Abstract
Background
Cardiometabolic multimorbidity (CMM) and depression are major health concerns, and the onset of either condition may heighten the risk of developing the other.
Objectives
The goal of this study was to characterize the reciprocal associations between CMM and depression among middle-aged and older adults.
Methods
This multicohort study used harmonized data from 5 prospective cohorts from China, South Korea, the United States, the United Kingdom, and Europe. Cardiometabolic diseases (CMDs) (including diabetes, heart diseases, and stroke) and depression were assessed at baseline and at 7 to 8 years’ follow-up. Lifestyle factors, including physical activity, alcohol consumption, and smoking status, were regarded as potential mediators. Two sets of analyses, CMM-depression analyses (n = 67,188) and depression-CMM analyses (n = 65,738), were conducted to explore the bidirectional associations between CMM and depression.
Results
In the CMM-depression analyses, 16,596 (24.7%) individuals developed depression. Participants with a single CMD (HR: 1.24; 95% CI:1.19-1.29) and CMM (HR: 1.52; 95% CI: 1.42-1.63) at baseline had higher risks of depression occurring. Physical activity and alcohol consumption significantly mediated 7.5% and 6.9% of the CMM-depression association, respectively. In the depression-CMM analyses, 1,461 (2.2%) participants developed CMM. The HR for developing CMM was 1.31 (95% CI: 1.14-1.50) in patients with depression, with increased risk of developing more CMDs. Physical activity and alcohol consumption mediated 12.0% and 7.1% of the depression-CMM association. The bidirectional relationships were more pronounced in Western countries than in Asian countries.
Conclusions
CMM and depression were bidirectionally associated. The mediated effects of lifestyle factors were larger in the depression-lifestyle-CMM pathway than in the CMM-lifestyle-depression pathway.
Key Words: cardiovascular diseases, diabetes, lifestyle modifications, mental health, reciprocal relationship
Central Illustration
Depression, a common and debilitating psychiatric disorder, affects approximately 6% of the global population.1,2 In 2017, the World Health Organization listed depression as a leading cause of disability worldwide.3 In addition to its psychological impact, depression is often accompanied by somatic conditions4 such as dyslipidemia, rheumatoid arthritis, sleep disorders, and cardiometabolic diseases (CMDs), including diabetes, heart diseases, and stroke.5, 6, 7 Recent trends, including population aging, increased life expectancy, and lifestyle changes, have contributed to the accumulation of chronic conditions, leading to the increase in multimorbidity.8 In particular, there has been significant research interest in cardiometabolic multimorbidity (CMM), which is defined as the co-existence of two or more CMDs.9 CMM increases the risk of activity limitation, cognitive dysfunction, dementia, and all-cause mortality,10,11 presenting an ongoing challenge to global health care systems.
Recent research has provided evidence on the relationship between depression and CMDs. A prospective study with 27-year follow-up found that depression increased the risk of cardiometabolic health problems (eg, diabetes, erectile dysfunction, sleep apnea).12 This finding was supported by two other cohort studies.13,14 The longitudinal association of CMDs with later depression has also been explored. For example, diabetes was found to be associated with increased risk of developing depression,15 and patients with stroke had a higher incidence of depression after hospitalization.16 However, these studies focused on single CMDs only, and, to the best of our knowledge, no current study has shed light directly on the reciprocal associations between depression and CMM. A population-based cohort study of 93,076 adults examined the bidirectional associations between depression and cardiovascular diseases (ischemic heart disease and stroke), but this study did not include diabetes and was exclusively conducted in Denmark, thus limiting the generalizability of its findings.17
CMM and depression are long-lasting and progressive, both of which could be partly attributable to modifiable indicators (eg, lifestyle factors).18,19 However, whether their bidirectional associations could be mediated by lifestyle factors remains unknown. To provide valuable insights for strategies to prevent and manage these significant physical and mental health challenges in the population,20 we leveraged harmonized data across 19 countries from Asia, North America, and Europe to generate robust evidence on the bidirectional associations between CMM and depression and the mediation effects of lifestyle factors among middle-aged and older adults.
Methods
Study Design and Participants
This prospective multicohort study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline. Data were pooled across 19 countries from 5 sister cohort studies from the Program on Global Aging, Health and Policy21: CHARLS (China Health and Retirement Longitudinal Study); KLoSA (Korean Longitudinal Study of Aging); HRS (U.S. Health and Retirement Study); ELSA (English Longitudinal Study on Ageing); and SHARE (Survey of Health, Ageing and Retirement in Europe). These studies followed similar protocols and administered surveys every 2 or 3 years, therefore allowing meaningful cross-study comparisons. Additional details for each study have been described elsewhere.22, 23, 24, 25, 26
We used data from 4 waves of a consistent time period in each study, including waves 1 to 4 of CHARLS (2011-2019), waves 4 to 7 of KLoSA (2012-2019), waves 11 to 14 of HRS (2012-2019), waves 6 to 9 of ELSA (2012-2019), and waves 5 to 8 of SHARE (2013-2020) (Supplemental Table 1). In our analyses, we included participants if they were middle-aged and older adults (aged ≥45 years) and reported complete information on CMDs and depression at baseline. Participants aged >85 years at baseline were excluded to minimize survival bias.
This study has been exempt from ethical review because it was a secondary analysis of a public data set.
CMDs and CMM
The range of CMDs included self-reported diabetes, heart diseases, and stroke,27 with definitions slight varied across studies. Heart diseases mainly referred to heart attack, coronary heart disease, angina, congestive heart failure, and other heart problems. The definition of stroke included ischemic attack in KLoSA and HRS; in ELSA and SHARE, it included cerebrovascular diseases. CMM was identified as the coexistence of ≥2 CMDs in an individual. In analyses of bidirectional associations, 2 variables were used: CMM status (no and yes) and CMD status (free of CMD, single CMD, and CMM). We additionally examined the number (0, 1, 2, and 3 CMDs) and combinations of CMDs, including the following groups: 1) none; 2) diabetes; 3) heart diseases; 4) stroke; 5) diabetes and heart diseases; 6) heart diseases and stroke; 7) diabetes and stroke; and 8) diabetes, heart diseases, and stroke.
Depression
In CHARLS and KLoSA, depression was assessed by using the 10-item version of the Center for Epidemiologic Studies Depression Scale. In HRS and ELSA, depression was assessed by using the 8-item version of the Center for Epidemiologic Studies Depression Scale. In SHARE, depression was assessed by using the 12-item version of the EURO-D. Individuals were then dichotomized into 2 groups of non-depression and depression using predefined study-specific cutoff values.28, 29, 30, 31, 32 Detailed description of the assessment in each study is provided in Supplemental Table 2.
Covariates
Covariates, including demographic characteristics (age, sex, and study), socioeconomic status (marital status, educational level, and total household wealth), lifestyle factors (smoking status, drinking status, physical activity, and body mass index), history of chronic conditions (hypertension, cancer, and lung diseases), and medication of chronic conditions (hypertension and lung diseases) were assessed. Lifestyle factors and medication of chronic conditions were regarded as time-dependent variables, according to previous research and empirical evidence.33 Total household wealth was calculated as the sum of all wealth components, including residence, business, vehicles, and savings account, excluding other debt or loans, and was categorized into quartiles. Further details of the measurement and harmonization of the covariates in each study are provided in Supplemental Table 2.
Statistical Analysis
The baseline characteristics of participants are described as number (percentage) according to CMD status and depression at baseline, and chi-square tests were used to compare the between-group differences. Two sets of analyses, CMM-depression analyses and depression-CMM analyses, were conducted to explore the bidirectional associations between CMM and depression.
