Sojka Z.; Lewiński J. A Case Study on the Desired Selectivity in Solid-State Mechano- and Slow-Chemistry, Melt, and Solution Methodologies. ChemSusChem 2021, 14, 3887–3894. 10.1002/cssc.202101269. [DOI] [PubMed] [Google Scholar]
- Edelmann F. T. Lanthanides and Actinides: Annual Survey of Their Organometallic Chemistry Covering the Year 2017. Coord. Chem. Rev. 2018, 370, 129–223. 10.1016/j.ccr.2018.05.013. [DOI] [Google Scholar]
- Edelmann F. T.; Farnaby J. H.; Jaroschik F.; Wilson B. Lanthanides and Actinides: Annual Survey of Their Organometallic Chemistry Covering the Year 2018. Coord. Chem. Rev. 2019, 398, 113005 10.1016/j.ccr.2019.07.002. [DOI] [Google Scholar]
- Aspinall H. C.Chemistry of the F-Block Elements; Routledge, 2018. 10.1201/9781315139258 [DOI] [Google Scholar]
- Lee J.; Zhang Q.; Saito F. Mechanochemical Synthesis of LaOX (X= Cl, Br) and Their Solid State Solutions. J. Solid State Chem. 2001, 160, 469–473. 10.1006/jssc.2001.9276. [DOI] [Google Scholar]
- Fetrow T. V.; Bhowmick R.; Achazi A. J.; Blake A. V.; Eckstrom F. D.; Vlaisavljevich B.; Daly S. R. Correction and Addition to Chelating Borohydrides for Lanthanides and Actinides: Structures, Mechanochemistry, and Case Studies with Phosphinodiboranates. Inor. Chem. 2022, 61, 5433–5434. 10.1021/acs.inorgchem.2c00443. [DOI] [PubMed] [Google Scholar]
- Fetrow T. V.; Daly S. R. Mechanochemical Synthesis and Structural Analysis of Trivalent Lanthanide and Uranium Diphenylphosphinodiboranates. Dalton Trans. 2021, 50, 11472–11484. 10.1039/D1DT01932E. [DOI] [PubMed] [Google Scholar]
- Salazar-Zertuche M.; Diaz-Guillen J.; Acosta-García J.; Diaz-Guillen J.; Montemayor S.; Burciaga-Diaz O.; Bazaldua-Medellin M.; Fuentes A. Ionic Conductivity of Ln4Zr3O12 Solid Electrolytes Synthesized by Mechanochemistry. International Journal of Hydrogen Energy 2019, 44, 12500–12507. 10.1016/j.ijhydene.2018.11.141. [DOI] [Google Scholar]
- Woen D. H.; Kotyk C. M.; Mueller T. J.; Ziller J. W.; Evans W. J. Tris (Pentamethylcyclopentadienyl) Complexes of Late Lanthanides Tb, Dy, Ho, and Er: Solution and Mechanochemical Syntheses and Structural Comparisons. Organometallics 2017, 36, 4558–4563. 10.1021/acs.organomet.7b00385. [DOI] [Google Scholar]
- Kantchev E. A. B.; O’Brien C. J.; Organ M. G. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions—A Synthetic Chemist’s Perspective. Angew. Chem., Int. Ed. 2007, 46, 2768–2813. 10.1002/anie.200601663. [DOI] [PubMed] [Google Scholar]
- Kubota K.; Ito H. Mechanochemical Cross-Coupling Reactions. Trends Chem. 2020, 2, 1066–1081. 10.1016/j.trechm.2020.09.006. [DOI] [Google Scholar]
- Cuccu F.; De Luca L.; Delogu F.; Colacino E.; Solin N.; Mocci R.; Porcheddu A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362 10.1002/cssc.202200362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu B. R.; Sruthi T.; Mitty R.; Venkateswarlu K. Catalyst-Free Mechanochemistry as a Versatile Tool in Synthetic Chemistry: A Review. Green Chem. 2023, 25, 6120–6148. 10.1039/D3GC01229H. [DOI] [Google Scholar]
- Usuki A.; Kojima Y.; Kawasumi M.; Okada A.; Fukushima Y.; Kurauchi T.; Kamigaito O. Synthesis of Nylon 6-Clay Hybrid. J. Mater. Res. 1993, 8, 1179–1184. 10.1557/JMR.1993.1179. [DOI] [Google Scholar]
- Virieux D.; Delogu F.; Porcheddu A.; García F.; Colacino E. Mechanochemical. Rearrangements. J. Org. Chem. 2021, 86, 13885–13894. 10.1021/acs.joc.1c01323. [DOI] [PubMed] [Google Scholar]
- Colacino E.; Porcheddu A.; Charnay C.; Delogu F. Engineering. From Enabling Technologies to Medicinal Mechanochemistry: An Eco-Friendly Access to Hydantoin-Based Active Pharmaceutical Ingredients. Reaction Chemistry 2019, 4, 1179–1188. 10.1039/C9RE00069K. [DOI] [Google Scholar]
- Mocci R.; Colacino E.; Luca L. D.; Fattuoni C.; Porcheddu A.; Delogu F. The Mechanochemical Beckmann Rearrangement: An Eco-Efficient “Cut-and-Paste” Strategy to Design the “Good Old Amide Bond. ACS Sustain Chem. Eng. 2021, 9, 2100–2114. 10.1021/acssuschemeng.0c07254. [DOI] [Google Scholar]
- Toda F.; Shigemasa T. Pinacol Rearrangement in the Solid State. Journal of the Chemical Society, Perkin Transactions 1 1989, 209–211. 10.1039/p19890000209. [DOI] [Google Scholar]
- Kaupp G.; Haak M.; Toda F. Atomic Force Microscopy and Solid-State Rearrangement of Benzopinacol. J. Phys. Org. Chem. 1995, 8, 545–551. 10.1002/poc.610080805. [DOI] [Google Scholar]
- Sekiya R.; Kiyo-oka K.; Imakubo T.; Kobayashi K. Intramolecular Migration of Bulky Substituents in the Solid State: Vinylogous Pinacol Rearrangements Induced Thermally and by Acid Catalysis. J. Am. Chem. Soc. 2000, 122, 10282–10288. 10.1021/ja000788l. [DOI] [Google Scholar]
- Bielski R.; Grynkiewicz G. Half a Century with Achmatowicz Rearrangement. Tetrahedron 2021, 85, 132058 10.1016/j.tet.2021.132058. [DOI] [Google Scholar]
- Achmatowicz O. Jr.; Bukowski P.; Szechner B.; Zwierzchowska Z.; Zamojski A. Synthesis of Methyl 2, 3-Dideoxy-DL-Alk-2-Enopyranosides from Furan Compounds: A General Approach to the Total Synthesis of Monosaccharides. Tetrahedron 1971, 27, 1973–1996. 10.1016/S0040-4020(01)98229-8. [DOI] [Google Scholar]
- Falenczyk C.; Pölloth B.; Hilgers P.; König B. Mechanochemically Initiated Achmatowicz Rearrangement. Synth. Commun. 2015, 45, 348–354. 10.1080/00397911.2014.963624. [DOI] [Google Scholar]
- Zhao G.; Tong R. A Solvent-Free Catalytic Protocol for the Achmatowicz Rearrangement. Green Chem. 2019, 21, 64–68. 10.1039/C8GC03030H. [DOI] [Google Scholar]
- Shi Y. X.; Xu K.; Clegg J. K.; Ganguly R.; Hirao H.; Friščić T.; García F. The First Synthesis of the Sterically Encumbered Adamantoid Phosphazane P 4 (N t Bu) 6 : Enabled by Mechanochemistry. Angew. Chem., Int. Ed. 2016, 55, 12736–12740. 10.1002/anie.201605936. [DOI] [PubMed] [Google Scholar]
- Ardila-Fierro K. J.; Lukin S.; Etter M.; Užarević K.; Halasz I.; Bolm C.; Hernández J. G. Direct Visualization of a Mechanochemically Induced Molecular Rearrangement. Angew. Chem., Int. Ed. 2020, 132, 13560–13564. 10.1002/ange.201914921. [DOI] [PubMed] [Google Scholar]
- Porcheddu A.; Delogu F.; De Luca L.; Colacino E. From Lossen Transposition to Solventless “Medicinal Mechanochemistry. ACS Sustain. Chem. Eng. 2019, 12044. 10.1021/acssuschemeng.9b00709. [DOI] [Google Scholar]
- Li Z.; Tong R. Catalytic Environmentally Friendly Protocol for Achmatowicz Rearrangement. J. Org. Chem. 2016, 81, 4847–4855. 10.1021/acs.joc.6b00469. [DOI] [PubMed] [Google Scholar]
- Sheldon R. A.; Arends I.; Hanefeld U.. Green Chem. and Catalysis; Wiley, 2007. 10.1002/9783527611003 [DOI] [Google Scholar]
- Anastas P. T.; Warner J. C.. Principles of Green Chem. Green Chem.: Theory practice; Oxford University Press, 1998; 29. [Google Scholar]
- McClenaghan N. D.; Absalon C.; Bassani D. M. Facile Synthesis of a Fullerene-Barbituric Acid Derivative and Supramolecular Catalysis of Its Photoinduced Dimerization. J. Am. Chem. Soc. 2003, 125, 13004–13005. 10.1021/ja0372098. [DOI] [PubMed] [Google Scholar]
