ABSTRACT
Feline mammary fibroadenomatous hyperplasia (FMFH) is an extensive proliferation of feline mammary tissues in response to endogenous or exogenous progestogens. The treatment of choice for FMFH is anti‐progestins or ovariohysterectomy (OVH). OVH if there is no intention of breeding the queen. This report describes a case of recurrent FMFH in an 11‐month‐old female Maine Coon cat. The condition was successfully treated with aglepristone during its first occurrence but recurred during the cat's subsequent gestation. OVH was performed along with aglepristone administration, resulting in complete regression of FMFH 21 days after aglepristone injection. The recurrence of FMFH successful treatment is well described; however, this case illustrates the necessity for further discussion regarding the utilisation of cats previously diagnosed with FMFH for breeding purposes.
Keywords: anti‐progestin, mammary glands hyperplasia, queen, recurrent
Therapeutic approaches for FMFH vary and should be discussed with the owner. For non‐breeding cats, OVH with or without aglepristone is recommended, whereas aglepristone alone is suitable for preserving reproductive functions. The authors advise that it is inadvisable to use a queen with a history of FMFH for breeding purposes.

1. Introduction
Feline mammary fibroadenomatous hyperplasia (FMFH) is a change in feline mammary tissues characterised by extensive proliferation of the ductal epithelium and mammary stroma. Also known as mammary hypertrophy or fibroadenomatous complex, it accounts for 13%–20% of mammary masses in queens (Payan‐Carreira 2013). The aetiology of FMFH is suspected to be an exaggerated response to endogenous or exogenous progesterone; however, the apparent predisposition of some cats to its development and the possibility of underlying genetic susceptibilities remain elusive. A previous study has reported the presence of both progesterone and oestrogen receptors in the fibroadenomatous tissue of the feline mammary gland, indicating that both hormones may be involved in the pathogenesis (Jurka and Max 2009). The condition typically occurs in young intact female cats during their first oestrous cycle. However, it has also been reported during gestation and pseudopregnancy and in intact and neutered males and females after treatment with exogenous or synthetic progesterone (Görlinger et al. 2002; Mayayo, Bo, and Pisu 2018; Vitasek and Dendisova 2006; Marino et al. 2021). Earlier reports suggest an age range for FMFH of 6 months to 13 years (Hayden, Barnes, and Johnson 1989; Wehrend, Hospes, and Gruber 2001; Payan‐Carreira 2013). The clinical signs are characterised by rapid enlargement of mammary tissue in one or more glands with localised erythema, inflammation, haemorrhages, ulceration and necrosis. The affected mammary gland shows a rapid increase in size and becomes swollen within a few weeks of the onset of signs. The diagnosis typically depends on historical data, clinical signs and histopathological examination. Serum progesterone levels are not a highly sensitive diagnostic indicator (Görlinger et al. 2002; Little 2011; de Melo et al. 2021; Vasiu et al. 2023). Mammary ultrasonography may offer valuable assistance in diagnosing FMFH, allowing for the assessment of mammary structure with efficiency and ease. Ultrasonography, FMFH lesions appear as a well‐circumscribed solid mass comprising a granular, slightly hyperechoic texture with regularly delimited margins, commonly interspersed with small cleft‐like structures (Payan‐Carreira 2013; Marino et al. 2021). Ultrasonography can also be useful to identify intramammary pockets filled with purulent exudate in cases with FMFH‐associated mastitis.
Treatment options for FMFH include ovariohysterectomy (OVH), ovariectomy, mastectomy and injections of anti‐progestin drugs. OVH or ovariectomy aims to remove the source of progesterone, usually leading to regression of the mammary tissue within 3–4 weeks. However, in some cases, regression is not achieved (Görlinger et al., 2002; de Melo et al., 2021). The profound mammary gland enlargement and swelling noted in many cats with FMFH make mastectomy a difficult procedure to perform (Marino et al. 2021). The use of the anti‐progestin, aglepristone, has been recommended and is often effective in treating FMFH, especially if persistent mammary growth is detected after OVH (de Melo et al. 2021; Marino et al. 2021; Vitasek and Dendisova 2006). Differences in treatment schedule and duration are related to the severity of the clinical signs (Payan‐Carreira 2013). However, OVH is recommended for definitive treatment when there is no intention of breeding the queen (de Melo et al. 2021; Payan‐Carreira 2013; Loretti et al. 2005).
The purpose of this report is to present and discuss a case of recurrent FMFH which regressed completely after treatment with aglepristone and recurred during subsequent gestation. This highlights the need for further discussion regarding the use of cats with a previous history of FMFH for breeding purposes. The risk of FMFH recurrence during gestation and potential consequences should be thoroughly discussed with breeders.