CMM-Depression Analyses
Cox regression models with time-dependent covariates were used to estimate the HRs and 95% CIs of the association between baseline CMM and depression incidence during follow-up. Participants were censored at interview month of depression or death, or end of follow-up of each cohort, whichever came first. Individuals were recorded as incident depression events if the depressive symptoms assessed by using study-specific instruments first appeared during follow-up. Considering the repeated measures on the same participants, we also applied generalized estimating equations (GEEs) for Poisson regression to estimate relative risks (RRs) and 95% CIs of the CMM-depression association. The mediation effects of lifestyles were further examined by using the mediation package in R (R Foundation for Statistical Computing). Physical activity, smoking status, and alcohol consumption were set as the mediators to estimate the total, indirect, and direct effects of CMM on depression, with the proportion mediated calculated via dividing indirect effect by total effect. Subgroup analyses were performed to compare the association of baseline CMM with incidence of depression among participants with different age (<65 years vs ≥65 years), sex, educational level, total household income, and studies. Considering the heterogeneity across studies, we conducted meta-analyses using random-effects models to summarize study-specific estimates for the CMM-depression association among the whole sample and sex-stratified samples, with the between-study heterogeneity tested using I2 statistics. The multivariable logistic regression models were conducted to examine ORs and 95% CIs of the association between CMM at baseline and depression trajectory during follow-up, using a group-based trajectory model implemented with the PROC TRAJ procedure in SAS (Supplemental Methods).
Depression-CMM Analyses
Cox regression models with time-dependent covariates and the GEEs for Poisson regression were conducted to estimate HRs, RRs, and 95% CIs of the association between depression and CMM incidence. In the Cox regression models, participants were censored at interview month of CMM or death, or end of follow-up of each cohort, whichever came first. Individuals were recorded as incident CMM events if they reported to have CMM for the first time. Correspondingly, the mediation effects of lifestyles (physical activity, smoking status, and alcohol consumption) for the depression-CMM analyses were further examined. Subgroup analyses and meta-analyses using random-effects models for the association between depression and CMM incidence were also performed.
All tests in this study were 2-sided with a significance level of P < 0.05. Statistical analyses were conducted by using SAS version 9.4 (SAS Institute, Inc) and R version 4.2.2.
Results
Baseline Characteristics
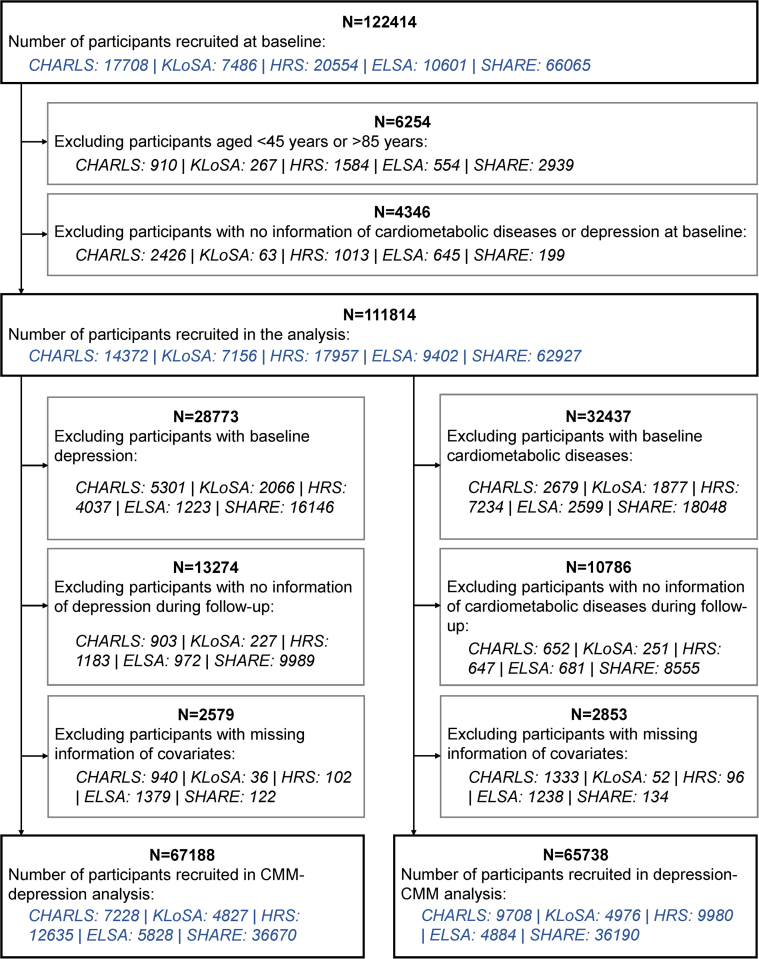
A total of 111,814 participants provided information on CMDs and depression at baseline. Participants already with depression at baseline (n = 28,773), lacking information on depression during follow-up (n = 13,274), or with missing information of covariates (n = 2,579) were excluded, yielding the final sample of 67,188 individuals for the CMM-depression analyses. The mean age of participants in the CMM-depression analyses was 64.36 ± 9.22 years, and 35,243 (52.5%) were female. Of these, 7,228 participants were from China, 4,827 from South Korea, 12,635 participants were from the United States, and 42,498 from the United Kingdom and 15 other European countries (Figure 1, Supplemental Table 1). At baseline, 49,892 (74.3%) of 67,188 individuals did not have any CMDs, 14,082 (21.0%) had a single CMD, and 3,214 (4.8%) had CMM. Table 1 shows the baseline characteristics of participants in the CMM-depression analyses according to CMD status.
Figure 1.
Flow Diagram of Participant Selection
Participants were selected according to the inclusion and exclusion criteria, yielding 2 sample sets for the bidirectional analyses. CHARLS = China Health and Retirement Longitudinal Study; CMM = cardiometabolic multimorbidity; ELSA = English Longitudinal Study on Ageing; HRS = U.S. Health and Retirement Study; KLoSA = Korean Longitudinal Study of Aging; SHARE = Survey of Health, Ageing and Retirement in Europe.
Table 1.
