Abstract
Background:
Acute spinal cord infarction (SCI) is a rare ischemic vascular lesion. It is difficult to diagnose during the acute phase because the clinical features can vary widely, and the diffusion-weighted imaging of spinal cord magnetic resonance imaging (MRI) often fails to detect any obvious abnormality. The first aim of this study was to describe the SCI patients’ characteristics, evaluate the accuracy of its diagnosis tools and management, and then find the strength of the effect of spinal surgical decompression on the patient’s outcome.
Methods:
A PubMed keyword and Boolean search using (“spinal cord infarction” OR “spinal cord ischemia” AND diagnosis OR management OR outcome) returned 221,571 results by applying filters. We added 17,400 results from Google Scholar. Fourteen studies were included in the quantitative meta-analysis of mean differences.
Results:
The Time to Nadir was <6 h (56.1%), 6–12 h (30.7%), 12–72 h (5.4%), and more than 72 h (7.8%). The higher proportion of Owl’s eye findings in the MRI was reported at the cervical level (39.6%) and thoracic level (22.9%) P = 0.031. The T2DWI has a moderate accuracy (area under the curve = 0.835) in detecting the T2 hypersignal intensity (T2HSI) at the hyperacute time to NADIR (<6 h). The median modified Rankin Scale (mRS) at admission was 3 (2–3), and after a follow-up duration of 12 months (6–15.5), the median mRS was reported to be 1 (1–2). About 68.9% benefited from medical treatment and physiotherapy, whereas spinal surgical decompression was done in 22.8%. Seventy percent of the overall studies favor spinal surgical decompression, with the estimated average standardized mean difference between medical and surgical treatment being = 1.2083 (95% confidence interval: 1.0250–1.3917).
Conclusion:
The T2DWI has moderate accuracy in detecting the T2HSI at the hyperacute time (NADIR <6 h). Even though surgical decompression favored good outcomes, medical treatment with physiotherapy was mostly used for the management of SCI.
Keywords: Diagnosis, Management and outcome, Patient characteristics, Spinal cord infarction, Spinal cord ischemia

INTRODUCTION
Spinal cord infarction (SCI) is an uncommon but devastating neurological disease.[14] Previously considered a stroke by the American Heart Association’s Stroke Council in the 21st century, SCI accounts for 0.3–1% of all strokes.[14,31]
It usually has an abrupt onset presenting in its severe form.[16] Few vascular disorders mimic the hyper-acute clinical presentation of spontaneous SCI, including spinal cord compression secondary to a spinal epidural or intramedullary hematoma.[37] CIs are either spontaneous or iatrogenic, with the spontaneous form having diverse etiologies. It could come from aortic or cardioembolic diseases, systemic hypoperfusion, vasculitis, or idiopathic, with atherosclerosis remaining the most common etiology of SCI.[37]
Spinal magnetic resonance imaging (MRI) remained the gold standard of the diagnosis, but in the hyperacute stage, MRI has low sensitivity for the diagnosis, as well as diffusion-weighted imaging (DWI) sensitivity because of the small size of the spinal cord and imaging-related artifacts.[7] Angiographic studies are not commonly indicated with SCI unless there is a suspicion of a spinal dural arteriovenous fistula when congestive myelopathy is seen on the MRI.[4,38,39] Of note, the time to NADIR of symptoms reached within 12 h is defined as hyperacute presentation and seems strongly associated with diagnosis accuracy of SCI.[5,45]
The management of SCI can be medical or surgical based on multiple factors.[14] Furthermore, patient outcomes following SCI are better with early diagnosis, timely management, and prevention of associated medical complications from neurological disorders such as motor impairment and bladder disorders.[37] However, despite its devastating consequences when poorly treated, the rarity of SCI coupled with its varied etiology and presentation, there are no standard guidelines for treatment regarding this condition.[15,17]
This study aimed to describe the SCI characteristics and identify the accuracy of diagnostic tools, treatment modalities, and outcomes of patients who suffered from SCI. We also aim to illustrate the strength of the effect of spinal surgical decompression on the patient’s outcome.
METHODOLOGY
This is a systematic review and meta-analysis of the diagnosis, management, and outcomes of SCI. Our study followed the guidelines of the Cochrane Handbook for Systematic Reviews and Meta-analysis of Diagnostic Test Accuracy when conducting this review.[9] Our study adhered to the Preferred Reporting Items for Systematic Review and Meta-analyses 2020 statement: an updated guideline for reporting systematic reviews.[36]
Search strategy
A PubMed keyword and Boolean search using (“spinal cord infarction” OR “spinal cord ischemia” AND diagnosis OR management OR outcome) returned 221,571 results by applying Filters for Free full text, Clinical Trial, Multicenter Study, Observational Study, Randomized Controlled Trial, English, French, from 1990 to 2022. (https://pubmed.ncbi. nlm.nih.gov/?term=%22spinal+cord+infarction%22+ OR+%22spinal+cord+ischemia%22+AND+diagnosis +OR+management+OR+outcome&filter=simsearch2.ffrft& filter=pubt.clinicaltrial&filter=pubt.multicenter study&filter=pubt.observationalstudy&filter=pubt. randomizedcontrolledtrial&filter=dates.1990-2022&filter=lang.english&filter=lang.french). We further used (spinal cord stroke, ischemic injury of the spinal cord, medullary infarction, diagnosis, management, and outcome) on Google Scholar with Filters for 1990– 2022 to have 17400 results. (https://scholar.google. com/scholar?hl=en&as_sdt=0%2C5&as_ylo=1990&as_ yhi=2022&q=Spinal+cord+stroke%2C+ischemic+injury +of+the+spinal+cord%2C+medullary+infarction%2C+ diagnosis%2C+management +and+outcome&btnG=).
