Abstract
In recent years, a wealth of clinical data has emerged regarding intravascular imaging involving either intravascular ultrasound or optical coherence tomography. This surge in data has propelled the adoption of intravascular imaging–guided percutaneous coronary intervention (PCI) in daily clinical practice. The findings of current randomized clinical trials regarding imaging guidance have lent strong support to the benefits of intravascular imaging–guided PCI. This holds especially true for the diagnosis and treatment of complex lesions, such as left main disease, diffuse long lesions, chronic total occlusion, severely calcified lesions, bifurcations, and in-stent restenosis, as well as in high-risk patients such as those with acute myocardial infarction or chronic kidney disease. During intravascular imaging–guided PCI, operators attempt to achieve stent optimization for maximized benefits of imaging guidance. This paper provides a comprehensive review on the updated clinical data of intravascular imaging–guided PCI and intravascular ultrasound/optical coherence tomography–derived stent optimization criteria.
Key Words: interventional, optical coherence, percutaneous coronary intervention, tomography, ultrasound
Central Illustration
Highlights
-
•
The intravascular imaging–guided PCI improves clinical outcomes in AMI and CKD, and complex lesions.
-
•
OCT-guided PCI provides clinical outcomes comparable to those of IVUS-guided PCI.
-
•
During intravascular imaging–guided PCI, stent optimization is desired to maximize benefits of imaging guidance.
Although coronary angiography remains an essential modality in the diagnosis and treatment of cardiovascular disease by assessing disease severity through the estimation of stenotic artery position and narrowed luminal diameter, its limitations stemming from 2-dimensional projected luminograms are well-recognized.1 Recent studies have highlighted the superior clinical outcomes of intravascular ultrasound (IVUS) and optical coherence tomography (OCT), both capable of 3-dimensional analysis, when compared with angiography.2,3 Therefore, current guidelines recommend that IVUS or OCT should be considered for stent optimization.4,5 The present review focuses on the clinical evidence and practical applications of intravascular imaging guidance during percutaneous coronary intervention (PCI), as well as strategies for achieving stent optimization including specific criteria for stent optimization.
Current Status of Intravascular Imaging–Guided PCI: Guidelines and Evidence
Updated guidelines regarding intravascular imaging–guided PCI
Regarding the recommendation for intravascular imaging evaluation, including IVUS or OCT use, recent European and American guidelines present similar positions, which are stated as follows4,5 [Class of recommendation/Level of Evidence]:
-
1)
IVUS or OCT should be considered to achieve PCI optimization in patients undergoing coronary stent implantation. [IIa/B]
-
2)
With respect to stent failure, such as stent thrombosis and in-stent restenosis (ISR), IVUS and/or OCT should be considered when assessing the mechanisms of stent restenosis and stent thrombosis. [IIa/C]
-
3)
IVUS should be considered to optimize PCI in patients with unprotected left main coronary artery (ULMCA) disease. [IIa/B]
In the recent European guideline, it was recommended that intravascular imaging guidance should be considered in the setting of acute coronary syndrome (ACS) [IIa/A].6 In addition, the use of intracoronary imaging with OCT or IVUS, preferably OCT, may be considered in patients with ACS with ambiguous culprit lesions [IIb/C] (Table 1).
Table 1.
Updated Guidelines Regarding Intravascular Imaging–Guided PCI
| European Guidelines4,6 |
American Guidelines5 |
|||
|---|---|---|---|---|
| Class of Recommendation | Level of Evidence | Class of Recommendation | Level of Evidence | |
| Stent optimization by IVUS or OCT guidance | IIa | B | IIa | B |
| Assessing the mechanisms of stent restenosis and stent thrombosis by IVUS and/or OCT guidance | IIa | C | IIa | C |
| PCI optimization for ULMCA disease by IVUS guidance | IIa | B | IIa | B |
| IVUS or OCT guidance in the setting of ACS | IIa | A | Not mentioned | |
| OCT or IVUS guidance, preferably OCT, when assessing ambiguous culprit lesions in the setting of ACS | IIb | C | Not mentioned | |
ACS = acute coronary syndrome; IVUS = intravascular ultrasound; OCT = optical coherence tomography; PCI = percutaneous coronary intervention; ULMCA = unprotected left main coronary artery.
Key clinical data of IVUS-guided PCI
Many randomized clinical trials investigating the role of intravascular imaging guidance, especially IVUS-guided PCI, have been conducted. The utilization of IVUS-guided drug-eluting stent (DES) implantation yielded notable improvements in patients with chronic total occlusion (CTO), significantly improving the 1-year clinical outcomes in comparison with angiography guidance.7 There were 2 pivotal randomized controlled trials that demonstrated the short- and long-term benefits of IVUS-guided PCI with DES for complex lesions compared with angiography guidance: the IVUS-XPL (Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions) and the ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions) trials.2,8, 9, 10 More recently, the IVUS-ACS (Intravascular Ultrasound-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Acute Coronary Syndromes), a multicenter randomized trial, demonstrated that IVUS-guided PCI improved the 1-year clinical outcomes in patients with ACS, with improvements mainly driven by target-vessel–related myocardial infarction and ischemic-driven target-vessel revascularization.11
Several recent registry studies have also demonstrated the clinical benefits of IVUS-guided PCI in patients with various complex lesion characteristics or high ischemic risks such as acute myocardial infarction (AMI) or chronic kidney disease (CKD).12, 13, 14, 15, 16, 17, 18 A summary of key clinical data pertaining to IVUS-guided PCI can be found in Table 2.
Table 2.
Key Clinical Data of IVUS vs Angiography-Guided PCI
| Study (IVUS vs Angiography) | Sample Size (IVUS vs Angiography) | Study Population | Center (Country) | Followed Duration, mo |
Primary and Key Secondary Endpoints (IVUS vs Angiography) | Definite or Probable Stent Thrombosis (IVUS vs Angiography) |
|---|---|---|---|---|---|---|
| Multicenter randomized trial regarding IVUS- vs angiography-guided PCI | ||||||
| CTO-IVUS7 2015 | 201 vs 201 | CTO lesion | 20 (Korea) | 12 | aMACE(CD/MI/TVR): 2.6% vs 7.1% (HR: 0.35; 95% CI: 0.13-0.97) | 0.5% vs 1.0% (P = 0.11) |
| IVUS-XPL2 2015 | 700 vs 700 | Long lesion (Stent ≥ 28 mm) | 20 (Korea) | 12 |
aMACE(CD/TL-MI/ID-TLR): 2.9% vs 5.8% (HR: 0.48; 95% CI: 0.28-0.83) ID-TLR: 2.5% vs 5.0% (HR: 0.51; 95% CI: 0.28-0.91) |
0.3% vs 0.3% (HR: 1.00; 95% CI: 0.14-7.10) |
| ULTIMATE8 2018 | 722 vs 722 | All comers | 8 (China) | 12 | aTVF(CD/TV-MI/ID-TVR): 2.9% vs 5.4% (HR: 0.53; 95% CI: 0.31-0.90) | 0.1% vs 0.7% (HR: 0.20; 95% CI: 0.02-1.70) |
| IVUS-XPL (extended)9 2020 | 589 vs 594 | Long lesion (Stent ≥ 28 mm) | 20 (Korea) | 60 |
aMACE(CD/TL-MI/ID-TLR): 5.6% vs 10.7% (HR: 0.50; 95% CI: 0.34-0.75) ID-TLR: 4.8% vs 8.4% (HR: 0.54; 95% CI: 0.33-0.89) |
0.3% vs 0.3% (HR: 1.00; 95% CI: 0.14-7.10) |
| ULTIMATE (extended)10 2021 | 714 vs 709 | All comers | 8 (China) | 36 |
aTVF(CD/TV-MI/ID-TVR): 6.6% vs 10.7% (HR: 0.60; 95% CI: 0.42-0.87) ID-TLR: 3.8% vs 6.3% (HR: 0.59; 95% CI: 0.36-0.94) |
0.1% vs 1.1% (HR: 0.12; 95% CI: 0.02-0.99) |
| IVUS-ACS11 2024 | 1,753 vs 1,752 | ACS | 58 (Global) | 12 |
aTVF(CD/TV-MI/ID-TVR): 4.0% vs 7.3% (HR: 0.55; 95% CI: 0.41-0.74) TV-MI: 2.5% vs 3.8% (HR: 0.63; 95% CI: 0.43-0.92) ID-TVR: 1.4% vs 3.2% (HR: 0.44; 95% CI: 0.27-0.72) |
0.6% vs 0.9% (HR: 0.82; 95% CI: 0.35-1.90) |
| Cohort or registry data regarding IVUS- vs angiography-guided PCI | ||||||
| Choi et al12 2019 | 1,674 vs 4,331 | Complex lesions | Single center (Korea) | 64 | aCD: 10.2% vs 16.9% (HR: 0.57; 95% CI: 0.46-0.71) | 3.1% vs 4.4% (HR: 0.60; 95% CI: 0.41-0.86) |
| BCIS database13 2020 | 6,208 vs 5,056 | ULMCA | 113 (England & Wales) | 12 | aDeath: 8.9% vs 12.9% (OR: 0.66; 95% CI: 0.57-0.77) | |
| MAIN-COMPARE (subgroup)14 2021 | 756 vs 219 | ULMCA | 12 (Korea) | 143 |
aDeath: 16.4% vs 31.0% (HR: 0.73; 95% CI: 0.53-1.02) MACE (death/Q-MI/stroke): 19.2% vs 32.9% (HR 0.71; 95% CI: 0.52-0.97) |
|
| KAMIR-NIH16 2022 | 1,887 vs 7,120 | AMI | 20 (Korea) | 36 |
aTLF(CD/TV-MI/ID-TLR):4.8% vs 8.0% (HR: 0.59; 95% CI: 0.47-0.73) CD: 3.1% vs 5.5% (HR: 0.56; 95% CI: 0.42-0.73) TV-MI: 0.6% vs 1.2% (HR: 0.46; 95% CI: 0.25-0.86) |
0.4% vs 0.8% (HR: 0.42; 95% CI: 0.19-0.92) |
| ULTIMATE (subgroup)17 2019 | 180 vs 169 | CKD | 8 (China) | 12 | aTVF(CD/TV-MI/ID-TVR): 3.9% vs 10.7% (HR: 0.35; 95% CI: 0.15-0.84) | 0.0% vs 1.2% (P = 0.14) |
ACS = acute coronary syndrome; AMI = acute myocardial infarction; BCIS = British Cardiovascular Intervention Society; CD = cardiac death; CKD = chronic kidney disease; CTO = chronic total occlusion; CTO-IVUS = Chronic Total Occlusion Intervention With Drug-eluting Stents; ID-TLR = ischemic-driven target-lesion revascularization; ID-TVR = ischemic-driven target-vessel revascularization; IVUS = intravascular ultrasound; IVUS-ACS = Intravascular Ultrasound-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Acute Coronary Syndromes; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; KAMIR-NIH = Korea Acute Myocardial Infarction-National Institutes of Health; MACE = major adverse cardiac event(s); MAIN-COMPARE = Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization; MI = myocardial infarction; PCI = percutaneous coronary intervention; Q-MI = Q-wave myocardial infarction; TLF = target-lesion failure; TL-MI = target-lesion myocardial infarction; TVF = target-vessel failure; TV-MI = target-vessel myocardial infarction; ULMCA = unprotected left main coronary artery; ULTIMATE = Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions.