Baseline Characteristics of Participants Included in the CMM-Depression Analyses According to CMD Status
| Total (N = 67,188) | CMD Status at Baseline |
Chi-Square | P Value | |||
|---|---|---|---|---|---|---|
| None (n = 49,892) | Single CMD (n = 14,082) | CMM (n = 3,214) | ||||
| Study | 1,434.75 | <0.001 | ||||
| CHARLS | 7,228 (10.8%) | 6,195 (12.4%) | 901 (6.4%) | 132 (4.1%) | ||
| KLoSA | 4,827 (7.2%) | 3,794 (7.6%) | 890 (6.3%) | 143 (4.4%) | ||
| HRS | 12,635 (18.8%) | 8,045 (16.1%) | 3,533 (25.1%) | 1,057 (32.9%) | ||
| ELSA | 5,828 (8.7%) | 4,361 (8.7%) | 1,276 (9.1%) | 191 (5.9%) | ||
| SHARE | 36,670 (54.6%) | 27,497 (55.1%) | 7,482 (53.1%) | 1,691 (52.6%) | ||
| Age, y | 3,867.40 | <0.001 | ||||
| 45-54 | 10,556 (15.7%) | 9,374 (18.8%) | 1,054 (7.5%) | 128 (4.0%) | ||
| 55-64 | 25,054 (37.3%) | 20,138 (40.4%) | 4,175 (29.6%) | 741 (23.1%) | ||
| 65-74 | 20,539 (30.6%) | 14,080 (28.2%) | 5,199 (36.9%) | 1,260 (39.2%) | ||
| ≥75 | 11,039 (16.4%) | 6,300 (12.6%) | 3,654 (25.9%) | 1,085 (33.8%) | ||
| Sex | 377.79 | <0.001 | ||||
| Male | 31,945 (47.5%) | 22,634 (45.4%) | 7,506 (53.3%) | 1,805 (56.2%) | ||
| Female | 35,243 (52.5%) | 27,258 (54.6%) | 6,576 (46.7%) | 1,409 (43.8%) | ||
| Education level | 44.58 | <0.001 | ||||
| Primary | 22,926 (34.1%) | 16,732 (33.5%) | 4,955 (35.2%) | 1,239 (38.6%) | ||
| Secondary | 26,127 (38.9%) | 19,526 (39.1%) | 5,409 (38.4%) | 1,192 (37.1%) | ||
| Tertiary | 18,135 (27.0%) | 13,634 (27.3%) | 3,718 (26.4%) | 783 (24.4%) | ||
| Total household wealtha | 621.61 | <0.001 | ||||
| Q1 (lowest) | 16,281 (25.0%) | 11,071 (23.0%) | 4,085 (29.5%) | 1,125 (35.3%) | ||
| Q2 | 16,351 (25.1%) | 11,910 (24.7%) | 3,596 (26.0%) | 845 (26.5%) | ||
| Q3 | 16,295 (25.0%) | 12,328 (25.6%) | 3,287 (23.8%) | 680 (21.3%) | ||
| Q4 (highest) | 16,306 (25.0%) | 12,911 (26.8%) | 2,859 (20.7%) | 536 (16.8%) | ||
| Marital status | 426.87 | <0.001 | ||||
| Married or partnered | 52,791 (78.6%) | 39,903 (80.0%) | 10,588 (75.2%) | 2,300 (71.7%) | ||
| Separated or divorced | 5,035 (7.5%) | 3,635 (7.3%) | 1,138 (8.1%) | 262 (8.2%) | ||
| Widowed | 6,846 (10.2%) | 4,429 (8.9%) | 1,883 (13.4%) | 534 (16.6%) | ||
| Never married | 2,484 (3.7%) | 1,904 (3.8%) | 466 (3.3%) | 114 (3.6%) | ||
| Physical activity | 529.61 | <0.001 | ||||
| No | 8,497 (13.4%) | 5,528 (11.9%) | 2,189 (16.1%) | 780 (24.8%) | ||
| Yes | 54,710 (86.6%) | 40,958 (88.1%) | 11,390 (83.9%) | 2,362 (75.2%) | ||
| Alcohol assumption | 410.87 | <0.001 | ||||
| Less than weekly drinking | 36,399 (54.9%) | 25,972 (52.8%) | 8,264 (59.3%) | 2,163 (67.8%) | ||
| Weekly drinking or more | 29,930 (45.1%) | 23,223 (47.2%) | 5,682 (40.7%) | 1,025 (32.2%) | ||
| Current smoking | 162.48 | <0.001 | ||||
| No | 55,349 (82.4%) | 40,559 (81.3%) | 11,998 (85.2%) | 2,792 (86.9%) | ||
| Yes | 11,834 (17.6%) | 9,329 (18.7%) | 2,084 (14.8%) | 421 (13.1%) | ||
| Body mass index, kg/m2 | 2,208.95 | <0.001 | ||||
| <18.5 | 958 (1.5%) | 802 (1.7%) | 142 (1.0%) | 14 (0.4%) | ||
| 18.5-24.9 | 23,780 (36.5%) | 19,602 (40.6%) | 3,580 (26.2%) | 598 (19.2%) | ||
| 25-29.9 | 25,525 (39.2%) | 18,709 (38.7%) | 5,613 (41.0%) | 1,203 (38.6%) | ||
| ≥30.0 | 14,849 (22.8%) | 9,206 (19.1%) | 4,341 (31.7%) | 1,302 (41.8%) | ||
| Hypertension | 5,101.87 | <0.001 | ||||
| No | 38,712 (57.6%) | 32,638 (65.4%) | 5,359 (38.1%) | 715 (22.2%) | ||
| Yes | 28,460 (42.4%) | 17,239 (34.6%) | 8,722 (61.9%) | 2,499 (77.8%) | ||
| Cancer | 313.60 | <0.001 | ||||
| No | 61,867 (92.1%) | 46,464 (93.1%) | 12,607 (89.5%) | 2,796 (87.0%) | ||
| Yes | 5,313 (7.9%) | 3,424 (6.9%) | 1,472 (10.5%) | 417 (13.0%) | ||
| Lung diseases | 492.44 | <0.001 | ||||
| No | 62,946 (93.7%) | 47,291 (94.8%) | 12,868 (91.4%) | 2,787 (86.7%) | ||
| Yes | 4,233 (6.3%) | 2,595 (5.2%) | 1,212 (8.6%) | 426 (13.3%) | ||
| Medication for hypertension | 4,879.99 | <0.001 | ||||
| No | 42,787 (63.8%) | 35,456 (71.2%) | 6,393 (45.5%) | 938 (29.3%) | ||
| Yes | 24,233 (36.2%) | 14,314 (28.8%) | 7,657 (54.5%) | 2,262 (70.7%) | ||
| Medication for lung disease | 231.69 | <0.001 | ||||
| No | 65,584 (97.7%) | 48,955 (98.2%) | 13,582 (96.6%) | 3,047 (95.0%) | ||
| Yes | 1,552 (2.3%) | 909 (1.8%) | 484 (3.4%) | 159 (5.0%) | ||
Values are n (%) unless otherwise indicated.
CHARLS = China Health and Retirement Longitudinal Study; CMD = cardiometabolic disease; CMM = cardiometabolic multimorbidity; ELSA = English Longitudinal Study on Ageing; HRS = U.S. Health and Retirement Study; KLoSA = Korean Longitudinal Study of Aging; Q = quartile; SHARE = Survey of Health, Ageing and Retirement in Europe.
Total household wealth was categorized into quartiles.
Similarly, for the depression-CMM analyses, this study excluded participants already with any CMDs at baseline (n = 32,437), lacking information on CMDs during follow-up (n = 10,786), or with missing information of covariates (n = 2,853), leaving 65,738 participants in the analyses. Their mean age was 63.02 ± 9.13 years, and 37,970 (57.8%) were female. There were 9,708 individuals from China, 4,976 from South Korea, 9,980 individuals from the United States, and 41,074 from the United Kingdom, and 15 from other European countries (Figure 1, Supplemental Table 1). A total of 14,673 (22.3%) participants had depression at baseline; Table 2 shows the baseline characteristics of participants in the depression-CMM analyses according to depression status.
Table 2.
