Abstract
Immunoglobulin-A nephropathy (IgAN) is the most common primary glomerulonephritis in the world, with up to 40% of patients progressing to end-stage kidney disease (ESKD) within 30 years of diagnosis. IgAN is characterized by elevated serum levels of galactose-deficient IgA1 (Gd-IgA1), which leads to immune complex formation and deposition in the glomerular mesangium, causing kidney injury. A diverse disease course and the long-term follow-up required for clinically relevant endpoints (e.g., ESKD) have been barriers to the development of novel therapies in IgAN. Disease management has focused on supportive care with inhibitors of the renin–angiotensin system and, more recently, sodium–glucose transporter inhibitors to control proteinuria. The recent acceptance of proteinuria as a surrogate endpoint by regulatory bodies and a better understanding of disease pathology have helped to initiate the development of several novel treatments. Subsequently, a targeted-release formulation of budesonide and a dual endothelin/angiotensin inhibitor (sparsentan) have received accelerated approval for patients with IgAN. However, additional therapies are needed to target the different pathogenic mechanisms and individualize patient care. Several compounds currently under investigation target various effectors of pathology. There are promising clinical results from emerging compounds that target the generation of Gd-IgA1 by B cells, including inhibitors of A PRoliferation-Inducing Ligand (APRIL) and dual inhibitors of APRIL and B-cell activating factor (BAFF). Other investigational therapies target the complement cascade by inhibiting proteins of the lectin or alternative pathways. As the therapeutic landscape evolves, it will be important to revise treatment guidelines and develop updated standards of care.
Keywords: IgA nephropathy, IgA pathogenesis, therapeutics APRIL, Gd-IgA1, surrogate endpoints
Introduction
Immunoglobulin-A (IgA) nephropathy (IgAN) is the most common primary glomerulonephritis (GN) in the world and one of the leading causes of kidney failure.1
The diagnostic hallmark of IgAN is the predominance of IgA deposits, either alone or with IgG, IgM, or both, in the glomerular mesangium.2,3 The estimated incidence of IgAN is 0.2–5 per 100,000 people per year.1 Its prevalence varies geographically and across ethnic/racial groups and is the highest in Asia, where IgAN accounts for 45% of primary GN cases.4,5 By contrast, the prevalence is 6–8% of primary GN cases in the Middle East.6 In India, IgAN prevalence is 16.5% and is the most commonly diagnosed primary GN; presentation is often severe, characterized by tubulointerstitial fibrosis and sclerosis.7-9
The progression of IgAN following initial clinical presentation also varies by region and ethnic/racial groups, possibly due in part to variability in diagnosis timing, patient characteristics, and access to treatments.8 Even though it was regarded as a benign disease when first described by Jean Berger in 1968,10 IgAN can vary from slowly to rapidly progressive.1 The most common presentation of IgAN in adults is asymptomatic microscopic hematuria with or without proteinuria and/or progressive kidney disease,1,2 and asymptomatic glomerular IgA deposits have been reported in populations without clinical manifestations, including in 6.8% of 756 forensic necropsies in Finland, in 16.1% of 510 kidney donor biopsies in Japan, and in 11.8% of 5751 kidney biopsies in India.11-13 For a significant proportion of patients, the disease has a progressive clinical course that eventually leads to end-stage kidney disease (ESKD); up to 40% of patients develop ESKD within 30 years of diagnosis.14 Patients with IgAN also experience a 6-year reduction in median life expectancy and a 69.6% patient survival rate within 30 years of diagnosis.15 By contrast, among a cohort of Indian patients receiving optimized supportive care and systemic glucocorticoids over 3 years, approximately 40% displayed rapid progression to chronic kidney disease (CKD), and 37% experienced the composite outcome of ≥50% fall in estimated glomerular filtration rate (eGFR), eGFR <15 mL/min/1.73 m2, commencement of kidney replacement therapy, or death.8 This variation in prevalence and presentation could be driven partly by genetic and environmental factors, as well as regional differences in screening and biopsy practices1; IgAN can be detected early in countries with national kidney screening programs, such as Japan, South Korea, and Taiwan.1
In addition to considerable mortality and morbidity, patients with IgAN also experience decreased quality of life. Although there is only a minimal impact on the overall quality of life in the early stages of the disease, patients progressing to advanced stages of kidney impairment experience anxiety, depression, sexual dysfunction, insomnia, fatigue, and anorexia, among other sequelae.14
A significant challenge in treating patients with IgAN is the lack of efficacious therapies that target pathogenic mechanisms. Accurately predicting an individual’s risk of progression and how that individual may respond to treatment is also an unmet need.16 Despite these challenges, there have been promising advances in IgAN, including non-invasive biomarkers,17,18 proteomics,19,20 and the development of prediction models to stratify the risk of kidney disease progression.21-23 With the acceptance of proteinuria as a surrogate clinical endpoint,24 there has been renewed interest in trialing existing medications with relevant mechanisms of action and developing novel therapies that specifically target the pathology of IgAN. There has also been an increased understanding of the mechanisms underlying IgAN pathogenesis, including the identification of the “four-hit hypothesis,”1 the role of complement activation,25 and the identification of key cytokines,26 which has aided in informed exploration of novel treatments.
This in-depth review describes the pathogenesis of IgAN and current treatment guidance and explores how recently approved and emerging therapies targeting specific mechanisms of disease may provide individualized treatment options.
IgAN Pathogenesis
IgAN is understood to develop as a result of sequential pathogenic changes, known as the “four-hit hypothesis.”1 In brief, hit 1 is increased levels of circulating galactose-deficient IgA1 (Gd-IgA1); hit 2 is the synthesis of antiglycan autoantibodies targeting Gd-IgA1; hit 3 is binding of Gd-IgA1 to these autoantibodies, forming circulating immune complexes; and hit 4 is the deposition of these immune complexes in the glomerular mesangium and the subsequent activation of downstream inflammatory processes, including the complement cascade and the renin–angiotensin system (RAS), which ultimately leads to fibrosis and progressive decline in kidney function.1,27
Hit 1: Increase in circulating Gd-IgA1
Patients with IgAN have elevated levels of circulating IgA1 that is galactose-deficient in the hinge region of the heavy chains (Gd-IgA1) [Figure 1].28 There is significant heritability of Gd-IgA1, observed across a diverse range of racial groups.29,30 Aberrant IgA1 glycosylation precedes clinical signs of disease, and not all patients with elevated serum levels of Gd-IgA1 go on to exhibit overt disease.25,30 This indicates that a high level of Gd-IgA1 is an important but not the only pathogenic factor in IgAN.
Figure 1:
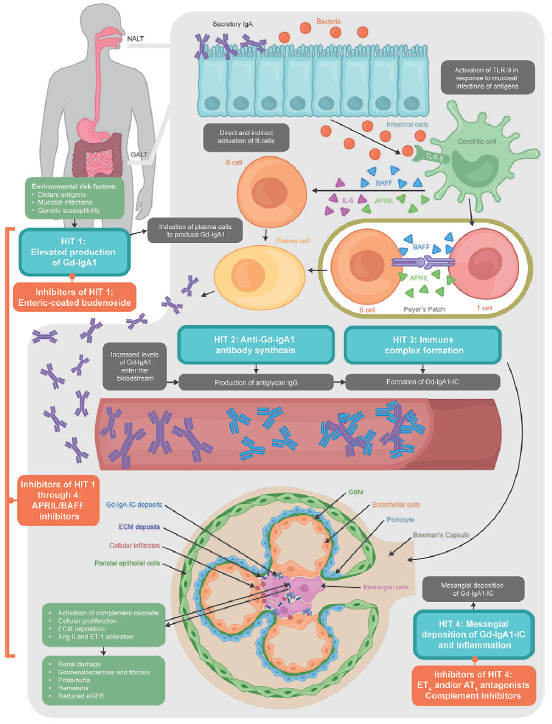
Pathogenesis of IgAN and emerging therapies. Ang II: angiotensin II; APRIL: a proliferation-inducing ligand; AT1: angiotensin II subtype 1; BAFF: B-cell activating factor; ECM: extracellular matrix; eGFR: estimated glomerular filtration rate; ET-1: endothelin 1; ETA: endothelin A; GALT: gut-associated lymphoid tissue; GBM: glomerular basement membrane; Gd-IgA1-IC: galactose-deficient-IgA1 immune complex; IgA: immunoglobulin A; IgG: immunoglobulin G; IL-6: interleukin 6; NALT: nasopharynx-associated lymphoid tissue; TLR-9: toll-like receptor 9.