Indicates the primary endpoint.
Key clinical data of OCT-guided PCI
Despite OCT possessing a higher resolution (10 μm) in power over IVUS, there is a paucity of data regarding the roles of OCT-guided PCI vs the conventional angiography-guided PCI. In a retrospective multicenter registry, CLI-OPCI (Centro per la Lotta contro l’Infarto–Optimization of Percutaneous Coronary Intervention), OCT-guided PCI was significantly associated with improved clinical outcomes.19 The DOCTORS (Does Optical Coherence Tomography Optimize Results of Stenting) study showed that OCT-guided PCI was significantly associated with a higher post-procedural fractional flow reserve than that of angiography-guided PCI in patients with non–ST-segment elevation myocardial infarction.20 However, a prospective propensity-matched cohort of the TOTAL (Thrombectomy Versus Percutaneous Coronary Intervention Alone) trial showed no significant difference in the ischemic risk at 1 year between OCT-guided and angiography-guided PCI in patients with ST-segment elevation myocardial infarction.21 Recently, 2 large-scale randomized trials have provided substantial evidence on the clinical benefits of OCT-guided PCI; the ILUMIEN IV: OPTIMAL PCI (Optimal Coherence Tomography Guided Coronary Stent Implantation Compared to Angiography: a Multicenter Randomized Trial in PCI) trial demonstrated that OCT-guided PCI resulted in a significant improvement in the acute minimal stent area (MSA) (5.72 ± 2.04 mm2 in the OCT group vs 5.36 ± 1.87 mm2 in the angiography group; P < 0.001), but there was no apparent between-group difference in the percentage of patients with target-vessel failure at 2 years.22 The OCTOBER (Optical Coherence Tomography Optimized Bifurcation Event Reduction) trial showed that OCT-guided PCI for complex bifurcation lesions was superior to angiography-guided PCI for the improvement of the 2-year target-lesion failure rate.3 Ongoing OCT-based randomized trials (OCCUPI [Optical Coherence Tomography-Guided Coronary Intervention in Patients With Complex Lesions: a Randomized Controlled Trial; NCT03625908]) are anticipated to further support the effectiveness of OCT-guided PCI for complex lesions compared with angiography-guided PCI. An overview of critical clinical data pertaining to OCT-guided PCI can be found in Table 3.
Table 3.
Key Clinical Data of OCT-Guided PCI
| Study Type | Sample size (OCT vs. Angiography) | Study Population | Center (Country) | Followed Duration, mo |
Primary and Key Secondary Endpoints (OCT vs Angiography) | |
|---|---|---|---|---|---|---|
| OCT- vs angiography-guided PCI | ||||||
| CLI-OPCI19 2012 | Retrospective matched | 335 vs 335 | IHD | 3 (Italy) | 12 | aMACE(CD/MI): 6.6% vs 13.0% (OR: 0.37; 95% CI: 0.10-0.90) |
| DOCTORS20 2016 | Random | 120 vs 120 | NSTEMI | 9 (France) |
aPost-PCI: FFR: 0.94 vs 0.92 (P = 0.005) Procedural complications: 5.8% vs 5.8% Final residual stenosis: 7.0% vs 8.7% (P = 0.01) |
|
| TOTAL (subgroup)21 2016 | Propensity matched | 214 vs 428 | STEMI | 12 |
aMACE(CD/MI/ST/TVR): 7.5% vs 9.8% (HR: 0.76; 95% CI: 0.43-1.34) Final in-stent angiographic minimal lumen diameter: 2.99 mm vs 2.79 mm (P < 0.0001) |
|
| ILUMIEN IV22 2023 | Random | 1,233 vs 1,254 | Complex lesions or diabetes | 80 (Global) | 24 |
aTVF(CD/TV-MI/ID-TVR): 7.4% vs 8.2% (HR: 0.90; 95% CI: 0.67-1.19) aPost-PCI: MSA: 5.72 mm2 vs 5.36 mm2 (95% CI: 0.21-0.51) Definite or probable ST: 0.5% vs 1.4% (HR: 0.36; 95% CI: 0.14-0.91) |
| OCTOBER3 2023 | Random | 600 vs 601 | True bifurcation lesions | 38 (Europe) | 24 |
aMACE(CD/TL-MI/ID-TLR): 10.1% vs 14.1% (HR: 0.70; 95% CI: 0.50-0.98) Procedure-related complications: 6.8% vs 5.7% |
| OCCUPI 2024 (NCT30625908) | Random | 803 vs 801 | Complex lesions | 20 (Korea) | 12 | aMACE(CD/MI/ST/ID-TVR) |
CD = cardiac death; CLI-OPCI = Centro per la Lotta contro l’Infarto–Optimization of Percutaneous Coronary Intervention; DOCTORS = Does Optical Coherence Tomography Optimize Results of Stenting; FFR = fractional flow reserve; ID-TLR = ischemic-driven target-lesion revascularization; ID-TVR = ischemic-driven target-vessel revascularization; IHD = ischemic heart disease; ILUMIEN IV = Optimal Coherence Tomography Guided Coronary Stent Implantation Compared to Angiography: a Multicenter Randomized Trial in PCI; MACE = major adverse cardiac event(s); MI = myocardial infarction; MSA = minimal stent area; NSTEMI = non–ST-segment elevation myocardial infarction; OCCUPI = Optical Coherence Tomography-Guided Coronary Intervention in Patients With Complex Lesions: a Randomized Controlled Trial; OCT = optical coherence tomography; OCTOBER = Optical Coherence Tomography Optimized Bifurcation Event Reduction; PCI = percutaneous coronary intervention; ST = stent thrombosis; STEMI = ST-segment elevation myocardial infarction; TL-MI = target-lesion myocardial infarction; TOTAL = Thrombectomy Versus Percutaneous Coronary Intervention Alone; TVF = target-vessel failure; TV-MI = target-vessel myocardial infarction; TVR = target-vessel revascularization.
Indicates the primary endpoint.
Key clinical data of intravascular imaging–guided PCI and IVUS-guided vs OCT-guided PCI
The RENOVATE-COMPLEX-PCI (Randomized Controlled Trial of Intravascular Imaging Guidance versus Angiography-Guidance on Clinical Outcomes after Complex Percutaneous Coronary Intervention) randomized trial involving patients with complex coronary lesions demonstrated that intravascular imaging–guided PCI, including IVUS (74%) and OCT (26%), was associated with a lower rate of a composite of cardiac death, target-vessel–related myocardial infarction, or target-vessel revascularization than angiography-guided PCI.23
Regarding the comparison of clinical outcomes between IVUS-guided and OCT-guided PCI, 2 randomized noninferiority trials, the OPINION (Optical Frequency Domain Imaging vs. Intravascular Ultrasound in Percutaneous Coronary Intervention) and the MISTIC-1 (The Multimodality Imaging Study in Cardiology Cohort 1) trials demonstrated that OCT-guided PCI was not inferior to IVUS-guided PCI in terms of mid- and long-term clinical outcomes.24,25 More recently, the randomized OCTIVUS (Optical Coherence Tomography versus Intravascular Ultrasound-Guided Percutaneous Coronary Intervention) trial demonstrated that OCT-guided PCI was noninferior to IVUS-guided PCI with respect to the incidence of a composite of cardiac death, target-vessel–related myocardial infarction, or target-vessel revascularization at 1 year.26 In a subgroup analysis of the RENOVATE and the OCTIVUS trials, OCT-guided PCI showed a similar risk of target-vessel failure as that of IVUS-guided PCI among patients with complex lesions.27,28 Details of these studies are provided in Table 4.
Table 4.
Key Clinical Data of Intravascular Imaging-Guided PCI
| Study | Study Type | Sample Size (Imaging vs Angiography) or (OCT vs IVUS) | Study Population | Center (Country) | Followed Duration, mo | Primary and Key Secondary Endpoints |
|---|---|---|---|---|---|---|
| Intravascular imaging- vs angiography-guided PCI | Imaging vs Angiography | |||||
| RENOVATE-COMPLEX-PCI23 2023 | Random | 1,092 vs. 547 Imaging (IVUS, 74%; OCT, 26%) vs Angio |
Complex lesions | 20 (Korea) | 25 |
aTVF(CD/TV-MI/ID-TVR): 7.7% vs 12.3% (HR: 0.64; 95% CI: 0.45-0.89) CD: 1.7% vs 3.8% (HR: 0.47; 95% CI: 0.24-0.93) |
| OCT-guided vs IVUS-guided PCI | OCT vs IVUS | |||||
| OPINION24 2017 | Random | 412 vs 405 | Stable or unstable angina | 42 (Japan) | 12 |
aTVF(CD/TV-MI/ID-TVR): 5.2% vs 4.9% (HR: 1.07; 95% CI: 0.24-1.80) In-stent restenosis on 8-month angiogram: 1.6% vs 1.6% |
| MISTIC-125 2020 | Random | 54 vs 55 | Stable angina | 2 (Japan) | 12 |
aIn-stent MLA on 8-month OCT: 4.56 mm2 vs 4.13 mm2 TLF (CD/TV-MI/TLR): 7.4% vs 7.3% (HR: 1.05; 95% CI: 0.26-4.18) |
| OCTIVUS26 2023 | Random | 1,005 vs 1,003 | Stable angina or NSTE-ACS | 9 (Korea) | 12 |
aTVF(CD/TV-MI/ID-TVR): 2.5% vs 3.1% (HR: 0.80; 95% CI: 0.47-1.36) Contrast-induced nephropathy: 1.4% vs 1.5% (OR: 0.93; 95% CI: 0.45-1.91) Procedure-related complications requiring active intervention: 2.2% vs 3.7% (P = 0.003) |
| OCTIVUS (substudy)27 2023 | Random | 719 vs 756 | Complex lesions | 9 (Korea) | 24 |
aTVF(CD/TV-MI/ID-TVR): 6.5% vs 7.4% (HR: 0.87; 95% CI: 0.59-1.29) Procedure-related complications requiring active intervention: 1.7% vs 3.4% (P = 0.03) |
| RENOVATE-COMPLEX PCI (substudy)28 2023 | Random | 278 vs 800 | Complex lesions | 20 (Korea) | 25 |
aTVF(CD/TV-MI/ID-TVR): 5.8% vs 8.0% (HR: 0.72; 95% CI: 0.41-1.26) Contrast-induced nephropathy: 0.7% vs 3.0% (HR: 0.36; 95% CI: 0.09-1.54) |
| Merged KAMIR-NIH and KAMIR V55 2024 | Registry | 535 vs 4,725 | AMI | 43 (Korea) | 12 | aTLF(CD/TV-MI/ID-TLR): 2.1% vs 3.4% (HR: 0.61; 95% CI: 0.33-1.12) |
AMI = acute myocardial infarction; CCS = chronic coronary syndrome; CD = cardiac death; ID-TLR = ischemic-driven target-lesion revascularization; ID-TVR = ischemic-driven target-vessel revascularization; KAMIR-NIH = Korea Acute Myocardial Infarction-National Institutes of Health; MISTIC-1 = The Multimodality Imaging Study in Cardiology Cohort 1; MLA = minimal lumen area; NSTE-ACS = non–ST-segment elevation acute coronary syndrome; OCTIVUS = Optical Coherence Tomography versus Intravascular Ultrasound-Guided Percutaneous Coronary Intervention; OPINION = Optical Frequency Domain Imaging vs. Intravascular Ultrasound in Percutaneous Coronary Intervention; PCI = percutaneous coronary intervention; RENOVATE-COMPLEX PCI = Randomized Controlled Trial of Intravascular Imaging Guidance versus Angiography-Guidance on Clinical Outcomes after Complex Percutaneous Coronary Intervention; ST = stent thrombosis; TLF = target-lesion failure; TLR = target-lesion revascularization; TVF = target-vessel failure; TV-MI = target-vessel myocardial infarction.