Baseline Characteristics of Participants Included in the Depression-CMM Analyses According to Depression Status
| Total (N = 65,738) | Depression at Baseline |
Chi-Square | P Value | ||
|---|---|---|---|---|---|
| No (n = 51,065) | Yes (n = 14,673) | ||||
| Study | 648.37 | <0.001 | |||
| CHARLS | 9,708 (14.8%) | 6,348 (12.4%) | 3,360 (22.9%) | ||
| KLoSA | 4,976 (7.6%) | 3,794 (7.4%) | 1,182 (8.1%) | ||
| HRS | 9,980 (15.2%) | 8,146 (16.0%) | 1,834 (12.5%) | ||
| ELSA | 4,884 (7.4%) | 4,392 (8.6%) | 492 (3.4%) | ||
| SHARE | 36,190 (55.1%) | 28,385 (55.6%) | 7,805 (53.2%) | ||
| Age, y | 62.45 | <0.001 | |||
| 45-54 | 12,753 (19.4%) | 9,623 (18.8%) | 3,130 (21.3%) | ||
| 55-64 | 26,274 (40.0%) | 20,574 (40.3%) | 5,700 (38.8%) | ||
| 65-74 | 18,017 (27.4%) | 14,376 (28.2%) | 36,41 (24.8%) | ||
| ≥75 | 8,694 (13.2%) | 6,492 (12.7%) | 2,202 (15.0%) | ||
| Sex | 535.75 | <0.001 | |||
| Male | 27,768 (42.2%) | 23,211 (45.5%) | 4,557 (31.1%) | ||
| Female | 37,970 (57.8%) | 27,854 (54.5%) | 10,116 (68.9%) | ||
| Education level | 660.38 | <0.001 | |||
| Primary | 24,569 (37.4%) | 17,237 (33.8%) | 7,332 (50.0%) | ||
| Secondary | 24,763 (37.7%) | 19,917 (39.0%) | 4,846 (33.0%) | ||
| Tertiary | 16,406 (25.0%) | 13,911 (27.2%) | 2,495 (17.0%) | ||
| Total household wealtha | 632.53 | <0.001 | |||
| Q1 (lowest) | 15,784 (24.9%) | 11,058 (22.4%) | 4,726 (33.9%) | ||
| Q2 | 15,829 (25.0%) | 12,205 (24.7%) | 3,624 (26.0%) | ||
| Q3 | 15,857 (25.1%) | 12,750 (25.8%) | 3,107 (22.3%) | ||
| Q4 (highest) | 15,830 (25.0%) | 13,355 (27.1%) | 2,475 (17.8%) | ||
| Marital status | 337.07 | <0.001 | |||
| Married or partnered | 51,339 (78.1%) | 40,837 (80.0%) | 10,502 (71.6%) | ||
| Separated or divorced | 5,046 (7.7%) | 3,700 (7.2%) | 1,346 (9.2%) | ||
| Widowed | 6,748 (10.3%) | 4,548 (8.9%) | 2,200 (15.0%) | ||
| Never married | 2,580 (3.9%) | 1,958 (3.8%) | 622 (4.2%) | ||
| Physical activity | 398.46 | <0.001 | |||
| No | 8,432 (14.0%) | 5,649 (11.9%) | 2,783 (21.7%) | ||
| Yes | 51,955 (86.0%) | 41,937 (88.1%) | 10,018 (78.3%) | ||
| Alcohol assumption | 409.26 | <0.001 | |||
| Less than weekly drinking | 36,275 (56.0%) | 26,651 (52.9%) | 9,624 (66.5%) | ||
| Weekly drinking or more | 28,555 (44.0%) | 23,710 (47.1%) | 4,845 (33.5%) | ||
| Current smoking | 119.01 | <0.001 | |||
| No | 52,656 (80.1%) | 41,448 (81.2%) | 11,208 (76.4%) | ||
| Yes | 13,076 (19.9%) | 9,613 (18.8%) | 3,463 (23.6%) | ||
| Body mass index, kg/m2 | 145.18 | <0.001 | |||
| <18.5 | 1,358 (2.1%) | 824 (1.7%) | 534 (3.8%) | ||
| 18.5-24.9 | 26,247 (41.3%) | 20,109 (40.7%) | 6,138 (43.8%) | ||
| 25-29.9 | 23,811 (37.5%) | 19,158 (38.7%) | 4,653 (33.2%) | ||
| ≥30.0 | 12,074 (19.0%) | 9,377 (19.0%) | 2,697 (19.2%) | ||
| Hypertension | 37.15 | <0.001 | |||
| No | 42,542 (64.7%) | 33,417 (65.5%) | 9,125 (62.2%) | ||
| Yes | 23,166 (35.3%) | 17,632 (34.5%) | 5,534 (37.8%) | ||
| Cancer | 9.53 | <0.001 | |||
| No | 61,119 (93.0%) | 47,578 (93.2%) | 13,541 (92.3%) | ||
| Yes | 4,610 (7.0%) | 3,483 (6.8%) | 1,127 (7.7%) | ||
| Lung diseases | 193.41 | <0.001 | |||
| No | 61,636 (93.8%) | 48,406 (94.8%) | 13,230 (90.2%) | ||
| Yes | 4,095 (6.2%) | 2,653 (5.2%) | 1,442 (9.8%) | ||
| Medication for hypertension | 9.73 | 0.001 | |||
| No | 46,536 (71.0%) | 36,307 (71.3%) | 10,229 (69.9%) | ||
| Yes | 19,042 (29.0%) | 14,631 (28.7%) | 4,411 (30.1%) | ||
| Medication for lung disease | 103.10 | <0.001 | |||
| No | 64,204 (97.7%) | 50,107 (98.2%) | 14,097 (96.1%) | ||
| Yes | 1,498 (2.3%) | 929 (1.8%) | 569 (3.9%) | ||
Values are n (%).
Abbreviations as in Table 1.
Total household wealth was categorized into quartiles.
CMM-Depression Analyses: the Association Between CMM and Incidence of Depression
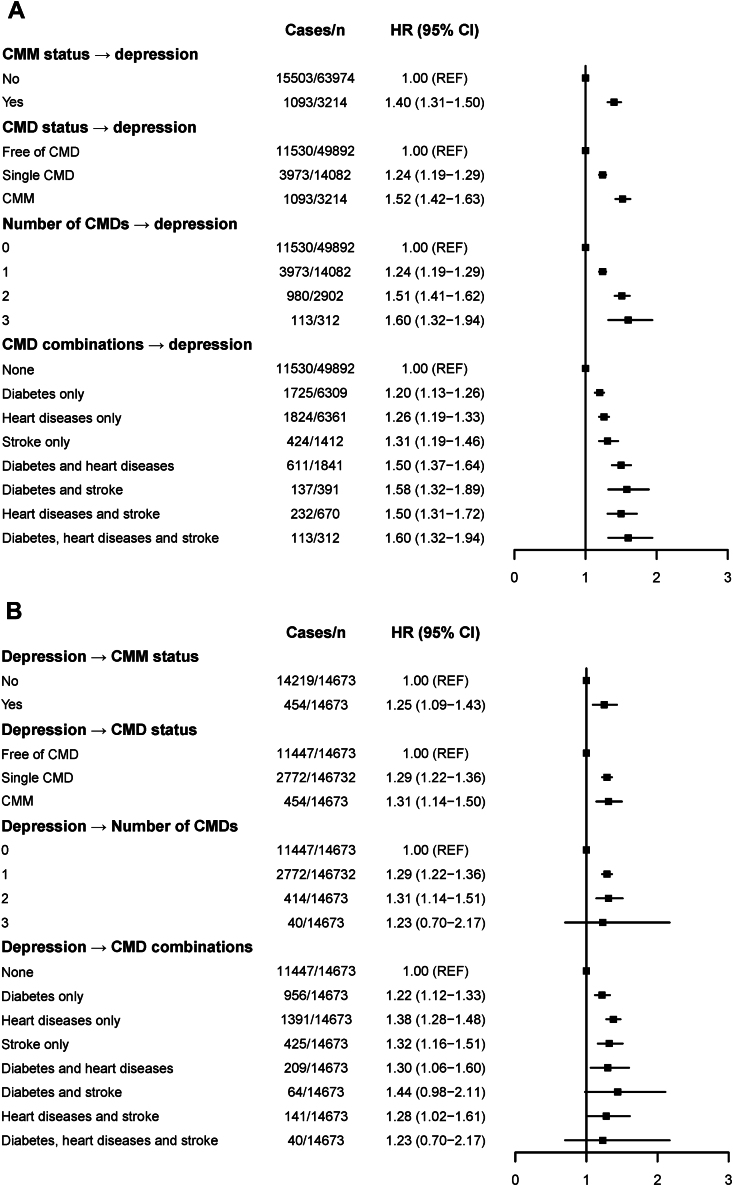
Of the 67,188 participants in the CMM-depression analyses, 16,596 (24.7%) progressed to depression during follow-up. Figure 2A displays the association between baseline CMM and depression incidence during follow-up using Cox regression models. Individuals with a single CMD (HR: 1.24; 95% CI: 1.19-1.29) and CMM (HR: 1.52; 95% CI: 1.42-1.63) at baseline had higher risks of developing depression. The risk of depression incidence rose as the number of baseline CMDs increased, with HRs ranging from 1.24 (95% CI: 1.19-1.29) to 1.60 (95% CI: 1.32-1.94). Those with combinations of diabetes, heart diseases, and stroke had the highest risk of developing depression (HR: 1.60; 95% CI: 1.32-1.94) (Central Illustration). Results from the GEE analyses also showed an increased risk of depression in those with a single CMD (RR: 1.34; 95% CI: 1.29-1.40) and CMM (RR: 1.78; 95% CI: 1.68-1.89), after adjusting for covariates (Supplemental Table 3).