The increased synthesis of IgA in plasma cells in patients with IgAN results from mucosal toll-like receptor 9 (TLR-9)-induced overexpression of A PRoliferation-Inducing Ligand (APRIL).26 TLR-9 also induces overexpression of B-cell activating factor (BAFF), which is thought to contribute to the synthesis of IgA in plasma cells.26 These B-cell growth factors are believed to promote T-cell independent B-cell activation and generation of Gd-IgA1, with APRIL thought to play a more dominant pathological role [Figure 1].26,31 Levels of serum APRIL protein, but not BAFF, are elevated in patients with IgAN compared with healthy controls and have been found to positively correlate with levels of proteinuria.32 Levels of APRIL and BAFF were shown to correlate with Gd-IgA1 production in a mouse model of IgAN, but only APRIL was associated with the formation of IgG-IgA immune complexes.26 In a study of IgAN recurrence after kidney transplant, APRIL but not BAFF levels were higher post-transplant in patients with recurrent disease.33 Furthermore, treating a grouped ddY [gddY] mouse, a spontaneous mouse model of IgAN, with an anti-BAFF monoclonal antibody (mAb) significantly decreased levels of serum IgA, IgG, and IgM compared with phosphate-buffered saline but did not affect urinary albumin excretion or serum levels of Gd-IgA1 and IgA-IgG immune complexes.34 This suggests that BAFF-dependent IgA production may not be pivotal to the pathogenesis of IgAN. As such, the role of BAFF in IgAN pathology remains unclear.26,32
IgA is mostly produced by mucosa-associated lymphoid tissue (MALT) along the mucosal surfaces, forming a selectively permeable barrier with the microbiota, namely gut-associated (GALT) and nasopharynx-associated lymphoid tissue (NALT).35 Antigens in the small intestine and tonsils are the most common sites of antigen-dependent priming of naïve B cells, via the actions of interleukins, transforming growth factor-β (TGF-β), BAFF, and APRIL.35 Therefore, it is theorized that mucosal immunity is a driver of the pathogenesis of IgAN and that genetic, environmental, and dietary factors could cause functional changes in the gut mucosal immune system, resulting in disease development and progression.35
Hit 2: Anti-Gd-IgA1 antibody synthesis
In the second hit, Gd-IgA1 is targeted by antiglycan autoantibodies, mostly of the IgG subtype. The hinge-region galactose-deficiency results in the exposure of terminal N-acetylgalactosamine (GalNAc) residues; serum IgG in patients with IgAN has been shown to have specificity for GalNAc, resulting in the binding of IgG antibodies to Gd-IgA1.36 Elevated levels of anti-Gd-IgA1 IgG correlate with proteinuria in patients with IgAN.36
Hit 3: Immune complex formation
In the third hit, these glycan-specific IgG antibodies form pathogenic immune complexes with Gd-IgA1 [Figure 1].36 In patients with IgAN, these immune complexes are relatively large (>800 kD) and may be incapable of entering the hepatic space of Disse in which circulating IgA1 would normally be broken down.25 Instead, the Gd-IgA1-antiglycan IgG immune complexes are deposited in the mesangium.37
Hit 4: Accumulation of immune complexes in the glomerular mesangium
Lastly, the fourth hit is the mesangial deposition of Gd-IgA1-antiglycan IgG immune complexes, leading to local inflammation, cell proliferation, and – when unchecked – glomerular and interstitial fibrosis [Figure 1].1,25,38
Gd-IgA1-containing complexes can activate the complement system via the alternative or lectin pathways, augmenting the inflammatory cascade [Figure 1].25 Both pathways result in the cleavage of C3 into C3a and C3b, leading to amplification of inflammation and, ultimately, kidney injury and loss of function.39 Components of the complement system have been found in the mesangial deposits of patients with IgAN, and studies indicate that complement activation may only occur via the alternative or lectin pathways at a ratio of approximately 3:1, but not both.40
Deposition of Gd-IgA1 also stimulates mesangial cells, promoting TGF-β synthesis via the activation of the RAS in podocytes.27 RAS has been historically associated with hypertension, but more recent studies show a role in the pathogenesis and continued pathology of IgAN. Endothelin-1 (ET-1), part of the RAS, activates endothelin A (ETA) receptors in podocytes and endothelial cells on the glomerular capillary wall, leading to podocyte dysfunction, vasoconstriction, kidney tubular injury, inflammation, and fibrosis.41,42 Angiotensin II (Ang II) – another part of the RAS – also increases the synthesis of TGF-β, leading to enhanced matrix formation and kidney fibrosis.43
Historic Approach to Treatment of IgAN
For years, it has been understood that the majority of adult patients undergoing treatment for IgAN have slowly progressive disease, characterized by mild-to-moderate proteinuria, persistent microhematuria, hypertension, and a gradual decline in GFR.44 However, recent data have shown more rapid progression than previously thought.45 A retrospective cohort study of a UK registry (enrolment from 2013 onward) showed that 50% of 2439 patients with IgAN experienced kidney failure or died after a median follow-up of 5.9 years, including 30% of patients at low risk of progression (proteinuria <0.88 g/g).45 These data underscore the need for treatments that alter the course of disease, preserve kidney function, and extend survival.
Long-term remission of proteinuria has been associated with a reduction in the risk of disease progression,45 and this relationship is consistent across demographic and clinical characteristics.46 In general, as recommended by the 2021 Kidney Disease Improving Global Outcomes (KDIGO) guidelines, the management of primary IgAN starts with optimized supportive care, such as controlling hypertension, and lifestyle recommendations, including a low-sodium diet.47
Patients with IgAN have higher blood pressure than matched healthy individuals, even when apparently normotensive.48 The 2021 KDIGO guidelines for the treatment of patients with IgAN recommend that all patients with proteinuria >0.5 g/day receive either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin-receptor blocker (ARB), but not both, to control hypertension [Table 1].47,49,50
Table 1:
Therapeutic options currently in use for the treatment of IgAN by their mechanism of action
| Treatment by mechanism of action | Examples | Indicated population | Populations not recommended |
|---|---|---|---|
| Hemodynamic therapies | |||
| RAS blockade (ACE inhibitors and ARB) | ACE inhibitors: Captopril, ramipril, lisinopril, benazepril ARB: Losartan, valsartan, irbesartan | All patients with proteinuria >0.5 g/day, irrespective of the presence of hypertension (no combination therapy of ACE inhibitors and ARB) | Not recommended in patients who have rapidly changing GFR |
| SGLT-2 inhibitor | Dapagliflozin, canagliflozin | Patients with CKD who are at risk of progression | Contraindicated in patients with eGFR <30 mL/min/1.73 m2 49,50 |
| Glucocorticoids | |||
| Untargeted glucocorticoids | Prednisone, methylprednisolone | Patients at high risk of progressive CKDa and eGFR ≥30 mL/min/1.73 m2 should be considered for a 6-month course and counseled on the risk of treatment-emergent toxicity | Not recommended in patients with eGFR <30 mL/min/1.73 m2 and patients at high risk of steroid-related toxicity, particularly patients with eGFR <50 mL/min/m2 |
| Other immunomodulatory therapies | |||
| MMF | Suggested as a steroid-sparing agent for Chinese patients receiving glucocorticoids | Not suggested for non-Chinese patients owing to no evidence for the efficacy of MMF monotherapy in randomized controlled trials | |
| Cyclophosphamide | Patients with rapidly progressive IgAN, in combination with glucocorticoids | Not recommended in non-rapidly-progressive IgAN | |
Unless otherwise stated, source: KDIGO 2021 guidelines.47 Proteinuria >0.75–1 g/day despite ≥3 months of supportive care with RAS blockade. ACE: angiotensin-converting-enzyme; ARB: angiotensin receptor blocker; CKD: chronic kidney disease; eGFR: estimated GFR; GFR: glomerular filtration rate; IgAN: Immunoglobulin A nephropathy; MMF: mycophenolate mofetil; RAS: renin-angiotensin system; SGLT-2: sodium-glucose cotransporter-2.
Patients at high risk of disease progression (proteinuria >1 g/day and GFR <50 mL/min/1.73 m2 despite ≥3 months of optimized supportive therapy including RAS blockade) may be considered for immunosuppressive treatment.47 The 2012 KDIGO guidelines on GN suggested a 6-month course of glucocorticoids for such patients.51 Based on evidence from subsequent studies that reported an increased risk of adverse events (AEs) with glucocorticoids, the 2021 guidelines were amended to suggest a more cautious approach. Patients who are at a high risk of progressive CKD despite maximal supportive care can be considered for a 6-month course of glucocorticoid therapy, but the important risk of treatment-emergent toxicity must be discussed with patients, particularly those with an eGFR <50 mL/min/1.73 m2.47 The 3-year, randomized STOP-IgAN trial in Germany compared the addition of immunosuppression (including methylprednisolone, prednisolone, cyclophosphamide, and azathioprine) with supportive care alone, and although patients in the immunosuppression group showed improved clinical outcomes based on proteinuria compared with the supportive-care group, patients in the immunosuppression group experienced more AEs, particularly infections of the gastrointestinal or respiratory tract, with one sepsis-related death.52 Similarly, the randomized, double-blind TESTING trial aimed to evaluate the efficacy and safety of methylprednisolone compared with placebo in patients with high-risk IgAN receiving supportive care; whereas methylprednisolone was efficacious compared with placebo, there were high rates of serious AEs (14.7% vs. 3.2%), including two fatal infections with methylprednisolone, which led to premature discontinuation of the trial.53 The study was recommenced 18 months later, as TESTING 2, following a reduced steroid-dose protocol: mean time-averaged proteinuria was lower in the reduced-dose methylprednisolone group than in the placebo group (1.6 vs. 2.4 g/day; P < 0.001).54 Results from both studies highlight the safety risks associated with steroid therapies.52,54
In line with these outcomes, the 2021 KDIGO guidelines only suggest a 6-month course of glucocorticoids in patients who remain at high risk of progressive CKD despite maximal supportive care and who are unwilling to participate in a clinical trial [Table 1].47 The guidelines also state that a detailed discussion of the risks and benefits should be undertaken first and that glucocorticoids should be avoided or administered with caution in patients with eGFR <30 mL/min/1.73 m2, diabetes, obesity (body mass index >30 kg/m2), latent infections, active peptic ulceration, uncontrolled psychiatric illness, severe osteoporosis, and other comorbidities. All patients with suspected IgAN should be assessed for secondary causes at diagnosis (e.g., cirrhosis, inflammatory bowel disease, infections, and autoimmune diseases) prior to treatment discussions.47,55 In patients for whom glucocorticoids are being considered, KDIGO suggests mycophenolate mofetil as an alternative for Chinese patients but notes that there is insufficient evidence to support its use in non-Chinese patients.47
Although immunosuppressive use is recommended only for patients classified as high risk of disease progression,47 emerging evidence suggests a substantial risk of progression in patients with urine protein-to-creatine ratio (UPCR) <0.88 g/g, who are traditionally regarded as being at low risk.45 In a retrospective cohort analysis, 30% of patients with UPCR 0.44–<0.88 g/g developed kidney failure within 10 years.45 Thus, there is a need for therapies to treat patients with IgAN who are not eligible for immunosuppressive treatment or are unable to achieve management goals with supportive care alone; in addition, there is a need for early interventions that may alter the course of the disease.