Indicates primary endpoint.
Clinical Data of Intravascular Imaging Guidance According to the Lesion and Patient Subsets
The prospective cohort and registry data on intravascular imaging modalities, including IVUS and/or OCT, supported the benefits of intravascular imaging–guided PCI for complex coronary lesions, including ULMCA disease, diffuse long lesions, CTO, severely calcified lesions, bifurcations, ISR, and for high-risk patients, such as those with AMI (Table 5).
Table 5.
Clinical Data of Intravascular Guidance by Lesion and Patients Subsets
| Study | Study Type | Study Population | Followed Duration, mo |
Primary Endpoint (Imaging vs Angiography) |
|---|---|---|---|---|
| Unprotected left main coronary artery disease | ||||
| NOBLE29 2020 | RCT subgroup | Post-PCI IVUS (n = 435) vs No post-PCI IVUS (n = 164) | 12 | TLR: 5.1% vs 11.6% (HR: 0.44; 95% CI: 0.24-0.82) |
| BCIS database13 2020 | Registry | Imaging (n = 5056) vs Angiography (n = 6208) | 12 | Death: 8.9% vs 12.9% (OR: 0.66; 95% CI: 0.57-0.77) |
| MAIN-COMPARE (subgroup)14 2021 | Registry | IVUS (n = 756) vs Angiography (n = 219) | 143 | MACE(Death/Q-MI/stroke): 22.2 vs 30.3% (HR: 0.71; 95% CI: 0.52-0.97) |
| RENOVATE-COMPLEX-PCI (substudy)30 2023 | RCT | Imaging (n = 138) vs angiography (n = 54) | 25 | TVF(CD/TV-MI/ID-TVR): 6.8% vs 25.1% (HR: 0.31; 95% CI: 0.13-0.76) |
| LEMON31 2021 | Pilot Study |
OCT-guided LM PCI (n = 70) | — | Procedural success: 86%a |
| Diffuse long lesions | ||||
| IVUS-XPL2 2015 | RCT | IVUS (n = 700) vs Angiography (n = 700) | 12 | MACE(CD/TL-MI/ID-TLR): 2.9% vs 5.8% (HR: 0.48; 95% CI: 0.28-0.83) |
| Merged IVUS-XPL and ULTIMATE32 2022 | Pooled analysis | IVUS (n = 1289) vs Angiography (n = 1,288) | 36 | CD: 1.0% vs 2.2% (HR: 0.43; 95% CI: 0.22-0.84) |
| RENOVATE-COMPLEX-PCI23 2023 | RCT subgroup | Imaging (n = 617) vs Angiography (n = 281) | 25 | TVF(CD/TV-MI/ID-TVR): 6.5% vs 11.9% (HR: 0.52; 95% CI: 0.32-0.83) |
| ILUMIEN IV22 2023 | RCT subgroup | OCT (n = 853) vs Angiography (n = 824) | 24 | TVF(CD/TV-MI/ID-TVR): 6.4% vs 7.9% (HR: 0.81; 95% CI: 0.56-1.16) |
| Chronic total occlusion lesions | ||||
| CTO-IVUS7 2015 | RCT | IVUS (n = 201) vs Angiography (n = 201) | 12 | MACE(CD/MI/TVR): 2.6% vs 7.1% (HR: 0.35; 95% CI: 0.13-0.97) |
| Calcified lesions | ||||
| IVUS-derived calcium score35 2021 | In superficial calcium >270°, 1) ≥5mm length; 2) 360° of calcium; 3) calcified nodule; 4) vessel size <3.5 mm | Cutoff value of stent expansion <70%: calcium score ≥2 | ||
| OCT-based calcium score39 2018 | 1) >180° of calcium (2 point); 2) >0.5 mm of calcium thickness (1 point); ≥5mm length (1 point) | Stent expansion at target-lesion calcium: 96% (score 0-3) vs 78% (score 4) (P < 0.01) | ||
| Bifurcation lesions | ||||
| COBIS40 2011 | Registry | IVUS (n = 487) vs Angiography (n = 487) | 36 | MACE(Death/MI): 3.8% vs 7.8% (HR: 0.44; 95% CI: 0.12-0.96) |
| Kim et al.41 2010 | Registry | IVUS (n = 473) vs Angiography (n = 285) | 48 | Death: 0.4% vs 3.6% (HR: 0.17; 95% CI: 0.04-0.81) |
| OCTOBER3 2023 | RCT | OCT (n = 600) vs Angiography (n = 601) | 24 | MACE(CD/TL-MI/ID-TLR): 10.1% vs 14.1% (HR: 0.70; 95% CI: 0.50-0.98) |
| In-stent restenosis lesions | ||||
| iOPEN-ISR45 2021 | Registry | IVUS (n = 1,003) vs Angiography (n = 519) | 12 | MACE(Death/Q-MI/TVR): 18.0% vs 24.5% (HR: 0.77; 95% CI: 0.60-0.98) |
| RESTENT-ISR46 2016 | RCT | EES (n = 158) vs ZES (n = 146) | 36 | Neointimal volume on 9-month IVUS: 0.51 vs 0.56 mm3/1 mm (EES vs ZES) (P = 0.47) MACE(Death/MI/TLR/ST): 15.8% vs 22.6% (EES vs ZES) (P = 0.276) |
| Acute myocardial infarction | ||||
| KAMIR-NIH16 2022 | Registry | IVUS (n = 1,887) vs Angiography (n = 7,120) | 36 | TLF(CD/TV-MI/ID-TLR): 4.8% vs 8.0% (HR: 0.59; 95% CI: 0.47-0.73) |
COBIS = Coronary Bifurcation Stenting; iOPEN-ISR = intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN for In-stent Restenosis; LEMON = Left Main OCT-Guided Interventions; NOBLE = Nordic-Baltic-British Left Main Revascularization; QCA = quantitative coronary angiography; RESTENT-ISR = Prospective, Single-blinded, Randomized Comparison of the Clinical and Angiographic Results With Intravascular Analysis of Everolimus-Eluting Versus Zotarolimus-Eluting Stents for In-Stent Restenosis Lesions: Volumetric Analysis With Intravascular Ultrasound: Phase IV Multicenter Trial; ZES = zotarolimus-eluting stent(s); other abbreviations as in Table 1, Table 2, Table 3, Table 4.
Procedural success defined as TIMI flow grade 3 in all vessels + residual stenosis <50% by QCA + adequate stent expansion (MSA ≥80% of reference minimal luminal area in both proximal and distal stent sections, respectively).
Left main disease
IVUS guidance for left main disease can provide accurate vessel sizes of the reference segments and the degree of plaque extension to distal left main bifurcation, which leads to PCI optimization by appropriate stent selection and post-stent dilation. Several studies support the benefit of intravascular imaging for ULMCA, especially IVUS guidance. The subgroup analysis of the NOBLE (Nordic-Baltic-British Left Main Revascularization) trial showed that the use of IVUS to prevent stent underexpansion in PCI for ULMCA improved target-lesion revascularization rates at 5 years (0% in MSA ≥13.4 mm2 vs 12.2% in MSA ≤10.8 mm2, P = 0.002).27 The BCIS (British Cardiovascular Intervention Society) registry demonstrated that intravascular imaging–guided PCI for ULMCA was associated with fewer immediate procedural complications and improved 1-month and 12-month survival compared with angiography guidance.13 In a subgroup analysis of the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) study, IVUS-guided PCI for ULMCA was associated with a lower risk of mortality at 10 years compared with angiography-guided PCI.14 More recently, a subgroup analysis of the RENOVATE-COMPLEX-PCI trial showed that intravascular imaging guidance was superior to angiography guidance for ULMCA in reducing the risk of target-vessel failure, primarily due to a reduction of cardiac death.30
OCT-guided PCI for ULMCA has some limitations in the assessment of the left main ostium due to the difficulty in obtaining optimal blood clearing. However, a pilot study demonstrated the feasibility and safety of OCT-guided mid/distal left main PCI.31 Randomized trials are needed to confirm whether OCT-guided PCI for ULMCA improves clinical outcomes.
Diffuse long lesion
Precise morphological evaluation using intravascular imaging modalities facilitates the selection of an appropriate stent size and length for diffuse long lesions. In addition, the post-stent evaluation, related to stent expansion and apposition, edge dissection, lesion coverage, and the remnant residual plaque, could reduce the risks associated with stent thrombosis after DES implantation. In the IVUS-XPL trial involving patients requiring long-length DESs (≥28 mm), IVUS-guided PCI resulted in a significantly lower rate of the composite of cardiac death, myocardial infarction, or target-lesion revascularization at 1 year, primarily due to a lower risk of target-lesion revascularization.2 On the extended follow-up, the benefit of IVUS guidance was maintained compared with conventional angiographic guidance.9 From a patient-level analysis from the IVUS-XPL and ULTIMATE trials, the use of IVUS-guided long DES implantation compared with angiography-guided PCI was associated with a significant reduction in cardiac death and the composite of cardiac death, myocardial infarction, or stent thrombosis at 3 years.32
There had been a lack of evidence regarding OCT guidance for diffuse long lesions. The RENOVATE-COMPLEX-PCI showed the benefits of both IVUS and OCT for long lesions (expected stent length of at least 38 mm) in the subgroup analysis.23 In a subgroup analysis of the ILUMIEN IV trial, the OCT-guided PCI was not associated with a reduction in target-vessel failure at 2 years in patients with diffuse long lesions (≥28 mm).22 Further studies will be required to confirm the benefit of OCT guidance for diffuse long lesions.