Study selection
All the articles from the search were exported to Zotero[47], where duplicates were identified and deleted. The de-duplicated search results were then exported into Rayyan for blinded and independent screening of articles.[27] The study selection process consisted of multiple steps and was guided by the inclusion and exclusion criteria. Studies of interest included those that discussed the diagnosis, management, and outcomes of SCI. We also included various study designs, including original articles, case reports, cohort studies, cross-sectional studies, case series, and trials. Only publications in English and French languages were considered. Editorials, reviews, opinion pieces, comments, letters, reviews, meta-analyses, and qualitative studies were excluded. Two reviewers independently screened the titles and abstracts of selected studies against the inclusion criteria. This was followed by a full-text screening to confirm the eligibility of articles to be extracted. Any disagreement between the reviewers’ decisions prompted further discussion, and if a disagreement persisted, a third reviewer (N.D.A.B. or Y.C.H.D.) resolved the conflict.
Data extraction
Data extraction was performed in two stages, a pilot extraction stage followed by a data extraction proper stage. The pilot stage consisted of having all the extractors go through the same 10 selected articles to extract data. This was to ensure that all data extractors could extract data accurately, ensure homogeneity in data reporting, and ensure the data collection sheet captured all relevant data from the included studies. Studies that met inclusion criteria after full text were extracted, summarized, and tabulated in an Excel pro forma sheet. We extracted data on the article title, original language of publication, journal title, year of publication, name of the first author, country of origin of the first author, the start date of participant recruitment in the study, the end date of participant recruitment in the study, study period, study design, site(s) of recruitment of participants, population size, participants demographics, patient clinical information on SCI, topography of the lesion, diagnosis tools for SCI, treatment modalities, and outcome of care.
Data analysis
Following data curation, the extracted data were transferred to Jamovi statistical software (version 2.3.18) for descriptive analyses. The measures of central tendency and spread were used to calculate pooled statistics, and frequencies and proportions reported categorical data. Variables about the two groups were compared with appropriate statistical tests to identify significant differences. Student’s t-test was used for normally distributed continuous data, and the Wilcoxon rank-sum test was used for nonparametric continuous data not meeting the normality assumption. The Pearson Chi-square test was used for categorical data, and the Fisher exact test was used for categorical data when the cells had an expected count of <5.
The analysis was carried out using the standardized mean difference as the outcome measure. A fixed-effects model was fitted to the data. We choose to evaluate the difference in SCI outcomes between medical treatment and surgical decompression groups. Treatment options are identical in the 14 studies included in this meta-analysis. We then assume that these studies have the same common true effect size. Therefore, the factors affecting the effect size are assumed to be the same across studies. These considerations led to the assumption that there is no heterogeneity (between-study differences in treatment effects), which anticipated the choice of the fixed-effect model. Studentized residuals and Cook’s distances are used to examine whether studies may be outliers and/or influential in the context of the model. Studies with a studentized residual larger than the 100 × (1–0.05/[2 × k])th percentile of a standard normal distribution are considered potential outliers (i.e., using a Bonferroni correction with two-sided alpha = 0.05 for k studies included in the meta-analysis). Studies with a Cook’s distance larger than the median plus 6 times the interquartile range of the Cook’s distances are considered to be influential. The rank correlation test and the regression test, using the standard error of the observed outcomes as predictors, are used to check for funnel plot asymmetry.
RESULTS
Characteristics of included studies
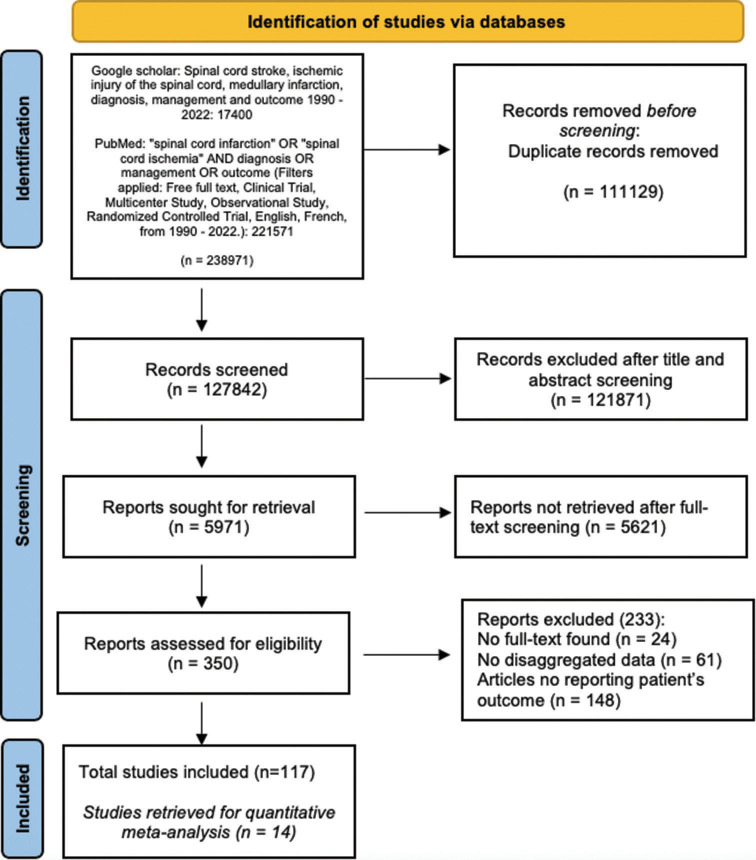
A total of 238,971 records were identified following the search on Google Scholar and PubMed. Following the exclusion of 111,129 de-duplicated records (46.5%), 121,871 (51%) articles were excluded at the title and abstract screening phase. 5621 (2.4%) more articles were removed at the full-text screening phase, and 117 (0.1%) articles were deemed eligible for inclusion and were extracted [Figure 1]. The articles consisted of 103 case reports (83 single case reports and 20 multiple case reports), and 14 were cohort, prospective, and retrospective studies, all constituting 876 patients. The included studies were published between 1990 and 2022, with the majority being in English (98.3%) and French (1.7%).
