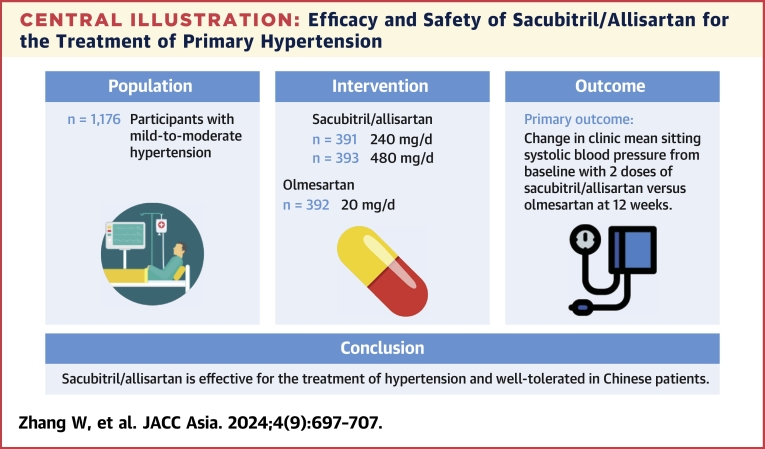
Abstract
Background
The prevalence of hypertension still increases with the very rapidly increasing longevity in some countries, such as China. The control rate remains low.
Objectives
This randomized, double-blind, phase 3 study assessed the efficacy and safety of sacubitril/allisartan, compared with olmesartan in Chinese patients with mild-to-moderate hypertension.
Methods
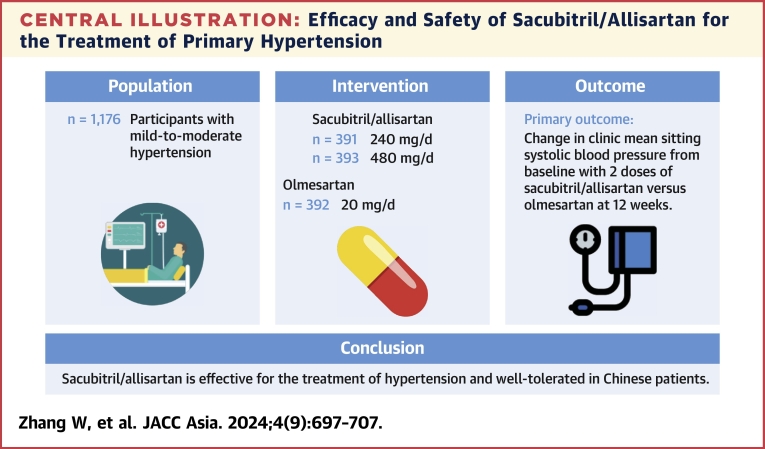
Eligible patients aged 18 to 75 years (n = 1,197) with mild-to-moderate hypertension were randomized to receive sacubitril/allisartan 240 mg (n = 399), sacubitril/allisartan 480 mg (n = 399), or olmesartan 20 mg (n = 399) once daily for 12 weeks. Patients who completed the 12-week treatment then received another 12-week extended treatment (n = 1,084) and 28-week prolonged treatment (n = 189). The primary end point was a reduction in clinic mean sitting systolic blood pressure (msSBP) from baseline at 12 weeks.
Results
Sacubitril/allisartan 240 mg/d provided a greater reduction in msSBP than olmesartan at 12 weeks (between-group difference: −1.9 mm Hg [95% CI: −4.2 to 0.4 mm Hg]; P = 0.0007, for noninferiority). Sacubitril/allisartan 480 mg/d provided a significantly greater reduction in msSBP than olmesartan at 12 weeks (between-treatment difference: −5.0 mm Hg [95% CI: −7.3 to −2.8 mm Hg]; P < 0.001, for superiority). Greater reductions in 24-hour, and daytime and nighttime systolic and diastolic blood pressure were also observed with both doses of sacubitril/allisartan compared with olmesartan (P ≤ 0.001 for 480 mg/d). The blood pressure reductions tended to be dose-dependent for sacubitril/allisartan. Sacubitril/allisartan was well tolerated, and no cases of angioedema or death were reported.
Conclusions
Sacubitril/allisartan is effective for the treatment of hypertension and well tolerated in Chinese patients.
Key Words: ambulatory blood pressure, angiotensin receptor neprilysin inhibitor, blood pressure reduction, hypertension, phase 3 randomized controlled trial
Central Illustration
High blood pressure remains a major global health burden, leading to 10.8 million deaths per year worldwide.1,2 The prevalence of hypertension in China (defined as ≥140/90 mm Hg) increased from 5.1% in 1958-1959 to 23.2% in 2012 to 2015.3,4 Treatment and control rates of hypertension have also increased substantially from 12.1% and 2.8%, respectively, in 1991, to 40.7% and 15.3%, respectively, in 2012 to 2015.3,4 Nonetheless, despite the availability of effective and safe antihypertensive drugs of various modes of action, only 21% of Chinese patients taking antihypertensive medication have their 24-hour blood pressure perfectly controlled.5
Concomitant neprilysin inhibition and renin-angiotensin system suppression have effects on blood pressure reductions to a greater extent compared with renin-angiotensin system inhibitors alone.6 Previous studies with sacubitril/valsartan, the first-in-class angiotensin receptor neprilysin inhibitor, showed superior reductions in clinic and ambulatory blood pressure in patients with mild-to-moderate hypertension compared with valsartan7 or placebo,8 especially in Asian patients who are more likely to have a higher salt sensitivity and salt consumption.9 Sacubitril/allisartan is developed as a new agent in the class of angiotensin-receptor neprilysin inhibitors, with allisartan as a replacement of valsartan in a single compound.10 In a phase 2, dose-finding study in patients with hypertension,11 sacubitril/allisartan 480 mg/d provided a significantly greater reduction in clinic systolic blood pressure than placebo at 8 weeks (between-treatment difference: −9.1 mm Hg [95% CI: −1.6 to −16.6 mm Hg]; P = 0.02). The present phase 3 study was subsequently designed to investigate the long-term antihypertensive efficacy and safety in Chinese patients with mild-to-moderate hypertension.