- Novaes L. F. T.; Liu J.; Shen Y.; Lu L.; Meinhardt J. M.; Lin S. Electrocatalysis as an Enabling Technology for Organic Synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002. 10.1039/D1CS00223F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg G.Catalysis: Concepts and Green Applications; Wiley, 2017. 10.1002/9783527621866 [DOI] [Google Scholar]
- Hernández J. G.; Friščić T. Metal-Catalyzed Organic Reactions Using Mechanochemistry. Tetrahedron Lett. 2015, 56, 4253–4265. 10.1016/j.tetlet.2015.03.135. [DOI] [Google Scholar]
- Porcheddu A.; Colacino E.; De Luca L.; Delogu F. Metal-Mediated and Metal-Catalyzed Reactions Under Mechanochemical Conditions. ACS Catal. 2020, 10, 8344–8394. 10.1021/acscatal.0c00142. [DOI] [Google Scholar]
- Fiss B. G.; Richard A. J.; Douglas G.; Kojic M.; Friščić T.; Moores A. Mechanochemical Methods for the Transfer of Electrons and Exchange of Ions: Inorganic Reactivity from Nanoparticles to Organometallics. Chem. Soc. Rev. 2021, 50, 8279–8318. 10.1039/D0CS00918K. [DOI] [PubMed] [Google Scholar]
- Amrute A. P.; De Bellis J.; Felderhoff M.; Schüth F. Mechanochemical Synthesis of Catalytic Materials. Chem.—Eur. J. 2021, 27, 6819–6847. 10.1002/chem.202004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. C.; Leitch J. A.; Raby-Buck S. E.; Browne D. L. Mechanochemical Techniques for the Activation and Use of Zero-Valent Metals in Synthesis. Nat. Synth. 2022, 1, 763–775. 10.1038/s44160-022-00106-4. [DOI] [Google Scholar]
- Egorov I. N.; Santra S.; Kopchuk D. S.; Kovalev I. S.; Zyryanov G. V.; Majee A.; Ranu B. C.; Rusinov V. L.; Chupakhin O. N. Ball Milling: An Efficient and Green Approach for Asymmetric Organic Syntheses. Green Chem. 2020, 22, 302–315. 10.1039/C9GC03414E. [DOI] [Google Scholar]
- Hernández J. G.; Bolm C. [Cp* RhCl 2] 2: Mechanosynthesis and Applications in C–H Bond Functionalisations under Ball-Milling Conditions. Chem. Commun. 2015, 51, 12582–12584. 10.1039/C5CC04423E. [DOI] [PubMed] [Google Scholar]
- Schmidt R.; Thorwirth R.; Szuppa T.; Stolle A.; Ondruschka B.; Hopf H. Fast, Ligand-and Solvent-Free Synthesis of 1, 4-Substituted Buta-1, 3-diynes by Cu-Catalyzed Homocoupling of Terminal Alkynes in a Ball Mill. Chem.—Eur. J. 2011, 17, 8129–8138. 10.1002/chem.201100604. [DOI] [PubMed] [Google Scholar]
- Tan D.; Mottillo C.; Katsenis A. D.; Štrukil V.; Friščić T. Development of C–N Coupling Using Mechanochemistry: Catalytic Coupling of Arylsulfonamides and Carbodiimides. Angew. Chem., Int. Ed. 2014, 53, 9321–9324. 10.1002/anie.201404120. [DOI] [PubMed] [Google Scholar]
- Dayaker G.; Tan D.; Biggins N.; Shelam A.; Do J.; Katsenis A. D.; Friščić T. Catalytic Room-Temperature C–N Coupling of Amides and Isocyanates by Using Mechanochemistry. ChemSusChem 2020, 13, 2966–2972. 10.1002/cssc.201902576. [DOI] [PubMed] [Google Scholar]
- Seo T.; Ishiyama T.; Kubota K.; Ito H. Solid-State Suzuki–Miyaura Cross-Coupling Reactions: Olefin-Accelerated C–C Coupling Using Mechanochemistry. Chem. Sci. 2019, 10, 8202–8210. 10.1039/C9SC02185J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proutiere F.; Schoenebeck F. Solvent Effect on Palladium-catalyzed Cross-coupling Reactions and Implications on the Active Catalytic Species. Angew. Chem., Int. Ed. 2011, 50, 8192–8195. 10.1002/anie.201101746. [DOI] [PubMed] [Google Scholar]
- Schmidt R.; Stolle A.; Ondruschka B. Aromatic Substitution in Ball Mills: Formation of Aryl Chlorides and Bromides Using Potassium Peroxomonosulfate and NaX. Green Chem. 2012, 14, 1673–1679. 10.1039/c2gc16508b. [DOI] [Google Scholar]
- Zhao Y.; Rocha S. V.; Swager T. M. Mechanochemical Synthesis of Extended Iptycenes. J. Am. Chem. Soc. 2016, 138, 13834–13837. 10.1021/jacs.6b09011. [DOI] [PubMed] [Google Scholar]
- Williams M. T.; Morrill L. C.; Browne D. L. Expedient Organocatalytic Aza-Morita–Baylis–Hillman Reaction through Ball-Milling. ACS Sustain Chem. Eng. 2020, 8, 17876–17881. 10.1021/acssuschemeng.0c07320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Dong X.; Lu A. Mechanochemical Synthesis of Porous Carbons and Their Applications in Catalysis. ChemPlusChem. 2020, 85, 866–875. 10.1002/cplu.202000191. [DOI] [PubMed] [Google Scholar]
- Fulmer D. A.; Shearouse W. C.; Medonza S. T.; Mack J. Solvent-Free Sonogashira Coupling Reaction via High Speed Ball Milling. Green Chem. 2009, 11, 1821–1825. 10.1039/b915669k. [DOI] [Google Scholar]
- Su W.; Yu J.; Li Z.; Jiang Z. Solvent-Free Cross-Dehydrogenative Coupling Reactions under High Speed Ball-Milling Conditions Applied to the Synthesis of Functionalized Tetrahydroisoquinolines. J. Org. Chem. 2011, 76, 9144–9150. 10.1021/jo2015533. [DOI] [PubMed] [Google Scholar]
- Cao Q.; Howard J. L.; Wheatley E.; Browne D. L. Mechanochemical Activation of Zinc and Application to Negishi Cross-Coupling. Angew. Chem., Int. Ed. 2018, 130, 11509–11513. 10.1002/ange.201806480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Li C.-J. Highly Efficient Copper-Catalyzed Nitro-Mannich Type Reaction: Cross-Dehydrogenative-Coupling between Sp 3 C–H Bond and Sp 3 C–H Bond. J. Am. Chem. Soc. 2005, 127, 3672–3673. 10.1021/ja050058j. [DOI] [PubMed] [Google Scholar]
- Čarný T.; Peňaška T.; Andrejčák S.; Šebesta R. Mechanochemical Pd-Catalyzed Cross-Coupling of Arylhalides and Organozinc Pivalates. Chem. – Eur. J. 2022, 28, e202202040 10.1002/chem.202202040. [DOI] [PubMed] [Google Scholar]
- Xiang S.-H.; Tan B. Advances in Asymmetric Organocatalysis over the Last 10 Years. Nat. Commun. 2020, 11, 1–5. 10.1038/s41467-020-17580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo S. Highly Efficient, General Procedure for the Preparation of Alkylzinc Reagents from Unactivated Alkyl Bromides and Chlorides. Org. Lett. 2003, 5, 423–425. 10.1021/ol0272693. [DOI] [PubMed] [Google Scholar]
- Berkessel A.; Gröger H.. Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis, 1st ed.; Wiley, 2005. [Google Scholar]
- Rodríguez B.; Rantanen T.; Bolm C. Solvent-Free Asymmetric Organocatalysis in a Ball Mill. Angew. Chem., Int. Ed. 2006, 45, 6924–6926. 10.1002/anie.200602820. [DOI] [PubMed] [Google Scholar]
- Rodríguez B.; Bruckmann A.; Bolm C. A Highly Efficient Asymmetric Organocatalytic Aldol Reaction in a Ball Mill. Chem.—Eur. J. 2007, 13, 4710–4722. 10.1002/chem.200700188. [DOI] [PubMed] [Google Scholar]
- Krištofíková D.; Mečiarová M.; Rakovský E.; Šebesta R. Mechanochemically Activated Asymmetric Organocatalytic Domino Mannich Reaction-Fluorination. ACS Sustain. Chem. Eng. 2020, 8, 14417–14424. 10.1021/acssuschemeng.0c04260. [DOI] [Google Scholar]
- Lei M.; Shi L.; Li G.; Chen S.; Fang W.; Ge Z.; Cheng T.; Li R. Dipeptide-Catalyzed Direct Asymmetric Aldol Reactions in the Presence of Water. Tetrahedron 2007, 63, 7892–7898. 10.1016/j.tet.2007.05.077. [DOI] [Google Scholar]
- Hernandez J. G.; Juaristi E. Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides: Improved Stereoinduction under Solvent-Free Conditions. J. Org. Chem. 2011, 76, 1464–1467. 10.1021/jo1022469. [DOI] [PubMed] [Google Scholar]