Our discussion thus far highlights the wealth of research into the pathology and management of IgAN; however, there remain some barriers to the development and approval of novel IgAN therapies. There is a lack of robust preclinical models that mimic human IgAN, particularly in the early stages of the disease.44 In addition, large-scale Phase 3 clinical trials to evaluate progression to ESKD as a primary endpoint require long-term follow-up owing to the rate of progression of IgAN and would be costly.24,56 In 2016, a partnership between the US Food and Drug Administration (FDA) and the American Society of Nephrology determined that due to its direct contribution to the decline in kidney function and its significant association with changes in kidney outcome, proteinuria reduction would be a reasonable surrogate endpoint for treatment effect on progression to ESKD in patients with IgAN.24 Proteinuria has since been used as the primary endpoint for trials in IgAN,56 but the progression rate of IgAN may still necessitate trials with relatively large patient populations and long follow-up to observe a treatment effect.24 Compounds granted accelerated approval on the basis of proteinuria results are still required to show benefit on eGFR decline to receive final approval.
To support the development of treatments for IgAN, the 2021 KDIGO guidelines recommend enrolment into clinical trials for patients with proteinuria >1 g/day despite ≥3 months of optimized supportive care.44,47
Emerging Therapies for IgAN
Two therapies have received accelerated approval for the treatment of patients with IgAN based on primary endpoints of proteinuria: enteric-coated, targeted-release budesonide, a glucocorticoid, and sparsentan, an ETA receptor antagonist.57,58 Targeted-release budesonide was designed to deliver glucocorticoid locally in the ileum to suppress mucosal B cells, which are responsible for the production of Gd-IgA1 [Figure 1].59 Based on its beneficial effect on UPCR, it was granted accelerated approval in 2021 for patients with IgAN in the Phase 3 NefIgArd study, supported by evidence from the Phase 2b NEFIGAN study.58,60,61 Initial results of the Phase 3 NefIgArd trial showed a 27% reduction in UPCR over 9 months with target-release budesonide compared with placebo (P = 0.0003) [Table 2].49,57,58,60,62-104 There were also encouraging preliminary eGFR results, with a significant 3.87 mL/min/1.73 m2 improvement in slope at 1 year with treatment compared with placebo (P = 0.0014); however, final eGFR data are awaited at the conclusion of the final part of the trial.60 Discontinuations due to treatment-emergent AEs (TEAE) were 9.3% in the targeted-release budesonide group compared with 1.0% in the placebo group.60 AEs related to steroid toxicity, including edema, hypertension, acne, and hirsutism, were more frequent in the budesonide group than in the control group.60 Although there was no difference in the rate of infections between the treatment arms, targeted-release budesonide should be avoided in patients at risk of infections and patients with tuberculosis or other existing infections (e.g., viral and fungal) because of its immunosuppressive mechanism of action.58,59
Table 2:
Emerging therapies for the treatment of IgAN by mechanism of action
| Compound | Mechanism of action | Route of administration and dose | Phase of development | Results (if available) |
|---|---|---|---|---|
| Hemodynamic therapies | ||||
| Sparsentan | Dual ETA/ AT1 receptor antagonist | 400 mg, oral, OD |
SPARTACUS, phase 2 open-label (Ongoing [not yet recruiting], NCT05856760)62 PROTECT, phase 3 RCT (Ongoing, NCT03762850)63,64 Accelerated approval for adults with IgAN granted by FDA (Feb 2023)a 57 |
UPCR: −49.8% at week 36 compared with −15.1% with irbesartan (n = 202 in both groups; P < 0.0001; PROTECT interim analysis)63 eGFR: −2.9 vs −3.9 mL/min/per 1.73 m2/ year in the sparsentan vs. irbesartan groups (n = 202 in both groups; 95% CI −0.03 to 1.94; p = 0.058) in 2-year total slope. eGFR total slope endpoint was not achieved.65 |
| Atrasentan | ETA antagonist | 0.75 mg, oral, OD |
AFFINITY, phase 2 open-label study (Ongoing, NCT04573920)66,67 ALIGN, phase 3 RCT (Ongoing; NCT04573478)68,69 |
UPCR: −43.6% at week 12 (n = 8; no comparator; AFFINITY interim analysis)67 |
| Dapagliflozin | SGLT-2 inhibitor | 10 mg, oral, OD | Approved for the treatment of CKD in patients at risk of progression with and without type 2 diabetes,49 but pre-specified analysis of a trial in CKD70 and a recent meta - analysis71 supports its use within the IgAN population |
UACR: −26% at month 36 compared with placebo (n = 137 vs. 133; P < 0.001; post-development CKD trial prespecified analysis)70 eGFR: −3.5 vs. −4.7 mL/min/1.73 m2/ year in the placebo group (n = 137 vs. 133; no difference; post-development CKD trial pre-specified analysis)70 |
| Empagliflozin | SGLT-2 inhibitor | 10 mg, oral, OD |
Not currently approved for use in non-diabetic kidney disease, but a recent meta-analysis71 supports its use within this population Part of an ongoing phase 3 trial in patients with CKD, where 12% of enrolled patients have IgAN72,73 |
eGFR: −2.16 in the empagliflozin group vs. −2.92 mL/min/1.73 m2 in the placebo group for total slope (n = 3304 vs. 3305, (95% CI: 0.54–0.96); exploratory endpoint)74 |
| Complement inhibitors | ||||
| Narsoplimab (OMS721) | MASP-2 inhibitor | 370 mg, IV, QW |
ARTEMIS-IgAN, phase 3 RCT (Ongoing; NCT03608033)75 Received FDA Breakthrough Therapy and Orphan Drug designations and EU Orphan Medical Product designation76 |
UPE: −18.0% vs. −18.4% in the vehicle-and narsoplimab-treated groups, respectively at week 12 (n = 5 vs. 4; phase 2 secondary endpoint)77 The primary endpoint of reduction in UPE from baseline compared to placebo at 36 weeks was not met and the program was terminated.78 |
| Iptacopan | Complement Factor B inhibitor | 200 mg, oral, BID |
Phase 2 study of safety and efficacy of LNP023 in patients with kidney disease caused by inflammation (Complete, NCT03373461)79-82 APPLAUSE-IgAN, phase 3 RCT (Ongoing, NCT04578834)39,83 |
UPCR: 23% reduction in 24h UPCR from baseline versus placebo at 3 months (n = 26 vs 25; 80% CI: 0.66-0.92).84 Clinically meaningful and statistically significant reduction in proteinuria with iptacopan versus placebo in APPLAUSE-IgAN interim analysis.85 |
|
Cemdisiran (ALN-CC5) |
Anti-complement C5 siRNA |
600 mg, SC, Q4W |
Phase 2 RCT in patients with IgAN (NCT03841448)86 |
UPCR: ≥50% reduction in 24 h UPCR in 31.8% of cemdisiran-treated patients at Week 32 versus 12.5% of placebo patients87 45.8% reduction in spot UPCR with cemdisiran versus placebo at Week 3287 |
| Compound | Mechanism of action | Route of administration and dose | Phase of development | Results (if available) |
| IONIS-FB-LRx (RO7434656) | siRNA against Complement Factor B | SC, Q4W |
Phase 2 open-label study (Ongoing, NCT04014335)88 IMAGINATION, phase 3 RCT (Ongoing, NCT05797610)89 |
UPCR: −44% at Week 29 (n = 10; no comparator; phase 2 primary endpoint)90 eGFR: no change from baseline to Week 29 (n = 10; no comparator; phase 2 secondary endpoint)90 |