Methods
Study design
This study was a phase 3, multicenter, randomized, double-blind, active (olmesartan)-controlled, parallel-group trial (ChiCTR2100051183) undertaken in 71 participating hospitals in China. It included a washout single-blind placebo run-in period (4 weeks), followed by a double-blind treatment period (12 weeks), and then entered into an open-label titration period (40 weeks). The study adhered to the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, and the ethics committees of all participating hospitals. All participants provided written informed consent before participation in the study.
Participants
Participants were recruited between December 17, 2021, and July 18, 2022. The study was completed on March 30, 2023. Men and women aged 18 to 75 years with mild-to-moderate hypertension were eligible if the following inclusion and exclusion criteria were also met. Previously untreated patients were required to have a systolic blood pressure of 150 to 179 mm Hg (mean of 3 readings at the screening clinic visit and after the run-in period, respectively). Patients with previously treated, but uncontrolled, hypertension (systolic blood pressure of 150 to 179 mm Hg after the run-in period) with antihypertensive monotherapy or combination therapy of 2 classes of drugs entered the trial if they were willing to switch their previous treatment to the study treatment.
Exclusion criteria included secondary hypertension; severe hypertension (systolic blood pressure ≥180 mm Hg and/or diastolic blood pressure ≥110 mm Hg); history of angioedema; acute cardiovascular events within 12 months before enrollment: congestive heart failure, unstable angina, or severe cardiac arrhythmia; hyperkalemia or hypokalemia (serum potassium >5.5 or <3.5 mmol/L); hepatic impairment (aspartate and/or alanine aminotransferase 2.5 or more times the upper limit of normal range of the respective institution); renal impairment (serum creatinine 1.5 times the upper limit of normal range of the respective institution); type 1 diabetes mellitus or uncontrolled type 2 diabetes mellitus (glycated hemoglobin >8%); obesity (body mass index >30 kg/m2); cancer; patients undergoing hemodialysis or salt restriction; allergy to sacubitril/valsartan, olmesartan, or other relevant drugs; use of any medication that may affect blood pressure (other antihypertensive drugs, antianginal drugs except trimetazidine, digitalis, glucocorticoids except topical glucocorticoids, estrogens, potassium supplements, and other drugs considered unsuitable by the investigator); taking contraceptives or being of childbearing potential; drug or alcohol abuse; participation in another investigational drug trial within 3 months before enrollment; poor adherence to antihypertensive treatment, defined as a percentage of <80% or >120% of pills that should be taken during the run-in period, as evaluated by the pill count method; and an investigator’s opinion that the patient would be inappropriate for the study.
Randomization and masking
Eligible patients underwent a 1-week screening/washout period to remove the effects of prior therapeutic agents. During this period, previously untreated patients remained free of any antihypertensive drug, and previously treated patients discontinued antihypertensive drugs. Patients were then enrolled into a 4-week, single-blind, placebo run-in period. After 4 weeks, all patients who fulfilled the criteria for enrollment were randomly assigned (1:1:1) to treatment with sacubitril/allisartan 240 mg/d (with sacubitril 116 mg and allisartan 124 mg, respectively), 480 mg/d (with sacubitril 232 mg and allisartan 248 mg, respectively), and olmesartan 20 mg/d, respectively. Treatment assignment was done with a computer-generated random sequence with a block size of 3. The system assigned a randomization number to each patient that linked the patient to a treatment group and specified a unique number for the study drug to be dispensed. Placebo and active treatments were identical in appearance. Both the study investigators and participants were masked to treatment for the duration of the trial.
Follow-up visits and procedures
Participants in the study experienced 2 consecutive study periods. Period 1 (12 weeks) was a double-blind, randomized, and active-controlled study in which patients received sacubitril/allisartan 240 mg, sacubitril/allisartan 480 mg, or olmesartan 20 mg in a 1:1:1 ratio. Period 2 (40 weeks) was an open-label titration study consisting of 2 sequential parts: part 1 was a 12-week extended treatment part, in which all patients received initially sacubitril/allisartan 240 mg and then were titrated according to achieved clinic blood pressure; part 2 was a 28-week prolonged treatment part, during which patients were initiated with the dosage of sacubitril/allisartan assessed at week 24, and then titrated according to achieved blood pressure.
Period 1 included 3 clinic visits, respectively, at 4, 8, and 12 weeks of follow-up during the randomized treatment period for the assessment of efficacy and safety. Safety and blood pressure control were assessed every 4 weeks during the 12-week extended treatment part, and every 4 or 12 weeks during the prolonged treatment part. During the study treatment period, patients were instructed to take their study medication before breakfast. Clinic blood pressure was measured at each of the clinic visits. Ambulatory blood pressure monitoring, laboratory tests for blood and urine, electrocardiography, and physical examinations were performed at the initial and terminating visits. The study medication was supplied free of charge for the entire study period by Salubris Pharma.
Efficacy and safety evaluations
The primary efficacy variable was the change from baseline in clinic mean sitting systolic blood pressure (msSBP) at 12 weeks of follow-up (period 1) in all dosages of sacubitril/allisartan, compared with olmesartan. Secondary efficacy variables included change from baseline in clinic mean sitting diastolic blood pressure (msDBP) and 24-hour ambulatory systolic/diastolic blood pressure. Safety evaluations included adverse events (AEs) and serious adverse events (SAEs), including any clinically significant abnormalities on physical examinations or laboratory tests or 12-lead electrocardiogram. Information about symptoms, severity, relation to the study medication, intervention, and outcome was documented for all AEs.