Figure 1:
Preferred Reporting Items for Systematic Review and Meta-analyses Flowchart for studies selection.
SCI patient’s characteristics
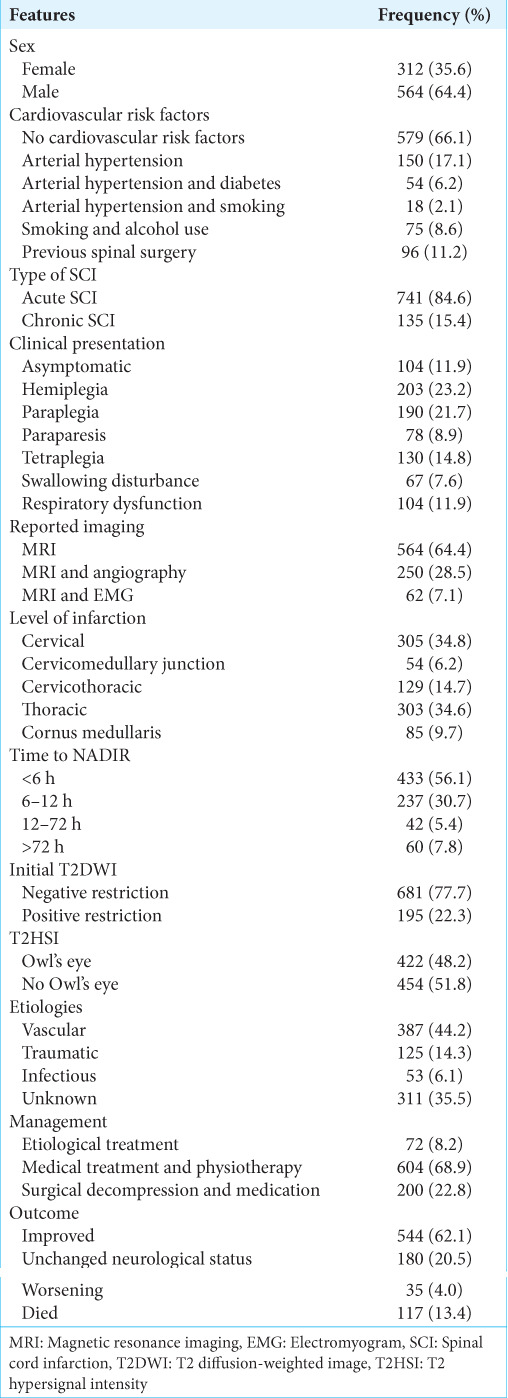
A total of 876 patients with SCI were included in this review. The mean age was 51.1 ± 19.4 years with a male predominance of 64.4% (n = 564). Most cases did not report any cardiovascular risk factor 66.1% (n = 579). However, arterial hypertension alone (n = 150, 17.1%) followed by arterial hypertension + diabetes (n = 54, 6.2%) were the most reported among patients with an underlying cardiovascular risk factor. Only 11.2% (n = 96) of patients with SCI reportedly had prior spine surgery, and 15.4% (n = 135) presented to the hospital more than 6 months after the onset of symptoms suggestive of SCI. Clinical presentation varied, and 11.9% (n = 104) of cases were asymptomatic. Others presented with hemiplegia (23.2%, n = 203), paraplegia (21.7%, n = 190), tetraplegia (14.8%, n = 130), respiratory dysfunction (11.9%, n = 104), swallowing dysfunction (7.6%, n = 67), and paraparesis (8.9%, n = 78) [Table 1].
Table 1:
Characteristics of patients with SCI.
Diagnosis of SCI
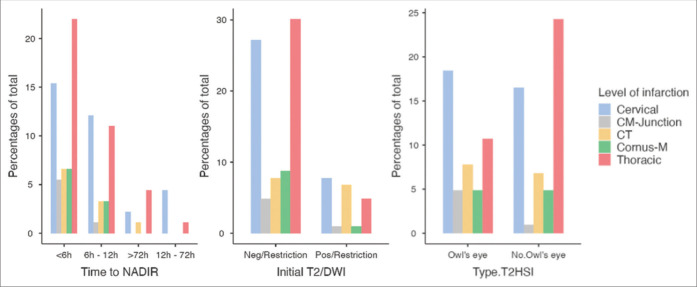
About the time from the onset of symptoms to NADIR, we considered the 772 symptomatic cases (876–104 asymptomatic). In most cases, the time to NADIR was <6 h (56.1%, n = 433), from 6 to 12h (30.7%, n = 237), from 12 h to 72 h (5.4%, n = 42), and more than 72 h (7.8%, n = 60). MRI alone was the most common imaging modality reportedly used in diagnosing SCI (n = 564, 64.4%) [Table 1]. The cervical (34.8%, n = 305) and thoracic (34.6%, n = 303) spinal cord levels were the most affected. The initial T2 diffusion weighted image (T2DWI) with diffusion extraction was positive in only 22.3% (n = 195) of cases with the pathognomonic T2 hypersignal intensity (T2HSI) Owl’s eye finding in 48.2% (n = 422). The hyperacute time to NADIR (<6 h) was the highest proportion at 38.8% (P < 0.001) in the group of patients with thoracic lesions, while the late time to NADIR (12–72 h) was higher when the lesions were at the cervical level at 81.0% (P < 0.001). It is worth mentioning the lower proportion (9.8%) of hyperacute time to NADIR (<6 h) when the lesion was at the cervico-medullary junction (P = 0.01) [Table 2]. Moreover, the initial positive T2 sequence with diffusion restriction was statistically significant (P = 0.015) higher in the proportion of 35.9%, 32.3%, and 22.6% when the lesion was at the cervical, cervicothoracic, and thoracic levels, respectively. The higher proportion of Owl’s eye findings in the MRI was reported to be at the cervical level (39.6%) and thoracic level (22.9%) (P < 0.001) [Figure 2].
Table 2:
Diagnosis of the SCI.