| Glucocorticoids | ||||
| Targeted-release budesonide | Glucocorticoid receptor agonist | 16 mg, OD |
NefIgArd, phase 3 RCT (Ongoing, NCT03643965)60,91 Accelerated approval for adults with IgAN granted by FDA (2021)b 58 |
UPCR: −33.6% at month 9, placebo −5.2% at month 9 (n = 182 in both groups, no P value provided; NefIgArd co-primary endpoint)92 eGFR: +5.89 mL/min/1.73 m2 compared with placebo at month 24 (−6.11 vs. −12.00 mL/min/m2, n = 182 in both groups, no P value provided; NefIgArd co-primary endpoint)92 |
| B cell-targeting therapies | ||||
| Atacicept | APRIL/BAFF inhibitor | 150 mg, SC, QW | ORIGIN 3, phase 2b/3 RCT (Ongoing, NCT04716231)93 |
UPCR: −34% at week 24 compared with placebo (n = 27 vs. 29, P = 0.025, prespecified per-protocol analysis)94 eGFR: +5.8 mL/min/1.73 m2 absolute difference at week 36 between atacicept and placebo (n = 30 vs. 31, P = 0.038, intent-to-treat analysis)95 |
| Sibeprenlimab | Anti-APRIL mAb | 400 mg, SC, Q4W |
ENVISION, phase 2 RCT (Ongoing, NCT04287985)86,96,97 VISIONARY, phase 3 RCT (Ongoing, NCT05248646)98 |
UPCR: Reduction in 24h UPCR of 47.2%, 58.8%, 62.0% and 20.0% in the sibeprenlimab 2-mg/kg (n = 38), 4-mg/kg (n = 41), and 8-mg/kg (n = 38) groups and the placebo group (n = 38), respectively99 eGFR: Reduction in eGFR of −2.7, 0.2, −1.5, and −7.4 ml/min/1.73 m2 in the sibeprenlimab 2-mg/kg, 4-mg/kg, and 8-mg/kg groups and the placebo group, respectively99 |
| Zigakibart (BION-1301) | Anti-APRIL mAb | 600 mg, SC, Q2W |
Phase 1/2 RCT (Ongoing, NCT03945318)100 BEYOND, phase 3 RCT (Ongoing [not yet recruiting], NCT05852938)101 |
UPCR: −67% at week 52 (n = 8 from Cohort 1 of Phase 1/2 RCT, no P value provided, no comparator)102 |
| Telitacicept | APRIL/BAFF inhibitor | 240 mg, SC, QW or Q2W | Phase 3 RCT (Ongoing [not yet recruiting], NCT05799287)103 |
Proteinuria: −49% to Week 24 (n = 14; LS mean difference vs. placebo: −0.88 g/d, P = 0.013; phase 2 primary endpoint)104 eGFR: +2.34 vs. −5.70 mL/min/1.73 m2 in the placebo group (n = 14; P = 0.015; phase 2 secondary endpoint)104 |
Continued approval may be contingent upon confirmation of a clinical benefit in the ongoing Phase 3 PROTECT study; topline results from the 2-year confirmatory endpoints are expected in the fourth quarter of 2023 and are intended to support traditional approval.57,58 bContinued approval may be contingent on further clinical trials to verify and describe the full clinical benefit.58 APRIL: a proliferation-inducing ligand; AT1: angiotensin II subtype 1; BAFF: B-cell activating factor; BID: twice a day; CKD: chronic kidney disease; ETA: endothelin A; eGFR: estimated glomerular filtration rate; FDA: food and drug administration; IgAN: Immunoglobulin A nephropathy; mAb: monoclonal antibody; MASP-2: mannan-associated lectin-binding serine protease-2; OD: once a day; QW: once a week; Q4W: every 4 weeks; Q8W: every 8 weeks; RCT: randomised controlled trial; CI: confidence interval; SC: subcutaneous; SGLT-2: sodium glucose cotransporter-2; siRNA: short interfering RNA; UACR: urine albumin-to-creatinine ratio; UPCR: urine protein-to-creatinine ratio; UPE: urine protein excretion.
Whereas budesonide has immunosuppressive properties, sparsentan has hemodynamic and anti-fibrotic properties, selectively targeting two important pathways to inflammation and kidney damage following mesangial deposition of immune complexes: ET-1 and Ang II [Figure 1].105,106 Sparsentan was granted accelerated approval in early 2023 on the basis of interim analyses from the international, randomized, double-blind, active-controlled, Phase 3 PROTECT study that showed that once-daily sparsentan resulted in a meaningful reduction in proteinuria compared with the ARB irbesartan in adults with IgAN.57,63 The PROTECT study also reported that 88% of patients in the sparsentan group experienced TEAEs compared with 78% in the irbesartan group; the most common TEAEs were peripheral edema (14% vs. 9%), hypotension (14% vs. 6%), and dizziness (13% vs. 5%).63 Of note, the US prescribing information for sparsentan includes a boxed warning with regard to the risk of liver toxicity and embryo-fetal toxicity.106
In addition to the recent approvals of targeted-release budesonide and sparsentan, a spectrum of novel therapies for IgAN are under clinical development to target the various pathologic pathways. These include agents that reduce proteinuria and fibrosis and immunomodulatory agents that target mucosal immunity, the immune response, and the activation of the complement system [Table 2].
In a similar family to sparsentan, the ETA antagonist atrasentan is currently being studied in Phase 2 and 3 trials.66,68 Interim results from the Phase 2 trial, without a control comparator, indicate that atrasentan may help reduce proteinuria [Table 2].67
Another class of drugs with hemodynamic properties that are being trialed in patients with IgAN is sodium glucose cotransporter-2 (SGLT-2) inhibitors. SGLT-2 inhibitors are approved for use in patients with diabetes and CKD.40,50 Dapagliflozin and empagliflozin were studied as adjuncts to standard therapy in large-scale trials of patients with CKD, including a significant proportion with IgAN.70-74,107 In the DAPA-CKD trial [Table 2], dapagliflozin was associated with reduced risk of disease progression (decline in the eGFR ≥50%, ESKD, or renal- or cardiovascular-related death) compared with placebo in patients with CKD in the overall study population (N = 4304, hazard ratio [HR]: 0.61, 95% confidence interval [CI]: 0.51‒0.72, P < 0.001),107 and in a prespecified subgroup analysis of patients with IgAN (N = 270, HR: 0.29, 95% CI: 0.12–0.73, P = 0.005).70 Similarly, primary analysis of the EMPA-kidney trial (N = 6609) revealed that empagliflozin reduced the risk of CKD progression or cardiovascular death in patients with CKD at risk of progression.74 Prespecified exploratory analysis demonstrated a reduced rate of annual decline in patients treated with empagliflozin versus placebo, including in all key subgroups.74 In the IgAN subgroup (n = 817), the relative risk of CKD progression was 0.56 (95% CI: 0.36‒0.89) for empagliflozin versus placebo.71 Although both SGLT-2 inhibitors and ET receptor antagonists have been shown to reduce proteinuria via a hemodynamic mechanism of action, there is no evidence that they affect the underlying immune dysfunction in IgAN; thus, they are considered therapies for the stabilization of clinical parameters as needed.108-110
There are also a number of immunomodulatory therapies under development that target the immunopathology of IgAN. Molecular targets include cytokines involved in the increased production of Gd-IgA1, such as APRIL, and effectors of the complement system in the mesangium, such as complement factor B and mannan-associated lectin-binding serine protease-2 [Table 2]. Although the treatments discussed so far are administered orally, these other therapies may be oral or require either intravenous or subcutaneous administration. Route of administration can also be a driver in patient selection of therapeutic options and can impact compliance.