Blood pressure measurement
Clinic blood pressure and pulse rate were measured with an automated electronic blood pressure monitor (A&D UA-651A) before medication and after the patients had rested for at least 5 minutes in the sitting position. At screening, blood pressure was measured in both arms by clinicians. If the between-arm difference of blood pressure was >10 mm Hg, then the arm with the higher systolic blood pressure reading was used at all subsequent clinic visits, otherwise the nondominant arm was used at all subsequent clinic visits. On each visit, 3 consecutive readings were taken with an interval of 1 minute and averaged for analysis.
Twenty-four–hour ambulatory blood pressure assessment (A&D TM-2430) was performed on the same arm as the clinic blood pressure measurement. The monitors were programmed to obtain blood pressure readings at 20-minute intervals during the daytime (06:00-22:00) and at 30-minute intervals during the nighttime (22:00-06:00) on regular working days outside the hospital. On the monitoring day, the study participants were instructed to follow their usual daily activities, avoid vigorous exercise, and remain still with the forearm extended during each blood pressure measurement. A recording was considered valid and included in the analysis if there were at least 20 and 7 blood pressure readings in the daytime and nighttime, respectively, and a total of at least 70% valid measurements.
Statistical analysis
We used SAS software version 9.4 (SAS Institute) for statistical analysis. To investigate the primary efficacy, a fixed sequential testing design was employed to evaluate the efficacy of the sacubitril/allisartan groups. Firstly, a noninferiority test was conducted comparing the sacubitril/allisartan 240 mg/d group with the olmesartan 20 mg/d group, using the change from baseline in msSBP as the primary efficacy variable. The noninferiority margin was set at −2 mm Hg. Noninferiority was considered to be achieved if the upper limit of the 95% CI for the between-group difference was below 2 mm Hg. If the sacubitril/allisartan 240 mg/d group demonstrated noninferiority compared with the olmesartan 20 mg/d group, we proceeded with a superiority design to compare the sacubitril/allisartan 480 mg/d group with the olmesartan group. According to the results of previous clinical trials on sacubitril/valsartan, olmesartan, and a phase 2 study of sacubitril/allisartan, we assumed a difference of −2 mm Hg in the primary efficacy variable between the sacubitril/allisartan 240 mg/d group and the olmesartan group, with a SD of ± 14 mm Hg. Additionally, for the primary efficacy, we anticipated a difference of 4 mm Hg between the sacubitril/allisartan 480 mg group and the olmesartan group, with a SD of ± 14 mm Hg. The statistical significance level (alpha) was set at 0.025 (1-sided), and the power was set at 90%. Considering a 20% dropout rate, the sample size for the sacubitril/allisartan 240 mg, 480 mg, and olmesartan groups was 376, for a total of at least 1,128 subjects.
For the primary efficacy variable, we performed analyses in the full analysis set, which included all randomized patients who had received at least 1 dose of the study medication and had efficacy evaluation data available, following the intention-to-treat principle. The safety analysis was performed in the patients who took at least 1 dose of the study treatment. Means and proportions in baseline characteristics were compared by the analysis of variance and the Fisher exact test, respectively. Mixed model for repeated measures analyses was used for the primary outcome in which the response variable was the least square mean ± SE change from baseline and the between-group differences (95% CI), with treatment, visit, and treatment-by-visit interaction as fixed-effect factors, and blood pressure at baseline and participating hospitals as covariates. The between-group differences were computed by subtracting the changes in the olmesartan group from those in the sacubitril/allisartan groups. Negative values therefore indicate a larger reduction in the sacubitril/allisartan groups.
Results
Flow of patients and baseline characteristics
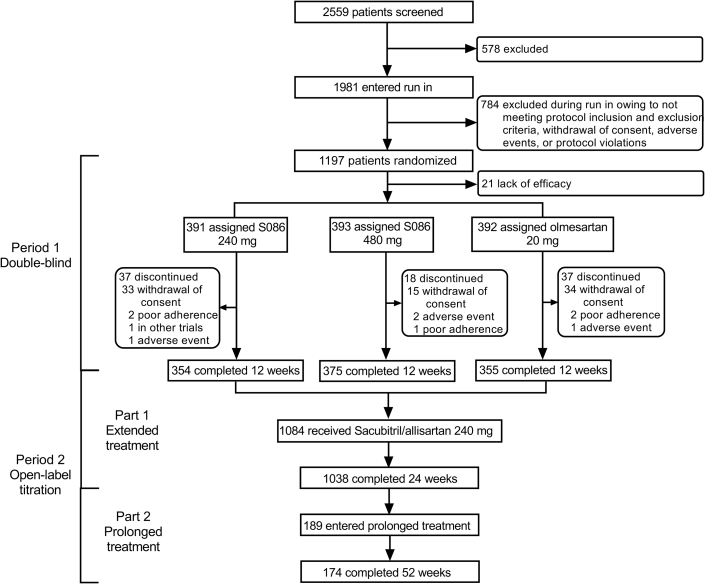
The study was done from December 17, 2021, to March 30, 2023. Overall, 2,559 individuals were screened at 71 sites in 26 provinces, and a total of 1,197 eligible patients were randomly assigned to 1 of the study groups: sacubitril/allisartan 240 mg/d (n = 399), sacubitril/allisartan 480 mg/d (n = 399), and olmesartan 20 mg/d (n = 399). All participants were included in the safety analysis set. Twenty-one patients were excluded from the full analysis set (n = 1,176) because of unavailable efficacy data. Ninety-two patients discontinued the study, of whom 82 withdrew on their own, and 10 were withdrawn by the investigators because of AEs (n = 4), poor adherence (n = 5), or participation in other trials (n = 1) (Figure 1). A total of 1,084 patients completed the 12-week double-blind treatment and were enrolled in the period 2 open-label titration study. A total of 1,038 patients completed the extended 12 weeks of treatment, and 189 patients who were willing and then entered the prolonged 28 weeks of treatment. A total of 175 patients completed the whole 52 weeks of treatment.
Figure 1.