CTO lesion
IVUS guidance during CTO-PCI plays an essential role for the success of CTO-PCI; in cases of stumpless CTO, it is recommended to cross a guidewire to the side branch (SB) located immediately proximal to the CTO lesion.33 The use of IVUS during antegrade or retrograde CTO-PCI also proves to be highly advantageous in terms of identifying the location of CTO wire, facilitating guidewire tracking, and delivering crucial information regarding the true lumen or subintimal space.34 In addition, IVUS is helpful for deciding the optimal stent sizing and landing and ensuring full expansion, and when treating complex long-calcified CTO lesions. In the randomized CTO-IVUS (Chronic Total Occlusion Intervention With Drug-eluting Stents) study, IVUS-guided CTO-PCI improved the 12-month major adverse cardiac event rate after DES implantation when compared with conventional angiography-guided CTO-PCI.7
OCT guidance for CTO remains limited in daily practice because of potential risks for expanding the false lumen or coronary artery rupture by contrast injection during imaging acquisition.
Calcified lesion
Imaging guidance is essential in managing calcified lesions, offering precise assessment of calcification severity, treatment efficacy, and informing decisions regarding the appropriate plaque modification strategy.12,23 The IVUS-derived calcium score considering calcium arc and length, presence of calcified nodule, and vessel diameter has been reported to predict stent underexpansion and guide calcium modification.35 However, IVUS is limited for assessing calcium thickness and vessel diameter at the site of calcified plaque due to poor penetration into the calcification.
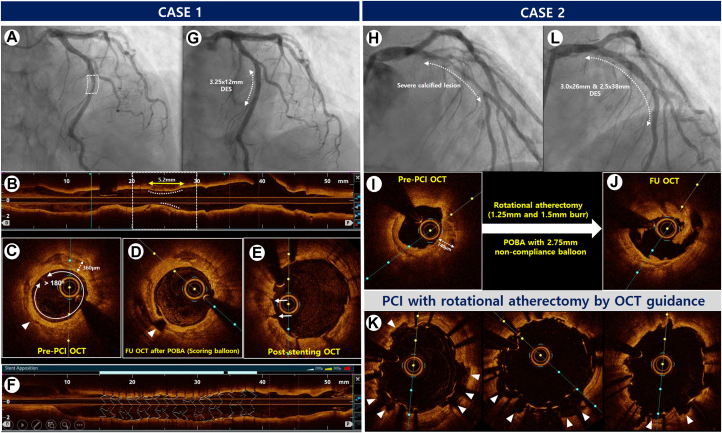
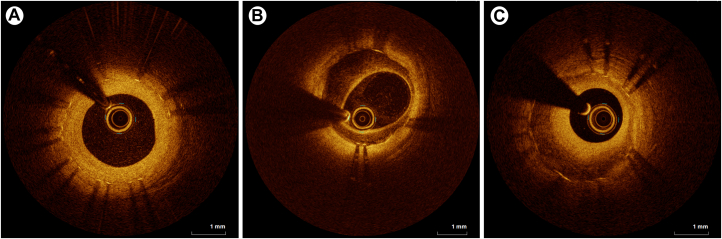
OCT can offer a superior delineation of calcified lesions because of its enhanced resolution, surpassing that of IVUS, and enables a quantitative analysis of calcified lesions, encompassing parameters such as calcium thickness, angle of the calcium layer, and length of calcified plaques.36 Several studies have suggested OCT thresholds, including calcium arc and thickness, to achieve successful calcium fracture in calcified plaques with various plaque modification strategies.37,38 Recently, an OCT-based calcium scoring system considering calcium arc, thickness, and length was introduced to aid in calcified plaque modification before stent implantation.39 Representative cases of OCT-guided PCI for heavily calcified plaques are shown in Figure 1.
Figure 1.
OCT-Guided PCI With DES Implantation for the Severe Calcified Lesions
Case 1. (A) Angiogram showing severe stenosis in the mid-portion of the LAD in a 67-year-old woman presenting with non–ST-segment elevation myocardial infarction. (B and C) Pre-PCI OCT assessment of a longitudinal circumferential calcified plaque with OCT-based calcium score of 3 points (maximal calcium angle >180° [2 points], calcium length >5.0 mm [1 point], and maximal calcium thickness [0 point]). (D) Post-ballooning OCT showing a calcium fracture after balloon angioplasty using a scoring balloon (arrowhead). (E) Post-stent cross-sectional OCT showing the presence of cracks (arrows). (F) 3-dimensional stent image and apposition index demonstrating a well-expanded stent without malapposition. (G) Final angiography showing good distal flow without residual stenosis after stent implantation, Case 2. (H) Angiogram showing diffuse severe stenosis with heavy calcification in the proximal to mid-portion of the LAD in a 54-year-old man presenting with stable angina. (I) Pre-PCI OCT assessment of diffuse circumferential plaques with thick calcifications leading to OCT-based calcium score of 4 points (maximal calcium angle >180° [2 points], calcium length >5.0 mm [1 point], and maximal calcium thickness [1 point]). (J) Post-rota and ballooning OCT showing good modification of the calcified plaque by rotational atherectomy and noncompliant balloon inflation. (K) Post-stent OCT showing multiple calcium fractures (arrowheads). (L) The final angiogram showing good distal flow without residual stenosis. DES = drug-eluting stent(s); LAD = left anterior descending artery; OCT = optical coherence tomography; PCI = percutaneous coronary intervention; POBA = plain old balloon angioplasty.
Bifurcation lesion
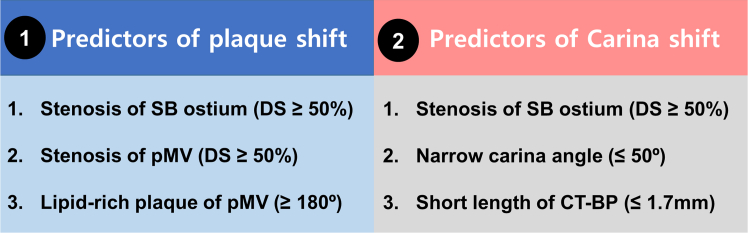
Intravascular imaging assessment for bifurcation lesions proves invaluable in discerning the extent of plaque within the main vessel, as well as in determining ostial involvement of SB. Previous registry data showed that IVUS-guided DES implantation was associated with improved clinical outcomes in patients with non–left main bifurcation lesions.40,41 Several studies have investigated IVUS predictors of SB occlusion after main vessel (MV) stenting in bifurcation lesions. Notably, findings indicate that the ostial plaque of the SB, especially if diffuse plaques, or aggressive stent expansion within the distal MV segment after stenting causing the carina shift, were predictors of SB occlusion during IVUS-guided PCI.42,43
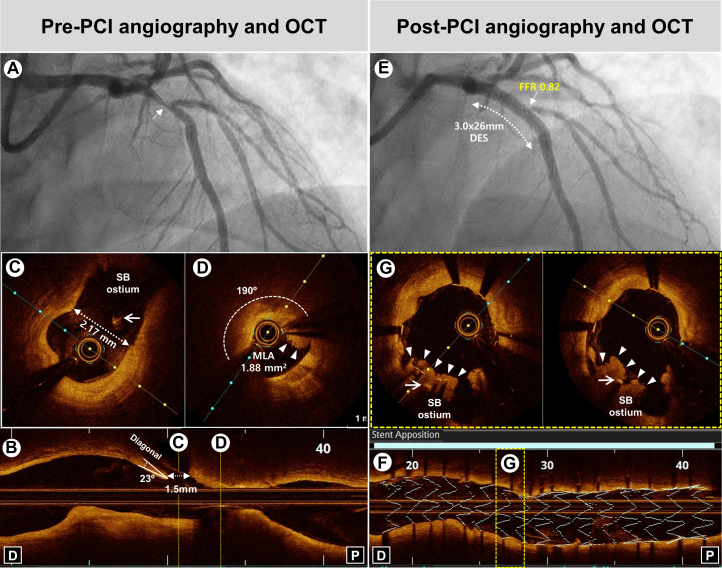
The OCTOBER, the first randomized OCT study for complex bifurcation lesions, demonstrated that OCT-guided PCI was associated with a significantly lower incidence of major adverse cardiac events (MACEs) at 2 years than angiography-guided PCI (10.1% vs 14.1%).3 OCT having a superior resolution power over IVUS could provide a longitudinal reconstruction image that clearly identifies both the carina and SB ostium, as well as the carina angle. A retrospective OCT study demonstrated that high lipid content of the proximal MV and SB ostial stenosis severity were independent predictors of SB occlusion after MV stenting.44 The angiographic, IVUS, and OCT factors associated with SB compromise during bifurcation PCI and representative case of bifurcation PCI by OCT guidance are shown in Figures 2 and 3.
Figure 2.
Risk Factors of SB Compromise
The predictors of plaque shift and carina shift are summarized at the boxes (① and ②). CT-BP = carina tip and branching point; DS = diameter stenosis; MV = main vessel; pMV = proximal main vessel; SB = side branch.
Figure 3.
Representative Case of Bifurcation PCI by OCT Guidance
(A) Baseline angiography showing severe stenosis (arrow) at the bifurcation lesion of the proximal LAD. (B) Pre-PCI OCT showing narrow carina angle (23°) and short carina tip to branching point (1.5 mm). (C and D) Cross-sectional OCT images demonstrating MLA of 1.88 mm2 with a large lipid-rich plaque (190°) and intraluminal thrombus (arrowheads) in proximal MV without the stenosis of SB ostium (diameter of SB ostium: 2.17 mm) (arrow: guidewire of SB). (E) Post-PCI angiography showing successful PCI using SB wire jailing technique in the proximal LAD with severe stenosis and TIMI 3 flow in the SB ostium, demonstrating functional nonsignificance (FFR: 0.82). (F) 3-dimensional stent image and apposition index demonstrating stent optimization. (G) Cross-sectional OCT images demonstrating plaque shift (arrowheads) to SB ostium (arrow: guidewire of SB). FFR = fractional flow reserve; MLA = minimal lumen area; SB = side branch; other abbreviations as in Figures 1 and 2.
ISR lesion
IVUS and OCT play a vital role in the management of ISR lesions by diagnosing and characterizing ISR, aiding in precise lesion measurement, identifying the causes of restenosis including the evaluation of stent underexpansion, and optimizing treatment strategies. The observation study for patients with ISR treatment reported that IVUS-guided PCI resulted in a lower rate of MACE at 1 year following PCI for ISR than angiography-guided treatment.45 In the randomized trial for patients with DES-ISR, re-stenting with new-generation DES was effective and safe in reducing neointima volume and late loss with a favorable MACE occurrence.46
The utilization of OCT with a high resolution allows precise characterization of neointimal tissue. Gonzalo et al47 delineated the neointimal characteristics on OCT into 3 differential patterns: homogeneous, heterogeneous, and layered (Figure 4). Retrospective OCT studies showed that the restenosis rate after drug-coated balloon (DCB) treatment was lower in ISR lesions with homogeneous neointima, whereas a heterogeneous tissue pattern with insufficient OCT-derived final minimal lumen area was significantly associated with restenosis after DCB treatment for DES-ISR.48,49 Furthermore, in the DCB treatment for ISR lesions, a large reduction in the mean neointimal volume on OCT was reported to lead to an improvement in clinical outcomes.50 Various qualitative or quantitative OCT analyses should be used to investigate clinical outcomes after ISR treatment using DCB or DES.
Figure 4.
