Abstract
Anshen Dingzhi prescription (ADP) is a classic prescription of traditional Chinese medicine, which has been used in the treatment of neuropsychiatric diseases. However, its treatment of breast cancer-related post-traumatic stress disorder (BC-PTSD) lacks clinical research evidence and its mechanism is not clear. The present study investigated the efficacy and action mechanism of ADP against BC-PTSD. The results of the clinical trial showed that after 4 weeks of treatment, both groups showed reduced post-traumatic stress disorder checklist-civilian version (PCL-C), Pittsburgh sleep quality index (PSQI), self-rating depression scale (SDS) and self-rating anxiety scale (SAS) scores, and increased functional assessment of cancer therapy-breast (FACT-B) scores. The serum cortisol (CORT), tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) levels were decreased and brain-derived neurotrophic factor (BDNF) level were increased, and the improvement of serum TNF-α, IL-1β, and BDNF in treatment group was better than that of the control group. The overall treatment efficacy in the treatment group (43.90%) was superior to that in the control group (23.81%), and the overall incidence of adverse effects was lower than that in the control group. The results of network analysis and molecular docking showed that ADP blood components could act on IL1B, TNF, and BDNF. ADP contributes to the treatment of BC-PTSD symptoms, with a mechanism possibly related to its regulatory effect on TNF-α, IL-1β, and BDNF levels.
Trial registration: Chinese Clinical Trial Registry, http://www.chictr.org.cn,ChiCTR2300077801
Keywords: BC-PTSD, Anshen Dingzhi prescription, network analysis, molecular docking, BDNF, inflammatory factor
Graphical abstract
Introduction
According to statistics, approximately 2.26 million women were diagnosed with breast cancer (BC) globally in 2020. 1 In 2022, the new incidence of female BC in China reached 340 000 cases, and the number of patients is still growing. 2 Breast cancer-related post-traumatic stress disorder (BC-PTSD) is a delayed and persistent psychological condition manifesting after a BC diagnosis and treatment. Symptoms include avoidance, nightmares, cognitive and emotional negativization, and hyper-arousal related to breast cancer content. This disorder significantly diminishes quality of life and, in severe cases, could lead to suicidal tendencies.3,4 Studies have shown that the prevalence of BC-PTSD could be as high as 25.3%, 5 and the risk of PTSD in patients diagnosed with cancer is increased by 1.66 times compared with the normal population. 6 Currently, PTSD treatment predominantly relies on chemical drugs such as paroxetine hydrochloride and sertraline, recommended by FDA guidelines. While paroxetine hydrochloride is effective short-term, prolonged use could result in drug dependence and adverse effects like drowsiness, headache, nausea, constipation, weight gain, and memory impairment. 7 Adverse drug reactions may cause psychological resistance in patients, which leads to lower treatment compliance and worse efficacy. Therefore, to find treatment methods with precise efficacy and fewer adverse effects for the treatment of BC-PTSD is of urgent need.
Anshen Dingzhi prescription (ADP) is a famous prescription of Xin’an medicine recorded in “Yi Xue Xin Wu,” which consists of ginseng radix et rhizome (Panax ginseng C. A. Mey.) (Chinese herbal name 人参), polygalae radix (Polygala tenuifolia Willd.) (Chinese herbal name远志), acori tatarinowii rhizome (Acorus tatarinowii Schott) (Chinese herbal name石菖蒲), poria (Poria cocos (Schw.) Wolf) (Chinese herbal name茯苓), poriae cum radicise pini (Poria cocos (Schw.) Wolf) (Chinese herbal name茯神), and dens draconis (Chinese herbal name龙齿), and has the effect of tranquilizing the mind and calming the spirit, benefiting the vital energy and suppressing fright. We previously have shown that ADP as well as its pharmacodynamic components could effectively intervene in PTSD behaviors in single prolonged stress (SPS) mice.8 -11 The multi-target and multi-pathway drug characteristics of ADP determine that it has a holistic modulation and synergistic therapeutic effect, which has certain advantages for the intervention and individualized treatment of BC-PTSD. However, there is still insufficient evidence from clinical trials and the mechanism of action of ADP for BC-PTSD is unclear.
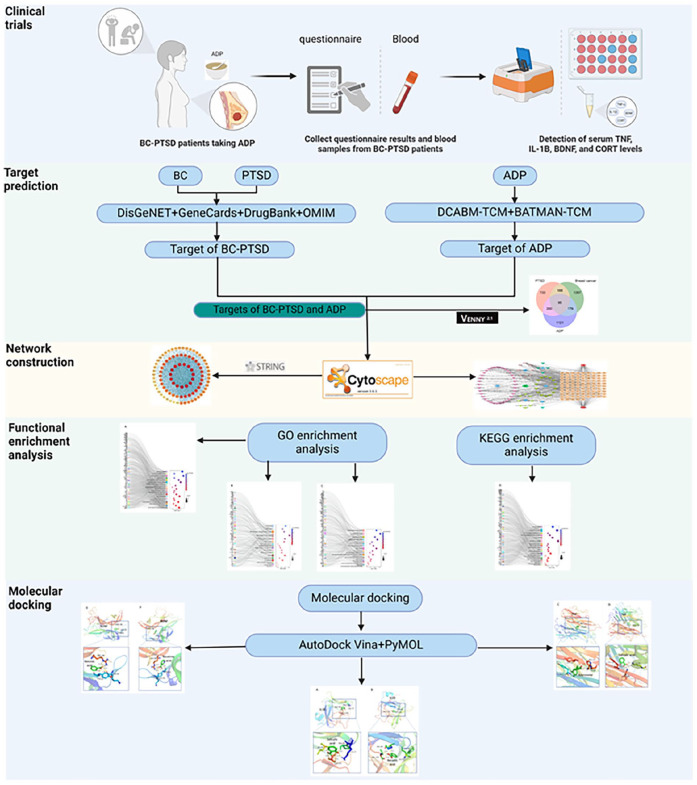
In this study, we designed a clinical trial to observe the efficacy of ADP in the treatment of BC-PTSD patients, explored the potential mechanisms of ADP for BC-PTSD using network analysis and molecular docking. This study will initiate the first clinical assessment of ADP for treating BC-PTSD.
Materials and Methods
Patients
From January 2021 to September 2023, 86 patients diagnosed with PTSD of breast cancer were selected from the inpatient and outpatient departments of the Department of Oncology at the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine. These patients were randomly divided into the treatment group (n = 43) and the control group (n = 43) using a randomized numerical table method. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (Ethics No. 2021AH-62). The clinical research process adheres to the principles of the Helsinki Declaration. All patients provided written informed consent before entering the study. The baseline characteristics of the included patients are shown in Table 1.
Table 1.
Baseline Information of the Patients.
| Control group (n = 43) | Treatment group (n = 43) | P-value | |
|---|---|---|---|
| Age | 57.63 ± 9.76 | 56.72 ± 10.26 | >.05 |
| Education level | >.05 | ||
| Below Junior High School | 6 (13.95%) | 5 (11.63%) | |
| Junior High School | 20 (46.51%) | 19 (44.19%) | |
| High School | 14 (32.56%) | 16 (37.21%) | |
| High school or above | 3 (6.98%) | 3 (6.98%) | |
| Body mass index (kg/m2) | 18.29 ± 1.65 | 18.03 ± 1.85 | >.05 |
| Marital status | >.05 | ||
| Married | 32 (74.42%) | 33 (76.74%) | |
| Unmarried/divorced/widowed | 11 (25.58%) | 10 (23.26%) |
Diagnostic Criteria
Breast cancer diagnostic criteria
patients with breast cancer were clearly confirmed by clinical manifestations, physical examination, imaging examination and histopathology according to the Chinese Association Against Cancer (CAC) Breast Cancer Diagnostic and Treatment Guidelines and Criteria (2021 Edition), 12 and the pathologic staging referred to the American Cancer Consortium (AJCC) TNM staging criteria.
Diagnostic criteria for PTSD
The diagnosis met the criteria for PTSD with reference to the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5). 4
The Inclusion Criteria Were as Follows
(1) All cases conformed to the aforementioned western medical diagnosis, are pathologically or cytologically confirmed as breast cancer, with clinical pathological staging criteria being below stage II, and have all undergone treatment combining surgery with radiotherapy or chemotherapy.
(2) All cases had PTSD symptoms as evaluated by post-traumatic stress disorder checklist-civilian version (PCL-C) for at least 1 month.
(3) The age was set between 18 and 70 years old, with elementary school education level or above.