Trial Profile
A total of 2,559 individuals were screened, and 1,197 eligible patients were randomly assigned. A total of 1,176 patients were included in the full analysis set; 1,084 patients completed the 12-week double-blind treatment; 1,038 patients completed the extended 12-week treatment; and 189 patients then entered the prolonged 28-week treatment. A total of 175 patients completed the whole 52-week treatment. S086 = sacubitril/allisartan.
Patient characteristics at baseline are reported in Table 1. Overall, 51.9% of patients were male. The mean age was 58.2 ± 8.7 years. The mean body mass index was 25.7 ± 2.6 kg/m2. Clinic systolic/diastolic blood pressure at baseline was 160.7 ± 6.8/92.3 ± 9.1 mm Hg. A total of 89.8% of patients had received prior treatment for hypertension. There were no remarkable differences between the randomized groups (P ≥ 0.14) (Table 1), except for greater body mass index in the study group of sacubitril/allisartan 240 mg/d (26.0 ± 2.5 kg/m2 vs 25.5 ± 2.6 kg/m2 vs 25.7 ± 2.6 kg/m2; P = 0.02).
Table 1.
Patient Characteristics at Baseline
| Sacubitril/Allisartan |
Olmesartan 20 mg (n = 392) |
||
|---|---|---|---|
| 240 mg (n = 391) | 480 mg (n = 393) | ||
| Male | 209 (53.5) | 198 (50.4) | 203 (51.8) |
| Age, y | 58.0 ± 8.8 | 58.6 ± 8.9 | 58.2 ± 8.5 |
| Body mass index, kg/m2 | 26.0 ± 2.5 | 25.5 ± 2.6 | 25.7 ± 2.6 |
| Blood pressure, mm Hg | |||
| Clinic systolic | 160.4 ± 6.6 | 160.8 ± 6.7 | 160.9 ± 6.9 |
| Clinic diastolic | 92.7 ± 9.1 | 91.8 ± 9.2 | 92.4 ± 8.8 |
| 24-h systolic | 146.0 ± 26.0 | 144.8 ± 25.8 | 146.2 ± 28.4 |
| 24-h diastolic | 87.6 ± 16.2 | 86.4 ± 15.8 | 87.1 ± 17.2 |
| Daytime systolic | 153.1 ± 13.5 | 152.0 ± 13.2 | 153.7 ± 13.6 |
| Daytime diastolic | 91.9 ± 9.1 | 90.7 ± 9.0 | 91.8 ± 9.0 |
| Nighttime systolic | 137.5 ± 27.1 | 136.0 ± 26.7 | 138.3 ± 29.6 |
| Nighttime diastolic | 82.4 ± 16.6 | 81.1 ± 16.0 | 82.4 ± 17.5 |
| Nondippers | 213 (59.2) | 215 (58.6) | 228 (62.3) |
| Clinic pulse rate, beat/min | 77.0 ± 12.5 | 76.5 ± 12.7 | 75.8 ± 14.0 |
| Previous antihypertensive treatment | 347 (88.8) | 359 (91.4) | 351 (89.5) |
| History of diabetes mellitus | 65 (16.6) | 55 (13.9) | 54 (13.7) |
| Current smoking | 98 (25.1) | 91 (23.2) | 98 (25.0) |
| Alcohol intake | 83 (21.2) | 66 (16.8) | 76 (19.4) |
| Blood biochemistry | |||
| Serum LDL cholesterol, mmol/L | 2.9 ± 0.8 | 3.1 ± 0.9 | 3.0 ± 0.8 |
| Serum total cholesterol, mmol/L | 4.9 ± 0.9 | 5.0 ± 1.0 | 4.9 ± 0.9 |
| Serum potassium, mmol/L | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.1 ± 0.3 |
| Plasma glucose, mmol/L | 5.7 ± 0.9 | 5.7 ± 0.9 | 5.8 ± 1.0 |
| Plasma HbA1c, % | 5.8 ± 0.6 | 5.8 ± 0.6 | 5.9 ± 0.7 |
| eGFR, mL/min/1.73 m2 | 93.9 ± 12.6 | 93.9 ± 13.3 | 93.1 ± 13.0 |
Values are n (%) or mean ± SD. The P value is for the comparison between all 3 groups.
eGFR = estimated glomerular filtration rate with creatinine; HbA1c = glycosylated hemoglobin; LDL = low-density lipoprotein.
Antihypertensive efficacy
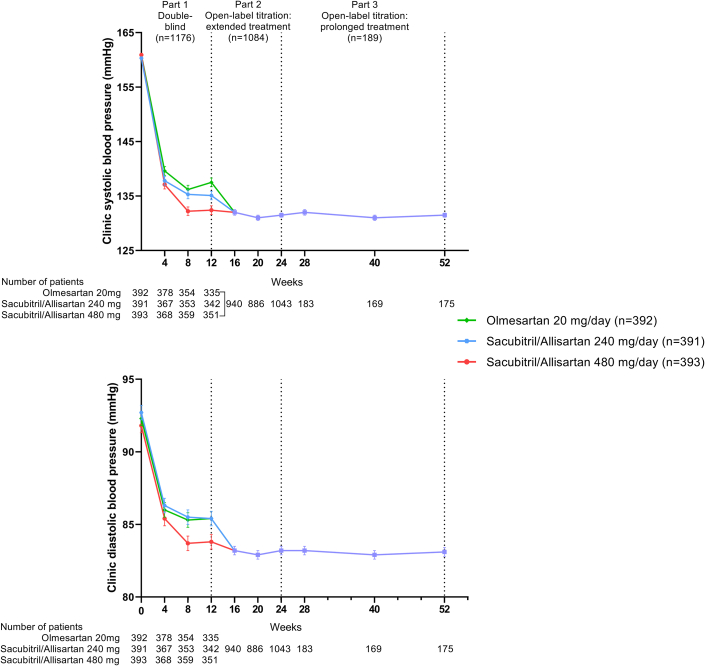
The time course of changes in clinic systolic and diastolic blood pressure during the 2 study periods illustrates the short-term (12 weeks) and sustained (up to 52 weeks) blood pressure–lowering effects of sacubitril/allisartan (Figure 2). During follow-up of the 12-week double-blind treatment, clinic systolic and diastolic blood pressure decreased continuously in all 3 groups. At 12 weeks, the least square mean ± SE change from baseline in msSBP/msDBP was −23.2 ± 0.9/−6.8 ± 0.5 mm Hg in the olmesartan group, −25.1 ± 0.9/−6.9 ± 0.5 mm Hg in the sacubitril/allisartan 240 mg/d group, and −28.2 ± 0.9/−8.0 ± 0.5 mm Hg in the sacubitril/allisartan 480 mg/d group. The least square mean reductions in msSBP were greater for sacubitril/allisartan 240 mg than olmesartan 20 mg by −1.9 (95% CI: −4.2 to 0.3) mm Hg, demonstrating the noninferiority of sacubitril/allisartan 240 mg to olmesartan 20 mg at the margin of 2 mm Hg (P < 0.001). The olmesartan-corrected reduction reached statistical significance in the sacubitril/allisartan 480 mg/d group for msSBP (−5.0 [95% CI: −7.3 to −2.8] mm Hg; P < 0.001) and msDBP (−1.3 [95% CI: −2.4 to −0.1] mm Hg; P = 0.04), demonstrating the superiority of sacubitril/allisartan 480 mg to olmesartan 20 mg. Clinic systolic and diastolic blood pressure were maintained during period 2 in patients who initially received sacubitril/allisartan 240 mg and were up-titrated to 480 mg according to clinic blood pressure during follow-up.