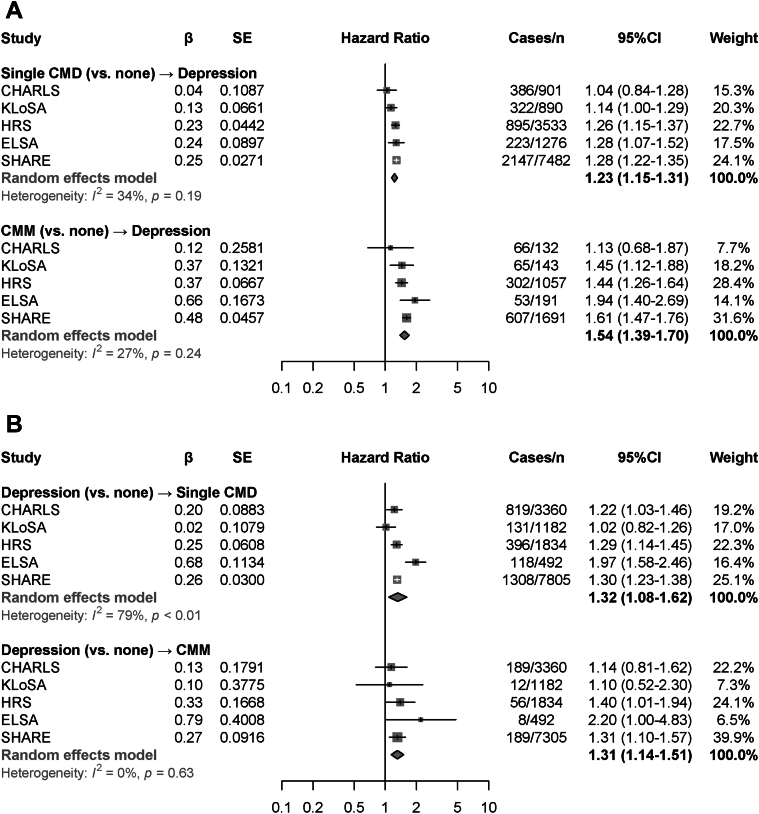
Figure 2.
Associations Between CMM and Depression
The forest plots show the HRs and 95% CIs for the cardiometabolic multimorbidity (CMM)-depression analyses (A) and the depression-CMM analyses (B) generated by Cox regression models with time-dependent covariates. Models were adjusted for age, sex, and study, marital status, educational level, and total household wealth, smoking status, drinking status, physical activity, body mass index (BMI), history of hypertension, cancer, and lung diseases, and medication of hypertension and lung diseases. CMD = cardiometabolic disease.
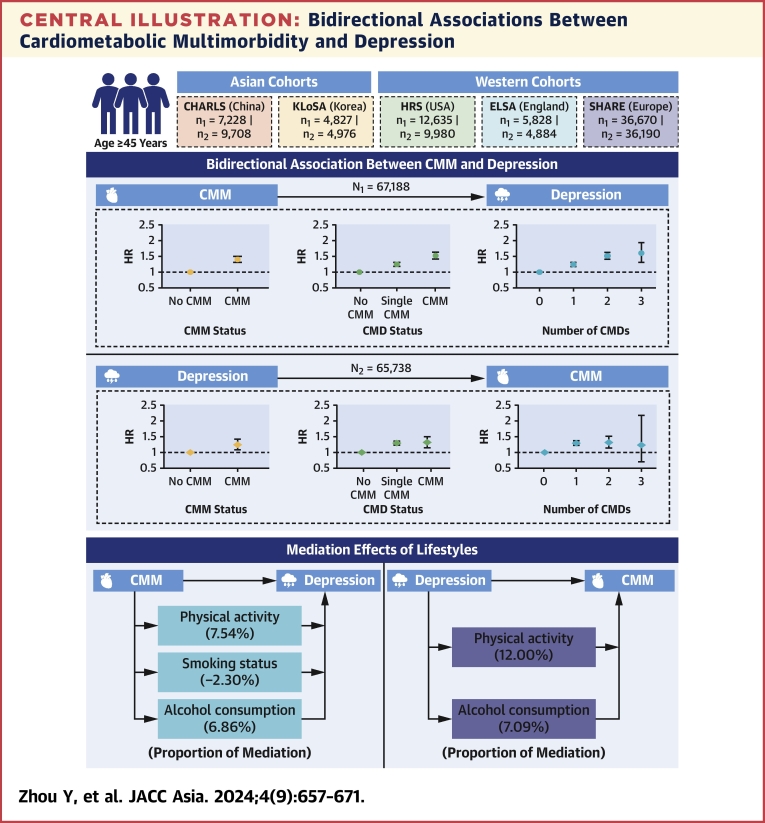
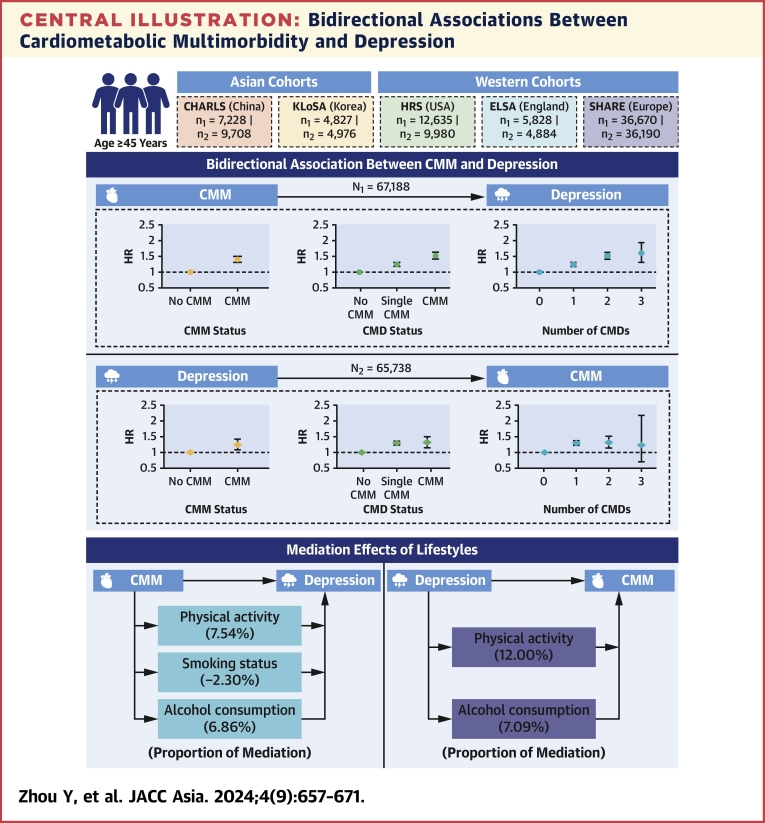
Central Illustration.
Bidirectional Associations Between Cardiometabolic Multimorbidity and Depression
Participants aged ≥45 years from 5 cohorts in Asian and Western countries were included to evaluate the bidirectional associations between cardiometabolic multimorbidity (CMM) and depression, and the mediation effects of lifestyles. CHARLS = China Health and Retirement Longitudinal Study; CMD = cardiometabolic disease; ELSA = English Longitudinal Study on Ageing; HRS = the U.S. Health and Retirement Study; KLoSA = Korean Longitudinal Study of Aging; SHARE = Survey of Health, Ageing and Retirement in Europe.
The mediation effects of lifestyle factors on the association of CMM with depression are presented in Table 3. Physical activity and alcohol consumption positively mediated the CMM-depression association (mediation proportions of physical activity: 7.5%; of alcohol consumption: 6.9%), while smoking status had a negative mediation effect (mediation proportion: –2.3%). Subgroup analyses according to age, sex, educational level, and total household income yielded broadly similar results (Supplemental Tables 4 to 6). The CMM-depression association from Cox regression models were stronger in Western countries than in Asian countries (Figure 3A), with no sex differences observed (Supplemental Figures 1A to 1B). The pooled HRs for depression incidence were 1.23 (single CMD vs none; 95% CI: 1.15-1.31) and 1.54 (CMM vs none; 95% CI: 1.39-1.70), with mild between-study heterogeneity observed (I2 = 27%-34%). The pooled RRs for depression incidence from GEE analyses showed no heterogeneity (Supplemental Figure 2A). Three trajectories for depression symptoms were identified: “persistently symptom-free,” “persistently low,” and “increasing” (Supplemental Table 7, Supplemental Figure 3). Increased risk for the “persistently low” and “increasing” depression trajectories were observed among those with CMM (Supplemental Table 8).