(4) The patients could understand and agree to participate in this study and have good compliance.
(5) The patients had stable condition, without serious heart, liver, brain, kidney and other organic damage or bone marrow hematopoietic dysfunction.
(6) The patient had some reading ability and could complete questionnaires.
The Exclusion Criteria Were as Follows
(1) Non-primary breast cancer.
(2) Prior history of primary or secondary psychiatric abnormality on psychiatric medication.
(3) Parents with psychiatric abnormalities within 3 generations of both parents.
(4) Receiving specific psychotherapy for PTSD (trauma-focused cognitive-behavioral therapy, oculomotor desensitization, and reprocessing).
(5) Experiencing a major traumatic event other than breast cancer.
(6) Those who have developed brain metastases from breast cancer.
(7) Pregnant women.
(8) Participants in other clinical studies within the last 1 month.
The Dropout Criteria Were as Follows
The patients enrolled in the study, received the intervention, but did not complete the intervention cycle and/or observation cycle as specified in the protocol were designated as dropouts.
Criteria for Termination of the Trial
(1) Serious adverse events or serious adverse reactions occurring in the trial should be stopped based on judgment.
(2) There is a serious deviation from the trial protocol in the implementation of the clinical treatment program, and the purpose of the study cannot be achieved.
(3) Subjects who develop serious complications during the course of the clinical trial and are not fit to continue the trial.
(4) The subject’s condition deteriorates and emergency treatment measures must be taken.
(5) Other diseases affecting the observation of the trial have appeared in the treatment, which should be stopped and treated as invalid cases according to the judgment.
Random Assignment and Blinding
A random number table was generated from the Research Randomizer website (https://www.randomizer.org/). Odd numbers from the table were allocated to the treatment group, while even numbers were assigned to the control group. Subsequently, the randomized grouping results were recorded in sealed envelopes, which were managed and stored by personnel not involved in patient treatment. When patients met the inclusion criteria, relevant personnel opened the envelopes and assigned patients to either the treatment or control group based on the random numbers inside. The data analyst had no knowledge of the participants’ group assignments.
Treatment Protocols
The control group received paroxetine hydrochloride. The dosing regimen started with an initial dose of 10 mg/day, incrementing by 10 mg weekly based on patient response, up to a maximum daily dose of 40 mg, administered as 20 mg in the morning and 20 mg in the evening. Paroxetine hydrochloride tablets are manufactured by Zhejiang Huahai Pharmaceutical Co. The treatment group was based on the treatment regimen of the control group, combined with ADP. The ADP consists of poria (10 g), poriae cum radicise pini (10 g), ginseng radix et rhizome (10 g), and polygalae radix (10 g), along with acori tatarinowii rhizoma (5 g) and dens draconis (5 g). The treatment group was given one dose per day of 200 ml each time, in the morning and in the evening. The Chinese medicine decoction was produced by the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine and were uniformly decocted by the hospital decoction room. Decoction method: 7 doses of ADP (1 week’s dose) were soaked for 30 minutes, put into the automatic decoction machine (Donghuayuan Pharmaceutical Company, Product No.: YJD20D-GL), and added 5000 ml of drinking water, turned on the decoction machine to heat up the decoction, and when the temperature reached 100°C to start timing the decoction for 40 minutes, and at the end of the decoction, the dregs of the medicine were removed through gauze to retain supernatant, and 14 bags were obtained. The course of treatment for both groups was 4 weeks. Fingerprint and main components were shown in previous publications.8,11
Clinical Research Process
In strict accordance with the diagnostic inclusion criteria, the patients were enrolled into the control group and the treatment group according to the principle of randomization, and the patients in the 2 groups did PCL-C, Pittsburgh sleep quality index (PSQI), self-rating anxiety scale (SAS) when they were enrolled in the group, self-rating depression scale (SDS), functional assessment of cancer therapy-breast (FACT-B), and blood was drawn for serum cortisol (CORT), brain-derived neurotrophic factor (BDNF), tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) levels. In strict accordance with the treatment protocol, after 4 weeks of continuous medication, patients in both groups did PCL-C, PSQI, SDS, SAS and FACT-B scale tests again, and blood was drawn to test serum CORT, BDNF, TNF-α and IL-1β levels, which were to be analyzed at the end of the trial.
During the study, we established a dedicated quiet area in the outpatient clinic for patients to fill out paper versions of assessment questionnaires. Research assistants provided the questionnaires to patients during their appointments and offered necessary support and explanations to ensure patients could accurately understand and complete the questionnaire content. All research assistants underwent specialized training, including how to standardize the filling-out process to ensure consistency and accuracy of data. Completed questionnaires were returned to the research assistants and stored in a password-protected lockbox to maintain confidentiality. We strictly adhered to relevant patient privacy regulations, and all collected information was used solely for research purposes.
Observation Indexes and Efficacy Evaluation
PCL-C
PCL-C is designed specifically for evaluating the experience of ordinary people after suffering a traumatic event, the scale consists of 17 entries, using a five-level scoring method (1-5 points), the higher the final composite score, the more pronounced the degree of PTSD, with a composite score of 17 to 37 for no obvious PTSD symptoms, a score of 38 to 49 for a certain degree of PTSD symptoms, and a score of 50 to 85 for obvious PTSD symptoms, which may be diagnosed as PTSD. 13
Efficacy evaluation criteria
The PCL-C score reduction rate was calculated as the clinical efficacy evaluation standard, and the PCL-C score reduction rate was adopted as the core efficacy index. The calculation method refers to the “Nimodipine method”, that is, PCL-C score reduction rate (%) = (total score before treatment − total score after treatment)/total score before treatment × 100%. The criteria were as follows.
Clinical cure: PCL-C score reduction rate ≥ 75%.
Significant effect: 50% ≤ PCL-C score reduction rate < 75%.
Effective: 30% ≤ PCL-C score reduction rate < 50%.
Ineffective: PCL-C score reduction rate < 30%.
Calculation of total clinical effectiveness rate: total effectiveness rate = (effective + obvious + clinically cured) number/total number × 100%.
SAS and SDS
Both scales consisted of 20 entries and were divided into 4 levels of scoring. A standardized score of <50 was classified as no anxiety/depression, 50 to 59 was classified as mild anxiety or depression, 60 to 69 was classified as moderate anxiety or depression, and >70 was classified as severe anxiety or depression. Anxiety and depression levels were assessed using SAS and SDS for both groups at 2 time points before and after treatment, respectively.14,15
PSQI
The scale consists of 18 scored entries categorized into 7 factors: sleep quality, time to sleep, sleep duration, sleep efficiency, sleep disorders, hypnotic medications, and daytime dysfunction. The factor hypnotic medication was not included in the scale. Each factor was scored from 0 to 3 points for a total of 6 factors, and the total score ranged from 0 to 18 points, with higher scores indicating poorer sleep. The PSQI was used to assess the sleep quality of patients in both groups at 2 time points before and after treatment. 16
FACT-B
FACT-B is a measurement scale specially set for breast cancer in the Cancer Therapy Functional Evaluation System. The scale covers 5 major items, namely physical health, social and family status, emotional status, functional status and other issues, and each item is scored on a five-point scale (0-4 points), with positive entries scoring 0 to 4 points and negative entries scoring the opposite. A higher score indicated a better quality of life. 17
Evaluation of laboratory-related indexes
Before and after the course of treatment, 4 ml of peripheral venous blood was collected from patients in the morning on an empty stomach, and the levels of serum IL-1β (Jianglai Bio, JL13662), TNF-α (Jianglai Bio, JL10208), CORT (Jianglai Bio, JL12114), and BDNF (Jianglai Bio, JL11683) were detected.
Safety indicators
Observe the occurrence of adverse reactions occurring during follow-up, including drowsiness, headache, nausea and vomiting, constipation, dry mouth, weight gain, and memory deterioration.
Network Analysis
Screening of ADP active ingredients and targets
The ADP was composed of poria, poriae cum radicise pini, ginseng radix et rhizome, polygalae radix, acori tatarinowii rhizoma, and dens draconis. The name of each Chinese medicine in the ADP was entered into the Bioactive Molecules Database of Traditional Chinese Medicine (BATMAN-TCM, http://bionet.ncpsb.org.cn/batman-tcm/) and the Chemical and Bioinformatics Database of Traditional Chinese Medicine (DCABM-TCM, http://bionet.ncpsb.org.cn/dcabm-tcm/) to obtain the blood components of each TCM as potential active ingredients of ADP, and to obtain the target information of each component.