- Do J.-L.; Mottillo C.; Tan D.; Štrukil V.; Friščić T. Mechanochemical Ruthenium-Catalyzed Olefin Metathesis. J. Am. Chem. Soc. 2015, 137, 2476–2479. 10.1021/jacs.5b00151. [DOI] [PubMed] [Google Scholar]
- Hermann G. N.; Unruh M. T.; Jung S.; Krings M.; Bolm C. Mechanochemical Rhodium(III)- and Gold(I)-Catalyzed C–H Bond Alkynylations of Indoles under Solventless Conditions in Mixer Mills. Angew. Chem., Int. Ed. 2018, 57, 10723–10727. 10.1002/anie.201805778. [DOI] [PubMed] [Google Scholar]
- Cao Q.; Nicholson W. I.; Jones A. C.; Browne D. L. Robust Buchwald–Hartwig Amination Enabled by Ball-Milling. Org. Biomol. Chem. 2019, 17, 1722–1726. 10.1039/C8OB01781F. [DOI] [PubMed] [Google Scholar]
- Ogba O. M.; Warner N. C.; O’Leary D. J.; Grubbs R. H. Recent Advances in Ruthenium-Based Olefin Metathesis. Chem. Soc. Rev. 2018, 47, 4510–4544. 10.1039/C8CS00027A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C.; Loh T. Rhodium-Catalyzed CH Alkynylation of Arenes at Room Temperature. Angew. Chem., Int. Ed. 2014, 53, 2722–2726. 10.1002/anie.201309198. [DOI] [PubMed] [Google Scholar]
- Calò V.; Nacci A.; Monopoli A.; Lopez L.; Di Cosmo A. Heck Reaction of β-Substituted Acrylates in Ionic Liquids Catalyzed by a Pd-Benzothiazole Carbene Complex. Tetrahedron 2001, 57, 6071–6077. 10.1016/S0040-4020(01)00528-2. [DOI] [Google Scholar]
- Crisp G. T. Variations on a Theme—Recent Developments on the Mechanism of the Heck Reaction and Their Implications for Synthesis. Chem. Soc. Rev. 1998, 27, 427–436. 10.1039/a827427z. [DOI] [Google Scholar]
- Le Bras J.; Muzart J. Intermolecular Dehydrogenative Heck Reactions. Chem. Rev. 2011, 111, 1170–1214. 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- Dorel R.; Grugel C. P.; Haydl A. M. The Buchwald–Hartwig Amination after 25 Years. Angew. Chem., Int. Ed. 2019, 58, 17118–17129. 10.1002/anie.201904795. [DOI] [PubMed] [Google Scholar]
- Heravi M. M.; Kheilkordi Z.; Zadsirjan V.; Heydari M.; Malmir M. Buchwald-Hartwig Reaction: An Overview. J. Organomet. Chem. 2018, 861, 17–104. 10.1016/j.jorganchem.2018.02.023. [DOI] [Google Scholar]
- Declerck V.; Colacino E.; Bantreil X.; Martinez J.; Lamaty F. Poly (Ethylene Glycol) as Reaction Medium for Mild Mizoroki–Heck Reaction in a Ball-Mill. Chem. Commun. 2012, 48, 11778–11780. 10.1039/c2cc36286d. [DOI] [PubMed] [Google Scholar]
- Tullberg E.; Peters D.; Frejd T. The Heck Reaction under Ball-Milling Conditions. J. Organomet. Chem. 2004, 689, 3778–3781. 10.1016/j.jorganchem.2004.06.045. [DOI] [Google Scholar]
- Yu J.; Shou H.; Yu W.; Chen H.; Su W. Mechanochemical Oxidative Heck Coupling of Activated and Unactivated Alkenes: A Chemo-, Regio- and Stereo-Controlled Synthesis of Alkenylbenzenes. Adv. Synth. Catal. 2019, 361, 5133–5139. 10.1002/adsc.201900965. [DOI] [Google Scholar]
- Lange J. H. M.; Coolen H. K. A. C.; Van Stuivenberg H. H.; Dijksman J. A. R.; Herremans A. H. J.; Ronken E.; Keizer H. G.; Tipker K.; McCreary A. C.; Veerman W.; Wals H. C.; Stork B.; Verveer P. C.; Den Hartog A. P.; De Jong N. M. J.; Adolfs T. J. P.; Hoogendoorn J.; Kruse C. G. Synthesis, Biological Properties, and Molecular Modeling Investigations of Novel 3,4-Diarylpyrazolines as Potent and Selective CB 1 Cannabinoid Receptor Antagonists. J. Med. Chem. 2004, 47, 627–643. 10.1021/jm031019q. [DOI] [PubMed] [Google Scholar]
- Walker S. E.; Lamb C. J. C.; Beattie N. A.; Nikodemiak P.; Lee A.-L. Oxidative Heck Desymmetrisation of 2,2-Disubstituted Cyclopentene-1,3-Diones. Chem. Commun. 2015, 51, 4089–4092. 10.1039/C5CC00407A. [DOI] [PubMed] [Google Scholar]
- Wiley P. F. The Reaction of Amides with Isocyanates. J. Am. Chem. Soc. 1949, 71, 1310–1311. 10.1021/ja01172a047. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Chai Z.; Zeng Q.; Zhang W.-X. Overview of 1,5-Selective Click Reaction of Azides with Alkynes or Their Synthetic Equivalents. Molecules 2023, 28, 1400. 10.3390/molecules28031400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.; Park K. T.; Kim J. G.; Chang S. Mechanistic Studies on the Rh(III)-Mediated Amido Transfer Process Leading to Robust C–H Amination with a New Type of Amidating Reagent. J. Am. Chem. Soc. 2015, 137, 4534–4542. 10.1021/jacs.5b01324. [DOI] [PubMed] [Google Scholar]
- Neumann S.; Biewend M.; Rana S.; Binder W. H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359 10.1002/marc.201900359. [DOI] [PubMed] [Google Scholar]
- Cook T. L.; Walker J. A.; Mack J. Scratching the Catalytic Surface of Mechanochemistry: A Multi-Component CuAAC Reaction Using a Copper Reaction Vial. Green Chem. 2013, 15, 617–619. 10.1039/c3gc36720g. [DOI] [Google Scholar]
- Hermann G. N.; Bolm C. Mechanochemical Rhodium(III)-Catalyzed C–H Bond Amidation of Arenes with Dioxazolones under Solventless Conditions in a Ball Mill. ACS Catal. 2017, 7, 4592–4596. 10.1021/acscatal.7b00582. [DOI] [Google Scholar]
- Haley R. A.; Zellner A. R.; Krause J. A.; Guan H.; Mack J. Nickel Catalysis in a High Speed Ball Mill: A Recyclable Mechanochemical Method for Producing Substituted Cyclooctatetraene Compounds. ACS Sustain. Chem. Eng. 2016, 4, 2464–2469. 10.1021/acssuschemeng.6b00363. [DOI] [Google Scholar]
- Wender P. A.; Christy J. P.; Lesser A. B.; Gieseler M. T. The Synthesis of Highly Substituted Cyclooctatetraene Scaffolds by Metal-Catalyzed [2 + 2+2 + 2] Cycloadditions: Studies on Regioselectivity, Dynamic Properties, and Metal Chelation. Angew. Chem., Int. Ed. 2009, 48, 7687–7690. 10.1002/anie.200903859. [DOI] [PubMed] [Google Scholar]
- Hernández J. G.; Bolm C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. 10.1021/acs.joc.6b02887. [DOI] [PubMed] [Google Scholar]
- Baron A.; Martinez J.; Lamaty F. Solvent-Free Synthesis of Unsaturated Amino Esters in a Ball-Mill. Tetrahedron Lett. 2010, 51, 6246–6249. 10.1016/j.tetlet.2010.09.069. [DOI] [Google Scholar]
- Zhang Z.; Peng Z.-W.; Hao M.-F.; Gao J.-G. Mechanochemical Diels-Alder Cycloaddition Reactions for Straightforward Synthesis of Endo-Norbornene Derivatives. Synlett 2010, 2010, 2895–2898. 10.1055/s-0030-1259030. [DOI] [Google Scholar]
- Wang G.-W. Mechanochemical Organic Synthesis. Chem. Soc. Rev. 2013, 42, 7668. 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- Lou S.-J.; Mao Y.-J.; Xu D.-Q.; He J.-Q.; Chen Q.; Xu Z.-Y. Fast and Selective Dehydrogenative C–H/C–H Arylation Using Mechanochemistry. ACS Catal. 2016, 6, 3890–3894. 10.1021/acscatal.6b00861. [DOI] [Google Scholar]
- Xu H.; Shang M.; Dai H.-X.; Yu J.-Q. Ligand-Controlled Para -Selective C–H Arylation of Monosubstituted Arenes. Org. Lett. 2015, 17, 3830–3833. 10.1021/acs.orglett.5b01802. [DOI] [PubMed] [Google Scholar]
- Fiore C.; Sovic I.; Lukin S.; Halasz I.; Martina K.; Delogu F.; Ricci P. C.; Porcheddu A.; Shemchuk O.; Braga D.; et al. Kabachnik–Fields Reaction by Mechanochemistry: New Horizons from Old Methods. ACS Sustain Chem. Eng. 2020, 8, 18889–18902. 10.1021/acssuschemeng.0c05744. [DOI] [Google Scholar]