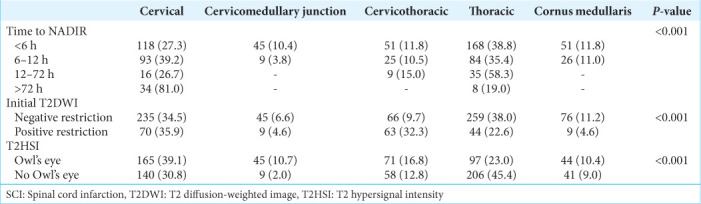
Figure 2:
Time to NADIR, initial T2DWI, and T2HSI by the level of the lesion. T2HSI: T2 hypersignal intensity, T2DWI:T2 diffusion weighted image.
Treatment modalities and outcomes of SCI
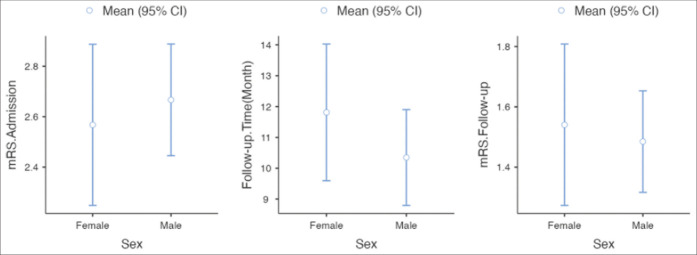
We found three main etiologies for SCI as follows: vascular 44.2% (n = 387), traumatic 14.3% (n = 125), and infectious 6.1% (n = 53). The etiology was unknown in 35.5% (n = 311) of cases. Only 72 cases (8.2%) reported etiological treatment of SCI. Of 68.9% (n = 604) benefited from medical treatment and physiotherapy, whereas spinal surgical decompression was done in 22.8% (n = 200) of cases [Table 1]. The median Modified Rankin Scale (mRS) at admission was 3 (2–3) and after a follow-up duration of 12 months (6–15.5), the median mRS was reported to be 1 (1–2). The mortality rate was 13.4% (n = 117). The variation of the mRS and the follow-up between males and females was not statistically significant (P = 0.184) [Figure 3].
Figure 3:
The outcome and follow-up duration of the spinal cord infarction. mRS: Modified Rankin scale.
Accuracy of the diagnosis tool
Through the binomial logistic regression, we assess the accuracy of the T2DWI with diffusion extraction for the detection of the T2HSI at the hyperacute time to NADIR (<6 h). The receiver operating characteristic (ROC) curve of Figure 4 shows an area under the curve (AUC) of 0.835 with a cutoff value set to 0.5. This AUC is between 0.75 and 0.85. This is the evidence that the T2DWI has moderate accuracy in detecting the T2HSI at the hyperacute time to NADIR (<6 h).
Figure 4:
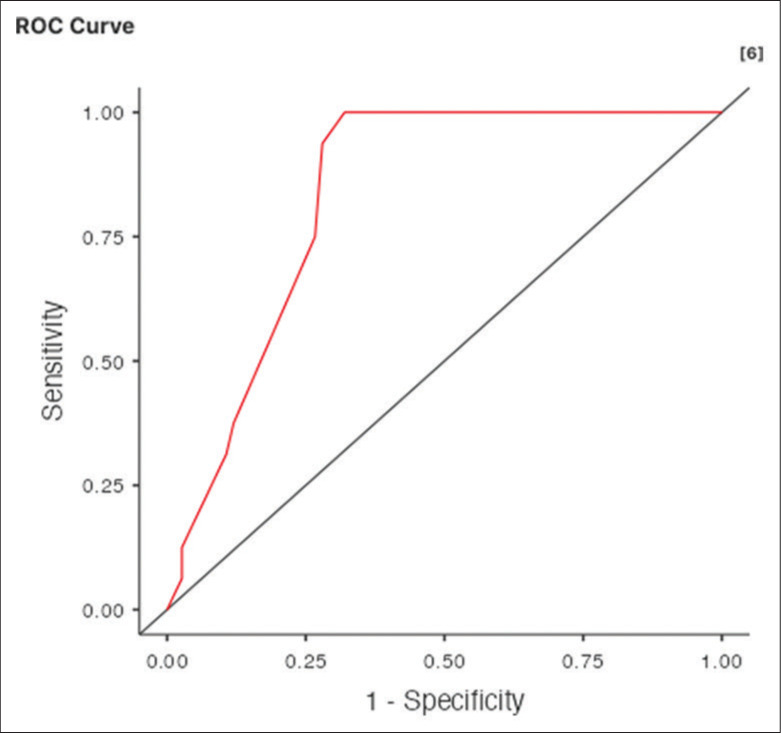
The binomial logistic regression for the accuracy of the MRI T2DWI for the detection of T2HSI at the hyperacute time to NADIR (<6 h). AUC = 0.835, the T2DWI has a moderate accuracy in detecting the T2HSI at the hyperacute time to NADIR (<6 h). MRI: Magnetic imaging imaging, T2DWI: T2 diffusion weighted image, T2HSI: T2 hypersignal intensity. AUC: Area under the curve
Medical treatment versus surgical decompression
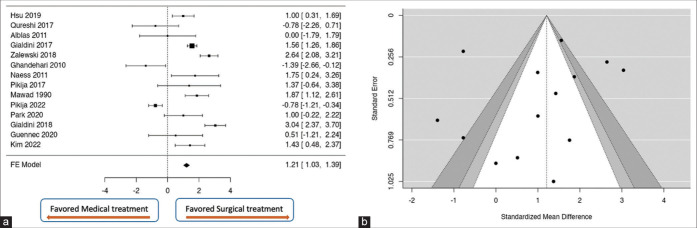
A total of k = 14 studies were included in the analysis. The observed standardized mean differences ranged from −1.3914 to 3.0379, with the majority of estimates being positive (71%). The estimated average standardized mean difference based on the fixed-effects model was 1.2083 (95% confidence interval [CI]: 1.0250–1.3917). Therefore, the average outcome differed significantly from zero (z = 12.9188, P < 0.0001). According to the Q-test, the true outcomes appear to be heterogeneous (Q[13] = 168.7521, P < 0.0001, I2 = 92.2964%). The overall pooled effect is located at the bottom left of Figure 5a. The forest plot did not cross the line of no effect, suggesting a statistically significant difference in the outcome between surgical and medical treatment of the SCI. Moreover, the majority of estimates are positive, about 71%. This tells us that 70% of the population of the overall studies favor spinal surgical decompression, with the estimated average standardized mean difference between medical and surgical treatment being 1.2083 (95% CI: 1.0250–1.3917) with z = 12.9188, P < 0.0001. Even though this result might not be clinically significant, the overall outcome does favor surgery over medical treatment for the management of SCI. Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (P = 0.2331 and P = 0.1396, respectively) [Figure 5b].