BC-PTSD disease target screening
We used “Breast cancer” and “Post-traumatic stress disorder” as keywords to search for BC and PTSD targets in the OMIM (https://www.omim.org/), GeneCards (https://www.genecards.org/), DrugBank (https://go.drugbank.com/) and DisGeNET (https://www.disgenet.org/) databases for the species “Homo sapiens.” Subsequently, we excluded duplicate targets of the same disease across different databases and took the intersection of BC and PTSD targets as the disease targets for BC-PTSD.
Construction and analysis of “drug-component-target-disease” network and protein interaction network
The common targets of ADP and BC-PTSD were screened as potential targets for the action of ADP in the treatment of BC-PTSD, and Venn diagrams were plotted. “Drug-component-target-disease” networks were built using Cytoscape software. The common targets were entered into the STRING database (https://cn.string-db.org/), with the biological species set as “Homo sapiens” and the minimum interaction threshold set as “medium confidence” (>0.4). The results of the protein-protein interaction (PPI) network were imported into Cytoscape 3.8.0 software, and the PPI network was visualized. Key targets were identified based on their degree values.
Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis
The potential target genes were uploaded into the metascape database (https://metascape.org/), and the species was selected as “Homo sapiens” for GO and KEGG signaling pathway enrichment analysis, and then the enrichment results were visualized.
Molecular Docking
Molecular docking was performed by selecting ADP blood components directly related to the clinical test indicators IL1B, TNF and BDNF in the network analysis, obtaining the 3D structures of IL1B, TNF, and BDNF proteins in the PDB database (https://www.rcsb.org/), and utilizing AutoDock Tools 1.5.6 software to perform the molecular docking of ADP blood components (ligands) with target proteins (receptors). Well-bound molecular docked conformations were imported into Pymol software for visualization of results.
Statistical Methods
SPSS 26.0 software was used for statistical analysis. Measurement data conforming to normal distribution were described by Mean ± SEM, inter-group comparisons were made by 2 independent samples t-test, and intra-group comparisons were made by paired samples t-test. Data not conforming to a normal distribution were described by median and interquartile range [M (P25, P75)]. Inter-group comparisons were conducted using the Mann-Whitney U test, and intra-group comparisons used the Wilcoxon test. Count data comparisons were conducted using the chi-square test, while rank information comparisons used the Mann-Whitney U test for between-group comparisons and the Wilcoxon test for within-group comparisons. P < .05 was taken as the difference being statistically significant.
Results
Comparison of the Efficacy of the 2 Groups of Patients After Treatment
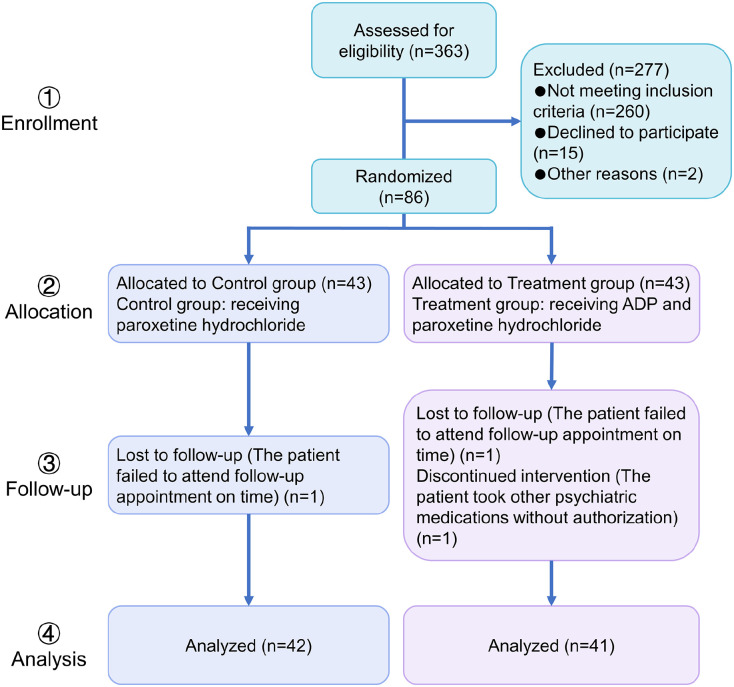
During the study period (January 2021-September 2023), out of 363 breast cancer patients, 86 patients met the inclusion criteria and agreed to participate in this trial. There were 3 dropout cases, 1 in the control group (one failed to follow up on time) and 2 in the treatment group (one failed to follow up on time and one unauthorized use of other psychiatric drugs). The detachment cases did not complete the treatment in this trial and therefore were not counted in the efficacy analysis. The study flow is shown in Figure 1. After treatment, the numbers of cured, markedly effective, effective, and ineffective cases in control group were 0, 2, 8, and 32, respectively. The numbers of cured, markedly effective, effective, and ineffective cases in the treatment group were 1, 4, 13, and 23, respectively (Table 2). There is a statistically significant difference between the groups (P < .05).
Figure 1.
Clinical trial design process.
Table 2.
Comparison of the Efficacy of the Two Groups After Treatment.
| Groups | n | Cured/case | Significant efficacy/case | Effective/case | Ineffective/case | Total effective rate (%) | P-value | Z |
|---|---|---|---|---|---|---|---|---|
| Control group | 42 | 0 | 2 | 8 | 32 | 23.81 | .048 | −1.98 |
| Treatment group | 41 | 1 | 4 | 13 | 23 | 43.90 |
Comparison of PCL-C Scores Between the 2 Groups
Before treatment, there was no statistically significant difference in the intergroup comparison of PCL-C scores between the 2 groups. After 4 weeks of treatment, the PCL-C scores of both groups were significantly lower than those before treatment (P < .05), and the PCL-C scores of the treatment group were lower than those of the control group (P < .05) (Figure 2).
Figure 2.
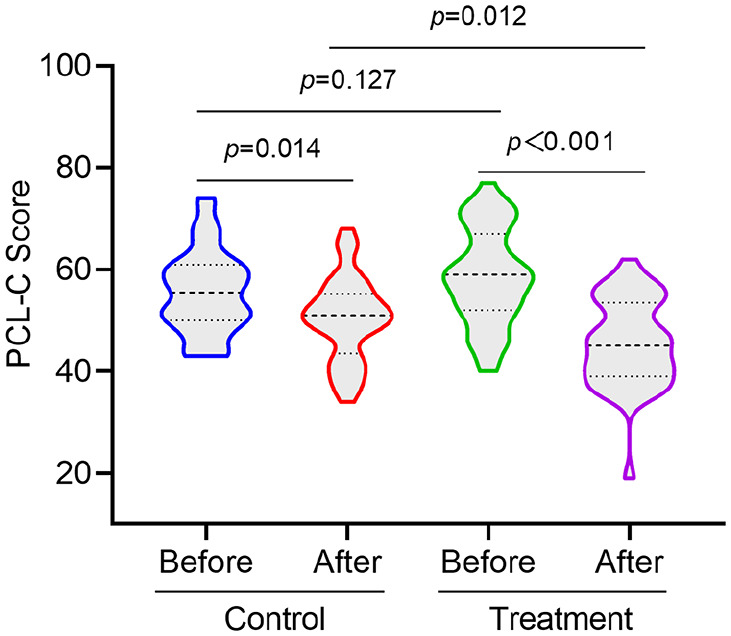
Comparison of PCL-C before and after treatment between the two groups of patients.
Comparison of SDS, SAS, PSQI and FACT-B Scores Between the 2 Groups of Patients
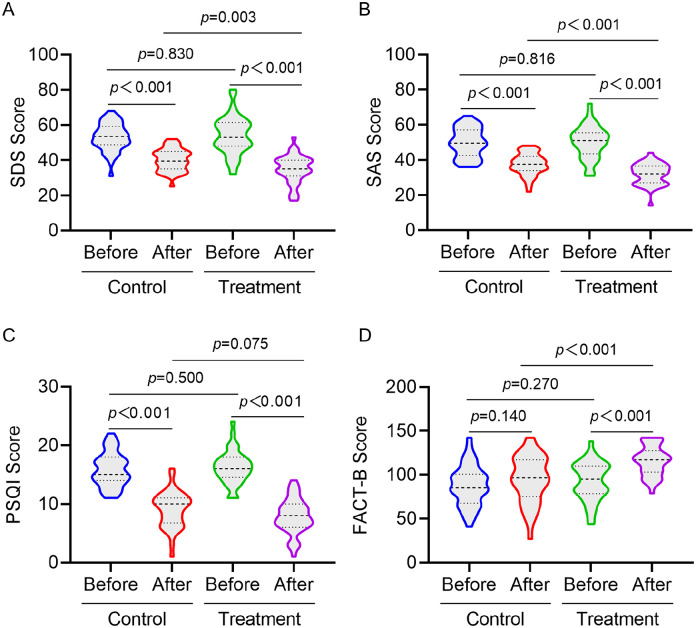
Comparison of SDS, SAS, PSQI and FACT-B scores of patients in the 2 groups before treatment showed no statistical difference. After 4 weeks of treatment, SDS, SAS and PSQI scores of patients in the 2 groups were significantly lower than those of before treatment (P < .05), and SDS and SAS scores of the treatment group were lower than those of the control group (P < .05). FACT-B scores of the treatment group increased after treatment, with a statistical difference (P < .05), and the FACT-B score of the treatment group was higher than that of the control group (P < .05), but there was no statistically significant difference between the FACT-B scores of the control group before and after the treatment. In addition, there was no statistically significant difference between the PSQI scores of the 2 groups of patients after the treatment (Figure 3).