Representative Optical Coherence Tomography Images of Neointimal Tissue
Homogeneous (uniform optical properties without focal variation in the backscattering patterns) (A), heterogeneous (focally changing optical properties and various backscattering patterns) (B), and layered (concentric layers with different optical properties) (C) types.
Fractional flow reserve–negative coronary stenotic lesion with vulnerable plaques
Guidelines do not recommend revascularization for the intermediate stenotic lesion with non-flow limiting, defined by fractional flow reserve (FFR) >0.80, but vulnerable plaque (also known as fibroatheroma, lipid-rich plaque, or necrotic core) is a potential factor in future adverse cardiac events.51 A prospective, double-blind study showed that OCT-detected thin-cap fibroatheroma in lesions with negative FFR value was associated with a 5-fold higher rate of adverse events in diabetic patients.52 Recently, the PREVENT (Preventive Coronary Intervention on Stenosis with Functionally Insignificant Vulnerable Plaque) randomized trial demonstrated that preventive PCI plus optimal medical therapy for FFR-negative stenotic lesions with IVUS- or OCT-detected high-risk vulnerable plaques was associated with a significant reduction of MACEs during long-term follow-up (up to 7.9 years) compared with optimal medical therapy alone.53
AMI
Despite the potential beneficial roles of IVUS in the setting of AMI, there has been a lack of large-scale random studies regarding the impact of intravascular imaging–guided PCI on the clinical outcomes following AMI-PCI. However, several registry data support the benefits of intravascular imaging guidance in the setting of AMI in terms of short- and long-term outcomes. The prospective nationwide registry J-MINUET (Japanese Registry of Acute Myocardial Infarction Diagnosed by Universal Definition) showed that IVUS-guided PCI was associated with lower in-hospital death compared with angiography guidance.15 In a nationwide prospective KAMIR-NIH (Korea Acute Myocardial Infarction-National Institutes of Health) registry, the use of IVUS or OCT guidance was associated with a reduction in 1-year clinical outcomes in patients with AMI who underwent PCI.54 Regarding the long-term benefit of IVUS guidance, IVUS-guided PCI was associated with a significantly lower risk of target-lesion failure at 3 years, mainly driven by hard endpoints including cardiac death or target-vessel myocardial infarction, compared with angiography-guided PCI.16
Regarding OCT-guided PCI in the setting of AMI, the pooled data showed that OCT-guided PCI provided comparable clinical outcomes for 1-year target-lesion failure compared with IVUS guidance.55 Further randomized studies are needed to determine the benefits of IVUS or OCT guidance in AMI.
Intravascular Imaging Predictors for Improved Clinical Outcomes: PCI Optimization Strategy
The achievement of stent optimization based on imaging evaluation has been reported to be associated with favorable clinical outcomes following stent implantation (Table 6). The IVUS-XPL trial showed that patients who met the IVUS criteria (54% of patients in the IVUS-guided PCI group) had a significantly lower incidence of MACE at 1 year than those who did not meet the IVUS criteria for stent optimization.2 Similarly, in the ULTIMATE trial, patients who met the optimization criteria (53% of patients in the IVUS-guided PCI group) had a significantly lower rate of target-vessel failure at 1 year compared with that in patients who did not meet the optimization criteria.8 The CLI-OPCI II OCT registry also showed that suboptimal stent deployment was an independent predictor of worse clinical outcomes.56 Therefore, the achievement of stent optimization criteria using IVUS or OCT guidance is essential for improved clinical outcomes when performing intravascular imaging–guided PCI. In this section, we review the data regarding stent optimization criteria using imaging guidance.
Table 6.
Key Clinical Data of Intravascular Imaging–Guided Stent Optimization
| Study | Imaging Modality Study Type |
Study Population | Optimization Criteria | % Patients Meeting the Optimization Criteria of the Total Image-Guided Patients | Followed Duration, mo | Key Endpoint (Optimal PCI vs Suboptimal PCI) |
|---|---|---|---|---|---|---|
| IVUS-XPL2 2015 | IVUS Random |
Long lesion (Stent ≥28 mm) | MSA ≥100% of the distal RLA | 54% (363/700 patients) | 12 | MACE(CD/TL-MI/ID-TLR): 1.5% vs 4.6% (HR: 0.31; 95% CI: 0.11-0.86) |
| 60 | MACE(CD/TL-MI/ID-TLR): 4.0% vs 7.4% (HR: 0.48; 95% CI: 0.28-0.83) | |||||
| ULTIMATE8 2018 | IVUS Random |
All comers |
|
53% (384/724 patients) | 12 | TVF(CD/TV-MI/ID-TVR): 1.6% vs 4.4% (HR: 0.35; 95% CI: 0.14-0.90) |
| 36 | TVF(CD/TV-MI/ID-TVR): 4.2% vs 9.2% (HR: 0.44; 95% CI: 0.24-0.81) | |||||
| Merged data of IVUS-XPL and ULTIMATE32 2022 | IVUS Pooled analysis |
Long lesion (Stent ≥28 mm) | Optimization criteria according to each trial’s definition | 52% (654/1,267 patients) | 36 | CD: 0.5% vs 1.5% (HR: 0.31; 95% CI: 0.08-1.15) MACE(CD/MI/ST): 0.5% vs 2.2% (HR: 0.22; 0.06-0.75) |
| Merged data of 4 randomized trials61 2020 | IVUS Pooled analysis |
Long lesion (Stent ≥26 mm) or CTO | MSA ≥4.5 mm2 or MSA ≥80% of mead RLA | 41% (578/1,396) | 12 | MACE(CD/MI/ST/TVR): 1.9% vs 4.8% (HR: 0.34; 95% CI: 0.17-0.70) |
| CLI-OPCI II56 2015 | OCT Registry |
All comers |
|
69% (679/984 lesions) | 10.4 | MACE(Death/MI/TLR): 7.1% vs 25.2% (HR: 0.23; 95% CI: 0.15-0.34) |
| IVUS-ACS11 2024 | IVUS Random |
ACS |
|
79.9% (1,392/1,743 patients) | 12 | TVF(CD/TV-MI/ID-TVR): 3.2% vs 7.1% (P < 0.001) |
Prestent evaluation
Pre-interventional intravascular imaging guidance enables the accurate evaluation of plaque characteristics and the cross-sectional and longitudinal measurements at the lesion and reference segments, and longitudinal assessment of the lesion length. Detailed information regarding the lesion and reference segments provides the operator with a stent landing zone and enables the precise selection of stent size and length for stent optimization.
Poststent evaluation
Intravascular imaging enables assessment of stent expansion, apposition, edge dissection, and intrastent evaluation after stenting. When stent underexpansion is found by intravascular imaging, the use of postdilation with high-pressure balloon by imaging guidance is recommended and is known to be associated with a lower risk of cardiac events.57 In addition, intravascular imaging–detected stent strut malapposition or edge dissection can lead to further procedures for stent optimization.58
Stent expansion
Stent expansion is the most important factor among the criteria of stent optimization. MSA as the absolute expansion (cross-sectional MSA with an absolute measurement) and relative stent expansion (ratio of MSA to the mean reference lumen area) are considered pivotal parameters for predicting adverse events after DES implantation.59, 60, 61 Pre- and post-PCI imaging guidance can lead to an appropriate and safe PCI strategy by the postdilation using a noncompliant balloon to achieve optimal stent expansion.57,58,61 Various MSA criteria were introduced; absolute expansion (MSA >4.5 or 5.0 mm2) was suggested according to the lesion or clinical characteristics, and relative expansion (MSA ≥80% or 90% of mean reference lumen area or ≥90% to 100% of distal reference lumen area) was suggested based on the mean reference or distal reference lumen area. Regarding the definition of inadequately expanded stent, Japanese expert consensus for IVUS suggested stent expansion <80% of the reference vessels or a single cutoff value of MSA (eg, <5.0 or 5.5 mm2).62,63
The values of OCT measurement are different from IVUS because faster pullback speed of OCT precludes selection of frames at maximum diastole in OCT image.36 Therefore, OCT-derived cross-sectional cutoff values were smaller than the IVUS criteria: OCT-derived MSA <4.5 mm2 vs IVUS-derived MSA <5.0 mm2 or 5.5 mm2 in non–left main lesions. OCT expansion criteria for relative expansion were similar to the those of IVUS: MSA ≥80% or 90% of mean reference lumen area or ≥100% of distal reference lumen area. Detailed values for optimal stent expansion are provided in Table 7.
Table 7.
Intravascular Imaging–Guided Drug-Eluting Stent Optimization Criteria in Randomized Trials
| Study | Imaging Tools | Optimization Criteria |
||||
|---|---|---|---|---|---|---|
| Stent Sizing | Landing Zone | Stent Expansion | Stent Apposition | Edge Dissection | ||
| CTO-IVUS7 2015 | IVUS | N/A | N/A | MSA ≥5 mm2 MSA ≥100% of the distal RLA |
Complete stent apposition | N/A |
| IVUS-XPL2 2015 | IVUS | N/A | N/A | MSA ≥100% of the distal RLA | N/A | N/A |
| OPINION24 2017 | IVUS OCT |
Lumen ϕ at the proximal and distal reference sites | OCT: No lipid-rich plaque IVUS: Plaque burden <50% |
MSA ≥90% of mean RLA | ASM ≤350 μm on OCT | No flow disturbance |
| ULTIMATE8 2018 | IVUS | Ratio of 0.8 to EEL ϕ or 1:1 to lumen ϕ of the distal reference site | Plaque burden <50% | MSA >5.0 mm2 MSA ≥90% of the distal RLA |
N/A | Flap length ≤3.0 mm No media or adventitia involvement |
| MISTIC-125 2020 | IVUS OCT |
IVUS: EEL ϕ at reference sites OCT: 10% or 0.25 mm larger than mean lumen ϕ at reference sites |
OCT: Lipid-rich plaque <180° IVUS: Plaque burden <50% |
MSA ≥80% of mean RLA | Malapposed strut <20% | Flap root thickness ≤300 μm Flap length ≤1.0 mm No media or adventitia involvement |
| RENOVATE-COMPLEX-PCI23 2023 | IVUS OCT |
IVUS: EEL ϕ at reference sites OCT: EEL ϕ (down to nearest 0.25 mm) or lumen ϕ (up to nearest 0.25 mm) at the distal reference sites. |
OCT: No lipid-rich plaque IVUS: Largest lumen with plaque burden <50% |
OCT: MSA >4.5 mm2 IVUS: MSA >5.5 mm2 (>7 mm2 in distal LM and >8 mm2 in LM shaft) MSA ≥90% of mean RLA |
ASM <400 μm with longitudinal extension ≤1mm | Circumferential angle <60º Flap length <3 mm No media or adventitia involvement |
| OCTIVUS26 2023 | IVUS OCT |
IVUS: EEL ϕ at reference sites OCT: EEL ϕ (down to nearest 0.25 mm) or lumen ϕ (up to nearest 0.25 mm) at the distal reference sites. |
No lipid-rich plaque with plaque burden ≤ 50% | MSA ≥80% of mean RLA | N/A | Circumferential angle ≤60º Flap length ≤2mm No media or adventitia involvement |
| ILUMIEN IV22 2023 | OCT | EEL ϕ (down to nearest 0.25 mm) or lumen ϕ (up to nearest 0.25 mm) at the distal reference sites. | Minimal lumen area ≥4.5 mm2 | MSA >4.5 mm2 MSA ≥90% of mean RLA in each proximal and distal segment, respectively |
ASM <200 μm combined with acceptable stent expansion (≥ 90%) | Circumferential angle <60º Flap length <3 mm |
| OCCUPI (NCT 30625908) | OCT | EEL ϕ (down to nearest 0.25 mm) or lumen ϕ (up to nearest 0.25 mm) at the distal reference sites. | Plaque burden <50% | MSA >4.5 mm2 MSA ≥80% of mean RLA MSA ≥100% of the distal RLA |
ASM <400 μm | Circumferential angle <60º Flap length <3 mm No media or adventitia involvement |
Stent apposition
Data regarding the clinical impacts of stent apposition between the stent struts and the vessel wall on intravascular imaging still showed conflicting results.36 Stent malapposition (SM), which was defined by the lack of contact between at least 1 stent strut and the underlying vessel wall in a segment not overlying SB, was classified into 3 types: 1) acute SM: immediately detected malapposition after DES implantation; 2) late-persistent SM: remained acute SM at the follow-up; and 3) late-acquired SM: newly developed SM identified during follow-up despite complete stent apposition on immediate post-stent intravascular imaging. Although acute SM was frequently observed, it has been reported to be well-resolved in up to 90% of cases on the serial OCT follow-up study.64 However, severe acute SM could prolong into the late-persistent SM. Regarding the clinical impact of acute SM, it has been reported to be frequently observed in patients with stent thrombosis, but it is not clear whether acute SM was directly associated with the development of stent thrombosis.65, 66, 67, 68 Detailed apposition criteria are provided in Table 7.