Figure 5:
(a) Forest plot comparing the mean differences of the effect of spinal surgical decompression as a treatment of SCI and the medical treatment. Zero is the line of no effect. The overall pooled effect did not cross the line of no effect, suggesting a statistically significant difference in the outcome between surgical and medical treatment of the SCI. More than 70% of the estimates are positive. Thus, the population of the overall studies favors spinal surgical decompression, with the estimated average standardized mean difference between medical and surgical treatment being = 1.2083 (95% CI: 1.0250–1.3917) with z = 12.9188, P < 0.0001. (b) Assessment of study bias with Funnel plots. There is no funnel plot asymmetry (Begg and Mazumdar Rank Correlation, P = 0.2331 and Egger’s Regression test, P = 0.1396). No study bias was found. SCI: Spinal cord infarction, CI: Confidence interval.
DISCUSSION
Key findings
The time from the onset of symptoms to NADIR was hyperacute in most cases, <6 h in 56.1% (n = 433), and was the highest (38.8%) when the lesions were at the thoracic level.
The initial positive T2 sequence with diffusion restriction was statistically significantly (P = 0.015) higher in the proportion of 35.9%, 32.3%, and 22.6% when the lesion was at the cervical, cervicothoracic, and thoracic level, respectively. The higher proportion of Owl’s eye findings in the MRI was reported to be at the cervical level (39.6%) and thoracic level (22.9%) P = 0.031. The T2DWI has a moderate accuracy (AUC = 0.835) in detecting the T2HSI at the hyperacute time to NADIR (<6 h)
Medical treatment and physiotherapy were done in 68.9% (n = 604), whereas spinal surgical decompression was done in 22.8% (n = 200) of cases. The median mRS at admission was 3 (2–3), and after a follow-up duration of 12 months (6–15.5), the median mRS was reported to be 1 (1–2). The death ratio was 13.6% (n = 14). Surgery allows 1.2083 times (95% CI: 1.0250–1.3917) faster recovery with a good prognosis compared to medical treatment alone.
Anatomy and physiological implications
While the dual PSAs supply the posterior third of the spinal cord, the ASA supplies the anterior two-thirds. Small penetrating vasocoronal arteries supply the circumference of the spinal cord, whereas the sulcal arteries originating from the ASA in the anterior fissure supply the central part of the ASA territory in varying numbers per segment at the cervical, thoracic, and lumbar levels.[13] The paired PSAs have frequent anastomoses on the spinal cord surface, supplying an anastomotic network of directly penetrating vessels. They run medially to the posterior roots. The amount of gray matter in a cross-section is directly proportional to the size of the area served by the central arteries. As a result, there is an intermediate area near the anterolateral part of the front horns and the central part of the dorsal horns, which are variably supplied by one system or the other. The central and peripheral areas are not clearly demarcated.[18,42] Neurological symptoms resulting from ischemia in the ASA territory include para- or tetraparesis, bladder dysfunction, bilateral dissociated sensory deficits, loss of temperature and pain perception below the infarct level, and bilateral lesions of the anterior horns, the anterolateral and crossing spinothalamic tracts, and the lateral corticospinal tracts tracks.[15] A neurological examination reveals variable temperatures of the limbs, although the vasoconstrictor tract in the lateral part of the spinal cord is also affected. Ipsilateral Horner syndrome can also be observed with a cervical infarct focus.[24] In the event of an infarction in the PSA area, light touch, the sense of vibration, and proprioception are impaired. In contrast to cerebral infarction, spinal cord ischemia is rare, and reliable epidemiological data are lacking.[10] The strong collateral vascular supply appears to be a factor contributing to the reduced incidence of medullary infarctions.[35]
Correlation with data in the literature
Similar to previous studies, our patients had an average age of about 50 years, which was lower than those who had suffered a cerebral infarction.[31] Furthermore, no vascular risk factors were reported in the majority of cases (66.1%). Furthermore, our patient’s neurological symptoms worsened gradually, unlike a stroke, in which neurological deficits occur suddenly. According to some authors, symptoms may not peak for 24 hours or, at most, 35–45 min. In addition, our analysis shows that 12.4% of patients arrived at the hospital more than 6 months after experiencing symptoms suggestive of spinal cord injury, with approximately 12% showing no symptoms. Pain was a common sign of SCI, presenting as spinal cord compression syndrome and radicular pain in 68.6% of cases, consistent with rates reported in the literature.[3,29] In the majority of patients, reflexes were absent or weak. This is explained by the fact that the assessment was carried out during the acute phase of the event. Symptoms of the acute phase include decreased or absent tendon reflexes and, in some cases, severe autonomic dysfunction. The acute phase can last up to 6 weeks. In fact, in our series, 11.9% of patients with cervical spine infarction suffered from severe hypotension and respiratory distress, requiring orotracheal intubation. Swallowing dysfunction was found in 7.6% of patients with upper cervical spine infarction. Up to 68% of cases of SCI cannot be clearly diagnosed due to the wide variation in etiology.[8,22] The most common causes are vertebral artery dissection, aneurysm rupture or dissection, and aortic surgery. Other less common causes include hypotension, cardioembolism, hypercoagulability, and fibrocartilaginous embolism.[1,21] In rare cases, sildenafil therapy can lead to a SCI.[12,33] Based on our study, we identified three primary etiologies: vascular (44.2%), traumatic (14.3%), and infectious (6.1%). However, the ultimate cause of medullary infarction remains unknown in almost 50% of patients.[12] Comparable to the results of Yuh et al.[44] in 35.5% of the cases in our study, the cause of the SCI was still unknown.