Table 3.
Mediation Effects of Lifestyle Factors on the Bidirectional Associations Between Cardiometabolic Multimorbidity and Depression
| Direct Effect |
Indirect Effect |
% Mediated | |||
|---|---|---|---|---|---|
| Coefficients (95% CI) | P Value | Coefficients (95% CI) | P Value | ||
| CMM-depression analyses | |||||
| CMD status → depression | |||||
| Physical activity | 4.13 (2.86-5.41) | <0.001 | 0.34 (0.25-0.43) | <0.001 | 7.5 |
| Smoking status | 4.14 (2.18-6.24) | <0.001 | −0.10 (−0.18 to −0.03) | 0.006 | −2.3 |
| Alcohol consumption | 4.39 (3.08-5.66) | <0.001 | 0.32 (0.24-0.42) | <0.001 | 6.9 |
| Depression-CMM analyses | |||||
| Depression → single CMD | |||||
| Physical activity | 7.13 (5.40-8.93) | <0.001 | 0.30 (0.18-0.44) | <0.001 | 4.0 |
| Smoking status | 7.37 (5.51-9.12) | <0.001 | 0.01 (−0.05 to 0.08) | 0.77 | 1.1 |
| Alcohol consumption | 7.04 (5.53-8.61) | <0.001 | 0.25 (0.16-0.35) | <0.001 | 3.5 |
| Depression → CMM | |||||
| Physical activity | 2.50 (−1.43 to 6.64) | <0.001 | 0.40 (0.14-0.65) | <0.001 | 12.0 |
| Smoking status | 4.38 (1.49-7.18) | <0.001 | −0.06 (−0.20 to 0.05) | 0.29 | −1.4 |
| Alcohol consumption | 4.06 (1.20-6.98) | 0.006 | 0.31 (0.15-0.48) | <0.001 | 7.1 |
Models were adjusted for age, sex, study, marital status, educational level, total household wealth, body mass index (BMI), history of hypertension, cancer and lung diseases, and medication of hypertension and lung diseases.
Abbreviations as in Table 1.
Figure 3.
Meta-Analyses of the Associations Between CMM and Depression
Random-effects meta-analyses were conducted to calculate the HRs and 95% CIs for CMM-depression analyses (A) and depression-CMM analyses (B). Heterogeneity was tested by using I2 statistics. Abbreviations as in Figures 1 and 2.
Depression-CMM Analyses: the Association Between Depression and Incidence of CMM
Of the 65,738 participants in the depression-CMM analyses, 10,082 (15.3%) participants developed a single CMD, and 1,461 (2.2%) developed CMM, including 103 (0.2%) with diabetes, heart diseases, and stroke. Figure 2B displays the association between baseline depression and incidence of CMM during follow-up using Cox regression models. Those with depression at baseline had higher risk of developing a single CMD (HR: 1.29; 95% CI: 1.22-1.36) and CMM (HR: 1.31; 95% CI: 1.14-1.50). Depression was associated with an elevated risk of developing more CMDs, although its association with 3 CMDs was imprecisely estimated. In addition, depression was significantly associated with specific combinations of CMDs (eg, HR: 1.30; 95% CI: 1.06-1.60 for diabetes and heart diseases) (Central Illustration). Results from GEE analyses yielded similar results, with RRs of 1.20 (95% CI: 1.14-1.26) and 1.53 (95% CI: 1.33-1.76) for developing a single CMD and CMM, respectively (Supplemental Table 3).
The mediation effects of lifestyle factors on the association of depression with CMM are presented in Table 3. Physical activity and alcohol consumption mediated the depression-CMM association, with mediation proportions of 12.0% and 7.1% respectively, while the mediation effect of smoking status was nonsignificant. The subgroups analyses showed that age, sex, educational level, and total household income did not substantially modify the depression-CMM association (Supplemental Tables 9 to 11). However, study-specific HRs indicated that the depression-CMM association attenuated to nonsignificance in Asian countries (eg, in CHARLS, HR for CMM: 1.10; 95% CI: 0.52-2.30) (Figure 3B), showing similar patterns between the sexes (Supplemental Figures 1C to 1D). The pooled HR was 1.32 (95% CI: 1.08-1.62: I2 = 79%) for developing a single CMD and 1.31 (95% CI: 1.14-1.51; I2 = 0%) for developing CMM among those with baseline depression (Figure 3B). The meta-analyses from GEEs for Poisson regression models also generated significant results (RR of developing a single CMD: 1.18 [95% CI: 1.12-1.25]; RR of developing CMM: 1.51 [95% CI: 1.31-1.74]), with between-study heterogeneity of 0% to 22% (Supplemental Figure 2B).
Discussion
In this study involving 5 aging cohorts across 19 countries from Asia, North America, and Europe, bidirectional associations between CMM and depression were found. Individuals with a single CMD and CMM at baseline had an elevated risk of developing depression. Alcohol consumption and physical activity significantly mediated a proportion of 6.9% to 7.5% of the association of CMM with depression. Similarly, individuals with depression had an increased risk of developing CMM, which rose with the number of CMDs, though this association was imprecise in some analyses. The mediation proportion of alcohol consumption and physical activity was 7.1% to 12.0% for the depression-CMM association.
Although the association between CMDs and depression has been widely explored in prior research,12,34,35 little attention has been paid to the longitudinal impact of CMM on the incidence of depression. Our study addressed this knowledge gap and found that individuals with CMM, regardless of specific CMD combinations, experienced a heightened risk of developing depression. Considering that the variables were collected over time as repeated measures, we further conducted GEEs for Poisson regression to longitudinally estimate the CMM-depression association, the results of which suggest that the longitudinal association of CMM with depression is robust. Our meta-analyses and additional analyses examining depression trajectories during follow-up showed a similar pattern of results, with no significant between-study heterogeneity.
We also observed a longitudinal association between depression and subsequent CMM from both Cox regression models with time-dependent covariates and GEE analyses. This finding concurs with a cohort study based on the UK Biobank14 but that study included only UK participants, limiting the generalization of its results. By using a broader range of nationally representative samples from 19 countries, our study confirmed the relationship between depression and CMM incidence, although the HRs for some categories of CMM were imprecisely estimated (eg, CMM of 3 CMDs), probably owing to the small participant numbers in these categories. In our meta-analyses, the main associations between depression and CMM incidence were significant but with significant between-study heterogeneity. The study-specific HRs suggested that the longitudinal association between depression and CMM incidence might be more pronounced in Western countries than in Asian countries, stressing the importance of considering large-sample populations with different cultural contexts in future research. However, the meta-analyses using random-effects models from GEEs observed nonsignificant between-study heterogeneity.
Our findings further included 3 lifestyle factors and found the bidirectional associations between CMM and depression were positively mediated by physical activity and alcohol consumption, suggesting that targeted interventions limiting drinking and improving physical activity would be more effective. Smoking status was found to negatively mediate the CMM-depression association, which might be explained by the short follow-ups and reverse causality. In addition, larger mediated proportions of lifestyle factors were observed in the depression-CMM pathway than otherwise, suggesting that lifestyle interventions might be more effective to prevent or delay CMM onset among individuals with depression.