Figure 3.
Comparison of SDS (A), SAS (B), PSQI (C), and FACT-B (D) scores before and after treatment between the 2 groups of patients.
Comparison of Serum CORT, BDNF, TNF-α and IL-1β Indexes in the 2 Groups of Patients
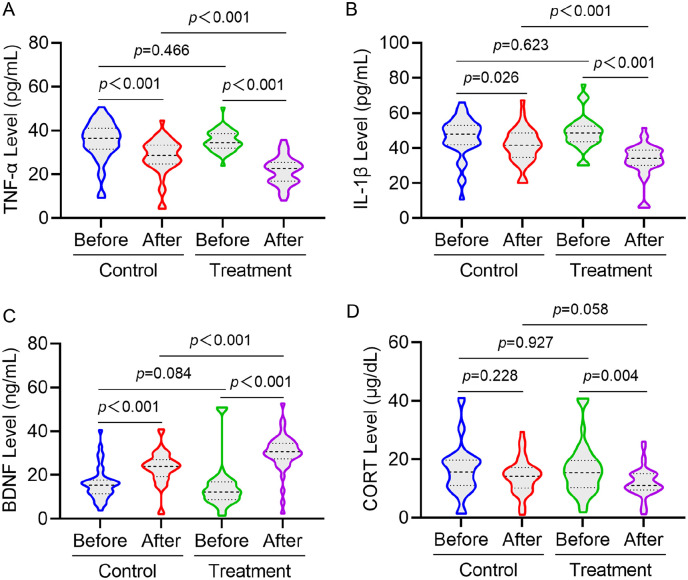
Before treatment, there were no statistically significant difference in serum CORT, BDNF, TNF-α and IL-1β levels between the 2 groups. After treatment, CORT, TNF-α and IL-1β levels of patients in the treatment group were significantly decreased (P < .05), and BDNF level was significantly increased (P < .05). After treatment of patients in the control group, TNF-α and IL-1β levels were significantly decreased (P < .05), and BDNF level was significantly increased (P < .05). After treatment and control group for intergroup comparison, TNF-α and IL-1β levels decreased significantly (P < .05) and BDNF level increased significantly (P < .05) in the treatment group (Figure 4).
Figure 4.
Comparison of serum TNF-α (A), IL-1β (B), BDNF (C), and CORT (D) indexes before and after treatment in 2 groups of patients.
Comparison of Adverse Reactions Between the 2 Groups
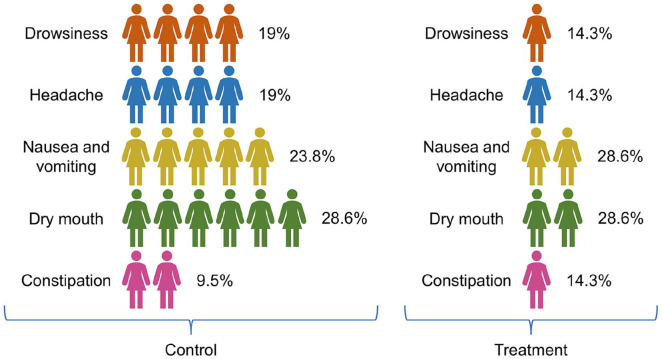
During the treatment period, adverse reactions in the 2 groups were documented (Figure 5). The incidence of adverse reactions in the treatment group was lower than that in the control group, and the difference is statistically significant (P < .05).
Figure 5.
Incidence of adverse reactions in control group (A) and treatment group (B).
Screening of Blood Components and Targets in ADP
The blood components and corresponding targets of ADP constituent drugs were searched and obtained from the DCABM-TCM and BATMAN-TCM databases, and a total of 69 blood components were obtained for traditional Chinese medicine of ADP, of which poria had 35, poriae cum radicise pini had 4, ginseng radix et rhizome had 13, polygalae radix had 8, acori tatarinowii rhizoma had 8, and dens draconis had 1 (Supplemental Table 1), and the targets of the blood components totaled 1595.
Target Screening of BC-PTSD
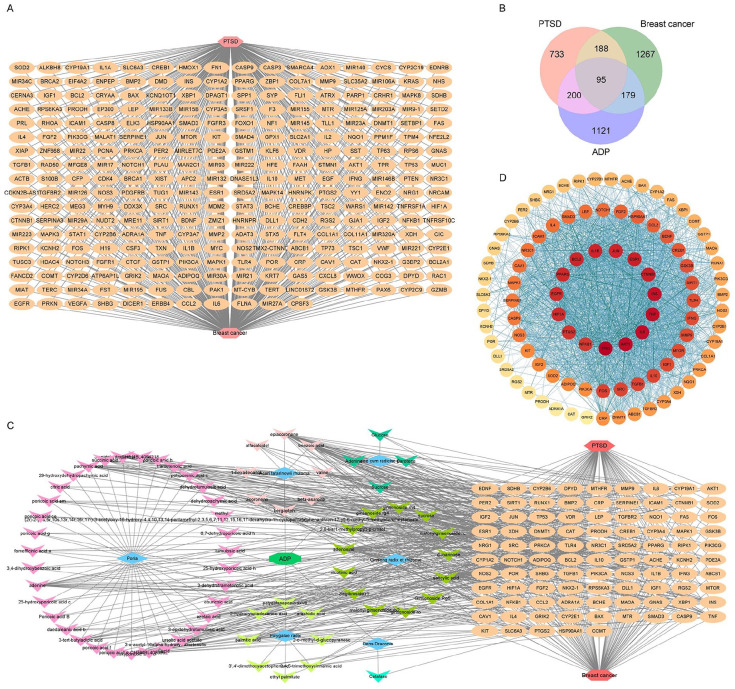
Through OMIM, GeneCards, DrugBank, and DisGeNET database search, screening and integration, a total of 1216 PTSD-related targets were obtained, and BC-related targets to 1729, of which there were 283 intersecting targets of BC and PTSD as BC-PTSD disease targets, as shown in Figure 6A.
Figure 6.
Network pharmacological analysis of ADP in the treatment of BC-PTSD. (A) BC-PTSD disease target. (B) Venn diagram of potential targets in ADP therapy for BC-PTSD. (C) Network analysis of “Drug-Component-Target-Disease”. (D) PPI network analysis of potential targets in ADP therapy for BC-PTSD.
“Drug-Component-Target-Disease” Network Construction
After screening the intersection of ADP blood component targets and BC-PTSD disease targets, a total of 95 potential targets of action for ADP treatment of BC-PTSD were obtained, as shown in Figure 6B. The “drug-component-target-disease” network was visualized by Cytoscape 3.8.0 software. Six Chinese medicines suggestive of ADP acted on 95 targets through 69 blood components to treat BC-PTSD, as shown in Figure 6C.