Figure 2.
Clinic Blood Pressure Over Time by Treatment Group
Symbols denote mean clinic blood pressure. Vertical lines denote standard error.
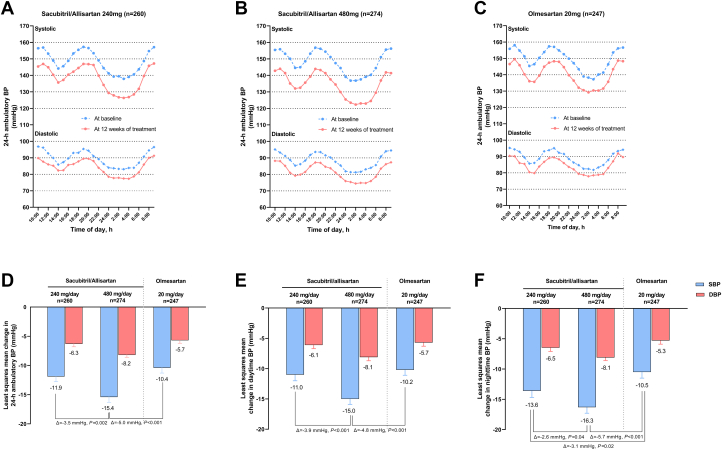
The average number of ambulatory blood pressure readings was 42.6 and 40.9 at baseline and 12 weeks of follow-up, respectively. At 12 weeks of follow-up, mean ambulatory blood pressure was reduced from baseline throughout the whole 24-hour period (Figure 3). The least square mean ± SE changes from baseline in 24-hour mean ambulatory systolic/diastolic blood pressure (maSBP/maDBP) in the sacubitril/allisartan 240 and 480 mg/d groups were −11.9 ± 0.9/−6.3 ± 0.5 mm Hg and −15.4 ± 0.9/−8.2 ± 0.5 mm Hg, respectively (Figure 3). The olmesartan-corrected reduction reached statistical significance only in the sacubitril/allisartan 480 mg/d group (maSBP −5.0 [95% CI: −7.2 to −2.8] mm Hg; P < 0.001, and maDBP −2.4 [95% CI: −3.7 to −1.1] mm Hg; P < 0.001). All 3 treatment regimens decreased the daytime and nighttime maSBP/maDBP from baseline to week 12. The reductions in nighttime maSBP were significantly greater with both doses of sacubitril/valsartan compared with olmesartan 20 mg (−3.1 [95% CI: −5.7 to −0.5] mm Hg; P = 0.02 for sacubitril/allisartan 240 mg and −5.7 [95% CI: −8.2 to −3.2] mm Hg; P < 0.001 for sacubitril/allisartan 480 mg).
Figure 3.
Absolute Change in Other Efficacy Parameters From Baseline
(A to C) 24-hour ambulatory blood pressure (BP) values at baseline and at 12 weeks of treatment; (D to F) Least square mean changes in 24 hours, daytime and nighttime ambulatory blood pressure from baseline to week 12. Symbols denote hourly mean. Vertical lines denote standard error.
Safety
Safety was assessed in patients who took at least 1 pill of the study medication (n = 1,197). Sacubitril/allisartan was well tolerated. Most of the AEs were infrequent, mild/moderate, and transient in nature. The most common AEs among all 3 groups were hyperuricemia, dyslipidemia, and urinary tract infection occurring during the first 12 weeks of treatment, upper respiratory tract infection, COVID-19, and urinary tract infection during the extended treatment, dyslipidemia, elevated plasma glucose, and SARS-CoV-2 positive during the prolonged treatment. There were no marked differences (P > 0.05) in the incidence of AEs between patients taking sacubitril/allisartan 240 mg/d (36.8% [147/399]), 480 mg/d (33.1% [132/399]), and olmesartan 20 mg/d (39.8% [159/399]) (Table 2) in the 12-week double-blind treatment.
Table 2.
