Abstract
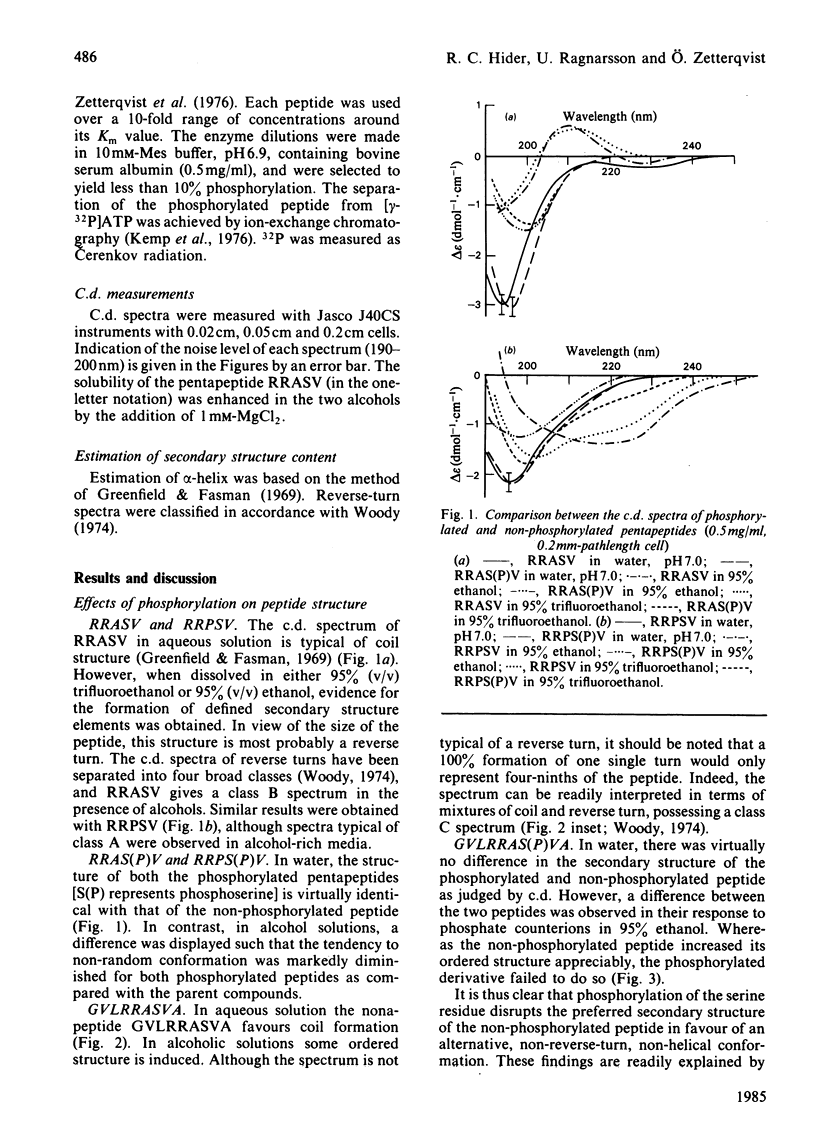
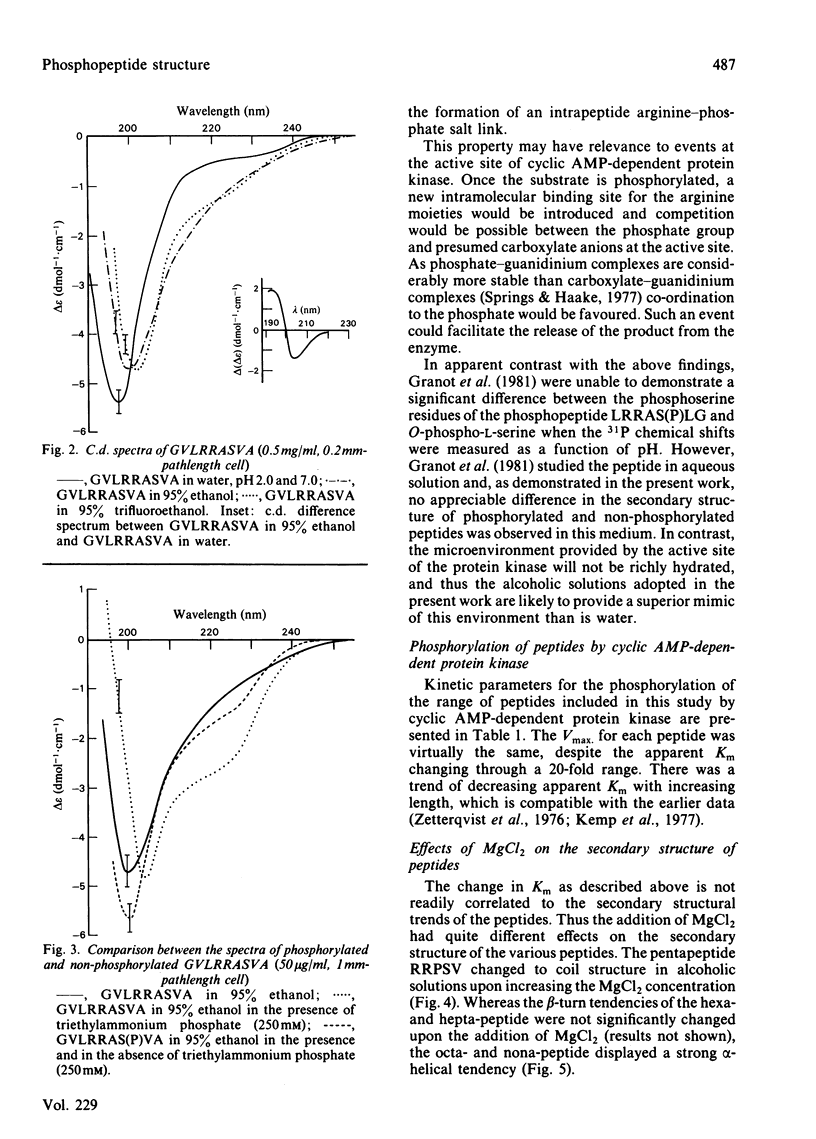
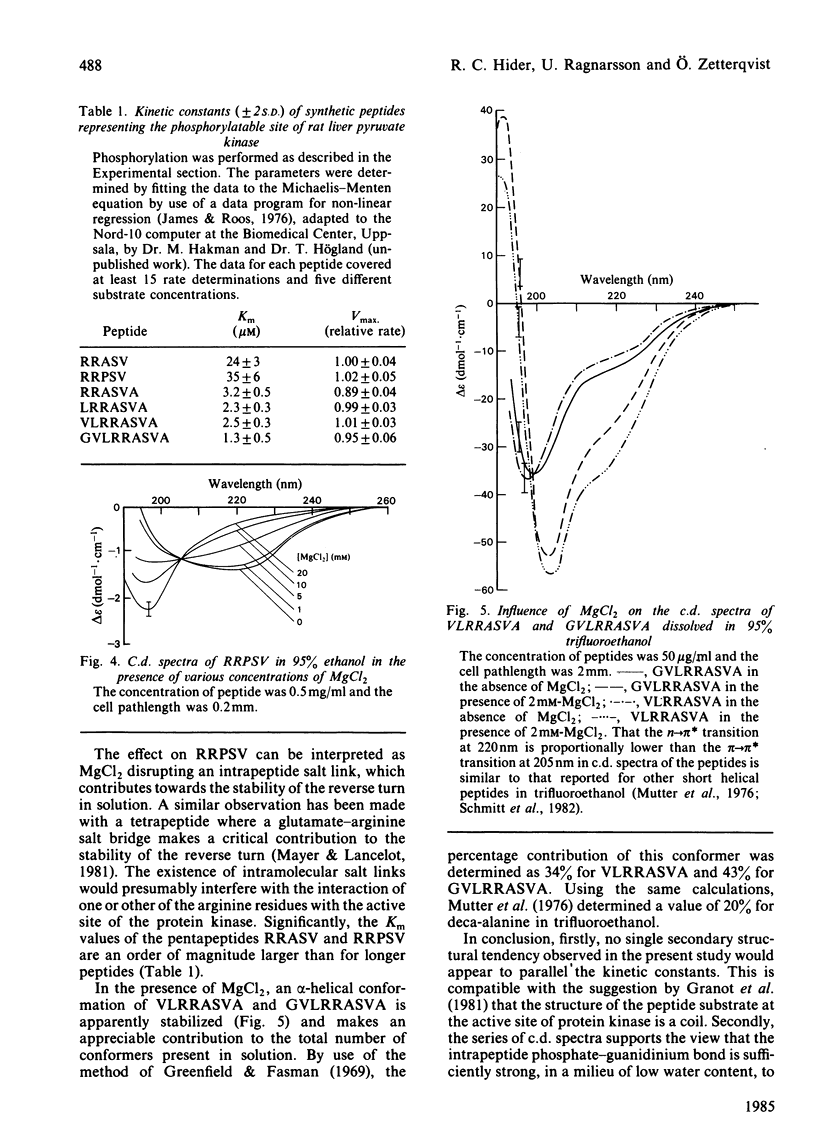
By c.d. studies it is shown that liver-pyruvate-kinase-related peptide substrates of cyclic AMP-dependent protein kinase have a high tendency towards non-random structures in non-aqueous media. When phosphorylated, the conformation tendencies decrease. This structural change is explained in terms of the formation of strong intrapeptide phosphate-guanidinium salt links. It is proposed that similar events occur at the catalytic site of protein kinase and that such an interaction could facilitate the removal of the phosphorylated products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cotton F. A., Day V. W., Hazen E. E., Jr, Larsen S., Wong S. T. Structure of bis(methylguanidinium) monohydrogen orthophosphate. A model for the arginine-phosphate interactions at the active site of staphylococcal nuclease and other phosphohydrolytic enzymes. J Am Chem Soc. 1974 Jul 10;96(14):4471–4478. doi: 10.1021/ja00821a020. [DOI] [PubMed] [Google Scholar]
- Cotton F. A., Hazen E. E., Jr, Legg M. J. Staphylococcal nuclease: proposed mechanism of action based on structure of enzyme-thymidine 3',5'-bisphosphate-calcium ion complex at 1.5-A resolution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2551–2555. doi: 10.1073/pnas.76.