Upstream in the pathogenesis of IgAN, emerging therapies are targeting B-cell activation to prevent the generation of Gd-IgA1. This would likely reduce the production of pathogenic autoantibodies and the deposition of circulating immune complexes. Two inhibitors of APRIL are currently being studied in Phase 3 trials.98,101 As with any immunosuppressive treatment, the impact of these B-cell-targeting agents on the immune response to infections is clinically relevant. Evidence from a Phase 1 trial of sibeprenlimab, an anti-APRIL mAb, showed that its administration in healthy individuals did not interfere with a participant’s ability to mount a substantial antigen-specific response to tetanus and diphtheria toxoid vaccinations, despite an overall reduction in the respective immunoglobulins.111 Interim analysis from a Phase 2 trial of sibeprenlimab showed that COVID-specific vaccine responses and infection-induced immune responses were not affected by 12 months of sibeprenlimab infusions in patients with IgAN.112 At 12 months, the geometric mean ratio reduction (±SE) from baseline in the 24-hour UPCR was 47.2 ± 8.2%, 58.8 ± 6.1%, 62.0 ± 5.7%, and 20.0 ± 12.6% in the sibeprenlimab 2-mg/kg, 4-mg/kg, and 8-mg/kg groups and the placebo group, respectively [Table 2].99
Other B-cell-targeting therapies under investigation inhibit BAFF alone or as dual APRIL/BAFF inhibition. As previously mentioned, evidence from tonsil microbial exposures of patients with IgAN, IgAN recurrence after kidney transplant, and gddY mice call into question the role of BAFF in the pathology of IgAN.32,34 This may provide an explanation for why compounds that solely target BAFF have yet to publish any encouraging results, and a number of programs, such as blisibimod, have been discontinued.113 The effectiveness of anti-APRIL versus anti-APRIL/BAFF treatments is still to be determined. In addition, dual APRIL/BAFF inhibition has a potential risk of infection due to the impact on almost all phases of B-cell maturation and function compared with APRIL inhibition alone, which only inhibits the plasma cells. For example, telitacicept, a dual APRIL/BAFF inhibitor, has been shown in a study of patients with systemic lupus erythematosus to result in an almost 50% reduction in CD19+ B cells, which may potentially impact humoral immunity.114 Nevertheless, in a Phase 2 trial of patients with IgAN, telitacicept had a tolerable safety profile and was associated with a reduction in proteinuria compared with placebo.104
Complement inhibition is an attractive target based on the pathogenesis of IgAN. Activation of lectin or the alternative pathway by Gd-IgA1, resulting in the cleavage of C3 into C3b, is an important driver of inflammatory amplification and kidney injury in IgAN.115 Given that IgAN is usually a slowly progressive disease, the risk: benefit profile of complement inhibition needs to be considered. Importantly, complement inhibition is associated with an increased risk of infection owing to downstream effects that reduce the formation of the membrane attack complex (C5b-9).116 Patients considering inhibitors of the complement cascade in clinical trials require vaccinations against Neisseria meningitidis, Streptococcus pneumoniae, and Hemophilus influenzae.39,83,116,117
Although limited clinical data have been reported with complement inhibitors, initial efficacy and safety results appear promising. Iptacopan (LNP023) is an oral, selective, small molecule, factor B inhibitor that targets alternative pathway activation. In a Phase 2, randomized, dose-ranging study (NCT03373461), patients with IgAN who were treated with the recommended dose of iptacopan (200 mg bid, n = 26) had a 31% decrease in UPCR from baseline after 3 months of treatment compared with a 12% decrease for those receiving placebo (n = 23).79-81 This was sustained over the course of treatment, with a 41% (n = 11) versus 2% (n = 10) decrease in UPCR from baseline after 6 months of treatment; no serious safety signals or serious infections were 3 APPLAUSE-IgAN trial (NCT04578834), in which patients with IgAN are randomized 1:1 to iptacopan 200 mg twice daily or to placebo.39,83 Interim analysis revealed that the study met its primary endpoint, with iptacopan demonstrating a superior reduction in proteinuria versus placebo.84
The complement pathway is also being targeted with RNA interference (RNAi)-based therapies.118 The efficacy and safety of two distinct anti-complement short interfering (si) RNA therapies, cemdisiran and IONIS-FB-LRx, are currently being assessed in separate Phase 2 studies of patients with IgAN.86,88 Cemdisiran (ALN-CC5) is a double-stranded siRNA directed against the mRNA of C5 of the complement pathway. Initial analyses of a randomized Phase 2 trial (NCT03841448)86 demonstrated that 31.8% of patients with IgAN treated with cemdisiran had ≥50% reduction in 24-h UPCR at week 32 versus 12.5% of placebo-treated patients; cemdisiran was generally well-tolerated.87 Despite these encouraging data, further development of cemdisiran was paused in December 2022 while the company considers the pathway for further clinical development.119 IONIS-FB-LRx (RO-7434656) is an antisense oligonucleotide directed against the mRNA of complement factor B and is being evaluated in a small, single-arm open-label study of patients with IgAN (NCT04014335).88 Initial results (n = 10) showed a 44% reduction in proteinuria from baseline (24-h urine collection) at week 29, with an acceptable safety profile.90 A Phase 3 trial was recently initiated (IMAGINATION; NCT05797610) and is ongoing.89
An important consideration when targeting the complement cascade is that patients with IgAN exhibit overexpression of the alternative or the lectin pathway, but not both.40 Thus, complement activation is not seen universally in all patients. Future studies should identify patients who would benefit most from these therapies and avoid using such potent immunosuppressive therapies in patients for whom the complement system is not activated.
Beyond drug development
In addition to developing new therapies, it is important to understand the clinical profile of a patient and their risk of disease progression to inform treatment decisions. Studies have shown promising results from prediction models based on Cox proportional hazards or machine learning algorithms, including the use of urine protein, global sclerosis, tubular atrophy/interstitial fibrosis, and eGFR, as well as demographics such as age, sex, and race/ ethnicity.21-23 However, more attention is required to ensure that such algorithms do not exacerbate biases in the captured data on which they are trained.120 Currently, the International IgAN Prediction Tool is the preferred method in the 2021 KDIGO guidelines for predicting risk of progression (reduced kidney function) at the time of biopsy and considers eGFR, hypertension, proteinuria, and pathology among other factors; the model has been developed further to predict risk at 1 or 2 years post biopsy.23,47
Conclusion
In recent years, research into the pathogenesis of IgAN and the development of compounds that selectively target its pathology have improved the outlook for patients. Adoption of proteinuria as a surrogate endpoint has accelerated the development of treatments beyond supportive care. Nevertheless, there remain important unmet needs for IgAN such that clinical trial participation is a recommended step for patients who remain at high risk of kidney disease progression despite receiving optimal treatment with currently available options. As our understanding of IgAN continues to grow, several novel therapies have entered clinical development, targeting various pathological steps, and may offer individualized treatment. The approval of targeted-release budesonide and sparsentan are important treatment advances in IgAN, but long-term efficacy and safety data are needed, as well as treatments that target different pathogenic mechanisms. Emerging evidence suggests that UPCR ≥0.44 g/g is associated with an elevated risk of progression to ESKD, suggesting that lowering the recommendation for clinical trial involvement to 0.44 g/g could benefit patients at risk of progression. Duration of treatment remains unclear, although recent evidence suggests that chronic therapy (longer or even life-long treatment duration) may be needed to prevent disease recurrence or progression to ESKD.
There are several additional challenges we will need to consider as our treatment armamentarium expands. These include better stratification of patients by disease risk, the efficacy and safety of treatments across the various patient subgroups for personalized patient care, the potential for use of different treatments in combination, and the duration of treatment (continuous versus intermittent to address exacerbations and stabilize disease) and its impact on ESKD and survival. The risk: benefit ratio of novel immunomodulatory drugs should also be assessed, particularly in patients with slowly progressive disease. Finally, the cost-effectiveness of immunosuppressive agents needs to be weighed against the benefit of reduced CKD progression, including the associated patient benefits (life expectancy and quality of life) and healthcare costs.
Acknowledgements
The authors would like to thank Dr. Cibele Pinto, Ph.D., and Dr. Alexandria Henry, PharmD, RPh, for their contributions to this manuscript. Medical writing support was provided by Tamsin Brown, MSc, of Fishawack Communications Inc., part of Avalere Health, and funded by Otsuka.
Footnotes
How to cite this article: Mathur M, Sahay M, Pereira BJG, Rizk DV. State-of-Art Therapeutics in IgA Nephropathy. Indian J Nephrol. 2024;34:417-30. doi: 10.25259/ijn_319_23
Financial support and sponsorship
Editorial support was funded by Otsuka.
Conflicts of interest
DVR reports research funding from Reata Pharmaceuticals, Travere Therapeutics (Retrophin), Achillion Pharmaceuticals, Pfizer Pharmaceuticals, Calliditas Therapeutics (Pharmalinks), Otsuka Pharmaceuticals (Visterra), and Chinook Therapeutics; consulting fee from Novartis, George Clinical, Otsuka Pharmaceuticals (Visterra), Calliditas Therapeutics (Pharmalinks), Angion, and Chinook Therapeutics; and ownership in Reliant Glycosciences LLC. MM and BJGP are employees of Visterra Inc.