PPI Network Construction and Pathway Enrichment Analysis of Potential Targets in ADP Therapy for BC-PTSD
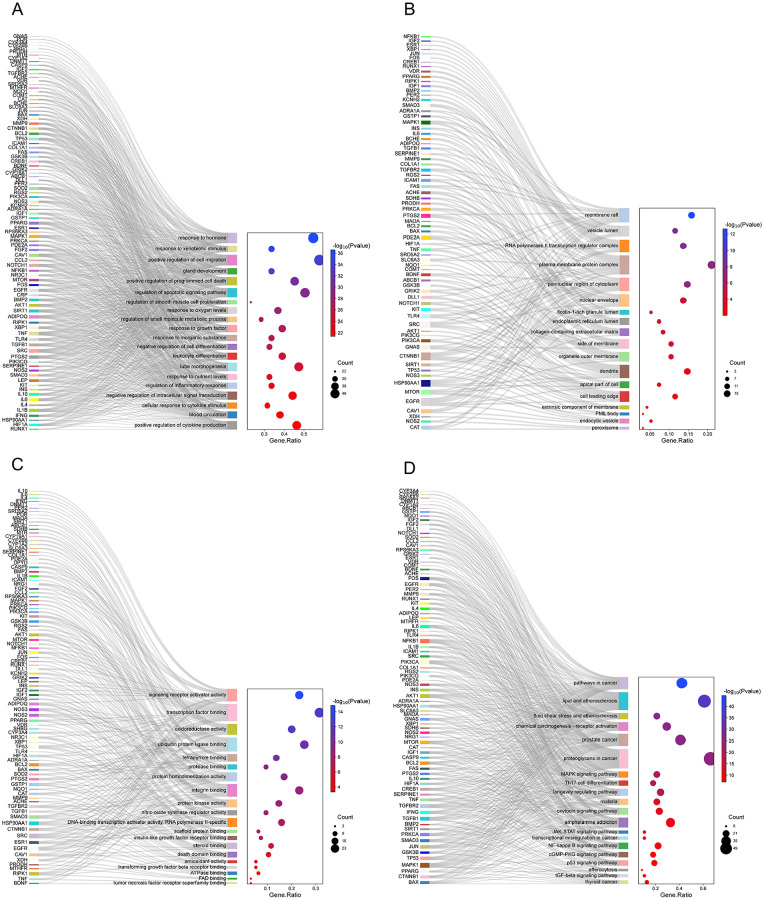
The potential targets of ADP for BC-PTSD were imported into the STRING database to construct protein interactions, and the results were imported into Cytoscape 3.8.0 software to map the PPI network of ADP for BC-PTSD based on the magnitude of node connectivity, and a total of 47 key targets were screened out, and the specific information is shown in Figure 6D. GO enrichment analysis and KEGG pathway analysis were performed on 95 potential targets for ADP treatment of BC-PTSD, and the results are shown in Figure 7. The GO analysis indicates that the biological processes (BP) related pathways include: response to hormone, response to external stimulus, positive regulation of cell migration, gland development, positive regulation of programed cell death, regulation of apoptotic signaling pathway, regulation of smooth muscle cell proliferation, response to oxygen levels, regulation of small molecule metabolic process, and response to growth factor (Figure 7A). Cellular components (CC) include: membrane raft, vesicle lumen, RNA polymerase II transcription regulatory complex, cell surface protein complex, cytoplasmic side of nuclear pore, nuclear membrane, ficolin-1-rich granule lumen, endoplasmic reticulum lumen, membrane raft, and organelle outer membrane (Figure 7B). Molecular functions (MF) include: activation of signal transducer activity, transcription factor binding, oxidoreductase activity, ubiquitin protein ligase binding, tetrapyrrole binding, protease binding, protein homodimerization activity, integrin binding, protein kinase activity, and nitric oxide synthase regulator activity (Figure 7C). KEGG pathway analysis results indicate that cancer pathways, lipid and atherosclerosis, fluid shear stress and atherosclerosis, chemical carcinogenesis-receptor activation, prostate cancer, protein glycan in tumors, MAPK signaling pathway, Th17 cell differentiation, longevity regulating pathway, and malaria play important roles in ADP treatment of BC-PTSD (Figure 7D).
Figure 7.
The results of GO and KEGG enrichment analysis of potential targets for ADP treatment of PTSD. (A) GO-BP analysis results indicated that differentially expressed genes are primarily associated with key biological processes such as response to xenobiotic stimulus and regulation of inflammatory responses. (B) GO-CC analysis results indicated that differentially expressed genes are mainly found in dendrites, perinuclear region of cytoplasm, and other regions. (C) GO-MF analysis results indicated that differentially expressed genes are primarily associated with functions such as transcription factor binding and ubiquitin protein ligase binding. (D) KEGG analysis indicated that differentially expressed genes are associated with signaling such as pathways in cancer and NF-kappaB signaling.
Molecular Docking Results
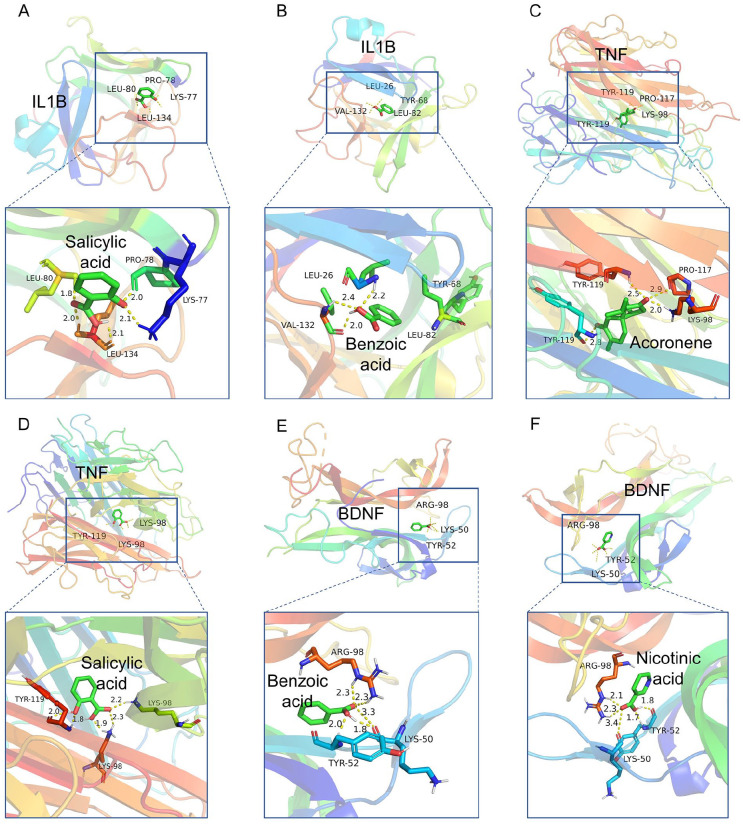
According to the “drug-component-target-disease” network, we found that the serum biochemical indicators IL1B, TNF, and BDNF detected in clinical trials were potential targets of ADP for the treatment of BC-PTSD with high degree of connectivity. That 2 blood components of ADP acted on BDNF, 8 blood components on IL1B, and 11 blood components on TNF (Table 3). The results indicated that salicylic acid could simultaneously interact with both IL1B and TNF, while benzoic acid could interact with both IL1B and BDNF. Acronene was identified as a blood component of ADP with numerous interaction targets, including TNF. Nicotinic acid was another blood component, apart from benzoic acid, that interacted with BDNF. Therefore, we performed molecular docking with the 4 ADP blood components as ligands and the BDNF or IL1B or TNF as receptors. The molecular docking results showed that both ligand molecules and receptor targets could spontaneously bind and were conformationally stable. Among them, the binding energy of acoronene with TNF was ≤−9 kcal/mol, forming several hydrogen bond bindings, and TNF with salicylic acid also had low binding energy, suggesting that there was a better binding activity between acoronene and salicylic acid and TNF. Similarly, the binding energies of IL1B with salicylic acid and benzoic acid, and BDNF with nicotinic acid and benzoic acid all had lower binding energies, indicating better binding activity between ligand and receptor (Figure 8 and Table 4). The molecular docking results indicated that blood components in ADP, such as acoronene, salicylic acid, nicotinic acid and benzoic acid, demonstrate strong binding interactions with potential targets IL1B, TNF, and BDNF related to BC-PTSD.
Table 3.
ADP Blood Components That Interact With IL1B, TNF, and BDNF Targets.
| Targets | |||
|---|---|---|---|
| BDNF | IL1B | TNF | |
| Interacting blood components | Nicotinic acid | Benzoic acid | Sucrose |
| Benzoic acid | Nicotinic acid | Adenosine | |
| Salicylic acid | Salicylic acid | ||
| 2,6-Bis(1-methylpropyl)-p-cresol | D-mannose | ||
| 16-Deoxyporicoic acid b | 2,6-Bis(1-methylpropyl)-p-cresol | ||
| Valine | 16-Deoxyporicoic acid b | ||
| Beta-asarone | Epiacoronene | ||
| Carotene | Acoronene | ||
| 3,4,5-Trimethoxycinnamic acid | |||
| 2-O-methyl-d-glucopyranose | |||
| Glucose | |||
Figure 8.