Stent edge dissection
Stent edge dissection is a well-known risk factor associated with MACE, including stent thrombosis after PCI.36 Because of the higher resolution of OCT compared with IVUS, edge dissection is more frequently observed by OCT. Furthermore, IVUS-derived criteria focus on its angle and length, whereas OCT-derived criteria focus on its depth and length as well. Therefore, the presence or absence of dissection as assessed by intravascular imaging is a major factor associated with stent optimization. Generally, the non–flow-limiting small stent edge dissection is known to have no effect during clinical follow-up; however, additional procedures, including the adding of stents, were recommend in cases of flow-limiting or large-sized edge dissection, which is defined as major edge dissection.56,69 For the assessment of major dissection, the evaluation of the lateral or longitudinal extension of edge dissection along with the involvement of medial or adventitia layers are strongly recommended. Various optimization criteria for edge dissection are detailed in Table 7.
Tissue prolapse
Tissue prolapse, defined as an intraluminal protrusion of tissue (plaque and/or thrombus) into the lumen through stent struts, is more frequently observed on OCT than on IVUS due to a superior resolution of OCT.36 Although tissue prolapse was associated with periprocedural myocardial infarction, or acute and subacute stent thrombosis in an IVUS analyses, it has been reported that tissue prolapse after successful PCI had no significant long-term clinical outcomes.70,71 However, a multicenter OCT registry showed that irregular tissue protrusion, not smooth or disrupted fibrous tissue protrusion, was an independent OCT predictor of poor clinical outcomes at 1 year.72 The impact of tissue prolapse on intravascular imaging on the short- and long-term outcomes and the characteristics of prolapse affecting the outcomes should be investigated and clarified.
Various stent optimization criteria by intravascular imaging guidance
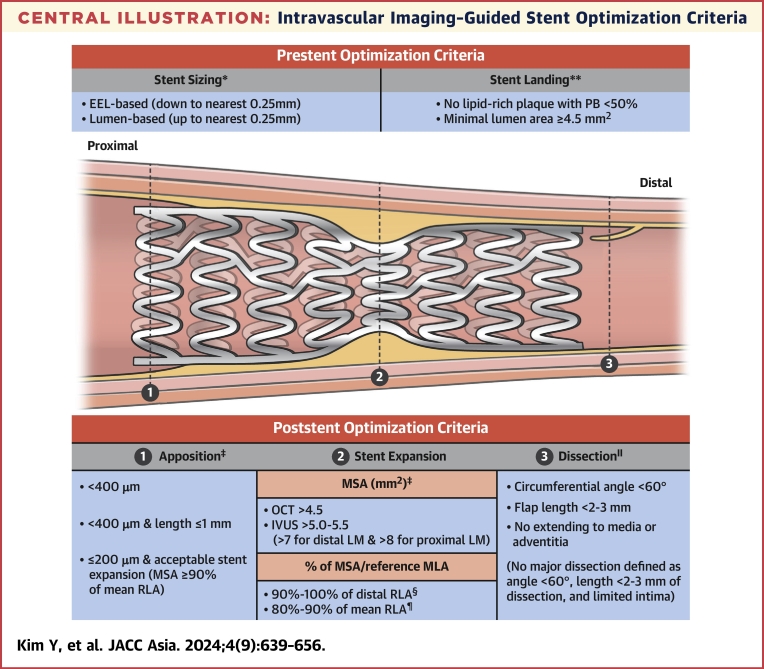
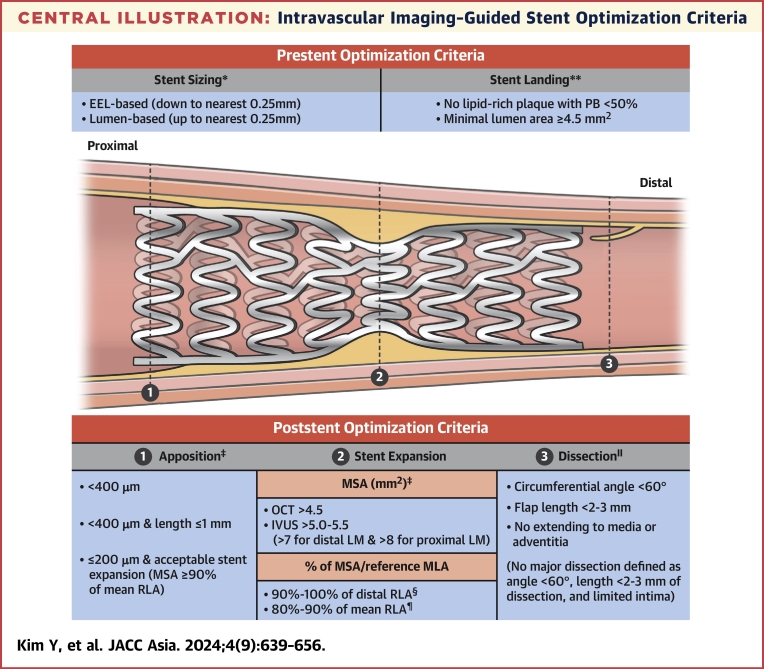
Major stent optimization criteria by intravascular imaging guidance includes the cutoff values or definitions regarding stent expansion, stent strut apposition, and presence/absence of edge dissection. Stent expansion as the criteria for stent optimization is described as various absolute (MSA) or relative expansion (MSA compared with a predefined reference area).36 Stent apposition criteria frequently use the maximal distance between the vessel wall and stent strut, with usually <200 to 400 μm considered acceptable criteria.23 Edge dissection is decided by the presence of major dissection assessed by the lateral and longitudinal extension, or depth involvement, and the flow disturbance related to edge dissection is also assessed.24 Criteria for stent optimization using intravascular imaging guidance in each randomized trial of imaging-guided PCI are summarized in the Central Illustration and Table 7.
Central Illustration.
Intravascular Imaging–Guided Stent Optimization Criteria
Summary of pre- and post-stenting optimization criteria derived from various randomized trials regarding intravascular imaging–guided percutaneous coronary intervention. EEL = external elastic lamina; IVUS = intravascular ultrasound; LM = left main; MSA = minimal stent area; OCT = optical coherence tomography; PB = plaque burden; RLA = reference lumen area. ∗Data from RENOVATE-COMPLEX PCI (Randomized Controlled Trial of Intravascular Imaging Guidance versus Angiography-Guidance on Clinical Outcomes after Complex Percutaneous Coronary Intervention), OCTIVUS (Optical Coherence Tomography versus Intravascular Ultrasound-Guided Percutaneous Coronary Intervention), ILUMIEN IV (Optimal Coherence Tomography Guided Coronary Stent Implantation Compared to Angiography: a Multicenter Randomized Trial in PCI), and OCCUPI (Optical Coherence Tomography-Guided Coronary Intervention in Patients With Complex Lesions: a Randomized Controlled Trial). ∗∗Data from OPINION (Optical Frequency Domain Imaging vs. Intravascular Ultrasound in Percutaneous Coronary Intervention), ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions), MISTIC-1 (The Multimodality Imaging Study in Cardiology Cohort 1), RENOVATE-COMPLEX-PCI, OCTIVUS, ILUMIEN IV, and OCCUPI. †Data from RENOVATE-COMPLEX-PCI, ILUMIEN IV, and OCCUPI. ‡Data from CTO-IVUS (Chronic Total Occlusion Intervention With Drug-eluting Stents), ULTIMATE, RENOVATE-COMPLEX-PCI, ILUMIEN IV, and OCCUPI. §Data from CTO-IVUS, IVUS-XPL (Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions), ULTIMATE, and OCCUPI. ¶Data from OPINION, MISTIC-1, RENOVATE-COMPLEX-PCI, OCTIVUS, ILUMIEN IV, and OCCUPI. ‖Data from ULTIMATE, RENOVATE-COMPLEX-PCI, OCTIVUS, ILUMIEN IV, and OCCUPI.
Follow-up Evaluation After Stenting
Neointimal hyperplasia and neointimal coverage
Follow-up intravascular imaging assessments after stenting are useful for the qualitative and quantitative evaluation of neointimal hyperplasia leading to late lumen loss, restenosis, or stent thrombosis. IVUS-based studies mainly evaluated the quantitative assessment of neointimal hyperplasia between post-intervention and follow-up.73 However, IVUS is limited in the qualitative assessment of neointimal types because of its low-resolution power. Contrastingly, high-resolution OCT enabled the qualitative analysis of neointima after stent implantation in several studies (Figure 4).47,74 Because endothelial coverage was known to be the most powerful histological predictor of stent thrombosis from the human autopsies of DES thrombosis, the role of OCT in assessing the neointimal coverage of various DES struts came into limelight.75 In particular, the uncovered strut, defined as a neointimal hyperplasia thickness of 0 μm on OCT, has been reported to be commonly observed in-stent thrombosis and to be reduced by OCT guidance in the randomized comparison with angiography guidance.66,76,77 In addition, a higher rate of % uncovered strut (cutoff value of ≥5.9%) on follow-up OCT was reported to be associated with the occurrence of adverse ischemic events after DES implantation in an OCT registry study, and subsequently, efforts have been made to tailor dual antiplatelet therapy regimens based on this threshold during short-term follow-up OCT assessments.78
Neoatherosclerosis
Pathologic studies have revealed the presence of a sizable necrotic core containing cholesterol crystals within neointima, resembling vulnerable plaque encountered in native coronary arteries, after DES implantation.79 With a higher resolution surpassing IVUS, OCT enabled the detection of newly developed atherosclerotic changes within stent segments in patients with stent restenosis, measurement of necrotic core cap thickness, and identification of thin-cap neoatherosclerosis, defined as fibrous cap thickness at the thinnest part with mean ≤65 μm within the stented vessel. With respect to DES failure, neoatherosclerosis is the most important mechanism for late thrombosis and restenosis and neointimal rupture is suggested as a contributing factor to stent thrombosis.80,81 The determinants for neoatherosclerosis have been known as the stent age, current smoking, and CKD.82 Therefore, the detection of neoatherosclerosis during follow-up OCT after DES implantation could help predict clinical outcomes of patients with DES implantation.