- Pickhardt W.; Grätz S.; Borchardt L. Direct Mechanocatalysis: Using Milling Balls as Catalysts. Chem. – Eur. J. 2020, 26, 12903–12911. 10.1002/chem.202001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C. G.; Grätz S.; Lukin S.; Halasz I.; Etter M.; Evans J. D.; Borchardt L. Direct Mechanocatalysis: Palladium as Milling Media and Catalyst in the Mechanochemical Suzuki Polymerization. Angew. Chem., Int. Ed. 2019, 58, 18942–18947. 10.1002/anie.201911356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C. G.; Oltermann M.; Pickhardt W.; Grätz S.; Borchardt L. Bronze Age of Direct Mechanocatalysis: How Alloyed Milling Materials Advance Coupling in Ball Mills. Adv. Energy Sustain. Res. 2021, 2, 2100011 10.1002/aesr.202100011. [DOI] [Google Scholar]
- Sprick R. S.; Bonillo B.; Clowes R.; Guiglion P.; Brownbill N. J.; Slater B. J.; Blanc F.; Zwijnenburg M. A.; Adams D. J.; Cooper A. I. Visible-Light-Driven Hydrogen Evolution Using Planarized Conjugated Polymer Photocatalysts. Angew. Chem., Int. Ed. 2016, 55, 1792–1796. 10.1002/anie.201510542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Leslie D.; Coleman M. G.; Mack J. Recyclable Heterogeneous Metal Foil-Catalyzed Cyclopropenation of Alkynes and Diazoacetates under Solvent-Free Mechanochemical Reaction Conditions. Chem. Sci. 2018, 9, 4650–4661. 10.1039/C8SC00443A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A. S.-K.; Todd M. H. Facile Synthesis of Vicinal Diamines via Oxidation of N-Phenyltetrahydroisoquinolines with DDQ. Tetrahedron Lett. 2009, 50, 1199–1202. 10.1016/j.tetlet.2008.12.101. [DOI] [Google Scholar]
- Ovalles S. R.; Hansen J. H.; Davies H. M. L. Thermally Induced Cycloadditions of Donor/Acceptor Carbenes. Org. Lett. 2011, 13, 4284–4287. 10.1021/ol201628d. [DOI] [PubMed] [Google Scholar]
- Hwang S.; Grätz S.; Borchardt L. A Guide to Direct Mechanocatalysis. Chem. Commun. 2022, 58, 1661–1671. 10.1039/D1CC05697B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Némethová V.; Krištofíková D.; Mečiarová M.; Šebesta R. Asymmetric Organocatalysis Under Mechanochemical Conditions. Chem. Rec. 2023, 23, e202200283 10.1002/tcr.202200283. [DOI] [PubMed] [Google Scholar]
- Mohr J. T.; Krout M. R.; Stoltz B. M. Natural Products as Inspiration for the Development of Asymmetric Catalysis. Nature 2008, 455, 323–332. 10.1038/nature07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. S.; Jacobsen E. N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem., Int. Ed. 2006, 45, 1520–1543. 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]
- Shen Z.; Sang Y.; Wang T.; Jiang J.; Meng Y.; Jiang Y.; Okuro K.; Aida T.; Liu M. Asymmetric Catalysis Mediated by a Mirror Symmetry-Broken Helical Nanoribbon. Nat. Commun. 2019, 10, 3976. 10.1038/s41467-019-11840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller K. M.; Wong C.-H. Enzymes for Chemical Synthesis. Nature 2001, 409, 232–240. 10.1038/35051706. [DOI] [PubMed] [Google Scholar]
- Wegman M. A.; Janssen M. H. A.; van Rantwijk F.; Sheldon R. A. Towards Biocatalytic Synthesis of β-Lactam Antibiotics. Adv. Synth. Catal. 2001, 343, 559–576. . [DOI] [Google Scholar]
- Otten L. G.; Hollmann F.; Arends I. W. C. E. Enzyme Engineering for Enantioselectivity: From Trial-and-Error to Rational Design?. Trends Biotechnol. 2010, 28, 46–54. 10.1016/j.tibtech.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Demirjian D. C.; Morís-Varas F.; Cassidy C. S. Enzymes from Extremophiles. Curr. Opin. Chem. Biol. 2001, 5, 144–151. 10.1016/S1367-5931(00)00183-6. [DOI] [PubMed] [Google Scholar]
- Van Den Burg B. Extremophiles as a Source for Novel Enzymes. Curr. Opin. Microbiol. 2003, 6, 213–218. 10.1016/S1369-5274(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Gupta M. N. Enzyme Function in Organic Solvents. Eur. J. Biochem. 1992, 203, 25–32. 10.1111/j.1432-1033.1992.tb19823.x. [DOI] [PubMed] [Google Scholar]
- Van Schie M. M. C. H.; Spöring J.-D.; Bocola M.; Domínguez De María P.; Rother D. Applied Biocatalysis beyond Just Buffers – from Aqueous to Unconventional Media. Options and Guidelines. Green Chem. 2021, 23, 3191–3206. 10.1039/D1GC00561H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. D.; Nichols J.; Kelly R. M.; Deiters A. Microwave Activation of Enzymatic Catalysis. J. Am. Chem. Soc. 2008, 130, 10048–10049. 10.1021/ja802404g. [DOI] [PubMed] [Google Scholar]
- Hudlicky T.; Reed J. W. Applications of Biotransformations and Biocatalysis to Complexity Generation in Organic Synthesis. Chem. Soc. Rev. 2009, 38, 3117. 10.1039/b901172m. [DOI] [PubMed] [Google Scholar]
- Sheldon R. A.; Brady D. The Limits to Biocatalysis: Pushing the Envelope. Chem. Commun. 2018, 54, 6088–6104. 10.1039/C8CC02463D. [DOI] [PubMed] [Google Scholar]
- Reetz M. T.; Jaeger K.-E. Enantioselective Enzymes for Organic Synthesis Created by Directed Evolution. Chem. - Eur. J. 2000, 6, 407–412. . [DOI] [PubMed] [Google Scholar]
- Hönig M.; Sondermann P.; Turner N. J.; Carreira E. M. Enantioselective Chemo- and Biocatalysis: Partners in Retrosynthesis. Angew. Chem., Int. Ed. 2017, 56, 8942–8973. 10.1002/anie.201612462. [DOI] [PubMed] [Google Scholar]
- Hernández J. G.; Frings M.; Bolm C. Mechanochemical Enzymatic Kinetic Resolution of Secondary Alcohols under Ball-Milling Conditions. ChemCatChem. 2016, 8, 1769–1772. 10.1002/cctc.201600455. [DOI] [Google Scholar]
- Pérez-Venegas M.; Juaristi E. Mechanoenzymology: State of the Art and Challenges towards Highly Sustainable Biocatalysis. ChemSusChem 2021, 14, 2682–2688. 10.1002/cssc.202100624. [DOI] [PubMed] [Google Scholar]
- Avila-Ortiz C. G.; Pérez-Venegas M.; Vargas-Caporali J.; Juaristi E. Recent Applications of Mechanochemistry in Enantioselective Synthesis. Tetrahedron Lett. 2019, 60, 1749–1757. 10.1016/j.tetlet.2019.05.065. [DOI] [Google Scholar]
- Bonnamour J.; Métro T.-X.; Martinez J.; Lamaty F. Environmentally Benign Peptide Synthesis Using Liquid-Assisted Ball-Milling: Application to the Synthesis of Leu-Enkephalin. Green Chem. 2013, 15, 1116. 10.1039/c3gc40302e. [DOI] [Google Scholar]
- Maurin O.; Verdié P.; Subra G.; Lamaty F.; Martinez J.; Métro T.-X. Peptide Synthesis: Ball-Milling, in Solution, or on Solid Support, What Is the Best Strategy?. Beilstein J. Org. Chem. 2017, 13, 2087–2093. 10.3762/bjoc.13.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weißbach U.; Dabral S.; Konnert L.; Bolm C.; Hernández J. G. Selective Enzymatic Esterification of Lignin Model Compounds in the Ball Mill. Beilstein J. Org. Chem. 2017, 13, 1788–1795. 10.3762/bjoc.13.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández J. G.; Ardila-Fierro K. J.; Crawford D.; James S. L.; Bolm C. Mechanoenzymatic Peptide and Amide Bond Formation. Green Chem. 2017, 19, 2620–2625. 10.1039/C7GC00615B. [DOI] [Google Scholar]
- Ardila-Fierro K. J.; Crawford D. E.; Körner A.; James S. L.; Bolm C.; Hernández J. G. Papain-Catalysed Mechanochemical Synthesis of Oligopeptides by Milling and Twin-Screw Extrusion: Application in the Juliá–Colonna Enantioselective Epoxidation. Green Chem. 2018, 20, 1262–1269. 10.1039/C7GC03205F. [DOI] [Google Scholar]