The best method for diagnosing a SCI is MRI. On T2-weighted sequences, most infarcts appear as pencil-like hyperintensities. On axial T2-weighted sequences, lesions affecting only gray matter exhibit an owl-eye pattern. On T1-weighted sequences, hyperintense lesions may be associated with hemorrhagic transformation in certain cases, while infarction of the adjacent vertebral body may be observed in other cases. In addition, gadolinium uptake could be observed.[6,26,46] However, a significant proportion of patients with SCI shows no changes on MRI, especially when the study is performed soon after the event. In these situations, diffusion-weighted sequences are more sensitive.[19,44] The pathognomonic T2HSI finding in the owl eye was limited on DWI/apparent diffusion coefficient maps in 48.2% of patients in our study who underwent DWI. In certain documented cases, changes appear within a few hours of symptoms appearing and disappear within a week. Due to flow artifacts, proximity to bone, longitudinal direction of white matter tracts, and curvature of the spinal cord, the quality of DWI sequences may not be optimal at the spinal cord level.[30,38,40] Although sensitivity results vary by series, they highlight the value of DWI in the early diagnostic process; therefore, this tool should be used whenever possible. Furthermore, our review shows that the sensitivity of DWI at the hyperacute time point to NADIR (<6 h) depends on the spinal location of the lesion. Patients with thoracic lesions had the highest proportion of this effect at 38.8% (P < 0.001), and the late time to NADIR (12–72 h) was higher at 81.0% (P < 0.001). Lesions were located at the cervical level. Owl’s MRI results showed that the cervical spine (39.6%) and thoracic spine (22.9%) had the highest percentage of ocular findings (P < 0.001). These results could be explained by the low degree of vascularization of the thoracic spinal cord. However, the high percentage (81.0%) of T2DWI findings at the late time point of NADIR could also be a direct consequence of the rich vascularization, as numerous anastomoses on the spinal cord surface supply a network of anastomotic vessels that penetrate directly into the cervical cord. The receiver operating characteristic curve of our study with a cutoff value of 0.5 shows an AUC of 0.835. This AUC is between 0 and 85 points. This shows that T2DWI can detect T2HSI at the hyperacute time point to NADIR (<6 h) with moderate accuracy.
Only autopsy studies can confirm the diagnosis of this rare cause of SCI; SCI should be suspected in young patients with a history of trauma, intense exercise, or Valsalva maneuver before the event; few or no vascular risk factors; and other causes.[25,43] There are currently no treatment recommendations. Some rare cases in the acute phase suggest intravenous fibrinolysis.[18,23,28,32,41] However, due to recruitment problems, the only clinical trial intended to provide data on this topic had to be stopped. Although this treatment has drawbacks, some studies support the use of lumbar CSF drainage in patients experiencing SCI following aortic surgery.[18,23] If clinically appropriate, some authors recommend initiating antiplatelet or anticoagulation therapy in remaining patients and reducing vascular risk factors.[20,34] Finally, although antiplatelet therapy for fibrocartilaginous embolism is controversial, some authors suggest it for patients with vascular risk factors. In our study, 22.8% of patients underwent surgical spinal decompression and started anticoagulation therapy (mainly in the group of patients with fibrocartilaginous embolism), whereas 68.9% of patients underwent medical treatment with physiotherapy at discharge. Increased morbidity and mortality rates are associated with SCI. According to certain studies, the prognosis of a SCI is better than that of a stroke.[2,11] The most important prognostic factor may be the intensity of neurological symptoms at baseline.[5,15,17,45] According to our review, the mean modified Rankin Scale (mRS) of the surgery group was 1 (1–2) (6–15.5) after 12 months of follow-up. The mean mRS at admission was 3 (2–3). The death rate was 13.4%.
Limitations
This study bears some limitations. Our review is based only on French or English language. Furthermore, not all the studies provided primary and long-term outcomes. In this review, most included papers reported a median follow-up duration of only 12 months. Meanwhile, long-term follow-up of 3 years or more could completely change the course of the reported outcome. Furthermore, there was no randomized controlled trial in our review. Such a high-quality study would have been preferable for this meta-analysis to highlight data discrepancies toward SCI guidelines. For these reasons, we systemically selected articles reporting identical treatment options to include in this meta-analysis. This makes our study the most current and informative in this field. In the future, we should increase our efforts toward interventional studies and nonrandomized/randomized controlled trials on SCI.
CONCLUSION
With the MRI T2DWI, the time to NADIR was short (<6 h) for SCI diagnosis. The T2DWI has moderate accuracy in detecting the T2HSI at the hyperacute time (NADIR <6 h). In the vast majority of cases, medical treatment with physiotherapy was the management of SCI. Surgical decompression with antiplatelet or anticoagulation therapy favored good outcomes. The presence of vascular risk factors, previous spinal surgery, and paraplegia at admission were the three factors associated with poor prognosis.
Footnotes
How to cite this article: Dokponou YC, Ontsi Obame FL, Takoutsing B, Mustapha MJ, Nyalundja AD, Saad MS, et al. Spinal cord infarction: A systematic review and meta-analysis of patient’s characteristics, diagnosis accuracy, management, and outcome. Surg Neurol Int. 2024;15:325. doi: 10.25259/SNI_477_2024
Contributor Information
Yao Christian Hugues Dokponou, Email: dokponou2407@gmail.com.
Fresnel Lutèce Ontsi Obame, Email: ontsiobamef1@gmail.com.