Molecular docking of ADP blood components acoronene, salicylic acid, nicotinic acid and benzoic acid with BC-PTSD disease targets IL1B, TNF, and BDNF. (A) Molecular docking of salicylic acid and IL1B (binding energy: −4.54 kcal/mol, binding sites: LYS-77, PRO-78, LEU-80, LEU-134). (B) Molecular docking of benzoic acid and IL1B (binding energy: −5.13 kcal/mol, binding sites: LEU-26, TYR-68, LEU-82, VAL-132). (C) Molecular docking of acoronene and TNF (binding energy: −9.60 kcal/mol, binding sites: LYS-98, PRO-117, TYR-119). (D) Molecular docking of salicylic acid and TNF (binding energy: −5.03 kcal/mol, binding sites: LYS-98, TYR-119);. (E) Molecular docking of nicotinic acid and BDNF (binding energy: −5.52 kcal/mol, binding sites: LYS-50, TYR-52, ARG-98). (F) Molecular docking of benzoic acid and BDNF (binding energy: −5.78 kcal/mol, binding sites: LYS-50, TYR-52, ARG-98).
Table 4.
Binding Energies of Selected ADP Blood Components to Key Targets.
| Target (receptor) | PDB ID | Docking molecule (ligand) | Binding energy (kcal/mol) |
|---|---|---|---|
| IL1B | 1I1B | Salicylic acid | −4.54 |
| IL1B | Benzoic acid | −5.13 | |
| TNF | 1A8M | Acoronene | −9.60 |
| TNF | Salicylic acid | −5.03 | |
| BDNF | 1B8M | Nicotinic acid | −5.52 |
| BDNF | Benzoic acid | −5.78 |
Discussion
This study explored the efficacy and mechanism of action of ADP in BC-PTSD. Clinical application of ADP effectively alleviated PTSD and depressive symptoms, improved sleep quality, reduced serum IL-1β, TNF-α, and CORT levels, and increased BDNF content, and network analysis, and molecular docking revealed that ADP blood components could act on IL1B, TNF, and BDNF. The study suggests that ADP may improve BC-PTSD symptoms by modulating inflammatory factors and BDNF levels.
PTSD is a common complication in breast cancer patients. 6 The traumatic stressors of BC-PTSD are persistent, cumulative, and uncertain, beginning with the “shock” and “fear” of the patient’s sudden, life-threatening diagnosis of breast cancer. 18 Patients experience the anxiety of cancer screening and diagnosis, the physiological damage to the body caused by radiotherapy, chemotherapy and surgical treatments for cancer, the concern that cancer requires long-term treatment, and the fear of cancer recurrence after recovery from treatment. 19 At the same time, the recurrence of cancer, metastasis and the uncertainty of shortened survival could all bring different degrees of mental stimulation, and these different traumatic events in turn have a cumulative effect on the patient’s spirit, and even trigger concurrent disorders, the most common being depression.19,20 Analysis of the results of the evaluation scales related to PTSD also reveals that the health status, physical functioning, social functioning, daytime energy, mental health, and emotional competence of patients with cancer-caused PTSD are significantly affected. 21 The PCL-C scale is a tool used to assess the symptoms of PTSD and can be used to assess the degree of PTSD in breast cancer patients. 22 In this study, ADP significantly reduced patients’ PCL-C scale scores and improved PTSD symptoms, and compared with paroxetine hydrochloride therapy alone, ADP combined with paroxetine hydrochloride could be a better treatment for BC-PTSD. Not only that, ADP had good efficacy on depression and anxiety as analyzed by SDS and SAS scale scores.23,24 In addition, PSQI scores showed that ADP had a significant improvement on sleep quality in BC-PTSD patients. In terms of the quality of survival for breast cancer patients, ADP enhanced quality of life, possibly due to its synergistic or adjunctive effects on PTSD and depression symptoms, as well as improved sleep quality.
From an inflammatory perspective, the inflammatory factors IL-1β and TNF-α are able to influence the morphology, function and cognitive level of the brain, which further affects neurodevelopment, synaptic plasticity and memory learning, and thus has an impact on the development of PTSD. 25 Patients suffering from breast cancer experiencce a physical and mental stress response that prompts the immune system to produce an inflammatory response and release excessive amounts of TNF-α. 26 These abnormal inflammatory responses and elevated TNF-α levels could adversely affect the brain and nervous system, leading to BC-PTSD development. 27 Meanwhile, TNF-α could affect the release and reuptake of neurotransmitters and alter signaling between neurons, which may be related to the symptoms of BC-PTSD such as fear, anxiety and sleep disturbances. 28 In addition, patients with BC-PTSD have abnormally high levels of TNF-α in their blood. 29 It suggests that TNF-α may be involved in regulating the inflammatory response and immune function during the development of BC-PTSD. IL-1β is one of the inflammatory factors associated with BC-PTSD, and it may participate in the pathogenesis of BC-PTSD. Elevated levels of IL-1β could cause abnormal neuronal development, impaired synaptic plasticity, and other changes. 30 Overactivation of IL-1β can trigger neuroinflammatory responses, which further affect normal brain function. 31 Additionally, IL-1β can also affect neurotransmitter release and neuronal excitability, thereby influencing emotions, memory, and stress responses. 32 BC-PTSD patients typically exhibit overactivation of the HPA axis, leading to elevated levels of CORT. 33 This excessive release may exacerbate inflammatory responses in the nervous system and disrupt immune system balance, potentially impairing normal neural function. 34 Additionally, heightened levels of serum CORT directly impact brain regions closely associated with emotional regulation and memory formation, such as the hippocampus and prefrontal cortex. 35 These physiological changes can intensify symptoms in BC-PTSD patients. Consistent with previous studies, in this study, serum IL-1β, TNF-α, and CORT levels were elevated in BC-PTSD patients, and ADP was able to reduce the levels of inflammatory factors and CORT.
Research indicates that the occurrence of PTSD is closely related to changes in BDNF. 36 BDNF plays a crucial role in the nervous system. It is involved in the survival, development, and functional regulation of nerve cells. After a traumatic event, BDNF levels decrease, leading to the onset of PTSD and pathophysiological changes. 37 BDNF could protect neurons and influence synaptic plasticity, and low levels of BDNF could result in neuronal damage and functional abnormalities. 38 Furthermore, BDNF is associated with behaviors such as memory, emotional regulation, and stress responses, leading to the development of PTSD symptoms. 39 This study also indicated that serum BDNF levels are reduced in BC-PTSD patients, while ADP could increase serum BDNF content.
Currently, the medications used for the treatment of PTSD are antidepressants, whose mechanism of action is primarily to alleviate the depression-related symptoms of the disease. The selective 5th reuptake inhibitors (SSRIs) sertraline, fluoxetine, paroxetine hydrochloride, norepinephrine, and the 5-hydroxytryptamine reuptake inhibitor venlafaxine are currently recognized as first-line therapeutic agents for the treatment of PTSD, with paroxetine hydrochloride and sertraline being the FDA-approved medications for the treatment of PTSD. 4 Despite the fact that SSRIs have improved PTSD symptoms to some extent, resistance still occurs in 20% to 30% of patients. 40 Moreover, these drugs are often accompanied by various adverse effects. For instance, the primary adverse effects of paroxetine hydrochloride include central nervous system issues (such as somnolence, insomnia, agitation, tremor, anxiety, and dizziness), gastrointestinal problems (such as constipation, nausea, diarrhea, dry mouth, vomiting, and flatulence), fatigue, and sexual dysfunction (including impotence and decreased libido). 7 Even though western medications have clear efficacy, they still cannot simultaneously treat or eliminate the core symptoms of PTSD. Therefore, the treatment of PTSD is also only for a certain symptom, for example, paroxetine hydrochloride is effective in improving depression in the type of PTSD. 41 Adverse effects and treatment specificity limit the widespread use of such drugs. The results of the statistical adverse reaction situation in this study showed that the administration of ADP could reduce the incidence of adverse reactions during treatment.
From clinical trials, we found that ADP could contribute to treatment of BC-PTSD, improve patients’ quality of life, and reduce the incidence of adverse effects. We conducted a reverse exploration of the ADP treatment mechanism for BC-PTSD through network analysis, and molecular docking. Results indicate that BDNF, IL-1β, and TNF-α, key biomarkers identified in clinical trials, are also critical targets in network pharmacological analysis, with ADP blood components binding well to these targets. The findings revealed that the treatment mechanism of ADP for BC-PTSD is associated with upregulating BDNF and inhibiting inflammatory cytokine levels. PPI results suggested that the targets of TP53, AKT1, IL6, TNF, INS, CTNNB1, ESR1, JUN, IL1B, and BCL2 have strong interactions and may also be the key mediators of ADP action. However, the mechanism of ADP on these targets needs further experimental verification.