Our findings are biologically plausible. The observed associations of stroke, heart diseases, and diabetes with elevated depression incidence in this study are consistent with at least 3 mechanistic hypotheses: the poststroke depression hypothesis, the vascular depression hypothesis,36 and the diabetes-depression hypothesis.37,38 Poststroke depression refers to depression that occurs in the context of a clinically apparent stroke36 and might be related to inflammatory processes, hypothalamic-pituitary-adrenal axis abnormalities, and alterations in neuroplasticity and glutamate neurotransmission.39 Vascular depression, in turn, refers to depression in the presence of vascular risk factors accompanied by neuropsychological deficit and distinct localized brain pathology.36 The longitudinal association between diabetes and depression might be driven by vascular and metabolic pathology.40 Microvascular dysfunction is common in people with diabetes, including effects on the brain, and its diabetes-related drivers are hyperglycemia, obesity and insulin resistance, and hypertension.37,38
There are also several plausible mechanisms for the effects of depression on CMDs, although the evidence is less robust. The depression-CMD association might be explained by depression-related alterations in the autonomic nervous system, platelet receptors and function, coagulopathic factors, proinflammatory cytokines, and neurohormonal factors.41 Chronic stress, a correlate of depression, increases the production of cortisol in the adrenal cortex and activates the sympathetic nervous system,42,43 while hypercortisolemia and sympathetic nervous system activation further contribute to insulin resistance, diabetes, and metabolic syndrome.44 Hypercortisolemia can induce tachyphylaxis of the reward system and the disturbed neurogenesis in the hippocampus, both related to depression.45,46
Our research has important implications for clinical practice and public health policy. The evidence highlights the likelihood of shared pathways between physical and mental conditions, suggesting that preventive interventions should commence early.47 Health care providers should be attentive to the heightened risk of depression in patients with CMM and implement timely interventions to safeguard their mental well-being. Similarly, there may be a need to enhance CMM prevention measures when treating patients with depression to prevent a cycle in which mental and physical health issues exacerbate each other. In summary, prioritizing integrated, person-centered care that addresses both CMM and depression is essential. This approach can help alleviate the burden of these conditions, enhance physical and mental health, and mitigate adverse health outcomes during middle and old age.
Study Limitations
First, the information of CMM, including diabetes, heart diseases, and stroke, was self-reported, and this was subject to recall bias. However, previous studies have shown that self-reported CMDs have good credibility.48, 49, 50, 51 Second, the included participants were from high-income countries or upper-middle income countries. Whether the bidirectional associations between CMM and depression apply to populations from lower-middle and low-income countries warrants further investigation. Third, the information of CMM symptoms and severity, and the diagnosis, medication, and subtypes of depression were unavailable, which could influence observed associations. Last, we included 4 waves of each cohort with only 8-year follow-up to capture the information of exposures and outcomes, which is subject to reverse causality effects. Therefore, future population-based studies with long-term follow-up are needed. Finally, because of data availability limitations and population differences among the included cohorts, measurement of depressive symptoms relied on study-specific multi-item instruments, rather than the same measures across all cohorts. Although we used predefined cutoffs for defining caseness, and all instruments were validated,52, 53, 54, 55 the variations in measurement tools across cohorts may have contributed to increased heterogeneity in cohort-specific results. Ideally, future research should use similar protocols and consistent cutoff thresholds across all included cohorts to confirm the validity of the findings.56,57
Conclusions
CMM and depression showed bidirectional associations, with the onset of either condition significantly increasing the risk of developing the other. Our findings underscore the importance of holistic and integrated approaches that address both CMM and depression to enhance health outcomes and mitigate the reciprocal impact of these conditions among middle-aged and older populations.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CMM and depression were bidirectionally associated, and the mediated effects of lifestyle factors were larger in the depression-lifestyle-CMM pathway than in the CMM-lifestyle-depression pathway.
TRANSLATIONAL OUTLOOK: Our findings underscore the importance of holistic and integrated approaches that address both CMM and depression to enhance health outcomes and mitigate the reciprocal impact of these conditions.
Funding Support and Author Disclosures
Dr Xu was supported by the Hundred Talents Program Research Initiation Fund from Zhejiang University and the Fundamental Research Funds for the Central Universities. Mr Kivimäki was supported by the Wellcome Trust (grant 221854/Z/20/Z), the UK Medical Research Council (grant MR/S011676/1 and MR/R024227/1), the National Institute on Aging (grant R01AG056477), and the Academy of Finland (grant 350426). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the manuscript. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all team members who have contributed to CHARLS, KLoSA, HRS, ELSA, and SHARE. This analysis uses data or information from the harmonized CHARLS, KLoSA, HRS, ELSA, and SHARE data sets and Codebooks developed by the Gateway to Global Aging Data and supported by the National Institute on Aging.
Footnotes
Kwang Kon Koh, MD, PhD, served as Guest Associate Editor for this paper. Nathan Wong, PhD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and figures, please see the online version of this paper.
Appendix
References
- 1.Kessler R.C., Bromet E.J. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida O.P. Prevention of depression in older age. Maturitas. 2014;79(2):136–141. doi: 10.1016/j.maturitas.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Depression and Other Common Mental Disorders. January 2017. Accessed 14 May 2024. https://www.who.int/publications/i/item/depression-global-health-estimates
- 4.Olver J.S., Hopwood M.J. Depression and physical illness. Med J Aust. 2013;199(S6):S9–S12. doi: 10.5694/mja12.10597. [DOI] [PubMed] [Google Scholar]
- 5.Khalfan A.F., Campisi S.C., Lo R.F., McCrindle B.W., Korczak D.J. The association between adolescent depression and dyslipidemia. J Affect Disord. 2023;338:239–245. doi: 10.1016/j.jad.2023.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Dickens C., Jackson J., Tomenson B., Hay E., Creed F. Association of depression and rheumatoid arthritis. Psychosomatics. 2003;44(3):209–215. doi: 10.1176/appi.psy.44.3.209. [DOI] [PubMed] [Google Scholar]
- 7.Frank P., Batty G.D., Pentti J., et al. Association between depression and physical conditions requiring hospitalization. JAMA Psychiatry. 2023;80(7):690–699. doi: 10.1001/jamapsychiatry.2023.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Shi L., Zheng X., et al. Patterns and determinants of multimorbidity in older adults: study in health-ecological perspective. Int J Environ Res Public Health. 2022;19(24) doi: 10.3390/ijerph192416756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busija L., Lim K., Szoeke C., Sanders K.M., McCabe M.P. Do replicable profiles of multimorbidity exist? Systematic review and synthesis. Eur J Epidemiol. 2019;34(11):1025–1053. doi: 10.1007/s10654-019-00568-5. [DOI] [PubMed] [Google Scholar]
- 10.Lyall D.M., Celis-Morales C.A., Anderson J., et al. Associations between single and multiple cardiometabolic diseases and cognitive abilities in 474 129 UK Biobank participants. Eur Heart J. 2017;38(8):577–583. doi: 10.1093/eurheartj/ehw528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishbook B.N., Brinton C.D., Siever J., Klassen T.D., Sakakibara B.M. Cardiometabolic multimorbidity and activity limitation: a cross-sectional study of adults using the Canadian Longitudinal Study on Aging data. Fam Pract. 2022;39(3):455–463. doi: 10.1093/fampra/cmab129. [DOI] [PubMed] [Google Scholar]
- 12.Ditmars H.L., Logue M.W., Toomey R., et al. Associations between depression and cardiometabolic health: a 27-year longitudinal study. Psychol Med. 2022;52(14):3007–3017. doi: 10.1017/S003329172000505X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng X., Ouyang F., Ma T., et al. The individual and joint associations of depression and sleep duration with cardiometabolic diseases and mortality: a prospective cohort study. Atherosclerosis. 2022;361:10–17. doi: 10.1016/j.atherosclerosis.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Qiao Y., Ding Y., Li G., Lu Y., Li S., Ke C. Role of depression in the development of cardiometabolic multimorbidity: findings from the UK Biobank study. J Affect Disord. 2022;319:260–266. doi: 10.1016/j.jad.2022.09.084. [DOI] [PubMed] [Google Scholar]