Adverse Events
| Sacubitril/Allisartan |
Olmesartan 20 mg |
||
|---|---|---|---|
| 240 mg | 480 mg | ||
| Period 1 double-blind | 399 | 399 | 399 |
| Patients with at least 1 event | 147 (36.8) | 132 (33.1) | 159 (39.8) |
| Hyperuricemia | 15 (3.8) | 19 (4.8) | 20 (5.0) |
| Hyperlipidemia | 13 (3.3) | 14 (3.5) | 17 (4.3) |
| Urinary tract infection | 14 (3.5) | 6 (1.5) | 14 (3.5) |
| Period 2 open-label titration: extended treatment | 1,084 | ||
| Patients with at least 1 event | 459 (42.3) | ||
| Upper respiratory tract infection | 54 (5.0) | ||
| COVID-19 | 36 (3.3) | ||
| Urinary tract infection | 31 (2.9) | ||
| Period 2 open-label titration: prolonged treatment | 189 | ||
| Patients with at least 1 event | 117 (61.9) | ||
| Hyperlipidemia | 14 (7.4) | ||
| Elevated plasma glucose | 12 (6.3) | ||
| SARS-CoV-2 positive | 11 (5.8) | ||
Values are n or n (%). COVID-19 refers to participants with infection symptoms and positive laboratory tests such as antigen or nucleic acid, whereas SARS-CoV-2 positive refers to participants who self-reported antigen positive but with no official diagnosis.
AEs necessitating patient withdrawal from the study occurred in 1.0%, 1.3%, and 2.3% of patients in the sacubitril/allisartan 240 mg/d, 480 mg/d, and olmesartan 20 mg/d groups, respectively, in the 12-week double-blind treatment. The incidence of SAEs was low and similar in all treatment groups. SAEs leading to discontinuation were cerebral infarction and hemorrhage (n = 5), bone fracture (n = 3), tumor (n = 2), atrial fibrillation (n = 1), acute myocardial infarction (n = 1), meniscus injury (n = 1), diarrhea (n = 1), virus infection (n = 1), and dizziness (n = 1). No cases of angioedema or death were reported in this study.
Discussion
This trial was the first large scale, randomized, comparator-controlled phase 3 study to evaluate the antihypertensive effect of sacubitril/allisartan in patients with mild-to-moderate hypertension (Central Illustration). The clinic and 24-hour ambulatory blood pressure–lowering effects of sacubitril/allisartan 240 mg/d were noninferior to those of the recommended dosage of olmesartan 20 mg/d, which was one of the most effective antihypertensive drugs and was also used as the positive control drug in previous clinical trials of sacubitril/valsartan. The antihypertensive effects of sacubitril/allisartan 480 mg/d were superior to those of sacubitril/allisartan 240 mg and olmesartan 20 mg/d. Hence, it was evident that the blood pressure–lowering effect of sacubitril/allisartan tended to be dose-dependent. In addition, significantly greater reductions in nighttime maSBP were observed with sacubitril/allisartan treatments compared with olmesartan. This blood pressure–lowering effect was maintained over a period of 52 weeks, suggesting long-term tolerability and efficacy of sacubitril/allisartan.
Central Illustration.
Efficacy and Safety of Sacubitril/Allisartan for the Treatment of Primary Hypertension
Efficacy and safety of sacubitril/allisartan in Chinese patients with mild-to-moderate hypertension. This phase 3 study was conducted across 71 participating hospitals in China.
In the phase 2 study of sacubitril/allisartan,11 we observed significant placebo-corrected decreases in clinic (−9.1/−2.8 mm Hg) and 24-hour ambulatory blood pressure (−9.8/−4.6 mm Hg) at the sacubitril/allisartan dosage of 480 mg/d during 8-week treatment. The blood pressure–lowering effect of sacubitril/allisartan, in the present study, was maintained over quite a long time (52 weeks). The dose response from this study indicated that 240 mg could be recommended as a standard dose and 480 mg as a maximal dose of sacubitril/allisartan offering a blood pressure–lowering effect. Our findings in this actively controlled trial are in line with the results of 2 recent dose-related blood pressure–lowering efficacy studies of sacubitril/valsartan, the first agent of this class.12,13 In a Chinese study of 1,438 patients with hypertension,12 2 dosages of sacubitril/valsartan (200 and 400 mg) were compared with olmesartan 20 mg for 8 weeks. The mean difference in clinic and 24-hour systolic blood pressure between sacubitril/valsartan 400 mg (n = 479) and olmesartan 20 mg (n = 486) were −3.5/−1.9 mm Hg and −2.5/−1.2 mm Hg, respectively. In a Japanese study with a similar design13 including 1,161 patients with hypertension, sacubitril/valsartan 400 mg (n = 385) showed a greater decrease of −6.97 mm Hg for clinic systolic blood pressure compared with olmesartan 20 mg (n = 389). By inhibiting neprilysin and angiotensin receptor simultaneously, this new class of antihypertensive drugs was effective in blood pressure–lowering action. In the current analysis, reductions from baseline in 24-hour, daytime, and nighttime ambulatory systolic blood pressure over 12 weeks of treatment with sacubitril/allisartan exceeded 10 mm Hg. Given the direct and continuous relationship between blood pressure and the risk of cardiovascular events,14 the magnitude of blood pressure reductions that occurred during treatment with sacubitril/allisartan in this study would be expected to have a positive impact on cardiovascular morbidity and mortality. Further studies on prevention of target organ damage and major cardiovascular events are needed.
Failure to control blood pressure with currently available drugs suggests that relevant pathophysiological pathways remain unopposed. Neprilysin inhibition offers an additional approach to reducing blood pressure. In a post hoc analysis of the PARAGON-HF (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction) trial,15 sacubitril/valsartan demonstrated a significant reduction in systolic blood pressure of −3.9 mm Hg at 16 weeks, compared with valsartan alone, in heart failure patients with apparent resistant hypertension. Another targeted approach to managing resistant hypertension involves the inhibition of aldosterone. Recent studies on aldosterone synthase inhibition with baxdrostat16 and lorundrostat17 reported placebo-corrected reductions of −11.0 mm Hg and −9.6 mm Hg in systolic blood pressure, respectively. Additionally, blocking the endothelin pathway represents a novel method for lowering blood pressure in resistant hypertension, with aprocitentan showing a significant reduction in office systolic blood pressure of −3.8 mm Hg compared with placebo.18 Furthermore, a recent study on zilebesiran,19 a novel investigational RNA interference therapeutic agent, has demonstrated a prolonged antihypertensive effect by inhibiting hepatic angiotensinogen synthesis, presenting a promising avenue for achieving more reliable and sustained control of blood pressure.