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström L., Ekman P., Humble E., Ragnarsson U., Zetterqvist O. Detection and identification of substrates for protein kinases: use of proteins and synthetic peptides. Methods Enzymol. 1984;107:130–154. doi: 10.1016/0076-6879(84)07008-7. [DOI] [PubMed] [Google Scholar]
- Fletterick R. J., Sprang S., Madsen N. B. Analysis of the surface topography of glycogen phosphorylase a: implications for metabolic interconversion and regulatory mechanisms. Can J Biochem. 1979 Jun;57(6):789–797. doi: 10.1139/o79-098. [DOI] [PubMed] [Google Scholar]
- Granot J., Mildvan A. S., Bramson H. N., Thomas N., Kaiser E. T. Nuclear magnetic resonance studies of the conformation and kinetics of the peptide-substrate at the active site of bovine heart protein kinase. Biochemistry. 1981 Feb 3;20(3):602–610. doi: 10.1021/bi00506a024. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Madsen N. B., KasvinskyPJ, Fletterick R. J. Allosteric transitions of phosphorylase a and the regulation of glycogen metabolism. J Biol Chem. 1978 Dec 25;253(24):9097–9101. [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Mutter M., Mutter H., Uhmann R., Bayer E. Konformationsuntersuchungen an Oligoalanin, Substanz P un der Myoglobinsequenz (66-73) Der Zirkulardichroismus von polyäthylenglykolgebundenen Peptiden. Biopolymers. 1976 May;15(5):917–927. doi: 10.1002/bip.1976.360150508. [DOI] [PubMed] [Google Scholar]
- Tatham A. S., Hider R. C., Drake A. F. The effect of counterions on melittin aggregation. Biochem J. 1983 Jun 1;211(3):683–686. doi: 10.1042/bj2110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titanji V. P., Ragnarsson U., Humble E., Zetterqvist O. Phosphopeptide substrates of a phosphoprotein phosphatase from rat liver. J Biol Chem. 1980 Dec 10;255(23):11339–11343. [PubMed] [Google Scholar]
- Williams R. J. The conformation properties of proteins in solution. Biol Rev Camb Philos Soc. 1979 Nov;54(4):389–437. doi: 10.1111/j.1469-185x.1979.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U., Humble E., Berglund L., Engström L. The minimum substrate of cyclic AMP-stimulated protein kinase, as studied by synthetic peptides representing the phosphorylatable site of pyruvate kinase (type L) of rat liver. Biochem Biophys Res Commun. 1976 Jun 7;70(3):696–703. doi: 10.1016/0006-291x(76)90648-3. [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U. The structural requirements of substrates of cyclic AMP-dependent protein kinase. FEBS Lett. 1982 Mar 22;139(2):287–290. doi: 10.1016/0014-5793(82)80872-7. [DOI] [PubMed] [Google Scholar]