Late SM
Several studies have highlighted the potential significance of late SM, encompassing both late-persistent and late-acquired forms, observed during follow-up IVUS after stenting might be associated with an increased incidence of cardiovascular events.83,84 In follow-up IVUS studies, late-acquired SM had been reported to be associated with a higher risk of very late stent thrombosis and is regarded as the most important factor for predicting DES failure.85
Regarding OCT data on late SM, although a higher incidence of late SM was reported because of the high-resolution power of OCT, its clinical correlation remains lacking.64,86 Because most studies regarding late SM using intravascular imaging had been conducted based on retrospective or registry data, large-scale, prospective long-term intravascular imaging studies are warranted to evaluate the clinical impact of LSM in the current DES era.
Conclusions
Many randomized controlled trials and registries have demonstrated that the routine use of intravascular imaging–guided PCI could substantially improve clinical outcomes in patients with coronary artery disease, especially those with high ischemic risks, such as AMI and CKD, and complex lesions, including ULMCA disease, CTO, severely calcified lesions, bifurcation, and ISR. Notably, recent data suggest that OCT-guided PCI provides clinical outcomes comparable to those of IVUS-guided PCI.
During intravascular imaging–guided PCI, operators attempt to achieve stent optimization to maximize the benefits of imaging guidance. To further enhance long-term patient outcomes using intravascular imaging guidance in daily clinical practice, step-by-step technical protocols for imaging guidance and dedicated imaging-guided stent optimization criteria are needed.
Funding Support and Author Disclosures
This work was supported by the Imaging and Physiology with Cardiovascular Disease (IPOP) and the Korean Society of Interventional Cardiology (KSIC). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Medical Illustration and Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Byeong-Keuk Kim, Email: kimbk@yuhs.ac.
Seung-Ho Hur, Email: shur@dsmc.or.kr.
References
- 1.Mintz G.S., Popma J.J., Pichard A.D., et al. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation. 1996;93:924–931. doi: 10.1161/01.cir.93.5.924. [DOI] [PubMed] [Google Scholar]
- 2.Hong S.J., Kim B.K., Shin D.H., et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 3.Holm N.R., Andreasen L.N., Neghabat O., et al. OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med. 2023;389:1477–1487. doi: 10.1056/NEJMoa2307770. [DOI] [PubMed] [Google Scholar]
- 4.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 5.Writing Committee Members. Lawton J.S., Tamis-Holland J.E., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Byrne R.A., Rossello X., Coughlan J.J., et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 7.Kim B.K., Shin D.H., Hong M.K., et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Gao X., Kan J., et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72:3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Hong S.J., Mintz G.S., Ahn C.M., et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13:62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Gao X.F., Ge Z., Kong X.Q., et al. 3-year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14:247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Li X., Ge Z., Kan J., et al. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet. 2024;403:1855–1865. doi: 10.1016/S0140-6736(24)00282-4. [DOI] [PubMed] [Google Scholar]
- 12.Choi K.H., Song Y.B., Lee J.M., et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12:607–620. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 13.Kinnaird T., Johnson T., Anderson R., et al. Intravascular imaging and 12-month mortality after unprotected left main stem PCI: an analysis from the British Cardiovascular Intervention Society Database. JACC Cardiovasc Interv. 2020;13:346–357. doi: 10.1016/j.jcin.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Kang D.Y., Ahn J.M., Yun S.C., et al. Long-term clinical impact of intravascular ultrasound guidance in stenting for left main coronary artery disease. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.121.011011. [DOI] [PubMed] [Google Scholar]
- 15.Okura H., Saito Y., Soeda T., et al. Frequency and prognostic impact of intravascular imaging-guided urgent percutaneous coronary intervention in patients with acute myocardial infarction: results from J-MINUET. Heart Vessels. 2019;34:564–571. doi: 10.1007/s00380-018-1285-3. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y., Bae S., Johnson T.W., et al. Role of intravascular ultrasound-guided percutaneous coronary intervention in optimizing outcomes in acute myocardial infarction. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.023481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Gao X., Ge Z., et al. Impact of intravascular ultrasound-guided drug-eluting stent implantation on patients with chronic kidney disease: results from ULTIMATE trial. Catheter Cardiovasc Interv. 2019;93:1184–1193. doi: 10.1002/ccd.28308. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K., Shiomi Morimoto T., et al. Optimal intravascular ultrasound-guided percutaneous coronary intervention in patients with multivessel disease. JACC Asia. 2023;3(2):211–225. doi: 10.1016/j.jacasi.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prati F., Di Vito L., Biondi-Zoccai G., et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. Eurointervention. 2012;8:823–829. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 20.Meneveau N., Souteyrand G., Motreff P., et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (Does Optical Coherence Tomography Optimize Results of Stenting) Circulation. 2016;134:906–917. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- 21.Sheth T.N., Kajander O.A., Lavi S., et al. Optical coherence tomography-guided percutaneous coronary intervention in ST-segment-elevation myocardial infarction: a prospective propensity-matched cohort of the thrombectomy versus percutaneous coronary intervention alone trial. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003414. [DOI] [PubMed] [Google Scholar]
- 22.Ali Z.A., Landmesser U., Maehara A., et al. Optical coherence tomography-guided versus angiography-guided PCI. N Engl J Med. 2023;389:1466–1476. doi: 10.1056/NEJMoa2305861. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.M., Choi K.H., Song Y.B., et al. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med. 2023;388:1668–1679. doi: 10.1056/NEJMoa2216607. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T., Shinke T., Okamura T., et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38:3139–3147. doi: 10.1093/eurheartj/ehx351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muramatsu T., Ozaki Y., Nanasato M., et al. Comparison between optical frequency domain imaging and intravascular ultrasound for percutaneous coronary intervention guidance in Biolimus A9-eluting stent implantation: a randomized MISTIC-1 non-inferiority trial. Circ Cardiovasc Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.120.009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang D.Y., Ahn J.M., Yun S.C., et al. Optical coherence tomography-guided or intravascular ultrasound guided percutaneous coronary intervention: the OCTIVUS randomized clinical trial. Circulation. 2023;148:1195–1206. doi: 10.1161/CIRCULATIONAHA.123.066429. [DOI] [PubMed] [Google Scholar]
- 27.Kang D.Y., Ahn J.M., Yun S.C., et al. Guiding intervention for complex coronary lesions by optical coherence tomography or intravascular ultrasound. J Am Coll Cardiol. 2024;83:401–413. doi: 10.1016/j.jacc.2023.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.M., Kim H., Lee J.Y., et al. Optical coherence tomography compared with intravascular ultrasound and angiography in complex coronary artery lesions. JACC Cardiovasc Imaging. 2024;17:336–338. doi: 10.1016/j.jcmg.2023.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Ladwiniec A., Walsh S.J., Holm N.R., et al. Intravascular ultrasound to guide left main stem intervention: a NOBLE trial substudy. EuroIntervention. 2020;16:201–209. doi: 10.4244/EIJ-D-19-01003. [DOI] [PubMed] [Google Scholar]
- 30.Kwon W., Lee J.M., Yun K.H., et al. Clinical benefit of intravascular imaging compared with conventional angiography in left main coronary artery intervention. Circ Cardiovasc Interv. 2023;16 doi: 10.1161/CIRCINTERVENTIONS.123.013359. [DOI] [PubMed] [Google Scholar]
- 31.Amabile N., Range G., Souteyrand G., et al. Optical coherence tomography to guide percutaneous coronary intervention of the left main coronary artery: the LEMON study. EuroIntervention. 2021;17:e124–e131. doi: 10.4244/EIJ-D-20-01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S.J., Zhang J.J., Mintz G.S., et al. Improved 3-year cardiac survival after IVUS-guided long DES implantation: a patient-level analysis from 2 randomized trials. JACC Cardiovasc Interv. 2022;15:208–216. doi: 10.1016/j.jcin.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Park Y., Park H.S., Jang G.L., et al. Intravascular ultrasound guided recanalization of stumpless chronic total occlusion. Int J Cardiol. 2011;148:174–178. doi: 10.1016/j.ijcard.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 34.Kimura M., Katoh O., Tsuchikane E., et al. The efficacy of a bilateral approach for treating lesions with chronic total occlusions the CART (controlled antegrade and retrograde subintimal tracking) registry. JACC Cardiovasc Interv. 2009;2:1135–1141. doi: 10.1016/j.jcin.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M., Matsumura M., Usui E., et al. Intravascular ultrasound-derived calcium score to predict stent expansion in severely calcified lesions. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.010296. [DOI] [PubMed] [Google Scholar]
- 36.Raber L., Mintz G.S., Koskinas K.C., et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–3300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- 37.Kubo T., Shimamura K., Ino Y., et al. Superficial calcium fracture after PCI as assessed by OCT. JACC Cardiovasc Imaging. 2015;8:1228–1229. doi: 10.1016/j.jcmg.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Fujino A., Mintz G.S., Lee T., et al. Predictors of calcium fracture derived from balloon angioplasty and its effect on stent expansion assessed by optical coherence tomography. JACC Cardiovasc Interv. 2018;11:1015–1017. doi: 10.1016/j.jcin.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Fujino A., Mintz G.S., Matsumura M., et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182–e2189. doi: 10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- 40.Kim J.S., Hong M.K., Ko Y.G., et al. Impact of intravascular ultrasound guidance on long-term clinical outcomes in patients treated with drug-eluting stent for bifurcation lesions: data from a Korean multicenter bifurcation registry. Am Heart J. 2011;161:180–187. doi: 10.1016/j.ahj.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.H., Kim Y.H., Kang S.J., et al. Long-term outcomes of intravascular ultrasound-guided stenting in coronary bifurcation lesions. Am J Cardiol. 2010;106:612–618. doi: 10.1016/j.amjcard.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Furukawa E., Hibi K., Kosuge M., et al. Intravascular ultrasound predictors of side branch occlusion in bifurcation lesions after percutaneous coronary intervention. Circ J. 2005;69:325–330. doi: 10.1253/circj.69.325. [DOI] [PubMed] [Google Scholar]