GO/KEGG pathway enrichment analysis pointed out that the cancer pathway was most closely related to BC-PTSD. Cancer is a complex class of diseases whose development and progression involve the abnormal regulation of several signaling pathways. Among them, PI3K/Akt/mTOR are very common cancer pathways. 42 The relationship between the PI3K/Akt/mTOR pathway and BC-PTSD involves several aspects. First, in terms of neuroendocrine regulation, this pathway regulates the levels of stress hormones in the body by affecting the activity of the HPA axis. 43 Abnormal levels of glucocorticoid hormones in patients with BC-PTSD may lead to an imbalance in the PI3K/Akt/mTOR pathway imbalance, which in turn affects neuroendocrine homeostasis. 43 Second, in terms of neuroplasticity, this pathway is involved in regulating neuronal growth, differentiation and synaptic plasticity, and studies have shown that neuroplasticity is impaired in the brains of BC-PTSD patients, and changes in molecules such as BDNF may lead to abnormal neural networks. 44 In addition, the PI3K/Akt/mTOR pathway is involved in regulating immune cell function and influencing the inflammatory response, and as neuroinflammation is present in BC-PTSD patients, the role of this pathway in the inflammatory process may contribute to the development of the disease. 45 Finally, the pathway has an anti-apoptotic effect, whereas neuronal apoptosis is increased in patients with BC-PTSD, and the pathway imbalance may exacerbate neuronal cell damage. 46 It suggests that BC-PTSD serum inflammatory factor production and abnormal BDNF levels are associated with pathways such as PI3K/Akt/mTOR.
Molecular docking results indicated that salicylic acid, benzoic acid, and acoronene could bind to IL-1B or TNF, suggesting their anti-inflammatory properties. Study showed that salicylic acid, benzoic acid, and their derivatives exhibit strong anti-inflammatory effects. 5-amino salicylic acid reduced TNF-α and IL-1β levels in the blood of inflamed mice. 47 The benzoic acid derivative 3-({[4-(4-methoxyphenyl)-6-methyl-2-pyrimidinyl]thio}methyl) benzoic acid decreased LPS-induced IL-1β levels in microglia, inhibiting inflammation. 48 Acoronene is a bioactive component of Acori tatarinowii rhizoma but its anti-inflammatory effects remain unclear, further validation of molecular docking results is needed. 49 Nicotinic acid and benzoic acid could bind to BDNF, suggesting they may enhance BDNF neuroprotective effects. Nicotinic acid has been shown to reduce NLRP3 inflammasome levels in depressed mice while increasing BDNF levels. 50 The benzoic acid derivative sodium benzoate enhances BDNF and protein kinase A (PKA) activity in the PFC of stressed rats, counteracting depressive behaviors. 51 Although there is no direct evidence that these components in ADP have a direct effect on PTSD, their anti-inflammatory properties and BDNF activation mechanisms may improve behaviors related to depression.
There were several limitations in this study. First, given that this study was conducted in a single center, future studies should expand the sample size and add multiple study centers. Second, in this experiment, due to the absence of a placebo for the ADP in the control group, it is necessary to include one in subsequent experimental designs to ensure more persuasive results. Finally, patients in the clinical study underwent surgery combined with radiation or chemotherapy, which was not accounted for in the network analysis due to variations in individual treatment regimens, resulting in idealized analyses.
Conclusion
ADP contributes to the treatment of BC-PTSD symptoms, with a mechanism possibly related to its regulatory effect on TNF-α, IL-1β, and BDNF levels. This study provides an effective traditional Chinese medicine treatment for BC-PTSD.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354241285435 for Clinical Efficacy and Mechanistic Insights of Anshen Dingzhi Prescription on Breast Cancer-Related PTSD Through Network Pharmacology and Molecular Docking by Hao Zhang, Yongfu Zhu, Guoqi Zhu and Shaojie Yang in Integrative Cancer Therapies
Abbreviations
| ADP | Anshen Dingzhi prescription |
| BC-PTSD | Breast cancer-related post-traumatic stress disorder |
| BDNF | Brain-derived neurotrophic factor |
| BP | Biological processes |
| CC | Cellular components |
| CORT | Cortisol |
| FACT-B | Functional assessment of cancer therapy-breast |
| GO | Gene Ontology |
| IL-1β | Interleukin-1 beta |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MF | Molecular functions |
| PCL-C | Post-traumatic stress disorder checklist-civilian version |
| PPI | Protein-protein interaction |
| PSQI | Pittsburgh sleep quality index |
| SAS | Self-rating anxiety scale |
| SDS | Self-rating depression scale |
| SPS | Single prolonged stress |
| TNF-α | Tumor necrosis factor-alpha |
Footnotes
CRediT Authorship Contribution Statement: Hao Zhang: Investigation, data curation, writing–original draft, preparation. Yongfu Zhu: Conceptualization, project administration. Guoqi Zhu: Conceptualization, project administration, writing–review & editing. Shaojie Yang: Investigation, project administration, writing–review & editing.
Data Availability: Data will be made available based on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Research Funds of Center for Xin’an Medicine and Modernization of Traditional Chinese Medicine of IHM (2023CXMMTCM013), Anhui Natural Science Foundation (2208085MH282, S202302a04021999), Excellent Funding for Academic and Scientific Research Activities for Academic and Technological Leaders in Anhui Province (2022D317), Key Research and Development Plan of Anhui Province (202104j07020004), Anhui University of Chinese Medicine Research Foundation (2021yfylc44), Talent Research Funding Program (0500-52-1), and Anhui Science Foundation of Colleges and Universities (2022AH050415).
ORCID iDs: Hao Zhang  https://orcid.org/0009-0000-3113-6355
https://orcid.org/0009-0000-3113-6355
Yongfu Zhu  https://orcid.org/0000-0002-8158-4102
https://orcid.org/0000-0002-8158-4102
Shaojie Yang  https://orcid.org/0009-0009-6767-1101
https://orcid.org/0009-0009-6767-1101
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95:20211033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinapoli L, Colloca G, Di Capua B, Valentini V. Psychological aspects to consider in breast cancer diagnosis and treatment. Curr Oncol Rep. 2021;23:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Vol 5. American Psychiatric Publishing, Inc. 2013. [Google Scholar]
- 5. Wu X, Wang J, Cofie R, Kaminga AC, Liu A. Prevalence of posttraumatic stress disorder among breast cancer patients: a meta-analysis. Iran J Public Health. 2016;45:1533-1544. [PMC free article] [PubMed] [Google Scholar]
- 6. Brown LC, Murphy AR, Lalonde CS, et al. Posttraumatic stress disorder and breast cancer: risk factors and the role of inflammation and endocrine function. Cancer. 2020;126:3181-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hutters CL, Giraldi A. [Sexual side effects from treatment with SSRI]. Ugeskr Laeger. 2022;184:V11210824. [PubMed] [Google Scholar]
- 8. Wang J, Zhao P, Cheng P, et al. Exploring the effect of Anshen Dingzhi prescription on hippocampal mitochondrial signals in single prolonged stress mouse model. J Ethnopharmacol. 2024;323:117713. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Gao F, Yang S, et al. Mechanism of Anshen Dingzhi prescription intervening against anxiety-like behavior in post-traumatic stress disorder: an analysis based on network pharmacology and molecular docking. J Anhui Univ Chin Med. 2023;42:74-80. [Google Scholar]
- 10. Zhang Z, Song Z, Shen F, et al. Ginsenoside rg1 prevents PTSD-Like behaviors in mice through promoting synaptic proteins, reducing kir4.1 and TNF-α in the hippocampus. Mol Neurobiol. 2021;58:1550-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang S, Qu Y, Wang J, et al. Anshen Dingzhi prescription in the treatment of PTSD in mice: Investigation of the underlying mechanism from the perspective of hippocampal synaptic function. Phytomedicine. 2022;101:154139. [DOI] [PubMed] [Google Scholar]
- 12. Association B-C-P-C. Chinese anti-cancer society breast cancer diagnosis and treatment guidelines and standards (2021 edition). China Oncol. 2021;31:954-1040. [Google Scholar]