Berjo Takoutsing, Email: takoutsingberjo@gmail.com.
Mubarak Jolayemi Mustapha, Email: mustaphamubarakjolayemi@gmail.com.
Arsène Daniel Nyalundja, Email: nyalu_arse.adn@outlook.com.
Moussa Elmi Saad, Email: saad.elmi@yahoo.com.
Omar Boladji Adebayo Badirou, Email: bboladji@gmail.com.
Dognon Kossi François de Paule Adjiou, Email: adjioudkfp@gmail.com.
Nicaise Agada Kpègnon, Email: nicaiseagada4@gmail.com.
Alngar Djimrabeye, Email: drdjimrabeye@gmail.com.
Nourou Dine Adeniran Bankole, Email: bankolenouroudine@yahoo.fr.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Alcanyis-Alberola M, Giner-Pascual M, Salinas-Huertas S, Gutiérrez-Delgado M. Iatrogenic spinal cord injury: An observational study. Spinal Cord. 2011;49:1188–92. doi: 10.1038/sc.2011.72. [DOI] [PubMed] [Google Scholar]
- 2.Algahtani AY, Bamsallm M, Alghamdi KT, Alzahrani M, Ahmed J. Cervical spinal cord ischemic reperfusion injury: A comprehensive narrative review of the literature and case presentation. Cureus. 2022;14:e28715. doi: 10.7759/cureus.28715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: A review of translational advances in spinal cord injury. J Neurosurg. 2018;30:1–18. doi: 10.3171/2018.9.SPINE18682. [DOI] [PubMed] [Google Scholar]
- 4.Bankole ND, Janot K, Listrat A, Travers N, Maldonado IL, Velut S. Child pial arteriovenous fistula of the conus medullaris presenting with spinal cord venous congestion: Case report and literature review. Interdiscip Neurosurg. 2021;25:101128. [Google Scholar]
- 5.Barreras P, Fitzgerald KC, Mealy MA, Jimenez JA, Becker D, Newsome SD, et al. Clinical biomarkers differentiate myelitis from vascular and other causes of myelopathy. Neurology. 2018;90:e12–21. doi: 10.1212/WNL.0000000000004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng MY, Lyu RK, Chang YJ, Chen CM, Chen ST, Wai YY, et al. Concomitant spinal cord and vertebral body infarction is highly associated with aortic pathology: A clinical and magnetic resonance imaging study. J Neurol. 2009;256:1418–26. doi: 10.1007/s00415-009-5126-2. [DOI] [PubMed] [Google Scholar]
- 7.Costamagna G, Meneri M, Abati E, Brusa R, Velardo D, Gagliardi D, et al. Hyperacute extensive spinal cord infarction and negative spine magnetic resonance imaging: A case report and review of the literature. Medicine. 2020;99:e22900. doi: 10.1097/MD.0000000000022900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotelli MS, Manelli F. Review on acute relapse of spinal cord infarction: A case report. Int J Integr Health Sci. 2021;9:73–8. [Google Scholar]
- 9.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faig J, Busse O, Salbeck R. Vertebral body infarction as a confirmatory sign of spinal cord ischemic stroke: Report of three cases and review of the literature. Stroke. 1998;29:239–43. doi: 10.1161/01.str.29.1.239. [DOI] [PubMed] [Google Scholar]
- 11.Fiani B, Arshad MA, Shaikh ES, Baig A, Farooqui M, Ayub MA, et al. Current updates on various treatment approaches in the early management of acute spinal cord injury. Rev Neurosci. 2021;32:513–30. doi: 10.1515/revneuro-2020-0148. [DOI] [PubMed] [Google Scholar]
- 12.Ge L, Arul K, Stoner M, Mesfin A. Etiology and outcomes of spinal cord infarct: A case series from a level 1 trauma center. Global Spine J. 2020;10:735–40. doi: 10.1177/2192568219877863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginos J, Mcnally S, Cortez M, Quigley E, Shah LM. Vertebral artery dissection and cord infarction-An uncommon cause of brown-séquard and horner syndromes. Cureus. 2015;7:e308. doi: 10.7759/cureus.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna Al-Shaikh R, Czervionke L, Eidelman B, Dredla BK. StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Spinal cord infarction. [PubMed] [Google Scholar]
- 15.Heldner MR, Arnold M, Nedeltchev K, Gralla J, Beck J, Fischer U. Vascular diseases of the spinal cord: A review. Curr Treat Options Neurol. 2012;14:509–20. doi: 10.1007/s11940-012-0190-9. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JL, Cheng MY, Liao MF, Hsu HC, Weng YC, Chang KH, et al. A comparison between spinal cord infarction and neuromyelitis optica spectrum disorders: Clinical and MRI studies. Sci Rep. 2019;9:7435. doi: 10.1038/s41598-019-43606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic J, Rey Bataillard V, Mercier N, Bonvin C, Michel P. Acute ischemic myelopathy treated with intravenous thrombolysis: Four new cases and literature review. Int J Stroke. 2019;14:893–7. doi: 10.1177/1747493019851289. [DOI] [PubMed] [Google Scholar]
- 18.Johkura K, Joki H, Johmura Y, Momoo T, Kuroiwa Y. Combination of infarctions in the posterior inferior cerebellar artery and anterior spinal artery territories. J Neurol Sci. 2003;207:1–4. doi: 10.1016/s0022-510x(02)00352-0. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka S, Terasawa H, Tohji H. Sequential bilateral medial medullary infarction due to vertebral artery dissection. Cerebrovasc Dis. 2007;24:309–12. doi: 10.1159/000106515. [DOI] [PubMed] [Google Scholar]
- 20.Khan MF, Jooma R, Hashmi FA, Raghib MF. Delayed spinal cord infarction following anterior cervical surgical decompression. BMJ Case Rep. 2017;2017:bcr-2017-21:98–63. doi: 10.1136/bcr-2017-219863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Lee HS, Jung YH, Kim YD, Nam HS, Nam CM, et al. Mechanism of medullary infarction based on arterial territory involvement. J Clin Neurol. 2012;8:116–22. doi: 10.3988/jcn.2012.8.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CW, Huang CC, Chen CH, Yang YH, Chen TW, Huang MH. Prediction of severe neurogenic bowel dysfunction in persons with spinal cord injury. Spinal Cord. 2010;48:554–9. doi: 10.1038/sc.2009.181. [DOI] [PubMed] [Google Scholar]