- 43.Xu J., Hahn J.Y., Song Y.B., et al. Carina shift versus plaque shift for aggravation of side branch ostial stenosis in bifurcation lesions: volumetric intravascular ultrasound analysis of both branches. Circ Cardiovasc Interv. 2012;5:657–662. doi: 10.1161/CIRCINTERVENTIONS.112.969089. [DOI] [PubMed] [Google Scholar]
- 44.Kini A.S., Yoshimura T., Vengrenyuk Y., et al. Plaque morphology predictors of side branch occlusion after main vessel stenting in coronary bifurcation lesions: optical coherence tomography imaging study. JACC Cardiovasc Interv. 2016;9:862–865. doi: 10.1016/j.jcin.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Shlofmitz E., Torguson R., Zhang C., et al. Impact of intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN for In-stent Restenosis (iOPEN-ISR study) Int J Cardiol. 2021;340:17–21. doi: 10.1016/j.ijcard.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Hong S.J., Ahn C.M., Kim B.K., et al. Prospective randomized comparison of clinical and angiographic outcomes between everolimus-eluting vs. zotarolimus-eluting stents for treatment of coronary restenosis in drug-eluting stents: intravascular ultrasound volumetric analysis (RESTENT-ISR trial) Eur Heart J. 2016;37:3409–3418. doi: 10.1093/eurheartj/ehw389. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalo N., Serruys P.W., Okamura T., et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–293. doi: 10.1016/j.ahj.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Tada T., Kadota K., Hosogi S., et al. Association between tissue characteristics evaluated with optical coherence tomography and mid-term results after paclitaxel-coated balloon dilatation for in-stent restenosis lesions: a comparison with plain old balloon angioplasty. Eur Heart J Cardiovasc Imaging. 2014;15:307–315. doi: 10.1093/ehjci/jet165. [DOI] [PubMed] [Google Scholar]
- 49.Miura K., Tada T., Habara S., et al. Optical coherence tomography predictors for recurrent restenosis after paclitaxel-coated balloon angioplasty for drug-eluting stent restenosis. Circ J. 2018;82:2820–2828. doi: 10.1253/circj.CJ-18-0464. [DOI] [PubMed] [Google Scholar]
- 50.Lee J.H., Kim U., Kim J.S., et al. Clinical implication of neointimal burden in in-stent restenosis treated with drug-coated balloon. Catheter Cardiovasc Interv. 2021;98:493–502. doi: 10.1002/ccd.29211. [DOI] [PubMed] [Google Scholar]
- 51.Gaba P., Gersh B.J., Muller J., et al. Evolving concepts of the vulnerable atherosclerotic plaque and the vulnerable patient: implications for patient care and future research. Nat Rev Cardiol. 2023;20:181–196. doi: 10.1038/s41569-022-00769-8. [DOI] [PubMed] [Google Scholar]
- 52.Kedhi E., Berta B., Roleder T., et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42(45):4671–4679. doi: 10.1093/eurheartj/ehab433. [DOI] [PubMed] [Google Scholar]
- 53.Park S.J., Ahn J.M., Kang D.Y., et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): a multicentre, open-label, randomised controlled trial. Lancet. 2024;403:1753–1765. doi: 10.1016/S0140-6736(24)00413-6. [DOI] [PubMed] [Google Scholar]
- 54.Kim N., Lee J.H., Jang S.Y., et al. Intravascular modality-guided versus angiography-guided percutaneous coronary intervention in acute myocardial infarction. Catheter Cardiovasc Interv. 2020;95:696–703. doi: 10.1002/ccd.28359. [DOI] [PubMed] [Google Scholar]
- 55.Lee O.H., Heo S.J., Johnson T.W., et al. Optical coherence tomography-guided versus intravascular ultrasound-guided percutaneous coronary intervention in patients with acute myocardial infarction. Rev Esp Cardiol (Engl Ed) 2024;77:607–617. doi: 10.1016/j.rec.2023.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Prati F., Romagnoli E., Burzotta F., et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. 2015;8:1297–1305. doi: 10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Park H., Ahn J.M., Kang D.Y., et al. Optimal stenting technique for complex coronary lesions: intracoronary imaging-guided pre-dilation, stent sizing, and post-dilation. JACC Cardiovasc Interv. 2020;13:1403–1413. doi: 10.1016/j.jcin.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Wijns W., Shite J., Jones M.R., et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36:3346–3355. doi: 10.1093/eurheartj/ehv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujii K., Carlier S.G., Mintz G.S., et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995–998. doi: 10.1016/j.jacc.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 60.Hong M.K., Mintz G.S., Lee C.W., et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–1310. doi: 10.1093/eurheartj/ehi882. [DOI] [PubMed] [Google Scholar]
- 61.Kim D., Hong S.J., Kim B.K., et al. Outcomes of stent optimisation in intravascular ultrasound-guided interventions for long lesions or chronic total occlusions. EuroIntervention. 2020;16:e480–e488. doi: 10.4244/EIJ-D-19-00762. [DOI] [PubMed] [Google Scholar]
- 62.Sonoda S., Hibi K., Okura H., et al. Current clinical use of intravascular ultrasound imaging to guide percutaneous coronary interventions (update) Cardiovasc Interv Ther. 2023;38:1–7. doi: 10.1007/s12928-022-00892-w. [DOI] [PubMed] [Google Scholar]
- 63.Saito Y., Kobayashi Y., Fujii K., et al. CVIT 2023 clinical expert consensus document on intravascular ultrasound. Cardiovasc Interv Ther. 2024;39:1–14. doi: 10.1007/s12928-023-00957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Im E., Kim B.K., Ko Y.G., et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014;7:88–96. doi: 10.1161/CIRCINTERVENTIONS.113.000797. [DOI] [PubMed] [Google Scholar]
- 65.Taniwaki M., Radu M.D., Zaugg S., et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–660. doi: 10.1161/CIRCULATIONAHA.115.019071. [DOI] [PubMed] [Google Scholar]
- 66.Adriaenssens T., Joner M., Godschalk T.C., et al. Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort) Circulation. 2017;136:1007–1021. doi: 10.1161/CIRCULATIONAHA.117.026788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Souteyrand G., Amabile N., Mangin L., et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37:1208–1216. doi: 10.1093/eurheartj/ehv711. [DOI] [PubMed] [Google Scholar]
- 68.Kyodo A., Watanabe M., Okamura A., et al. Post-stent optical coherence tomography findings at index percutaneous coronary intervention- characteristics related to subsequent stent thrombosis. Circ J. 2021;85:857–866. doi: 10.1253/circj.CJ-20-0759. [DOI] [PubMed] [Google Scholar]
- 69.Radu M.D., Raber L., Heo J., et al. Natural history of optical coherence tomography-detected non-flow-limiting edge dissections following drug-eluting stent implantation. EuroIntervention. 2014;9:1085–1094. doi: 10.4244/EIJV9I9A183. [DOI] [PubMed] [Google Scholar]
- 70.Hong Y.J., Jeong M.H., Choi Y.H., et al. Impact of tissue prolapse after stent implantation on short- and long-term clinical outcomes in patients with acute myocardial infarction: an intravascular ultrasound analysis. Int J Cardiol. 2013;166:646–651. doi: 10.1016/j.ijcard.2011.11.092. [DOI] [PubMed] [Google Scholar]
- 71.Sohn J., Hur S.H., Kim I.C., et al. A comparison of tissue prolapse with optical coherence tomography and intravascular ultrasound after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2015;31:21–29. doi: 10.1007/s10554-014-0540-7. [DOI] [PubMed] [Google Scholar]
- 72.Soeda T., Uemura S., Park S.J., et al. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation. 2015;132:1020–1029. doi: 10.1161/CIRCULATIONAHA.114.014704. [DOI] [PubMed] [Google Scholar]
- 73.Mintz G.S., Weissman N.J. Intravascular ultrasound in the drug-eluting stent era. J Am Coll Cardiol. 2006;48:421–429. doi: 10.1016/j.jacc.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 74.Kim J.S., Afari M.E., Ha J., et al. Neointimal patterns obtained by optical coherence tomography correlate with specific histological components and neointimal proliferation in a swine model of restenosis. Eur Heart J Cardiovasc Imaging. 2014;15:292–298. doi: 10.1093/ehjci/jet162. [DOI] [PubMed] [Google Scholar]
- 75.Finn A.V., Joner M., Nakazawa G., et al. Pathological correlates of late drug-eluting stent thrombosis - Strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 76.Kim J.S., Shin D.H., Kim B.K., et al. Randomized comparison of stent strut coverage following angiography- or optical coherence tomography-guided percutaneous coronary intervention. Rev Esp Cardiol (Engl Ed) 2015;68:190–197. doi: 10.1016/j.rec.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 77.Lee S.Y., Kim J.S., Yoon H.J., et al. Early strut coverage in patients receiving drug-eluting stents and its implications for dual antiplatelet therapy: a randomized trial. JACC Cardiovasc Imaging. 2018;11:1810–1819. doi: 10.1016/j.jcmg.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Won H., Shin D.H., Kim B.K., et al. Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2013;29:1255–1263. doi: 10.1007/s10554-013-0223-9. [DOI] [PubMed] [Google Scholar]
- 79.Kang S.J., Mintz G.S., Akasaka T., et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954–2963. doi: 10.1161/CIRCULATIONAHA.110.988436. [DOI] [PubMed] [Google Scholar]
- 80.Park S.J., Kang S.J., Virmani R., Nakano M., Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. Journal of the American College of Cardiology. 2012;59:2051–2057. doi: 10.1016/j.jacc.2011.10.909. [DOI] [PubMed] [Google Scholar]
- 81.Nakazawa G., Otsuka F., Nakano M., et al. The Pathology of Neoatherosclerosis in Human Coronary Implants Bare-Metal and Drug-Eluting Stents. J Am Coll Cardiol. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yonetsu T., Kato K., Kim S.J., et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging. 2012;5:660–666. doi: 10.1161/CIRCIMAGING.112.976167. [DOI] [PubMed] [Google Scholar]
- 83.Cook S., Eshtehardi P., Kalesan B., et al. Impact of incomplete stent apposition on long-term clinical outcome after drug-eluting stent implantation. Eur Heart J. 2012;33:1334–1343. doi: 10.1093/eurheartj/ehr484. [DOI] [PubMed] [Google Scholar]
- 84.Boden H., van der Hoeven B.L., Liem S.S., et al. Five-year clinical follow-up from the MISSION! Intervention Study: sirolimus-eluting stent versus bare metal stent implantation in patients with ST-segment elevation myocardial infarction, a randomised controlled trial. EuroIntervention. 2012;7:1021–1029. doi: 10.4244/EIJV7I9A164. [DOI] [PubMed] [Google Scholar]
- 85.Lee S.Y., Ahn J.M., Mintz G.S., et al. Ten-year clinical outcomes of late-acquired stent malapposition after coronary stent implantation. Arterioscler Thromb Vasc Biol. 2020;40:288–295. doi: 10.1161/ATVBAHA.119.313602. [DOI] [PubMed] [Google Scholar]
- 86.Im E., Hong S.J., Ahn C.M., et al. Long-term clinical outcomes of late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]