- Kaabel S.; Therien J. P. D.; Deschênes C. E.; Duncan D.; Friščić T.; Auclair K. Enzymatic Depolymerization of Highly Crystalline Polyethylene Terephthalate Enabled in Moist-Solid Reaction Mixtures. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2026452118 10.1073/pnas.2026452118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero Acero E.; Ribitsch D.; Steinkellner G.; Gruber K.; Greimel K.; Eiteljoerg I.; Trotscha E.; Wei R.; Zimmermann W.; Zinn M.; Cavaco-Paulo A.; Freddi G.; Schwab H.; Guebitz G. Enzymatic Surface Hydrolysis of PET: Effect of Structural Diversity on Kinetic Properties of Cutinases from Thermobifida. Macromolecules 2011, 44, 4632–4640. 10.1021/ma200949p. [DOI] [Google Scholar]
- List B. Enamine Catalysis Is a Powerful Strategy for the Catalytic Generation and Use of Carbanion Equivalents. Acc. Chem. Res. 2004, 37, 548–557. 10.1021/ar0300571. [DOI] [PubMed] [Google Scholar]
- Song R.; Jin Z.; Chi Y. R. NHC-Catalyzed Covalent Activation of Heteroatoms for Enantioselective Reactions. Chem. Sci. 2021, 12, 5037–5043. 10.1039/D1SC00469G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion N.; Díez-González S.; Nolan S. P. N-Heterocyclic Carbenes as Organocatalysts. Angew. Chem., Int. Ed. 2007, 46, 2988–3000. 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]
- Guo H.; Fan Y. C.; Sun Z.; Wu Y.; Kwon O. Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049–10293. 10.1021/acs.chemrev.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H.; Chan W.-L.; Lu Y. Phosphine-Catalyzed Asymmetric Organic Reactions. Chem. Rev. 2018, 118, 9344–9411. 10.1021/acs.chemrev.8b00261. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Schreiner P. R. Thio)Urea Organocatalysis—What Can Be Learnt from Anion Recognition?. Chem. Soc. Rev. 2009, 38, 1187. 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]
- Dong S.; Feng X.; Liu X. Chiral Guanidines and Their Derivatives in Asymmetric Synthesis. Chem. Soc. Rev. 2018, 47, 8525–8540. 10.1039/C7CS00792B. [DOI] [PubMed] [Google Scholar]
- Qian D.; Sun J. Recent Progress in Asymmetric Ion-Pairing Catalysis with Ammonium Salts. Chem. – Eur. J. 2019, 25, 3740–3751. 10.1002/chem.201803752. [DOI] [PubMed] [Google Scholar]
- Zong L.; Tan C.-H. Phase-Transfer and Ion-Pairing Catalysis of Pentanidiums and Bisguanidiniums. Acc. Chem. Res. 2017, 50, 842–856. 10.1021/acs.accounts.6b00604. [DOI] [PubMed] [Google Scholar]
- Shirakawa S.; Maruoka K. Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem., Int. Ed. 2013, 52, 4312–4348. 10.1002/anie.201206835. [DOI] [PubMed] [Google Scholar]
- Kennedy C. R.; Lin S.; Jacobsen E. N. The Cation−π Interaction in Small-Molecule Catalysis. Angew. Chem., Int. Ed. 2016, 55, 12596–12624. 10.1002/anie.201600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckmann A.; Krebs A.; Bolm C. Organocatalytic Reactions: Effects of Ball Milling, Microwave and Ultrasound Irradiation. Green Chem. 2008, 10, 1131. 10.1039/b812536h. [DOI] [Google Scholar]
- Veverková E.; Modrocká V.; Šebesta R. Organocatalyst Efficiency in the α-Aminoxylation and α-Hydrazination of Carbonyl Derivatives in Aqueous Media or in a Ball-Mill. Eur. J. Org. Chem. 2017, 2017, 1191–1195. 10.1002/ejoc.201601357. [DOI] [Google Scholar]
- André V.; Hardeman A.; Halasz I.; Stein R. S.; Jackson G. J.; Reid D. G.; Duer M. J.; Curfs C.; Duarte M. T.; Friščić T. Mechanosynthesis of the Metallodrug Bismuth Subsalicylate from Bi2O3 and Structure of Bismuth Salicylate without Auxiliary Organic Ligands. Angew. Chem., Int. Ed. 2011, 50, 7858–7861. 10.1002/anie.201103171. [DOI] [PubMed] [Google Scholar]
- Konnert L.; Dimassi M.; Gonnet L.; Lamaty F.; Martinez J.; Colacino E. Poly(Ethylene) Glycols and Mechanochemistry for the Preparation of Bioactive 3,5-Disubstituted Hydantoins. RSC Adv. 2016, 6, 36978–36986. 10.1039/C6RA03222B. [DOI] [Google Scholar]
- Jayasankar A.; Somwangthanaroj A.; Shao Z. J.; Rodríguez-Hornedo N. Cocrystal Formation during Cogrinding and Storage Is Mediated by Amorphous Phase. Pharm. Res. 2006, 23, 2381–2392. 10.1007/s11095-006-9110-6. [DOI] [PubMed] [Google Scholar]
- Trask A. V.; Haynes D. A.; Motherwell W. D. S.; Jones W. Screening for Crystalline Salts via Mechanochemistry. Chem. Commun. 2006, (1), 51–53. 10.1039/B512626F. [DOI] [PubMed] [Google Scholar]
- Huang J.; Zhang X.; Armstrong D. W. Highly Efficient Asymmetric Direct Stoichiometric Aldol Reactions on/in Water. Angew. Chem., Int. Ed. 2007, 46, 9073–9077. 10.1002/anie.200703606. [DOI] [PubMed] [Google Scholar]
- Hernández J. G.; Juaristi E. Efficient Ball-Mill Procedure in the ‘Green’ Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Dipeptides in the Presence of Water. Tetrahedron 2011, 67, 6953–6959. 10.1016/j.tet.2011.06.042. [DOI] [Google Scholar]
- Machuca E.; Juaristi E. Organocatalytic Activity of α,α-Dipeptide Derivatives of (S)-Proline in the Asymmetric Aldol Reaction in Absence of Solvent. Evidence for Non-Covalent π–π Interactions in the Transition State. Tetrahedron Lett. 2015, 56, 1144–1148. 10.1016/j.tetlet.2015.01.079. [DOI] [Google Scholar]
- Hernández J. G.; García-López V.; Juaristi E. Solvent-Free Asymmetric Aldol Reaction Organocatalyzed by (S)-Proline-Containing Thiodipeptides under Ball-Milling Conditions. Tetrahedron 2012, 68, 92–97. 10.1016/j.tet.2011.10.093. [DOI] [Google Scholar]
- Machuca E.; Rojas Y.; Juaristi E. Synthesis and Evaluation of (S)-Proline-Containing α,β-Dipeptides as Organocatalysts in Solvent-Free Asymmetric Aldol Reactions Under Ball-Milling Conditions. Asian J. Org. Chem. 2015, 4, 46–53. 10.1002/ajoc.201402170. [DOI] [Google Scholar]
- Klare H.; Neudörfl J. M.; Goldfuss B. New Hydrogen-Bonding Organocatalysts: Chiral Cyclophosphazanes and Phosphorus Amides as Catalysts for Asymmetric Michael Additions. Beilstein J. Org. Chem. 2014, 10, 224–236. 10.3762/bjoc.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatiuk Ż. A.; Janicki M. J.; Góra R. W.; Konieczny K.; Kowalczyk R. Applications of Thermal Activation, Ball-milling and Aqueous Medium in Stereoselective Michael Addition of Nitromethane to Enynones Catalyzed by Chiral Squaramides. Adv. Synth. Catal. 2019, 361, 1108–1116. 10.1002/adsc.201801498. [DOI] [Google Scholar]
- Larionov V. A.; Feringa B. L.; Belokon Y. N. Enantioselective “Organocatalysis in Disguise” by the Ligand Sphere of Chiral Metal-Templated Complexes. Chem. Soc. Rev. 2021, 50, 9715–9740. 10.1039/D0CS00806K. [DOI] [PubMed] [Google Scholar]
- Doyle A. G.; Jacobsen E. N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007, 107, 5713–5743. 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]
- Jörres M.; Mersmann S.; Raabe G.; Bolm C. Organocatalytic Solvent-Free Hydrogen Bonding-Mediated Asymmetric Michael Additions under Ball Milling Conditions. Green Chem. 2013, 15, 612. 10.1039/c2gc36906k. [DOI] [Google Scholar]
- Mack J.; Shumba M. Rate Enhancement of the Morita–Baylis–Hillman Reaction through Mechanochemistry. Green Chem. 2007, 9, 328–330. 10.1039/B612983H. [DOI] [Google Scholar]