- 13. Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE. Psychometric properties of the PTSD checklist-Civilian version. J Trauma Stress. 2003;16:495-502. [DOI] [PubMed] [Google Scholar]
- 14. Biggs JT, Wylie LT, Ziegler VE. Validity of the Zung self-rating depression scale. Br J Psychiatry. 1978;132:381-385. [DOI] [PubMed] [Google Scholar]
- 15. Kellner R, Uhlenhuth EH. The rating and self-rating of anxiety. Br J Psychiatry. 1991;159:15-22. [PubMed] [Google Scholar]
- 16. Mollayeva T, Thurairajah P, Burton K, et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52-73. [DOI] [PubMed] [Google Scholar]
- 17. Chonghua W, Dongmei Z, Xueliang T, et al. Revision of the Chinese version of the FACT-B for patients with breast cancer. Chin Ment Heal J. 2003;5:298-300. [Google Scholar]
- 18. Yang S, Chen Y, Zhu G. The Pathogenesis of post-traumatic stress disorder and the prevention and treatment of TCM were analyzed from heart and kidney. Clin J Tradit Chin -med. 2021;33:1011-1015. [Google Scholar]
- 19. Hajj A, Hachem R, Khoury R, et al. Clinical and genetic factors associated with anxiety and depression in breast cancer patients: a cross-sectional study. BMC Cancer. 2021;21:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Wang N, Zhong L, et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: a systematic review and meta-analysis of 282,203 patients. Mol Psychiatry. 2020;25:3186-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. Lancet Psychiatry. 2017;4:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LeardMann CA, McMaster HS, Warner S, et al. Comparison of posttraumatic stress disorder checklist instruments from diagnostic and statistical manual of mental disorders, fourth edition vs fifth edition in a large cohort of US military service members and veterans. JAMA Netw Open. 2021;4:e218072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong H. Clinical observation on the treatment of insomnia of heart and gallbladder qi deficiency type with the addition and subtraction of Anshen Dingzhi Wan. J Tradit Chin Med. 2020;36:48. [Google Scholar]
- 24. Zhang F. Clinical analysis of the treatment of heart-gallbladder-qi deficiency type depression with Tranquilizing Spirit and Tingzhi Pill. Guid China Med. 2019;17:164-165. [Google Scholar]
- 25. Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019;73:143-153. [DOI] [PubMed] [Google Scholar]
- 26. Jain M, Mishra A, Yadav V, et al. Long-term yogic intervention improves symptomatic scale and quality of life by reducing inflammatory cytokines and oxidative stress in breast cancer patients undergoing chemotherapy and/or radiotherapy: a randomized control study. Cureus. 2023;15:e33427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben-Azu B, Adebayo OG, Moke EG, et al. Geraniol attenuates behavioral and neurochemical impairments by inhibitions of HPA-axis and oxido-inflammatory perturbations in mice exposed to post-traumatic stress disorder. J Psychiatr Res. 2023;168:165-175. [DOI] [PubMed] [Google Scholar]
- 28. Wieck A, Grassi-Oliveira R, Hartmann do, Prado C, Teixeira AL, Bauer ME. Neuroimmunoendocrine interactions in post-traumatic stress disorder: focus on long-term implications of childhood maltreatment. Neuroimmunomodulation. 2014;21:145-151. [DOI] [PubMed] [Google Scholar]
- 29. Gottshall JL, Guedes VA, Pucci JU, et al. Poor sleep quality is linked to elevated extracellular vesicle-associated inflammatory cytokines in warfighters with chronic mild traumatic brain injuries. Front Pharmacol. 2021;12:762077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Puma DD, Colussi C, Bandiera B, et al. Interleukin 1β triggers synaptic and memory deficits in herpes simplex virus type-1-infected mice by downregulating the expression of synaptic plasticity-related genes via the epigenetic MeCP2/HDAC4 complex. Cell Mol Life Sci. 2023;80:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soltani Khaboushan A, Yazdanpanah N, Rezaei N. Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol Neurobiol. 2022;59:1724-1743. [DOI] [PubMed] [Google Scholar]
- 32. Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry. 2006;39:88-99. [DOI] [PubMed] [Google Scholar]
- 33. Murphy F, Nasa A, Cullinane D, et al. Childhood trauma, the HPA axis and psychiatric illnesses: A targeted literature synthesis. Front Psychiatry. 2022;13:748372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan J, Lu Y, Wang S, et al. Synergistic neuroprotective effects of two natural medicinal plants against CORT-induced nerve cell injury by correcting neurotransmitter deficits and inflammation imbalance. Phytomedicine. 2023;121:155102. [DOI] [PubMed] [Google Scholar]
- 35. Govindula A, Ranadive N, Nampoothiri M, et al. Emphasizing the crosstalk between inflammatory and neural signaling in post-traumatic stress disorder (PTSD). J Neuroimmune Pharmacol. 2023;18:248-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251-2274. [DOI] [PubMed] [Google Scholar]
- 37. Mojtabavi H, Saghazadeh A, van den Heuvel L, Bucker J, Rezaei N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): a systematic review and meta-analysis. PLoS One. 2020;15:e0241928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Domitrovic Spudic S, Nikolac Perkovic M, Uzun S, et al. Reduced plasma BDNF concentration and cognitive decline in veterans with PTSD. Psychiatry Res. 2022;316:114772. [DOI] [PubMed] [Google Scholar]
- 39. Bazzari AH, Bazzari FH. BDNF therapeutic mechanisms in neuropsychiatric disorders. Int J Mol Sci. 2022;23:8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatr Clin Pract. 2012;16:77-84. [DOI] [PubMed] [Google Scholar]
- 41. Dutt R, Shankar N, Srivastava S, Yadav A, Ahmed RS. Cardiac autonomic tone, plasma BDNF levels and paroxetine response in newly diagnosed patients of generalised anxiety disorder. Int J Psychiatr Clin Pract. 2020;24:135-142. [DOI] [PubMed] [Google Scholar]
- 42. Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 2021;9:1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, Li H, Fang F, et al. Geniposide improves repeated restraint stress-induced depression-like behavior in mice by ameliorating neuronal apoptosis via regulating GLP-1R/AKT signaling pathway. Neurosci Lett. 2018;676:19-26. [DOI] [PubMed] [Google Scholar]
- 44. Li H, Xue X, Li L, et al. Aluminum-induced synaptic plasticity impairment via PI3K-Akt-mTOR signaling pathway. Neurotox Res. 2020;37:996-1008. [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Li Z, Sun R, et al. Zunyimycin C enhances immunity and improves cognitive impairment and its mechanism. Front Cell Infect Microbiol. 2022;12:1081243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Wang B, Liu Y, et al. Inhibition of PI3K/Akt/mTOR signaling by NDRG2 contributes to neuronal apoptosis and autophagy in ischemic stroke. J Stroke Cerebrovasc Dis. 2023;32:106984. [DOI] [PubMed] [Google Scholar]
- 47. Jhundoo HD, Siefen T, Liang A, et al. Hyaluronic acid increases anti-inflammatory efficacy of rectal 5-amino salicylic acid administration in a murine colitis model. Biomol Ther. 2021;29:536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng LT, Chen J, Zhang L, et al. Inhibition of neuroinflammation by MIF inhibitor 3-({[4-(4-methoxyphenyl)-6-methyl-2-pyrimidinyl]thio}1methyl)benzoic acid (Z-312). Int Immunopharmacol. 2021;98:107868. [DOI] [PubMed] [Google Scholar]
- 49. Ni G, Yu DQ. [Chemical constituents from rhizomes of Acorus tatarinowii]. Zhongguo Zhong Yao Za Zhi. 2013;38:569-573. [PubMed] [Google Scholar]
- 50. Abdel Rasheed NO, Shiha NA, Mohamed SS, Ibrahim WW. SIRT1/PARP-1/NLRP3 cascade as a potential target for niacin neuroprotective effect in lipopolysaccharide-induced depressive-like behavior in mice. Int Immunopharmacol. 2023;123:110720. [DOI] [PubMed] [Google Scholar]
- 51. Guo F, Zhang Z, Liang Y, Yang R, Tan Y. Exploring the role and mechanism of sodium benzoate in CUMS-induced depression model of rats. Neuro Endocrinol Lett. 2020;41:205-212. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354241285435 for Clinical Efficacy and Mechanistic Insights of Anshen Dingzhi Prescription on Breast Cancer-Related PTSD Through Network Pharmacology and Molecular Docking by Hao Zhang, Yongfu Zhu, Guoqi Zhu and Shaojie Yang in Integrative Cancer Therapies