References
- 1.Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: Core curriculum 2021. Am J Kidney Dis. 2021;78:429–41. doi: 10.1053/j.ajkd.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–14. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez E, Carvaca-Fontán F, Luzardo L, Morales E, Alonso M, Praga M. A personalized update on IgA nephropathy: A new vision and new future challenges. Nephron. 2020;144:555–71. doi: 10.1159/000509997. [DOI] [PubMed] [Google Scholar]
- 4.Rajasekaran A, Julian BA, Rizk DV. IgA nephropathy: An interesting autoimmune kidney disease. Am J Med Sci. 2021;361:176–94. doi: 10.1016/j.amjms.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–3. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 6.Woo KT, Chan CM, Chin YM, Choong HL, Tan HK, Foo M, et al. Global evolutionary trend of the prevalence of primary glomerulonephritis over the past three decades. Nephron Clin Pract. 2010;116:c337–46. doi: 10.1159/000319594. [DOI] [PubMed] [Google Scholar]
- 7.Khairwa A. Indian scenario of IgA nephropathy: A systematic review and meta-analysis. Afr Health Sci. 2021;21:159–65. doi: 10.4314/ahs.v21i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander S, Varughese S, Franklin R, Rebekah G, Roy S, Yusuf S, et al. Three-year clinical outcomes of the first South Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2022;7:305–18. doi: 10.1016/j.ekir.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhat M, Sofi I, Sheikh R, Wani I. Incidence, demographic, biochemical, and clinicopathological profile of primary IgAN in a tertiary care center from Northern India. Egypt J Intern Med. 2022;34:1–6. doi: 10.1186/s43162-022-00109-9. doi: 10.1186/s43162-022-00109-9. [DOI] [Google Scholar]
- 10.Berger J, Hinglais N. [Intercapillary deposits of IgA-IgG] J Urol Nephrol (Paris) 1968;74:694–5. doi: 10.1681/ASN.V11101957. Les ddpots intercapillaires d’IgA-IgG. [DOI] [PubMed] [Google Scholar]
- 11.Varis J, Rantala I, Pasternack A, Oksa H, Jäntti M, Paunu ES, et al. Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol. 1993;46:607–10. doi: 10.1136/jcp.46.7.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286–94. doi: 10.1046/j.1523-1755.63.6s.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Prasad N, Khurana M, Behera M, Yaccha M, Bhadauria D, Agarwal V, et al. Clinicopathologic manifestations of immunoglobulin a nephropathy in a Northern Indian Cohort: A mute assassin with delayed diagnosis. Indian J Nephrol. 2023;33:12–21. doi: 10.4103/ijn.ijn_351_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. doi: 10.1038/nrdp.2016.1. doi: 10.1038/ nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 15.Jarrick S, Lundberg S, Welander A, Carrero JJ, Höijer J, Bottai M, et al. Mortality in IgA nephropathy: A nationwide population-based cohort study. J Am Soc Nephrol. 2019;30:866–76. doi: 10.1681/ASN.2018101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvaskandan H, Cheung CK, Muto M, Barratt J. New strategies and perspectives on managing IgA nephropathy. Clin Exp Nephrol. 2019;23:577–88. doi: 10.1007/s10157-019-01700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H. Biomarkers for IgA nephropathy on the basis of multi-hit pathogenesis. Clin Exp Nephrol. 2019;23:26–31. doi: 10.1007/s10157-018-1582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvaskandan H, Pawluczyk I, Barratt J. MicroRNAs: A new avenue to understand, investigate and treat immunoglobulin A nephropathy? Clin Kidney J. 2018;11:29–37. doi: 10.1093/ckj/sfx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julian BA, Suzuki H, Spasovski G, Suzuki Y, Tomino Y, Novak J. Application of proteomic analysis to renal disease in the clinic. Proteomics Clin Appl. 2009;3:1023–28. doi: 10.1002/prca.200800244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudnicki M, Siwy J, Wendt R, Lipphardt M, Koziolek MJ, Maixnerova D, et al. Urine proteomics for prediction of disease progression in patients with IgA nephropathy. Nephrol Dial Transplant. 2021;37:42–52. doi: 10.1093/ndt/gfaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Li X, Li Y, Xia E, Qin Y, Liang S, et al. Prediction and risk stratification of kidney outcomes in IgA nephropathy. Am J Kidney Dis. 2019;74:300–9. doi: 10.1053/j.ajkd.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179:942–52. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, et al. Application of the international IgA nephropathy prediction tool one or two years post-biopsy. Kidney Int. 2022;102:160–72. doi: 10.1016/j.kint.2022.02.042. [DOI] [PubMed] [Google Scholar]
- 24.Thompson A, Carroll K, L AI, Floege J, Perkovic V, Boyer-Suavet S, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:469–81. doi: 10.2215/CJN.08600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makita Y, Suzuki H, Kano T, Takahata A, Julian BA, Novak J, et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int. 2020;97:340–49. doi: 10.1016/j.kint.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Liu X, Peng H, Tang Y, Tang H, Chen Z, et al. Mesangial cells stimulated by immunoglobin A1 from IgA nephropathy upregulates transforming growth factor-beta1 synthesis in podocytes via renin-angiotensin system activation. Arch Med Res. 2010;41:255–60. doi: 10.1016/j.arcmed.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–54. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 29.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–14. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin X, Ding J, Zhu L, Shi S, Jiang L, Zhao M, et al. Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant. 2009;24:3372–5. doi: 10.1093/ndt/gfp294. [DOI] [PubMed] [Google Scholar]
- 31.Muto M, Manfroi B, Suzuki H, Joh K, Nagai M, Wakai S, et al. Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgA nephropathy. J Am Soc Nephrol. 2017;28:1227–38. doi: 10.1681/ASN.2016050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Currie EG, Coburn B, Porfilio EA, Lam P, Rojas OL, Novak J, et al. Immunoglobulin A nephropathy is characterized by anticommensal humoral immune responses. JCI Insight. 2022;7:e141289. doi: 10.1172/jci.insight.141289. doi: 10.1172/jci.insight.141289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Penagos L, Benito-Hernández A, San Segundo D, Sango C, Azueta A, Gómez-Román J, et al. A proliferation-inducing ligand increase precedes IgA nephropathy recurrence in kidney transplant recipients. Clin Transplant. 2019;33:e13502. doi: 10.1111/ctr.13502. doi: 10.1111/ctr.13502. [DOI] [PubMed] [Google Scholar]
- 34.Kim JS, Suzuki H, Kano T, Fukao Y, Nakayama M, Suzuki Y. Anti-BAFF antibody is effective to inhibit the production of immunoglobulins, but not nephritogenic IgA in murine IgA nephropathy. Kidney Int Rep. 2022;7(2) doi: 10.1016/j.ekir.2022.01.421. Supplement, Abstract POS-399. doi: 10.1016/j.ekir.2022.01.421. [DOI] [Google Scholar]
- 35.Gesualdo L, Di Leo V, Coppo R. The mucosal immune system and IgA nephropathy. Semin Immunopathol. 2021;43:657–68. doi: 10.1007/s00281-021-00871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–77. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan el RM, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–53. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 38.Rizk DV, Saha MK, Hall S, Novak L, Brown R, Huang ZQ, et al. Glomerular immunodeposits of patients with IgA nephropathy are enriched for igg autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol. 2019;30:2017–26. doi: 10.1681/ASN.2018111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizk DV, Rovin BH, Zhang H, Kashihara N, Maes B, Trimarchi H, et al. Targeting the alternative complement pathway with iptacopan to treat IgA nephropathy: Design and rationale of the APPLAUSE-IgAN Study. Kidney Int Rep. 2023;8:968–79. doi: 10.1016/j.ekir.2023.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–34. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 41.De Miguel C, Speed JS, Kasztan M, Gohar EY, Pollock DM. Endothelin-1 and the kidney: New perspectives and recent findings. Curr Opin Nephrol Hypertens. 2016;25:35–41. doi: 10.1097/MNH.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daehn IS, Duffield JS. The glomerular filtration barrier: A structural target for novel kidney therapies. Nat Rev Drug Discov. 2021;20:770–88. doi: 10.1038/s41573-021-00242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ketteler M, Noble NA, Border WA. Increased expression of transforming growth factor-beta in renal disease. Curr Opin Nephrol Hypertens. 1994;3:446–52. doi: 10.1097/00041552-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Floege J, Rauen T, Tang SCW. Current treatment of IgA nephropathy. Semin Immunopathol. 2021;43:717–28. doi: 10.1007/s00281-021-00888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitcher D, Braddon F, Hendry B, Mercer A, Osmaston K, Saleem MA, et al. Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2023;18:727–38. doi: 10.2215/CJN.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canney M, Barbour SJ, Zheng Y, Coppo R, Zhang H, Liu ZH, et al. Quantifying duration of proteinuria remission and association with clinical outcome in IgA nephropathy. J Am Soc Nephrol. 2021;32:436–47. doi: 10.1681/ASN.2020030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–s276. doi: 10.1016/j.kint.2021.05.021. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–7. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.AstraZeneca FARXIGA prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s003lbl.pdf. [Last accessed on 2023 Jul 18].
- 50.Janssen Pharmaceuticals Inc INVOKANA prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204353s000lbl.pdf. [Last accessed on 2023 Jul 18].
- 51.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2:S139–S274. doi: 10.1038/kisup.2012. doi: 10.1038/kisup.2012. [DOI] [Google Scholar]
- 52.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–36. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 53.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA. 2017;318:432–42. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv J, Wong MG, Hladunewich MA, Jha V, Hooi LS, Monaghan H, et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA. 2022;327:1888–98. doi: 10.1001/jama.2022.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saha MK, Julian BA, Novak J, Rizk DV. Secondary IgA nephropathy. Kidney Int. 2018;94:674–81. doi: 10.1016/j.kint.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barratt J, Rovin B, Diva U, Mercer A, Komers R. Implementing the kidney health initiative surrogate efficacy endpoint in patients with IgA nephropathy (the PROTECT Trial) Kidney Int Rep. 2019;4:1633–37. doi: 10.1016/j.ekir.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Travere Therapeutics Inc Travere Therapeutics announces FDA accelerated approval of FILSPARIᵀᴹ (sparsentan), the first and only non-immunosuppressive therapy for the reduction of proteinuriain IgA nephropathy. Updated February 17, 2023. Available from: https://ir.travere.com/news-releases/news-release-details/travere-therapeutics-announces-fda-accelerated-approval?_gl=1*3kagf*_ga*NzYwODg5ODA0LjE2ODU5NzI4M zI.*_ga_B1C89VFQRS*MTY4NTk3MjgzMi4xLjAuMTY4NTk3MjgzO S41My4wLjA. [Last accessed on 2023 Jun 07].
- 58.US Food and Drug Administration FDA approves first drug to decrease urine protein in IgA nephropathy, a rare kidney disease. Available from: https://www.fda.gov/drugs/fda-approves-first-drug-decrease-urine-protein-iga-nephropathy-rare-kidney-disease. [Last accessed on 2023 Jun 07].
- 59.Calliditas Therapeutics TARPEYO prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215935s000lbl.pdf. [Last accessed on 2023 Jul 18].