- De Souza R. O. M. A.; Pereira V. L. P.; Esteves P. M.; Vasconcellos M. L. A. A. The Morita–Baylis–Hillman Reaction in Aqueous–Organic Solvent System. Tetrahedron Lett. 2008, 49, 5902–5905. 10.1016/j.tetlet.2008.07.140. [DOI] [Google Scholar]
- Breslow R. On the Mechanism of Thiamine Action. IV. 1 Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. 10.1021/ja01547a064. [DOI] [Google Scholar]
- Nun P.; Pérez V.; Calmès M.; Martinez J.; Lamaty F. Preparation of Chiral Amino Esters by Asymmetric Phase-Transfer Catalyzed Alkylations of Schiff Bases in a Ball Mill. Chem. – Eur. J. 2012, 18, 3773–3779. 10.1002/chem.201102885. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S.; Banerjee S.; Shlain M. A.; Bardin A. A.; Ulijn R. V.; Nannenga B. L.; Rappe A. M.; Braunschweig A. B. Photomechanochemical Control over Stereoselectivity in the [2 + 2] Photodimerization of Acenaphthylene. Faraday Discuss. 2023, 241, 266–277. 10.1039/D2FD00122E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakoudakis D. A.; Chatel G.; Colmenares J. C. Mechanochemical Forces as a Synthetic Tool for Zero- and One-Dimensional Titanium Oxide-Based Nano-Photocatalysts. Top. Curr. Chem. 2020, 378, 2. 10.1007/s41061-019-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štrukil V.; Sajko I. Mechanochemically-Assisted Solid-State Photocatalysis (MASSPC). Chem. Commun. 2017, 53, 9101–9104. 10.1039/C7CC03510A. [DOI] [PubMed] [Google Scholar]
- Hernández J. G. Mechanochemical Borylation of Aryldiazonium Salts; Merging Light and Ball Milling. Beilstein J. Org. Chem. 2017, 13, 1463–1469. 10.3762/bjoc.13.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Cong T.; Liu P.; Sun P. Synthesis of 1,2-Diketones via a Metal-Free, Visible-Light-Induced Aerobic Photooxidation of Alkynes. J. Org. Chem. 2016, 81, 7256–7261. 10.1021/acs.joc.6b00097. [DOI] [PubMed] [Google Scholar]
- Sokolov A. N.; Bučar D.; Baltrusaitis J.; Gu S. X.; MacGillivray L. R. Supramolecular Catalysis in the Organic Solid State through Dry Grinding. Angew. Chem., Int. Ed. 2010, 49, 4273–4277. 10.1002/anie.201000874. [DOI] [PubMed] [Google Scholar]
- Stojaković J.; Farris B. S.; MacGillivray L. R. Vortex Grinding for Mechanochemistry: Application for Automated Supramolecular Catalysis and Preparation of a Metal–Organic Framework. Chem. Commun. 2012, 48, 7958–7960. 10.1039/c2cc33227b. [DOI] [PubMed] [Google Scholar]
- Egami H.; Ito Y.; Ide T.; Masuda S.; Hamashima Y. Simple Photo-Induced Trifluoromethylation of Aromatic Rings. Synthesis 2018, 50, 2948–2953. 10.1055/s-0037-1609759. [DOI] [Google Scholar]
- Kubota K.; Pang Y.; Miura A.; Ito H. Redox Reactions of Small Organic Molecules Using Ball Milling and Piezoelectric. Materials. Science 2019, 366, 1500–1504. 10.1126/science.aay8224. [DOI] [PubMed] [Google Scholar]
- Pang Y.; Lee J. W.; Kubota K.; Ito H. Solid-State Radical C–H Trifluoromethylation Reactions Using Ball Milling and Piezoelectric Materials. Angew. Chem., Int. Ed. 2020, 59, 22570–22576. 10.1002/anie.202009844. [DOI] [PubMed] [Google Scholar]
- Schumacher C.; Hernández J. G.; Bolm C. Electro-Mechanochemical Atom Transfer Radical Cyclizations Using Piezoelectric BaTiO 3. Angew. Chem., Int. Ed. 2020, 59, 16357–16360. 10.1002/anie.202003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari D. P.; Schroll P.; König B. Metal-Free, Visible-Light-Mediated Direct C–H Arylation of Heteroarenes with Aryl Diazonium Salts. J. Am. Chem. Soc. 2012, 134, 2958–2961. 10.1021/ja212099r. [DOI] [PubMed] [Google Scholar]
- Amer M. M.; Hommelsheim R.; Schumacher C.; Kong D.; Bolm C. Electro-Mechanochemical Approach towards the Chloro Sulfoximidations of Allenes under Solvent-Free Conditions in a Ball Mill. Faraday Discuss. 2023, 241, 79–90. 10.1039/D2FD00075J. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang Z.; Deng L.; Lao T.; Su Z.; Yu Y.; Cao H. Mechanochemical Synthesis of 1,2-Diketoindolizine Derivatives from Indolizines and Epoxides Using Piezoelectric Materials. Org. Lett. 2021, 23, 7171–7176. 10.1021/acs.orglett.1c02575. [DOI] [PubMed] [Google Scholar]
- Liang Y.; Teng L.; Wang Y.; He Q.; Cao H. A Visible-Light-Induced Intermolecular [3 + 2] Alkenylation–Cyclization Strategy: Metal-Free Construction of Pyrrolo[2,1,5- Cd ]Indolizine Rings. Green Chem. 2019, 21, 4025–4029. 10.1039/C9GC01766F. [DOI] [Google Scholar]
- Rodríguez-Padrón D.; Puente-Santiago A. R.; Balu A. M.; Muñoz-Batista M. J.; Luque R. Environmental Catalysis: Present and Future. ChemCatChem. 2019, 11, 18–38. 10.1002/cctc.201801248. [DOI] [Google Scholar]
- Kwiczak-Yigitbaşı J.; Demir M.; Ahan R. E.; Canlı S.; Şafak Şeker U. Ö.; Baytekin B. Ultrasonication for Environmentally Friendly Preparation of Antimicrobial and Catalytically Active Nanocomposites of Cellulosic Textiles. ACS Sustain. Chem. Eng. 2020, 8, 18879–18888. 10.1021/acssuschemeng.0c05493. [DOI] [Google Scholar]
- Fiss B. G.; Hatherly L.; Stein R. S.; Friščić T.; Moores A. Mechanochemical Phosphorylation of Polymers and Synthesis of Flame-Retardant Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 7951–7959. 10.1021/acssuschemeng.9b00764. [DOI] [Google Scholar]
- Jiang Y.; He C.; Qiu S.; Zhang J.; Wang X.; Yang Y. Scalable Mechanochemical Coupling of Homogeneous Co3O4 Nanocrystals onto In-Situ Exfoliated Graphene Sheets for Asymmetric Supercapacitors. Chem. Eng. J. 2020, 397, 125503 10.1016/j.cej.2020.125503. [DOI] [Google Scholar]
- Baláž P.; Achimovičová M.; Baláž M.; Chen K.; Dobrozhan O.; Guilmeau E.; Hejtmánek J.; Knížek K.; Kubíčková L.; Levinský P.; Puchý V.; Reece M. J.; Varga P.; Zhang R. Thermoelectric Cu–S-Based Materials Synthesized via a Scalable Mechanochemical Process. ACS Sustain. Chem. Eng. 2021, 9, 2003–2016. 10.1021/acssuschemeng.0c05555. [DOI] [Google Scholar]
- Gennari F. C.; Castro F. J.; Urretavizcaya G. Hydrogen Desorption Behavior from Magnesium Hydrides Synthesized by Reactive Mechanical Alloying. J. Alloys Compd. 2001, 321, 46–53. 10.1016/S0925-8388(00)01460-2. [DOI] [Google Scholar]
- Baum L.; Meyer M.; Mendozazelis L. Complex Mg-Based Hydrides Obtained by Mechanosynthesis: Characterization and Formation Kinetics. Int. J. Hydrog. Energy 2008, 33, 3442–3446. 10.1016/j.ijhydene.2007.10.045. [DOI] [Google Scholar]
- Deledda S.; Hauback B. C. The Formation Mechanism and Structural Characterization of the Mixed Transition-Metal Complex Hydride Mg 2 (FeH 6) 0.5 (CoH 5) 0.5 Obtained by Reactive Milling. Nanotechnology 2009, 20, 204010 10.1088/0957-4484/20/20/204010. [DOI] [PubMed] [Google Scholar]
- Balcerzak M.; Ternieden J.; Felderhoff M. Synthesis, Thermal Stability, and Hydrogen Storage Properties of Poorly Crystalline TiVFeCuNb Multi-Principal Element Alloy. J. Alloys Compd. 2023, 943, 169142 10.1016/j.jallcom.2023.169142. [DOI] [Google Scholar]
- Muñoz-Batista M. J.; Rodriguez-Padron D.; Puente-Santiago A. R.; Luque R. Mechanochemistry: Toward Sustainable Design of Advanced Nanomaterials for Electrochemical Energy Storage and Catalytic Applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. 10.1021/acssuschemeng.8b01716. [DOI] [Google Scholar]
