Assessment of right ventricular (RV) systolic function with 2-dimensional echocardiography is limited, and calculation of RV ejection fraction (RVEF) is not recommended in guidelines1 due to inaccuracy. Instead, surrogate echocardiographic markers such as RV free wall longitudinal strain (FWS), fractional area change (FAC), and tricuspid annular plane systolic excursion (TAPSE) are recommended but poorly correlate with RVEF.1 Advances in artificial intelligence (AI)-enabled waveform analysis can quantify RVEF from 12-lead electrocardiograms (ECG).2 We hypothesized that combination of ECG waveform and echocardiographic markers would outperform echocardiographic markers alone for low RVEF determination (RVEF < 40% by gold standard cardiac MRI [CMRI]). Furthermore, we hypothesized that ECG data are robust to factors causing imaging artifact, and incorporation of ECG would be informative in patients with poor imaging windows.
What is the clinical question being addressed?
Can AI-enabled ECG analysis of RVEF aid in RV assessment by echocardiography?
What is the main finding?
Combination of RV systolic assessment from ECG with echocardiography improves prediction of low RVEF and is robust to poor imaging windows compared to echocardiography alone.
We performed automated measurements of FWS, TAPSE, and FAC from RV-focused apical 4-chamber echo video using an AI software package we previously validated in patients with paired echocardiogram, ECG, and CMRI data.3 We categorized image quality through physician review as A, B, or C; where A is highest quality with full visualization of the RV free wall and apex, B has some areas of poor visualization, and C is very limited visualization or non RV-focused.3 We utilized our published method2 to predict the probability of RVEF <40% from ECG waveforms using a convolutional neural network (CNN). We then trained 2 models to predict RVEF <40% utilizing a gradient boosted decision trees algorithm (XGBoost): 1) an “Echo-only” model combining echocardiographic measures FWS, FAC, and TAPSE; and 2) an “Echo+ECG” model in which prediction probabilities from the ECG CNN model were added to the features of the echo-only model to create a stacked ensemble model. XGBoost is a flexible nonlinear supervised-learning algorithm that works by combining predictions from multiple decision trees to efficiently achieve accurate prediction.4 Evaluation metrics were average ± standard deviation of area under the receiver-operating characteristic curve (AUROC) and area under the precision-recall curve from 200 repeats of 5-fold cross-validation with outcome-stratified splitting. Due to small sample size, we did not reserve a single holdout test set because a random unfavorable split might diminish estimates of generalizability. It was ensured that none of the patients were included in ECG CNN model training set, no patient was included within both test and train sets in a single cross-validation fold, and that hyperparameter selection was nested within each training fold to avoid data leakage. Model AUROCs across test-set cross-validation folds were compared with corrected resampled t-test.5 Mount Sinai Hospital Institutional Review Board approved this study.
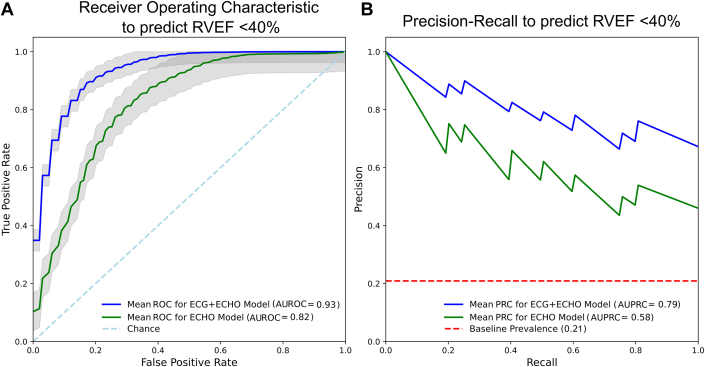
We included 220 independent patients with paired echo, ECG, and CMRI data. Cardiac diagnoses included 55% with hypertension, 41% heart failure with reduced LVEF, 11% heart failure with preserved LVEF, 35% with coronary artery disease, 25% with pulmonary hypertension, 21% with a history of atrial fibrillation/flutter, and 94% of patients were in sinus rhythm at imaging. The indication for CMRI was cardiomyopathy in 76% of patients, and post-myocardial infarction in 12%. Median RVEF was 52% (IQR: 43%-60%) and 46/220 (21%) had RVEF<40%. Image quality grades were grade A (highest) in 42 (19%), grade B in 76 (35%), and 102 (46%) grade C (lowest). There was no difference in the proportion with low RVEF across imaging grades (P = 0.91). There was a significant univariate difference in median RVEF between normal vs decreased RVEF for all included features: FAC (29% vs 19%; P < 0.001), FWS (18% vs 12%; P < 0.001), TAPSE (1.3 cm vs 0.85 cm; P < 0.001), and ECG predicted probability (0.4 vs 0.27; P < 0.001). AI model performances are shown in Figure 1. The Echo+ECG model outperformed the Echo-only model by AUROC (0.93 ± 0.04 vs 0.82 ± 0.07; P = 0.001) and area under the precision-recall curve (0.77 ± 0.13 vs 0.53 ± 0.14). At 90% sensitivity, ECHO+ECG achieved higher specificity (82% vs 59%), positive predictive value (57% vs 37%), and negative predictive value (97% vs 96%) compared to Echo-only. When stratified by imaging grade, the Echo-only AUC for grade C decreased by 17% compared to grade A, but the Echo+ECG model decreased by only 3%. The most important feature in Echo+ECG model was the ECG model probability (46%) followed by FAC (21%), FWS (17%), and TAPSE (17%).