2. Case Description
An intact, female Maine Coon cat, weighing 3.6 kg, first presented at 8 months of age, was referred to the Veterinary Teaching Hospital due to progressive marked enlargement of multiple mammary glands over the last 10 days. The cat had not previously received any drugs for oestrus prevention. On clinical examination, the cat had asymmetrical enlargement of six mammary glands, including the caudal thoracic (T2), cranial abdominal (A1) and caudal abdominal (A2) mammary glands. The sizes of the mammary glands ranged from 2 to 10 cm in diameter. A necrotic skin lesion and signs of mammary infection were observed on one caudal abdominal (A2) mammary gland (Figure 1). Haematology and serum biochemistry profiles were within the normal range, as shown in Table 1. Serum progesterone levels were elevated at 20.76 ng/mL (baseline concentrations P4 < 1 ng/mL, during gestation/pseudopregnancy P4 13.5–57 ng/mL) (Schmidt, Chakraborty, and Wildt 1983). Ultrasonographic examination showed enlargement of the aforementioned mammary glands, which had a heterogeneous echotexture with mixed echogenicity and smooth margins (Figure 1). The ovaries and uterus were normal in size, shape and echogenicity. A tissue biopsy was performed with a biopsy gun. Histopathology of the affected mammary tissue revealed diffuse, fibroepithelial hyperplasia characterised by varying degrees of proliferation of both intralobular mammary ductal epithelium and interlobular stroma. The hyperplastic, glandular epithelial cells were medium‐cuboidal in shape, with deep basophilic cytoplasm and small round nuclei arranged in 1–2 layers and with ductal and tubular structure. Multifocal areas of mammary ductal and glandular hyperplasia were also observed. The histological features were supportive of fibroadenomatous changes (Figure 1). Treatment with aglepristone was chosen on the basis of the owner's intention to breed the cat in the future.
FIGURE 1.
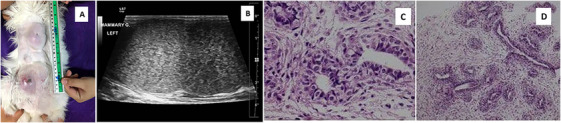
(A) Clinical appearance of the cat's mammary on the first day of the visit. (B) Ultrasonographic image of an enlarged mammary gland. (C and D) Histopathology of the mammary gland revealed diffused fibroepithelial hyperplasia of both intralobular mammary ductal epithelium and interlobular stroma. The hyperplastic glandular epithelial cells were medium cuboidal shape, arranges 1–2 layers ductal and tubular structure.
TABLE 1.
Haematology and serum biochemical of an 8‐month‐old female Maine Coon cat at the first visit.
| Haematology parameters | Results | Conventional unit | Feline reference ranges |
|---|---|---|---|
| PCV | 33 | % | 31–48 |
| Hb | 11 | g% | 10.–15.7 |
| RBC | 7.29 | ×103/µL | 6.9–10.1 |
| WBC | 10.88 | ×103/µL | 5.1–16.2 |
| Segmented neutrophils | 8.16 | ×103/µL | 2.3–11.6 |
| Band neutrophils | — | ×103/µL | 0–0.1 |
| Lymphocytes | 1.41 | ×103/µL | 0.9–6 |
| Monocytes | 0.76 | ×103/µL | 0–0.7 |
| Eosinophils | 0.54 | ×103/µL | 0.1–1.8 |
| Basophils | — | ×103/µL | 0–0.2 |
| Platelet | 215 | ×103/µL | 195–624 |
| Creatinine | 0.87 | mg/dL | 0.8–2.1 |
| ALT | 29 | U/L | 28–109 |
| Total protein | 6.6 | g/dL | 6.6–8.4 |
| Albumin | 3.7 | g/dL | 3.2–4.3 |
| Globulin | 3.9 | g/dL | 2.9–4.7 |
Treatment with subcutaneous injections of aglepristone (Alizin; Virbac, France) at a dosage of 10 mg/kg on Days 1, 2, 8, 15 and 22 (modified from Vitasek and Dendisova (2006)) was instigated, along with an empirically chosen broad‐spectrum antibiotic, amoxicillin trihydrate‐potassium clavulanate (Ranclav, Sun Pharmaceutical, India), dosed at 15 mg/kg orally, twice a day, for 14 days. A painkiller, tolfenamic acid (Tolfedine, Vetoquinol, Queensland, Australia), was orally administered at a dose of 4 mg/kg once a day for 4 days. A marked reduction in the size of the affected mammary glands was observed after the third aglepristone injection, and complete regression was achieved within 10 days of the last aglepristone injection.