- 60.Barratt J, Lafayette R, Kristensen J, Stone A, Cattran D, Floege J, et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023;103:391–402. doi: 10.1016/j.kint.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 61.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117–27. doi: 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
- 62.ClinicalTrials A study to investigate safety and effect of sparsentan in combination With SGLT2 inhibition in participants with IgAN (SPARTACUS) ClinicalTrials.gov identifier: NCT05856760. Available from: https://clinicaltrials.gov/ct2/show/NCT05856760. [Updated 2023 May 16. Last accessed on 2023 Jun 07].
- 63.Heerspink HJL, Radhakrishnan J, Alpers CE, Barratt J, Bieler S, Diva U, et al. Sparsentan in patients with IgA nephropathy: A prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet. 2023;401:1584–94. doi: 10.1016/S0140-6736(23)00569-X. [DOI] [PubMed] [Google Scholar]
- 64.ClinicalTrials A study of the effect and safety of sparsentan in the treatment of patients with IgA nephropathy (PROTECT) ClinicalTrials.gov identifier: NCT03762850. Available from: https://clinicaltrials.gov/ct2/show/NCT03762850. [Updated on 2023 Apr 27. Last accessed on 2023 Jun 07].
- 65.Rovin BH, Barratt J, Heerspink HJL, Alpers CE, Bieler S, Chae D-W, et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet. 2023;402:2077–90. doi: 10.1016/S0140-6736(23)02302-4. [DOI] [PubMed] [Google Scholar]
- 66.ClinicalTrials Atrasentan in patients With proteinuric glomerular diseases (AFFINITY) ClinicalTrials.gov identifier: NCT04573920. Updated May 1, 2023. Available from: https://www.clinicaltrials. gov/ct2/show/NCT04573920. [Last accessed on 2023 Jun 07].
- 67.Kim S-G, Vo N, Lee S-H, Ranganathan D, Inker L, El-Shahawy M, et al. Atrasentan for the treatment of IgA nephropathy: Interim results from the Affinity Study. Nephrol Dial Transplant. 2022;37(Suppl 3) doi: 10.1093/ndt/gfac107.004. Abstract FC052. doi: 10.1093/ndt/gfac107.004. [DOI] [Google Scholar]
- 68.ClinicalTrials Atrasentan in patients with IgA nephropathy (ALIGN) ClinicalTrials.gov identifier: NCT04573478. Available from: https://clinicaltrials.gov/ct2/show/NCT04573478. [Updated on 2023 May 30. Last accessed on 2023 Jun 07].
- 69.Lambers Heerspink H, Jardine M, Kohan DE, Lafayette R, Levin A, Liew A, et al. A phase 3, randomized, double-blind, placebo-controlled study of atrasentan in patients with IgA nephropathy -The Align Study. Kidney Int Rep. 2023;8(3) doi: 10.1016/j.ekir.2023.02.630. Suppl, Abstract WCN23-1085. doi: 10.1016/j.ekir.2023.02.630. [DOI] [Google Scholar]
- 70.Wheeler DC, Toto RD, Stefánsson BV, Jongs N, Chertow GM, Greene T, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215–24. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 71.Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–801. doi: 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ClinicalTrials EMPA-KIDNEY (The study of heart and kidney protection with empagliflozin) ClinicalTrials.gov identifier: NCT03594110. Available from: https://clinicaltrials.gov/ct2/show/NCT03594110. [Updated on 2023 May 16. Last accessed on 2023 Jun 07].
- 73.EMPA-KIDNEY Collaborative Group Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022;37:1317–29. doi: 10.1093/ndt/gfac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–27. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ClinicalTrials Study of the safety and efficacy of OMS721 in patients with immunoglobulin A (IgA) nephropathy. ClinicalTrials.gov identifier: NCT03608033. Available from: https://clinicaltrials.gov/ct2/show/NCT03608033. [Updated on 2019 May 30. Last accessed on 2023 Jun 07].
- 76. Omeros. Narsoplimab. Available from: https://www.omeros.com/narsoplimab/. [Last accessed on 2023 Jun 19].
- 77.Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int Rep. 2020;5:2032–41. doi: 10.1016/j.ekir.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Omeros Corporation provides update on interim analysis of ARTEMIS-IGAN Phase 3 trial of narsoplimab in IgA nephropathy. https://investor.omeros.com/news-releases/news-release-details/omeros-corporation-provides-update-interim-analysis-artemis-igan [Last accessed on 2023 Nov 14].]
- 79.Barratt J, Rovin BH, Zhang H, Rizk DV, Kashihara N, Maes BD, et al. Effect of iptacopan on proteinuria and complement biomarkers over time in IgA nephropathy. Kidney Int Rep. 2023;8 doi: 10.1016/j.ekir.2023.02.612. Abstract WCN23-0412. doi: 10.1016/j.ekir.2023.02.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barratt J, Rovin BH, Zhang H, Rizk DV, Kashihara N, Maes BD, et al. Effect of iptacopanon proteinuria and complement biomarkers over time in IgA nephropathy. Presented at: ISN World Congress of Nephrology 2023, Thailand. Available from: https://medcommshydhosting.com/CRM/Iptacopan/WCN/presentations/posters/WCN23-0412_Effect_of_iptacopan_on_ proteinuria_abstract.pdf. [Last accessed on 2023 Oct 10].
- 81.Barratt J, Rovin B, Zhang H, Kashihara N, Maes B, Rizk DV, et al. Efficacy and safety of iptacopan in IgA nephropahty: Results of a randomized double-blind placebo-controlled phase 2 study at 6 months. Kidney Int Rep. 2022;7(2) Suppl, Abstract POS-546. [Google Scholar]
- 82.ClinicalTrials Study of safety and efficacy of LNP023 in patients with kidney disease caused by inflammation. ClinicalTrials. gov identifier: NCT03373461. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT03373461. [Updated on 2023 Jun 30. Last accessed on 2023 Jul 14].
- 83.ClinicalTrials Study of efficacy and safety of LNP023 in primary IgA nephropathy patients (APPLAUSE-IgAN) ClinicalTrials.gov identifier: NCT04578834. Available from: https://clinicaltrials.gov/ct2/show/NCT04578834. [Updated on 2023 May 23. Last accessed on 2023 Jun 07].
- 84.Zhang H, Rizk DV, Perkovic V, Maes B, Kashihara N, Rovin B, et al. Targeting the alternative complement pathway with iptacopan to treat IgA nephropathy: Design and rationale of the APPLAUSE-IgAN Study. Kidney Int. 2023;8:968–79. doi: 10.1016/j.ekir.2023.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Novartis investigational iptacopan Phase III study demonstrates clinically meaningful and highly statistically significant proteinuria reduction in patients with IgA nephropathy (IgAN) Oct 02, 2023. Available from: https://www.novartis.com/dashboard/news/media-releases/novartis-investigational-iptacopan-phase-iii-study-demonstrates-clinically-meaningful-and-highly-statistically-significant-proteinuria-reduction-patients-iga-nephropathy-igan . [Last accessed on 2023 Oct 10].
- 86.ClinicalTrials A study of cemdisiran in adults with immunoglobulin A nephropathy (IgAN) Available from: https://clinicaltrials.gov/study/NCT03841448. [Updated 2023 Mar 21. Last accessed on 2023 Sep 25].
- 87.Barratt J, Yeo SC, Fernström A, Barbour SJ, Sperati SC, Villanueva R, et al. Results from the phase 2 study of cemdisiran in adult patients with IgA nephropathy. Presented at: European Meeting on Complement in Human Disease, 26 August 2022. Available from: https://capella.alnylam.com/wp-content/uploads/2022/08/Cemdisiran_EMCHD_Poster_FINAL.pdf. [Last accessed on 2023 Sep 25].
- 88.ClinicalTrials A study to evaluate the effectiveness and safety of IONIS-FB-LRx, an antisense inhibitor of complement factorB, in adult participants with primary IgA nephropathy. ClinicalTrials.gov identifier: NCT04014335. Available from: https://clinicaltrials.gov/ct2/show/NCT04014335. [Updated on 2022 Aug 12. Last accessed on Jun 07].
- 89.ClinicalTrials A study to evaluate the efficacy and safety of RO7434656 in participants with primary immunoglobulin A (IgA) nephropathy at high risk of progression (IMAGINATION) ClinicalTrials.gov identifier: NCT05797610. Available from: https://clinicaltrials.gov/ct2/show/NCT05797610. [Updated on 2023 May 17. Last accessed on 2023 Jun 07].
- 90.Barbour S, Hladunewich M, Irvine J, Makris A, Robson RA, Tan S-J, et al. An exploratory trial of an investigational RNA therapeutic, IONIS-FB-LRx, for treatment of IgA nephropathy. Presented at: ASN Kidney Week, 2022, Orlando, FL, USA. Available from: https://www.asn-online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3766973. [Last accessed on 2023 Oct 10].
- 91.ClinicalTrials Efficacy and safety of nefecon in patients with primary IgA (Immunoglobulin A) nephropathy (Nefigard) ClinicalTrials.gov identifier: NCT03643965. Available from: https://clinicaltrials.gov/ct2/show/NCT03643965. [Updated on 2019 Feb 24. Last accessed on 2023 Jun 09].