- 15.Wium-Andersen I.K., Osler M., Jørgensen M.B., Rungby Jr, Wium-Andersen M.K. Diabetes, antidiabetic medications and risk of depression—a population-based cohort and nested case-control study. Psychoneuroendocrinology. 2022;140 doi: 10.1016/j.psyneuen.2022.105715. [DOI] [PubMed] [Google Scholar]
- 16.Allan L.M., Rowan E.N., Thomas A.J., Polvikoski T.M., O'Brien J.T., Kalaria R.N. Long-term incidence of depression and predictors of depressive symptoms in older stroke survivors. Br J Psychiatry. 2013;203(6):453–460. doi: 10.1192/bjp.bp.113.128355. [DOI] [PubMed] [Google Scholar]
- 17.Wium-Andersen M.K., Wium-Andersen I.K., Prescott E.I.B., Overvad K., Jørgensen M.B., Osler M. An attempt to explain the bidirectional association between ischaemic heart disease, stroke and depression: a cohort and meta-analytic approach. Br J Psychiatry. 2020;217(2):434–441. doi: 10.1192/bjp.2019.130. [DOI] [PubMed] [Google Scholar]
- 18.Han Y., Hu Y., Yu C., et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur Heart J. 2021;42(34):3374–3384. doi: 10.1093/eurheartj/ehab413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarris J., Thomson R., Hargraves F., et al. Multiple lifestyle factors and depressed mood: a cross-sectional and longitudinal analysis of the UK Biobank (N = 84,860) BMC Med. 2020;18(1):354. doi: 10.1186/s12916-020-01813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornicroft G., Ahuja S., Barber S., et al. Integrated care for people with long-term mental and physical health conditions in low-income and middle-income countries. Lancet Psychiatry. 2019;6(2):174–186. doi: 10.1016/S2215-0366(18)30298-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee J., Phillips D., Wilkens J. Gateway to Global Aging Data: resources for cross-national comparisons of family, social environment, and healthy aging. J Gerontol B Psychol Sci Soc Sci. 2021;76(suppl 1):S5–S16. doi: 10.1093/geronb/gbab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnega A., Faul J.D., Ofstedal M.B., Langa K.M., Phillips J.W.R., Weir D.R. Cohort profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steptoe A., Breeze E., Banks J., Nazroo J. Cohort profile: the English Longitudinal Study of Ageing. Int J Epidemiol. 2013;42(6):1640–1648. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Börsch-Supan A., Brandt M., Hunkler C., et al. Data resource profile: the Survey of Health, Ageing and Retirement in Europe (SHARE) Int J Epidemiol. 2013;42(4):992–1001. doi: 10.1093/ije/dyt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin C. In: Encyclopedia of Gerontology and Population Aging. Gu D., Dupre M.E., editors. Springer International Publishing; 2019. Korean Longitudinal Study of Ageing; pp. 1–4. [Google Scholar]
- 27.Xu X., Mishra G.D., Dobson A.J., Jones M. Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: a 20-year cohort study. PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingo T.S., Gerasimov E.S., Canon S.M., Lah J.J., Levey A.I., Wingo A.P. Alzheimer's disease genetic burden is associated with mid-life depression among persons with normal cognition. Alzheimers Dement. 2023;19(3):868–874. doi: 10.1002/alz.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank P., Kaushal A., Poole L., Lawes S., Chalder T., Cadar D. Systemic low-grade inflammation and subsequent depressive symptoms: is there a mediating role of physical activity? Brain Behav Immun. 2019;80:688–696. doi: 10.1016/j.bbi.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Hamer M., Batty G.D., Kivimaki M. Risk of future depression in people who are obese but metabolically healthy: the English Longitudinal Study of Ageing. Mol Psychiatry. 2012;17(9):940–945. doi: 10.1038/mp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Bueno R., Calatayud J., Andersen L.L., et al. Dose-response association of handgrip strength and risk of depression: a longitudinal study of 115 601 older adults from 24 countries. Br J Psychiatry. 2023;222(3):135–142. doi: 10.1192/bjp.2022.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince M.J., Reischies F., Beekman A.T.F., et al. Development of the EURO–D scale—a European Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174(4):330–338. doi: 10.1192/bjp.174.4.330. [DOI] [PubMed] [Google Scholar]
- 33.Ng C.D., Weiss J. Association of genetic and behavioral characteristics with the onset of diabetes. BMC Public Health. 2019;19(1):1297. doi: 10.1186/s12889-019-7618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasserre A.M., Strippoli M.P.F., Glaus J., et al. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Mol Psychiatry. 2017;22(7):1026–1034. doi: 10.1038/mp.2016.178. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong N.M., Meoni L.A., Carlson M.C., et al. Cardiovascular risk factors and risk of incident depression throughout adulthood among men: the Johns Hopkins Precursors Study. J Affect Disord. 2017;214:60–66. doi: 10.1016/j.jad.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newberg A.R., Davydow D.S., Lee H.B. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18(5):433–441. doi: 10.1080/09540260600935447. [DOI] [PubMed] [Google Scholar]
- 37.van Sloten T.T., Sedaghat S., Carnethon M.R., Launer L.J., Stehouwer C.D.A. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabák A.G., Akbaraly T.N., Batty G.D., Kivimäki M. Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol. 2014;2(3):236–245. doi: 10.1016/S2213-8587(13)70139-6. [DOI] [PubMed] [Google Scholar]
- 39.Robinson R.G., Jorge R.E. Post-stroke depression: a review. Am J Psychiatry. 2016;173(3):221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 40.Bruce D.G., Casey G., Davis W.A., et al. Vascular depression in older people with diabetes. Diabetologia. 2006;49(12):2828–2836. doi: 10.1007/s00125-006-0478-y. [DOI] [PubMed] [Google Scholar]
- 41.Hare D.L., Toukhsati S.R., Johansson P., Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 42.Kyrou I., Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787–793. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Kivimäki M., Bartolomucci A., Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. 2023;19(1):10–27. doi: 10.1038/s41574-022-00746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrousos G.P. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 45.Herbert J., Goodyer I.M., Grossman A.B., et al. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18(6):393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 46.Moulton C.D., Costafreda S.G., Horton P., Ismail K., Fu C.H.Y. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 2015;9(4):651–662. doi: 10.1007/s11682-014-9348-2. [DOI] [PubMed] [Google Scholar]
- 47.Launders N., Kirsh L., Osborn D.P.J., Hayes J.F. The temporal relationship between severe mental illness diagnosis and chronic physical comorbidity: a UK primary care cohort study of disease burden over 10 years. Lancet Psychiatry. 2022;9(9):725–735. doi: 10.1016/S2215-0366(22)00225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilsanz P., Walter S., Tchetgen Tchetgen E.J., et al. Changes in depressive symptoms and incidence of first stroke among middle-aged and older US adults. J Am Heart Assoc. 2015;4(5) doi: 10.1161/JAHA.115.001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratliff S., Mezuk B. Depressive symptoms, psychiatric medication use, and risk of type 2 diabetes: results from the Health and Retirement Study. Gen Hosp Psychiatry. 2015;37(5):420–426. doi: 10.1016/j.genhosppsych.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierce M.B., Zaninotto P., Steel N., Mindell J. Undiagnosed diabetes—data from the English Longitudinal Study of Ageing. Diabet Med. 2009;26(7):679–685. doi: 10.1111/j.1464-5491.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 51.Xiaojing Y., Tingting L., Lang W., Zhi-Yong Z., Changwei L. Validity of self-reported diabetes among middle-aged and older Chinese adults: the China Health and Retirement Longitudinal Study. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andresen E.M., Malmgren J.A., Carter W.B., Patrick D.L. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 53.Prince M.J., Reischies F., Beekman A.T., et al. Development of the EURO-D scale—a European Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174:330–338. doi: 10.1192/bjp.174.4.330. [DOI] [PubMed] [Google Scholar]
- 54.Boey K.W. Cross-validation of a short form of the CES-D in Chinese elderly. Int J Geriatr Psychiatry. 1999;14(8):608–617. doi: 10.1002/(sici)1099-1166(199908)14:8<608::aid-gps991>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 55.Kessler R.C., Ustün T.B. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Díaz-Mardomingo M.D.C., García-Herranz S., Rodríguez-Fernández R., Venero C., Peraita H. Problems in classifying mild cognitive impairment (MCI): one or multiple syndromes? Brain Sci. 2017;7(9):111. doi: 10.3390/brainsci7090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jak A.J., Preis S.R., Beiser A.S., et al. Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J Int Neuropsychol Soc. 2016;22(9):937–943. doi: 10.1017/S1355617716000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.