Notably, our study showed that nighttime blood pressure reductions were prominently greater with sacubitril/allisartan than with olmesartan in Chinese patients for both dosages. This observation is consistent with the results of the phase 2 study and previous studies on sacubitril/valsartan.20 This feature might be of particular clinical significance for the Chinese and other Eastern populations.21,22 It has been suggested that nighttime blood pressure is a better predictor of adverse cardiovascular outcomes than daytime blood pressure.23, 24, 25 In the recent European hypertension guideline, assessment of nighttime blood pressure and nocturnal blood pressure variability was recommended.26 Considering the high prevalence of “non-dippers” among Asian patients, probably because of salt sensitivity,27,28 our study suggests that treatment with sacubitril/allisartan might be beneficial in Asian patients with hypertension.
Both doses of sacubitril/allisartan provided greater reductions from baseline in ambulatory nighttime blood pressure, compared with olmesartan. Moreover, statistically significant reductions (−2.6/−1.7 mm Hg) in ambulatory nighttime blood pressure were observed with sacubitril/allisartan 480 mg compared with 240 mg, whereas in the phase 3 study of sacubitril/valsartan in Chinese hypertensive patients,12 numeric, but not statistically significant, reductions (−0.31/−0.18 mm Hg) in ambulatory nighttime blood pressure were achieved with sacubitril/valsartan 400 mg compared with 200 mg. This indicates that sacubitril/allisartan could be especially suited for the management of nocturnal hypertension in Chinese population. Greater reductions in nighttime blood pressure in the high-dose sacubitril/allisartan group might be attributed to a larger dose of sacubitril29 and a longer half-life of allisartan as well.10 Further comparison of the blood pressure–lowering effects of sacubitril/allisartan between patients with a dipper vs nondipper profile of nighttime blood pressure are required.
In this study, there were no marked between-group differences in the incidence of AEs and treatment-related AEs. Both doses of sacubitril/allisartan were well tolerated. No association was found between the dose of sacubitril/allisartan and the incidence rate of AE. Of particular note, there was no report of angioedema in the sacubitril/allisartan groups.
Study limitations
First, the inclusion criteria of our study included clinic blood pressure, but not ambulatory blood pressure. Second, central blood pressure and arterial stiffness were not assessed during the trial, precluding a more thorough assessment of the antihypertensive properties of sacubitril/allisartan. Third, the impact of sacubitril/allisartan on target organ damage, such as left ventricular mass or urinary albumin excretion, and cardiovascular events was not assessed in the present study. Finally, caution is required when generalizing the findings of our study to other patient groups, given that the study population comprised Chinese patients only.
Conclusions
This phase 3 study showed that sacubitril/allisartan 240 mg and 480 mg once daily provided significantly greater blood pressure reductions, particularly nighttime blood pressure reductions, vs olmesartan 20 mg in Chinese patients with mild-to-moderate hypertension. In addition, sacubitril/allisartan was safe and well tolerated in Chinese population. The results suggest that sacubitril/allisartan could be particularly suitable for the management of hypertension in Chinese patients, and supports the rationale to prospectively study the effect of sacubitril/allisartan on cardiovascular events in Chinese patients with hypertension.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In Chinese patients with mild-to-moderate hypertension, we investigated the long-term antihypertensive efficacy and safety of 2 dosages of a novel angiotensin receptor neprilysin inhibitor, sacubitril/allisartan, during 52 weeks of treatment.
TRANSLATIONAL OUTLOOK: This phase 3 study showed that sacubitril/allisartan 240 mg and 480 mg once daily provided significantly greater blood pressure reductions, particularly nighttime blood pressure reductions, vs olmesartan 20 mg in Chinese patients with mild-to-moderate hypertension. In addition, sacubitril/allisartan was safe and well-tolerated in Chinese population. The results suggest that sacubitril/allisartan could be particularly suitable for the management of hypertension in Asian patients, and supports the rationale to prospectively study the effect of sacubitril/allisartan on cardiovascular events in Chinese patients with hypertension.