- 92.Lafayette R KJ, Stone A, Floege J, Tesar V, Trimarchi V, Zhang H, et al. Long-term renal benefit over 2 years with Nefecon verified: The NefiGard Phase 3 full trial results. Presented at: European Renal Association, 2023, Milan, Italy. Available from: https://www.calliditas.se/en/wp-content/uploads/sites/2/2023/06/Presentation-European-Renal-Association-ERA-Congress-2023.pdf. [Last accessed on 2023 Oct 10].
- 93.ClinicalTrials Atacicept in subjects with IgA nephropathy (ORIGIN 3). ClinicalTrials.gov identifier: NCT04716231. Available from: https://clinicaltrials.gov/ct2/show/NCT04716231. [Updated 2023 Jun 07. Last accessed on 2023 Jun 07].
- 94.Lafayette R, Maes B, Lin C, Barbour S, Phoon R, Kim S, et al. ORIGIN Trial: 24-week primary analysis of a randomized, double-blind, placebo-controlled phase 2b study of atacicept in patients with IgAN. Nephrol Dial Transplant. 2023;38 doi: 10.1093/ndt/gfad063a_3848. Abstract 3848, doi: 10.1093/ndt/gfad063a_3848. [DOI] [Google Scholar]
- 95.Lafayette R MB, Lin C, Barbour S, Phoon R, Kim SG, Tesar V, et al. 36-week efficacy & safety of atacicept 150 mg in the ORIGIN randomized, double-blind, placebo-controlled phase 2b study in IgAN and persistent proteinuria. Presented at: European Renal Association, 2023, Milan, Italy. Available from: https://veratx.com/wp-content/uploads/2023/06/ERA-presentation-LB-June-17-2023-IR-final.pdf. [Last accessed on 2023 Oct 10].
- 96.ClinicalTrials Safety and efficacy study of VIS649 for IgA Nephropathy. ClinicalTrials.gov identifier: NCT04287985. Available from: https://clinicaltrials.gov/ct2/show/NCT04287985. [Updated on 2023 Apr 28. Last accessed on 2023 Jun 07].
- 97.Chan DTM, Kanjanabuch T, Liew A, Mathur M, Yarbrough J, Wang X, et al. Interim biomarker analysis from a randomized, double-blind, placebo-controlled, phase 2 trial of sibeprenlimab (Vis649) in participants with immunoglobulin A nephropathy. Kidney Int Rep. 2023;8 doi: 10.1016/j.ekir.2023.02.169. Abstract WCN23-0684. doi: 10.1016/j.ekir.2023.02.169. [DOI] [Google Scholar]
- 98.ClinicalTrials Visionary Study: Phase 3 trial of sibeprenlimab in immunoglobulin A nephropathy (IgAN). ClinicalTrials.gov identifier: NCT05248646. Available from: https://clinicaltrials.gov/ct2/show/NCT05248646. [Updated on 2023 Mar 10. Last accessed on 2023 Jun 07].
- 99.Mathur M, Barratt J, Chacko B, Chan TM, Kooienga L, Oh K-H, et al. A phase 2 trial of sibeprenlimab in patients with IgA nephropathy. N Engl J Med. 2024;390:20–31. doi: 10.1056/NEJMoa2305635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.ClinicalTrials Safety and tolerability of BION-1301 in healthy volunteers and adults with IgA nephropathy (IgAN) ClinicalTrials.gov identifier: NCT03945318. Available from: https://clinicaltrials.gov/ct2/show/NCT03945318. [Updated on 2023 May 24. Last accessed on 2023 Jun 07].
- 101.ClinicalTrials A study of BION-1301 in adults with IgA nephropathy. ClinicalTrials.gov identifier: NCT05852938. Available from: https://clinicaltrials.gov/ct2/show/NCT05852938. [Updated on 2023 May 10. Last accessed on 2023 Jun 15].
- 102.Barratt J, Kooienga L, Agha I, Ruiz-Ramon P, Madan A, Thomas H, et al. Updated interim results of a phase 1/2 study of Bion-1301 in patients with Iga nephropathy. Nephrol Dial Transplant. 2023;38(Suppl 1) doi: 10.1093/ndt/gfad063c_4337. Abstract 4337. doi: 10.1093/ndt/ gfad063c_4337. [DOI] [Google Scholar]
- 103.ClinicalTrials A Study of telitacicept in patients with primary IgA nephropathy. ClinicalTrials.gov identifier: NCT05799287. Available from: https://clinicaltrials.gov/ct2/show/NCT05799287. [Updated on 2023 Apr 05. Last accessed on 2023 Jun 07].
- 104.Lv J, Liu L, Hao C, Li G, Fu P, Xing G, et al. Randomized phase 2 trial of telitacicept in patients with IgA nephropathy with persistent proteinuria. Kidney Int Rep. 2023;8:499–506. doi: 10.1016/j.ekir.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barratt J, Rovin B, Wong MG, Alpers CE, Bieler S, He P, et al. IgA nephropathy patient baseline characteristics in the sparsentan PROTECT Study. Kidney Int Rep. 2023;8:1043–56. doi: 10.1016/j.ekir.2023.02.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Travere Therapeutics FILSPARI prescribing information. Available from: https://travere.com/wp-content/uploads/2023/02/filspari-final-uspi-20230217.pdf. [Last accessed on 2023 Jul 18].
- 107.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 108.Vasquez-Rios G. SGLT2 inhibitors for the management of IgA nephropathy: A new therapeutic paradigm for an old entity? Kidney News. 2022;14:22. [Google Scholar]
- 109.Martínez-Díaz I, Martos N, Llorens-Cebrià C, Álvarez FJ, Bedard PW, Vergara A, et al. Endothelin receptor antagonists in kidney disease. Int J Mol Sci. 2023;24:3427. doi: 10.3390/ijms24043427. doi: 10.3390/ ijms24043427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kohan DE, Pollock DM. Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol. 2013;76:573–9. doi: 10.1111/bcp.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mathur M, Barratt J, Suzuki Y, Engler F, Pasetti MF, Yarbrough J, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of VIS649 (sibeprenlimab), an APRIL-neutralizing IgG(2) monoclonal antibody, in healthy volunteers. Kidney Int Rep. 2022;7:993–1003. doi: 10.1016/j.ekir.2022.01.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCafferty K, Follman K, Pasetti M, Schachter A, Mathur M, MA D, et al. Covid vaccine responses during sibeprenlimab treatment of IgA nephropathy (IgAN): An interim analysis. Nephrol Dial Transplant. 2023;38(Suppl 1) doi: 10.1093/ndt/gfad063a_3347. Abstract 3347. doi: 10.1093/ndt/gfad063a_3347. [DOI] [Google Scholar]
- 113.ClinicalTrials BRIGHT-SC: Blisibimod response in IgA nephropathy following at-home treatment by subcutaneous administration. ClinicalTrials.gov identifier: NCT02062684. Available from: https://clinicaltrials.gov/ct2/show/NCT02062684. [Updated on 2017 Sep 18. Last accessed on 2023 Jun 15].
- 114.Wang L, Li J, Xu D, Fang J, Vollenhoven RV, Zhang F. Efficacy and safety of telitacicept, a novel BLYS/APRIL dual inhibitor, in patients with systemic lupus erythematosus: A phase 3, randomized, placebo-conrtolled 52-week study. Ann Rheum Dis. 2023;82(Suppl 1) doi: 10.1136/annrheumdis-2023-eular.1727. Abstract OP0137. doi: 10.1136/annrheumdis-2023-eular.1727. [DOI] [Google Scholar]
- 115.Chiu YL, Lin WC, Shu KH, Fang YW, Chang FC, Chou YH, et al. Alternative complement pathway is activated and associated with galactose-deficient IgA(1) antibody in IgA nephropathy patients. Front Immunol. 2021;12:638309. doi: 10.3389/fimmu.2021.638309. doi: 10.3389/fimmu. 2021.638309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheung CK, Rajasekaran A, Barratt J, Rizk DV. An update on the current state of management and clinical trials for IgA nephropathy. J Clin Med. 2021;10:2493. doi: 10.3390/jcm10112493. doi: 10.3390/ jcm10112493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.ClinicalTrials Phase II study assessing safety and efficacy of APL-2 in glomerulopathies. ClinicalTrials.gov identifier: NCT03453619. Available from: https://clinicaltrials.gov/ct2/show/NCT03453619. [Updated on 2023 Apr 06. Last accessed on 2023 Jun 15].
- 118.Selvaskandan H, Pawluczyk I, Barratt J. Clinical application of microRNAs in glomerular diseases. Nephrol Dial Transplant. 2023;38:1375–84. doi: 10.1093/ndt/gfac230. [DOI] [PubMed] [Google Scholar]
- 119.Bayer M. Alnylam culls 3 programs, including nephropathy med that was slated for phase 3. Fierce Biotech 2022. Available from: https://www.fiercebiotech.com/biotech/alnylam-culls-three-programs-including-nephropathy-med-was-slated-ph-3. [Last accessed on 2023 Oct 10].
- 120.Nadkarni GN, Chaudhary K, Coca SG. Machine learning in glomerular diseases: Promise for precision medicine. Am J Kidney Dis. 2019;74:290–92. doi: 10.1053/j.ajkd.2019.04.011. [DOI] [PubMed] [Google Scholar]