Three months after the initial diagnosis of FMFH and treatment, the cat represents due to clinical signs consistent with FMFH recurrence. The ultrasonographic appearance of the mammary tissue was consistent with FMFH, and ultrasonography of the reproductive organs revealed the presence of gestational sacs with live foetuses. Along with mammary enlargement and increased soft tissue opacity, a radiographic study revealed at least six foetal skeletons in the uterus (Figure 2). The six kittens were born naturally at 64 days of gestation. Among them, two were born with congenital defects: one with a cleft palate and the other with spina bifida. Due to the queen's persistent mammary gland enlargement, accompanied by ulceration and inflammation (Figure 3), the kittens were hand reared with commercial feline replacement milk (KMR Kitten Milk Replacer, Pet‐Ag, Inc., United States). Regrettably, all six kittens died within 1 week of parturition.
FIGURE 2.
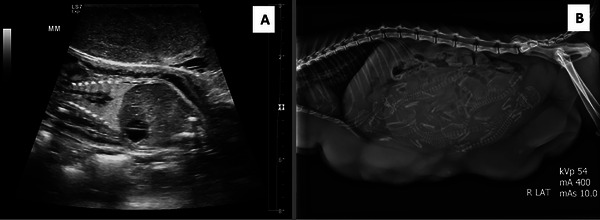
(A) Ultrasonographic image of Day 48 of gestation. The image shows an enlarged mammary gland with heterogeneous echotexture with smooth margins. The foetal structures are visible in the gestational sac, which is surrounded by amniotic fluid. This image is of the sagittal plain of the foetus. (B) 60 days of gestation, a radiographic image shows at least six foetuses in the uterus and the enlargement of multiple mammary glands.
FIGURE 3.
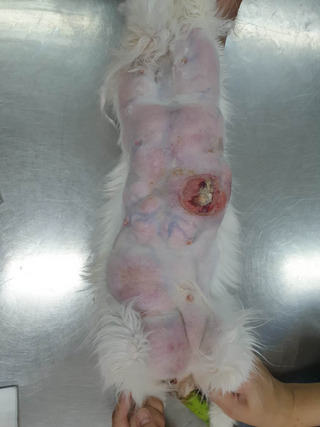
Clinical appearance of queen's mammary 1 day after parturition, first day of the second treatment. It showed enlargement of all eight glands with an ulceration lesion on left gland of cranial abdominal (A1).
OVH (performed via a left flank incision to avoid the enlarged mammary gland) was performed the day following parturition, revealing nine ovarian corpora lutea on the ovaries. A course of aglepristone injections was initiated 1 day after OVH; the dosage, route and frequency of the aglepristone injection were the same as during the first treatment. During the second treatment, a marked reduction in the size of the mammary tissue was observed after the fourth injection, and complete regression was reported by the owner within 21 days of the last aglepristone injection.
3. Discussion
This case report describes a cat with FMFH that was successfully treated with aglepristone but then recurred during a subsequent gestation. The progesterone produced by the ovarian corpus luteum to maintain the gestation is likely to have resulted in overstimulation of the mammary tissue and subsequent FMFH. FMFH is a progesterone‐associated disorder; thus, therapeutic approaches should focus on removing progesterone influences to alleviate symptoms (Marino et al. 2021). OVH, or ovariectomy, is typically recommended as the treatment of choice (Wehrend, Hospes, and Gruber 2001); however, as the owner intended to breed their cat, anti‐progestin was used to treat the cat described in this report. Treatment with anti‐progestin has a success rate of nearly 100% with minimal side effects, such as short‐term skin irritation at the injection site. Regression of mammary tissue can start as early as 3 days after the first injection and, in the cat described here, was observed on Day 8 of the first treatment course. Complete remission of FMFH is achieved on average 3.9 weeks after the first injection (range 1–11 weeks) (Görlinger et al. 2002; Vitasek and Dendisova 2006; Jurka and Max 2009; Gogny and Fieni 2016; Wehrend, Hospes, and Gruber 2001), consistent with the 4.6 weeks during our initial treatment course.
Notably, as has previously been described (Gogny and Fieni 2016; Jurka and Max 2009), the regression rate of the mammary enlargement following the second treatment course was appeared slower compared to the first, a significant reduction in size was observed after 2 weeks, and complete remission was reported 6 weeks after initiation of treatment. Cats with FMFH that have previously been treated with long‐acting progestogens also appear to have a longer duration of clinical signs (Jurka and Max 2009), and it is possible that modified or longer treatment protocols might be beneficial in these subsets of queens.