- Huot J.; Ravnsbæk D. B.; Zhang J.; Cuevas F.; Latroche M.; Jensen T. R. Mechanochemical Synthesis of Hydrogen Storage Materials. Prog. Mater. Sci. 2013, 58, 30–75. 10.1016/j.pmatsci.2012.07.001. [DOI] [Google Scholar]
- Yun S.; Kirakosyan A.; Yoon S.-G.; Choi J. Scalable Synthesis of Exfoliated Organometal Halide Perovskite Nanocrystals by Ligand-Assisted Ball Milling. ACS Sustain. Chem. Eng. 2018, 6, 3733–3738. 10.1021/acssuschemeng.7b04092. [DOI] [Google Scholar]
- Baláž P.; Achimovičová M.; Baláž M.; Billik P.; Cherkezova-Zheleva Z.; Criado J. M.; Delogu F.; Dutková E.; Gaffet E.; Gotor F. J.; Kumar R.; Mitov I.; Rojac T.; Senna M.; Streletskii A.; Wieczorek-Ciurowa K. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571. 10.1039/c3cs35468g. [DOI] [PubMed] [Google Scholar]
- Beyer M. K.; Clausen-Schaumann H. Mechanochemistry: The Mechanical Activation of Covalent Bonds. Chem. Rev. 2005, 105, 2921–2948. 10.1021/cr030697h. [DOI] [PubMed] [Google Scholar]
- Stolar T.; Užarević K. Mechanochemistry: An Efficient and Versatile Toolbox for Synthesis, Transformation, and Functionalization of Porous Metal–Organic Frameworks. CrystEngComm 2020, 22, 4511–4525. 10.1039/D0CE00091D. [DOI] [Google Scholar]
- Głowniak S.; Szczęśniak B.; Choma J.; Jaroniec M. Mechanochemistry: Toward Green Synthesis of Metal–Organic Frameworks. Mater. Today 2021, 46, 109–124. 10.1016/j.mattod.2021.01.008. [DOI] [Google Scholar]
- Szczęśniak B.; Borysiuk S.; Choma J.; Jaroniec M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horiz. 2020, 7, 1457–1473. 10.1039/D0MH00081G. [DOI] [Google Scholar]
- Jodlowski A. D.; Yépez A.; Luque R.; Camacho L.; de Miguel G. Benign-by-Design Solventless Mechanochemical Synthesis of Three-, Two-, and One-Dimensional Hybrid Perovskites. Angew. Chem., Int. Ed. 2016, 55, 14972–14977. 10.1002/anie.201607397. [DOI] [PubMed] [Google Scholar]
- Liu X.; Li Y.; Zeng L.; Li X.; Chen N.; Bai S.; He H.; Wang Q.; Zhang C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327 10.1002/adma.202108327. [DOI] [PubMed] [Google Scholar]
- Palazon F.; El Ajjouri Y.; Bolink H. J. Making by Grinding: Mechanochemistry Boosts the Development of Halide Perovskites and Other Multinary Metal Halides. Adv. Energy Mater. 2020, 10, 1902499 10.1002/aenm.201902499. [DOI] [Google Scholar]
- Yusuf V. F.; Malek N. I.; Kailasa S. K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. 10.1021/acsomega.2c05310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei M.; Foroughi M. M.; Ebrahimpoor N.; Jahani S.; Omidi A.; Khatami M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. 10.1016/j.trac.2019.06.007. [DOI] [Google Scholar]
- Zhou H.-C.; Long J. R.; Yaghi O. M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- Lee J.; Farha O. K.; Roberts J.; Scheidt K. A.; Nguyen S. T.; Hupp J. T. Metal–Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Yaghi O. M.; O’Keeffe M.; Ockwig N. W.; Chae H. K.; Eddaoudi M.; Kim J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- Wang G.-W.; Chen Z.-X.; Murata Y.; Komatsu K. [60]Fullerene Adducts with 9-Substituted Anthracenes: Mechanochemical Preparation and Retro Diels–Alder Reaction. Tetrahedron 2005, 61, 4851–4856. 10.1016/j.tet.2005.02.089. [DOI] [Google Scholar]
- Wang G.-W.; Komatsu K.; Murata Y.; Shiro M. Synthesis and X-Ray Structure of Dumb-Bell-Shaped C120. Nature 1997, 387, 583–586. 10.1038/42439. [DOI] [Google Scholar]
- Friščić T.; Reid D. G.; Halasz I.; Stein R. S.; Dinnebier R. E.; Duer M. J. Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal–Organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem., Int. Ed. 2010, 49, 712–715. 10.1002/anie.200906583. [DOI] [PubMed] [Google Scholar]
- Martinez V.; Karadeniz B.; Biliškov N.; Lončarić I.; Muratović S.; Žilić D.; Avdoshenko S. M.; Roslova M.; Popov A. A.; Užarević K. Tunable Fulleretic Sodalite MOFs: Highly Efficient and Controllable Entrapment of C 60 Fullerene via Mechanochemistry. Chem. Mater. 2020, 32, 10628–10640. 10.1021/acs.chemmater.0c03796. [DOI] [Google Scholar]
- Thorne M. F.; Gómez M. L. R.; Bumstead A. M.; Li S.; Bennett T. D. Mechanochemical Synthesis of Mixed Metal, Mixed Linker, Glass-Forming Metal–Organic Frameworks. Green Chem. 2020, 22, 2505–2512. 10.1039/D0GC00546K. [DOI] [Google Scholar]
- Salehipour M.; Rezaei S.; Rezaei M.; Yazdani M.; Mogharabi-Manzari M. Opportunities and Challenges in Biomedical Applications of Metal–Organic Frameworks. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4443–4462. 10.1007/s10904-021-02118-7. [DOI] [Google Scholar]
- Friščić T. Metal-organic Frameworks: Mechanochemical Synthesis Strategies. Encyclopedia of Inorganic and BioInor. Chem. 2014, 1–19. 10.1002/9781119951438.eibc2202. [DOI] [Google Scholar]
- Klimakow M.; Klobes P.; Thünemann A. F.; Rademann K.; Emmerling F. Mechanochemical Synthesis of Metal–Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas. Chem. Mater. 2010, 22, 5216–5221. 10.1021/cm1012119. [DOI] [Google Scholar]
- Yuan W.; Garay A. L.; Pichon A.; Clowes R.; Wood C. D.; Cooper A. I.; James S. L. Study of the Mechanochemical Formation and Resulting Properties of an Archetypal MOF: Cu3(BTC)2 (BTC = 1,3,5-Benzenetricarboxylate). CrystEngComm 2010, 12, 4063. 10.1039/c0ce00486c. [DOI] [Google Scholar]
- Xu W.; Chen H.; Jie K.; Yang Z.; Li T.; Dai S. Entropy-Driven Mechanochemical Synthesis of Polymetallic Zeolitic Imidazolate Frameworks for CO 2 Fixation. Angew. Chem., Int. Ed. 2019, 131, 5072–5076. 10.1002/ange.201900787. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Mao M. M.; Wang J.; Gludovatz B.; Zhang Z.; Mao S. X.; George E. P.; Yu Q.; Ritchie R. O. Nanoscale Origins of the Damage Tolerance of the High-Entropy Alloy CrMnFeCoNi. Nat. Commun. 2015, 6, 10143. 10.1038/ncomms10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadeniz B.; Howarth A. J.; Stolar T.; Islamoglu T.; Dejanović I.; Tireli M.; Wasson M. C.; Moon S.-Y.; Farha O. K.; Friščić T.; Užarević K. Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal–Organic Frameworks by Water-Assisted Mechanochemistry. ACS Sustain. Chem. Eng. 2018, 6, 15841–15849. 10.1021/acssuschemeng.8b04458. [DOI] [Google Scholar]
- Ray P. C.; Yu H.; Fu P. P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S. K.; Vasu V. Synthesis and Characterization of Copper Nanoparticles, Using Combination of Two Different Sizes of Balls in Wet Ball Milling. InternationaJournal of Emerging Trend in Science Technology 2016, 3, 2348–9480. 10.18535/ijetst/v3i04.07. [DOI] [Google Scholar]
- Musza K.; Szabados M.; Ádám A. A.; Kónya Z.; Kukovecz Á.; Sipos P.; Pálinkó I. Mechanochemically Modified Hydrazine Reduction Method for the Synthesis of Nickel Nanoparticles and Their Catalytic Activities in the Suzuki–Miyaura Cross-Coupling Reaction. React. Kinet. Mech. Catal. 2019, 126, 857–868. 10.1007/s11144-018-1509-7. [DOI] [Google Scholar]