- 23.Mateen FJ, Monrad PA, Leep Hunderfund AN, Robertson CE, Sorenson EJ. Clinically suspected fibrocartilaginous embolism: Clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218–25. doi: 10.1111/j.1468-1331.2010.03200.x. [DOI] [PubMed] [Google Scholar]
- 24.Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys Ther. 2000;80:673–87. [PubMed] [Google Scholar]
- 25.Nentwich LM. Diagnosis of acute ischemic stoke. Emerg Med Clin North Am. 2016;34:837–59. doi: 10.1016/j.emc.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: Clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113–20. doi: 10.1001/archneur.63.8.1113. [DOI] [PubMed] [Google Scholar]
- 27.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Over DR, Deaver J, Pumphery CY. Acute aortic occlusion with spinal cord infarction. Fed Pract. 2018;35:32–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Park D, Kim BH, Lee SE, Park JK, Cho JM, Kwon HD, et al. Spinal cord infarction: A single center experience and the usefulness of evoked potential as an early diagnostic tool. Front Neurol. 2020;11:563553. doi: 10.3389/fneur.2020.563553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pikija S, Mutzenbach JS, Kunz AB, Nardone R, Leis S, Deak I, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143. doi: 10.3389/fneur.2017.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romi F, Naess H. Spinal cord infarction in clinical neurology: A review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95–8. doi: 10.1159/000446700. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai T, Wakida K, Nishida H. Cervical posterior spinal artery syndrome: A case report and literature review. J Stroke Cerebrovasc Dis. 2016;25:1552–6. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Salamon E, Patsalides A, Gobin YP, Santillan A, Fink ME. Dural arteriovenous fistula at the craniocervical junction mimicking acute brainstem and spinal cord infarction. JAMA Neurol. 2013;70:796–7. doi: 10.1001/jamaneurol.2013.1946. [DOI] [PubMed] [Google Scholar]
- 34.Salvador de la Barrera S, Barca-Buyo A, Montoto-Marqués A, Ferreiro-Velasco ME, Cidoncha-Dans M, Rodriguez-Sotillo A. Spinal cord infarction: Prognosis and recovery in a series of 36 patients. Spinal Cord. 2001;39:520–5. doi: 10.1038/sj.sc.3101201. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Kawaguchi S, Takebayashi T, Yokogushi K, Takada J, Yamashita T. Vertebral body ischemia in the posterior spinal artery syndrome: Case report and review of the literature. Spine (Phila Pa 1976) 2003;28:E260–4. doi: 10.1097/01.BRS.0000067285.39466.FB. [DOI] [PubMed] [Google Scholar]
- 36.The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. EQUATOR Network. Available from: https://www.equator-network.org/reporting-guidelines/prisma [Last accessed on 2022 Nov 14] [DOI] [PMC free article] [PubMed]
- 37.Thomas G, Alakbarzade V, Sammaraiee Y, Cociasu I, Dalton C, Pereira AC. Spontaneous spinal cord infarction: A practical approach. Pract Neurol. 2022;22:497–502. doi: 10.1136/pn-2022-003441. [DOI] [PubMed] [Google Scholar]
- 38.Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006;48:795–801. doi: 10.1007/s00234-006-0130-z. [DOI] [PubMed] [Google Scholar]
- 39.Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, Lovblad KO, et al. Spinal cord ischemia: Practical imaging tips, pearls, and pitfalls. AJNR Am J Neuroradiol. 2015;36:825–30. doi: 10.3174/ajnr.A4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuilleumier P, Bogousslavsky J, Regli F. Infarction of the lower brainstem. Clinical, aetiological and MRI-topographical correlations. Brain. 1995;118:1013–25. doi: 10.1093/brain/118.4.1013. [DOI] [PubMed] [Google Scholar]
- 41.Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: Aetiology, clinical syndromes and imaging features. Neuroradiology. 2015;57:241–57. doi: 10.1007/s00234-014-1464-6. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg LA, Rowland LP. Merritts Textbook of Neurology. 9th. Baltimore: Williams-Wilkins Company; 1995. Vascular disease of the spinal cord; pp. 290–4. [Google Scholar]
- 43.Yoo DH, Cho YD, Boonchai T, Kim KM, Kim JE, Cho WS, et al. Endovascular treatment of medullary bridging vein-draining dural arteriovenous fistulas: Foramen magnum vs. craniocervical junction lesions. Neuroradiology. 2022;64:333–42. doi: 10.1007/s00234-021-02790-z. [DOI] [PubMed] [Google Scholar]
- 44.Yuh WT, Marsh EE, 3rd, Wang AK, Russell JW, Chiang F, Koci TM, et al. MR imaging of spinal cord and vertebral body infarction. AJNR Am J Neuroradiol. 1992;13:145–54. [PMC free article] [PubMed] [Google Scholar]
- 45.Zalewski NL, Rabinstein AA, Krecke KN, Brown RD, Jr, Wijdicks EF, Weinshenker BG, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76:56–63. doi: 10.1001/jamaneurol.2018.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zalewski NL, Rabinstein AA, Krecke KN, Brown RD, Wijdicks EF, Weinshenker BG, et al. Spinal cord infarction: Clinical and imaging insights from the periprocedural setting. J Neurol Sci. 2018;388:162–7. doi: 10.1016/j.jns.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Zotero Start. Available from: https://www.zotero.org/start_standalone [Last accessed on 2018 Apr 10]