Figure 1.
Ensemble Model Performance
(A) Receiver-operating characteristic for ECG+ECHO model (blue line) and echo-only model (green line), displayed as average ± 1 standard deviation (gray) of repeated 5-fold cross-validation test sets. (B) Average precision recall curve displayed similarly to above. Baseline prevalence demarcated by red dotted line. AUROC = area under the receiver-operating characteristic curve; AUPRC = area under precision-recall curve; ECG = electrocardiogram; ECHO = echocardiogram; RVEF = right ventricular ejection fraction.
This novel approach to RV systolic functional assessment combines multiple independent echocardiographic parameters and anchors them to quantitative gold standard CMRI, rather than evaluating them in isolation compared to reference standards. Integration of ECG data with echocardiographic data further improved performance, especially in patients with poor echocardiographic windows. This significant conceptual advancement addresses a gap in assessment of RV function and implies that integration of information across multiple data domains improves prediction. Promising applications of this precision medicine approach include screening for RV dysfunction to identify those in need of further evaluation, or to evaluate those with poor imaging windows and/or those unable to obtain advanced diagnostics such as 3-dimensional echocardiography or CMRI, which is resource- and personnel-intensive. Our model utilized AI methods for echocardiogram and ECG analysis that one day could possibly integrate into existing workflows for improved RV assessment (recognizing present-day technical challenges in integration across multiple data sources). The strength of this work is the novel conceptual approach to integrate multiple traditional diagnostics (ECG and 2-dimensional echocardiogram) to improve quantification of RV function measured by an advanced diagnostic (CMRI). However, this work is limited by its single-center nature without external validation to confirm the generalizability of the reported model accuracy, which is essential for clinical applicability. Future work will externally validate these findings and test this precision approach in subpopulations in which RV monitoring is crucial such as those with idiopathic pulmonary hypertension and congenital heart disease.
Funding support and author disclosures
This study was approved by the Icahn School of Medicine Institutional Review Board. This work was supported by NHLBI R01HL167050-02 (GNN). Dr Nadkarni has consultancy agreements with Renalytix and Heart Test Laboratories; has received research funding from Renalytix and Heart Test Laboratories; has received honoraria from Lexicon, Daiichi Sankyo, Menarini Health and Reata; has patents or royalties with Renalytix and Heart Test Laboratories; owns equity and stock options in Heart Test Laboratories and Renalytix; owns equity in Verici Dx; has received financial compensation as a scientific board member and advisor to Renalytix and Heart Test Laboratories; and serves in an advisory or leadership role for Pensieve Health and Renalytix. Grant support from ASE Foundation EDGES grant (SQD), United States. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Duong S.Q., Vaid A., My V.T.H., et al. Quantitative prediction of right ventricular size and function from the ECG. J Am Heart Assoc. 2024;13 doi: 10.1161/JAHA.123.031671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsia B.C., Lai A., Singh S., et al. Validation of American society of echocardiography guideline-recommended parameters of right ventricular dysfunction using artificial intelligence compared with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2023;36:967–977. doi: 10.1016/j.echo.2023.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Chen T., Guestrin C. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. ACM Digital Library; 2016. XGBoost: a scalable tree boosting system; pp. 785–794. [DOI] [Google Scholar]
- 5.Nadeau C., Bengio Y. In: Solla S., Leen T., Müller K., editors. Vol. 12. MIT Press; 1999. Inference for the generalization error. (Advances in Neural Information Processing Systems). [Google Scholar]