- Muñoz J. E.; Cervantes J.; Esparza R.; Rosas G. Iron Nanoparticles Produced by High-Energy Ball Milling. J. Nanoparticle Res. 2007, 9, 945–950. 10.1007/s11051-007-9226-6. [DOI] [Google Scholar]
- Tung D. K.; Manh D. H.; Phong L. T. H.; Nam P. H.; Nam D. N. H.; Anh N. T. N.; Nong H. T. T.; Phan M. H.; Phuc N. X. Iron Nanoparticles Fabricated by High-Energy Ball Milling for Magnetic Hyperthermia. J. Electron. Mater. 2016, 45, 2644–2650. 10.1007/s11664-016-4457-x. [DOI] [Google Scholar]
- Kumar N.; Biswas K.; Gupta R. K. Green Synthesis of Ag Nanoparticles in Large Quantity by Cryomilling. RSC Adv. 2016, 6, 111380–111388. 10.1039/C6RA23120A. [DOI] [Google Scholar]
- Rak M. J.; Friščić T.; Moores A. One-Step, Solvent-Free Mechanosynthesis of Silver Nanoparticle-Infused Lignin Composites for Use as Highly Active Multidrug Resistant Antibacterial Filters. RSC Adv. 2016, 6, 58365–58370. 10.1039/C6RA03711A. [DOI] [Google Scholar]
- Tavakoli M. M.; Tress W.; Milić J. V.; Kubicki D.; Emsley L.; Grätzel M. Addition of Adamantylammonium Iodide to Hole Transport Layers Enables Highly Efficient and Electroluminescent Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 3310–3320. 10.1039/C8EE02404A. [DOI] [Google Scholar]
- Liu T.; Zong Y.; Zhou Y.; Yang M.; Li Z.; Game O. S.; Zhu K.; Zhu R.; Gong Q.; Padture N. P. High-Performance Formamidinium-Based Perovskite Solar Cells via Microstructure-Mediated δ-to-α Phase Transformation. Chem. Mater. 2017, 29, 3246–3250. 10.1021/acs.chemmater.7b00523. [DOI] [Google Scholar]
- Lozano G. The Role of Metal Halide Perovskites in Next-Generation Lighting Devices. J. Phys. Chem. Lett. 2018, 9, 3987–3997. 10.1021/acs.jpclett.8b01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidikoudi M.; Fresta E.; Costa R. D. White Perovskite Based Lighting Devices. Chem. Commun. 2018, 54, 8150–8169. 10.1039/C8CC03166E. [DOI] [PubMed] [Google Scholar]
- Wang H.; Kim D. H. Perovskite-Based Photodetectors: Materials and Devices. Chem. Soc. Rev. 2017, 46, 5204–5236. 10.1039/C6CS00896H. [DOI] [PubMed] [Google Scholar]
- Deng H.; Yang X.; Dong D.; Li B.; Yang D.; Yuan S.; Qiao K.; Cheng Y.-B.; Tang J.; Song H. Flexible and Semitransparent Organolead Triiodide Perovskite Network Photodetector Arrays with High Stability. Nano Lett. 2015, 15, 7963–7969. 10.1021/acs.nanolett.5b03061. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Su R.; Du W.; Liu X.; Zhao L.; Ha S. T.; Xiong Q. Advances in Small Perovskite-Based Lasers. Small Methods 2017, 1, 1700163 10.1002/smtd.201700163. [DOI] [Google Scholar]
- Veldhuis S. A.; Boix P. P.; Yantara N.; Li M.; Sum T. C.; Mathews N.; Mhaisalkar S. G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. 10.1002/adma.201600669. [DOI] [PubMed] [Google Scholar]
- Leijtens T.; Bush K. A.; Prasanna R.; McGehee M. D. Opportunities and Challenges for Tandem Solar Cells Using Metal Halide Perovskite Semiconductors. Nature Energy 2018, 3, 828–838. 10.1038/s41560-018-0190-4. [DOI] [Google Scholar]
- Khatun S.; Maiti A.; Pal A. J. Bowing of Transport Gap in Hybrid Halide Perovskite Alloys (CH3NH3Sn1– x Pb x I3): Which Band Is Responsible?. Appl. Phys. Lett. 2020, 116, 012104 10.1063/1.5134749. [DOI] [Google Scholar]
- Ono L. K.; Juarez-Perez E. J.; Qi Y. Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. 10.1021/acsami.7b06001. [DOI] [PubMed] [Google Scholar]
- Prochowicz D.; Yadav P.; Saliba M.; Saski M.; Zakeeruddin S. M.; Lewiński J.; Grätzel M. Mechanosynthesis of Pure Phase Mixed-Cation MA x FA 1–x PbI 3 Hybrid Perovskites: Photovoltaic Performance and Electrochemical Properties. Sustain. Energy Fuels 2017, 1, 689–693. 10.1039/C7SE00094D. [DOI] [Google Scholar]
- Saski M.; Prochowicz D.; Marynowski W.; Lewiński J. Mechanosynthesis, Optical, and Morphological Properties of MA, FA, Cs-SnX 3 (X = I, Br) and Phase-Pure Mixed-Halide MASnI x Br 3–x Perovskites. Eur. J. Inorg. Chem. 2019, 2019, 2680–2684. 10.1002/ejic.201801506. [DOI] [Google Scholar]
- Protesescu L.; Yakunin S.; Nazarenko O.; Dirin D. N.; Kovalenko M. V. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. 10.1021/acsanm.8b00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey N.; Kaal S.; Sebastia-Luna P.; Birkhölzer Y. A.; Ledinsky M.; Palazon F.; Bolink H. J.; Morales-Masis M. Pulsed Laser Deposition of Cs 2 AgBiBr 6 : From Mechanochemically Synthesized Powders to Dry, Single-Step Deposition. Chem. Mater. 2021, 33, 7417–7422. 10.1021/acs.chemmater.1c02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ajjouri Y.; Locardi F.; Gélvez-Rueda M. C.; Prato M.; Sessolo M.; Ferretti M.; Grozema F. C.; Palazon F.; Bolink H. J. Mechanochemical Synthesis of Sn(II) and Sn(IV) Iodide Perovskites and Study of Their Structural, Chemical, Thermal, Optical, and Electrical Properties. Energy Technol. 2020, 8, 1900788 10.1002/ente.201900788. [DOI] [Google Scholar]
- Martínez-Sarti L.; Palazon F.; Sessolo M.; Bolink H. J. Dry Mechanochemical Synthesis of Highly Luminescent, Blue and Green Hybrid Perovskite Solids. Adv. Opt. Mater. 2020, 8, 1901494 10.1002/adom.201901494. [DOI] [Google Scholar]
- Palazon F.; El Ajjouri Y.; Sebastia-Luna P.; Lauciello S.; Manna L.; Bolink H. J. Mechanochemical Synthesis of Inorganic Halide Perovskites: Evolution of Phase-Purity, Morphology, and Photoluminescence. J. Mater. Chem. C 2019, 7, 11406–11410. 10.1039/C9TC03778K. [DOI] [Google Scholar]
- Prochowicz D.; Yadav P.; Saliba M.; Saski M.; Zakeeruddin S. M.; Lewiński J.; Grätzel M. Reduction in the Interfacial Trap Density of Mechanochemically Synthesized MAPbI 3. ACS Appl. Mater. Interfaces 2017, 9, 28418–28425. 10.1021/acsami.7b06788. [DOI] [PubMed] [Google Scholar]
- Karmakar A.; Askar A. M.; Bernard G. M.; Terskikh V. V.; Ha M.; Patel S.; Shankar K.; Michaelis V. K. Mechanochemical Synthesis of Methylammonium Lead Mixed–Halide Perovskites: Unraveling the Solid-Solution Behavior Using Solid-State NMR. Chem. Mater. 2018, 30, 2309–2321. 10.1021/acs.chemmater.7b05209. [DOI] [Google Scholar]
- Chen D.; Chen X.; Wan Z.; Fang G. Full-Spectral Fine-Tuning Visible Emissions from Cation Hybrid Cs 1–m FA m PbX 3 (X = Cl, Br, and I, 0 ≤ m ≤ 1) Quantum Dots. ACS Appl. Mater. Interfaces 2017, 9, 20671–20678. 10.1021/acsami.7b05429. [DOI] [PubMed] [Google Scholar]
- Chen D.; Li J.; Chen X.; Chen J.; Zhong J. Grinding Synthesis of APbX3 (A = MA, FA, Cs; X = Cl, Br, I) Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 10059–10067. 10.1021/acsami.8b19002. [DOI] [PubMed] [Google Scholar]
- Leupold N.; Schötz K.; Cacovich S.; Bauer I.; Schultz M.; Daubinger M.; Kaiser L.; Rebai A.; Rousset J.; Köhler A.; Schulz P.; Moos R.; Panzer F. High Versatility and Stability of Mechanochemically Synthesized Halide Perovskite Powders for Optoelectronic Devices. ACS Appl. Mater. Interfaces 2019, 11, 30259–30268. 10.1021/acsami.9b09160. [DOI] [PubMed] [Google Scholar]
- Tan D.; García F. Main Group Mechanochemistry: From Curiosity to Established Protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. 10.1039/C7CS00813A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are available in the published article.