Funding Support and Author Disclosures
The study was funded by Salubris Pharma (Shenzhen, Guangdong Province, China). The study investigators were also financially supported by National Natural Science Foundation of China (grants 82070432, 82070435, 82270469, and 82370426); Ministry of Science and Technology (grants 2018YFC1704902 and 2022YFC3601302); Shanghai Municipal Commissions of Science and Technology (grant 19DZ2340200) and Health (a special grant for “leading academics”); the Three-year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System (GWV-10.1-XK05) Big Data and Artificial Intelligence Application; the Clinical Research Program, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (grant 2018CR010); and the Shenzhen Science and Technology Program (grant KQTD20200820151701004). Prof Wang has received lecture and consulting fees from Novartis, Omron, Servier, and Viatris. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all investigators at the participating centers and all patients for their commitment to the study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y.K., Lu C.Q., Gao R.C., Yu J.S., Liu G.C. Nation-wide hypertension screening in China during 1979-1980. Chin Med J. 1982;95:101–108. [PubMed] [Google Scholar]
- 4.Wang Z.W., Chen Z., Zhang L.F., et al. Status of hypertension in China: results from the China Hypertension Survey, 2012-2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 5.Li M.X., Zhang D.Y., Tang S.T., et al. Control status of ambulatory blood pressure and its relationship with arterial stiffness in the China nationwide registry of treated hypertensive patients: the REACTION-ABP study. Hypertens Res. 2023:2302–2311. doi: 10.1038/s41440-023-01336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusaka H., Sueta D., Koibuchi N., et al. LCZ696, angiotensin II receptor neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens. 2015;28:1409–1417. doi: 10.1093/ajh/hpv015. [DOI] [PubMed] [Google Scholar]
- 7.Ruilope L.M., Dukat A., Bohm M., Lacourciere Y., Gong J., Lefkowitz M.P. Blood pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 8.Kario K., Sun N., Chiang F.T., Supasyndh O., Baek S.H., Inubushi-Molessa A. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63:698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002. [DOI] [PubMed] [Google Scholar]
- 9.Sun N.L., Jiang Y.N., Wang H.Y., et al. Survey on sodium and potassium intake in patients with hypertension in China. J Clin Hypertens (Greenwich) 2021;23:1957–1964. doi: 10.1111/jch.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y., Zhang H., Li X.J., et al. A randomized, double-blind, placebo-controlled, single, and multiple dose-escalation Phase I clinical trial to investigate the safety, pharmacokinetic, and pharmacodynamic profiles of oral S086, a novel angiotensin receptor neprilysin inhibitor, in healthy Chinese volunteers. Expert Opin Investig Drugs. 2022;31:977–985. doi: 10.1080/13543784.2021.1985464. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Zhang W., Yan J., et al. Efficacy and safety of sacubitril/allisartan for the treatment of primary hypertension: a phase 2 randomized, double-blind study. Hypertens Res. 2023;46:2024–2032. doi: 10.1038/s41440-023-01326-7. [DOI] [PubMed] [Google Scholar]
- 12.Huo Y., Li W.M., Webb R., Zhao L., Wang Q., Guo W.N. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: a randomized, double-blind, 8-week study. J Clin Hypertens (Greenwich) 2019;21:67–76. doi: 10.1111/jch.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakugi H., Kario K., Yamaguchi M., Sasajima T., Gotou H., Zhang J. Efficacy of sacubitril/valsartan versus olmesartan in Japanese patients with essential hypertension: a randomized, double-blind, multicenter study. Hypertens Res. 2022;45:824–833. doi: 10.1038/s41440-021-00819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bundy J.D., Li C., Stuchlik P., et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775–781. doi: 10.1001/jamacardio.2017.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson A.M., Jhund P.S., Anand I.S., et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur Heart J. 2021;42:3741–3752. doi: 10.1093/eurheartj/ehab499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman M.W., Halvorsen Y.D., Marshall W., et al. BrigHTN Investigators Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388:395–405. doi: 10.1056/NEJMoa2213169. [DOI] [PubMed] [Google Scholar]
- 17.Laffin L.J., Rodman D., Luther J.M., et al. Target-HTN Investigators Aldosterone synthase inhibition with lorundrostat for uncontrolled hypertension: the Target-HTN randomized control trial. JAMA. 2023;330:1140–1150. doi: 10.1001/jama.2023.16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaich M.P., Bellet M., Weber M.A., et al. PRECISION Investigators Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022;400:1927–1937. doi: 10.1016/S0140-6736(22)02034-7. [DOI] [PubMed] [Google Scholar]
- 19.Desai A.S., Webb D.J., Taubel J., et al. Zilebesiran, an RNA interference therapeutic agent for hypertension. N Engl J Med. 2023;389:228–238. doi: 10.1056/NEJMoa2208391. [DOI] [PubMed] [Google Scholar]
- 20.Kario K., Rakugi H., Yarimizu D., Morita Y., Eguchi S., Lekushi K. Twenty-four-hour blood pressure-lowering efficacy of sacubitril/calsartan versus olmesartan in Japanese patients with essential hypertension based on nocturnal blood pressure dipping status: a post hoc analysis of data from a randomized, double-blind multicenter study. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.027612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supasyndh O., Sun N.L., Kario K., Hafeez K., Zhang J. Long-term (52-week) safety and efficacy of Sacubitril/valsartan in Asian patients with hypertension. Hypertens Res. 2017;40:472–476. doi: 10.1038/hr.2016.151. [DOI] [PubMed] [Google Scholar]
- 22.Supasyndh O., Wang J.A., Hafeez K., Zhang Y., Zhang J., Rakugi H. Efficacy and safety of Sacubitril/valsartan (LCZ696) compared with olmesartan in elderly Asian patients (≥65 Years) with systolic hypertension. Am J Hypertens. 2017;30:1163–1169. doi: 10.1093/ajh/hpx111. [DOI] [PubMed] [Google Scholar]
- 23.Kario K., Hoshide S., Mizuno H., et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142:1810–1820. doi: 10.1161/CIRCULATIONAHA.120.049730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Wang J.G. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278–283. doi: 10.1161/HYPERTENSIONAHA.111.00217. [DOI] [PubMed] [Google Scholar]
- 25.Staplin N., Sierra A., Ruilope L.M., et al. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59,124 patients. Lancet. 2023;401:2041–2050. doi: 10.1016/S0140-6736(23)00733-X. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G., Kreutz R., Brunström M., et al. 2023 ESH guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA) J Hypertens. 2023;41:1874–2071. doi: 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 27.Du S.F., Wang H.J., Zhang B., Popkin B.M. Dietary potassium intake remains low and sodium intake remains high, and most sodium is derived from home food preparation for Chinese adults, 1991-2015 trends. J Nutr. 2020;150:1230–1239. doi: 10.1093/jn/nxz332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshide S., Yamamoto K., Katsurada K., et al. Agreement regarding overcoming hypertension in the Asian Hypertension Society Network 2022. Hypertens Res. 2023;46:3–8. doi: 10.1038/s41440-022-00994-1. [DOI] [PubMed] [Google Scholar]
- 29.Lzzo J.L., Jr., Zappe D.H., Jia Y., Hafeez K., Zhang J. Efficacy and safety of crystalline valsartan/sacubitril (LCZ696) compared with placebo and combinations of free valsartan and sacubitril in patients with systolic hypertension: the RATIO study. J Cardiovasc Pharmacol. 2017;69:374–381. doi: 10.1097/FJC.0000000000000485. [DOI] [PubMed] [Google Scholar]