An advantage of using aglepristone is that it provides preservation of reproductive function and is non‐invasive compared to surgical methods such as OVH; however, FMFH may recur (Jurka and Max 2009; Wehrend, Hospes, and Gruber 2001; Payan‐Carreira 2013; Görlinger et al. 2002). Wehrend, Hospes, and Gruber (2001) reported recurrence of FMFH only 13 days after the start of an aglepristone treatment in a cat that had been previously treated with long‐acting progesterone. Jurka and Max (2009) reported two cats with recurrent FMFH a few months of aglepristone treatment; one recurrence was attributed to the use of exogenous steroids and the other following mating and ovulation (Jurka and Max 2009). However, there are many reports of queens going on to have normal gestations and no recurrence of FMFH after treatment with aglepristone (Jurka and Max 2009; Gogny and Fieni 2016).
As far as the authors are aware, there is no published evidence documenting an association between anti‐progestin treatment and congenital defects in cats. The congenital defects of the kittens reported in this case are therefore assumed to be unrelated to the aglepristone treatment. Indeed, the reported side effects of aglepristone injection are limited to short‐term skin irritation at the injection site (Gogny and Fieni 2016; Fieni et al. 2006).
4. Conclusion
Therapeutic approaches for FMFH differ among cases depending on individual conditions. The recommended treatment for intact female cats that are not intended for breeding is OVH with or without aglepristone treatment. Should an owner want to preserve reproductive function, aglepristone provides an alternative treatment option; however, it is essential to inform the owner of the possibility of disease recurrence. In cases of FMFH associated with gestation, natural lactation may not be possible, leading to the necessity of feeding kittens with commercial milk. Thus, in the author's opinion, it is inadvisable to use a queen with a history of FMFH for breeding purposes.
Author Contributions
Panisara Kunkitti: conceptualisation, investigation, surgeon, review and editing final draft. Nichanan Maneeganondh: investigation, writing–original draft. Nitaya Boonbal: ultrasonologist, investigation, review and editing.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/vms3.70060.
Acknowledgements
The authors would like to thank the owner of the cats for the constant come for the treatment and useful information.
Funding: The authors received no specific funding for this work.
Data Availability Statement
Data are available on request from the authors.
References
- de Melo, E. H. , Camara D. R., Notomi M. K., et al. 2021. “Effectiveness of Ovariohysterectomy on Feline Mammary Fibroepithelial Hyperplasia Treatment.” Journal of Feline Medicine and Surgery 23: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieni, F. , Martal J., Marnet P. G., Siliart B., and Guittot F.. 2006. “Clinical, Biological and Hormonal Study of Mid‐Pregnancy Termination in Cats With Aglepristone.” Theriogenology 66: 1721–1728. 10.1016/j.theriogenology.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Gogny, A. , and Fieni F.. 2016. “Aglepristone: A Review on Its Clinical Use in Animals.” Theriogenology 85: 555–566. 10.1016/j.theriogenology.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Görlinger, S. , Kooistra H. S., van den Broek A., and Okkens A. C.. 2002. “Treatment of Fibroadenomatous Hyperplasia in Cats With Aglepristone.” Journal of Veterinary Internal Medicine 16: 710–713. 10.1111/j.1939-1676.2002.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Hayden, D. W. , Barnes D. M., and Johnson K. H.. 1989. “Morphologic Changes in the Mammary Gland of Megestrol Acetate‐Treated and Untreated Cats: A Retrospective Study.” Veterinary Pathology 26: 104–113. 10.1177/030098588902600202. [DOI] [PubMed] [Google Scholar]
- Jurka, P. , and Max A.. 2009. “Treatment of Fibroadenomatosis in 14 Cats With Aglepristone—Changes in Blood Parameters and Follow‐Up.” The Veterinary Record 165: 657–660. 10.1136/vr.165.22.657. [DOI] [PubMed] [Google Scholar]
- Kaneko, J. J. , Harvey J. W., and Bruss M. L., eds. 2008. Clinical Biochemistry of Domestic Animals. Cambridge: Academic press. [Google Scholar]
- Latimer, K.S. , ed. 2011. Duncan and Prasse's Veterinary Laboratory Medicine: Clinical Pathology. Hoboken: John Wiley & Sons. [Google Scholar]
- Little, S. 2011. “Feline Reproduction: Problems and Clinical Challenges.” Journal of Feline Medicine and Surgery 13: 508–515. 10.1016/j.jfms.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loretti, A. P. , da Silva Ilha M. R., Ordas J., and de las Mulas J. M.. 2005. “Clinical, Pathological and Immunohistochemical Study of Feline Mammary Fibroepithelial Hyperplasia Following a Single Injection of Depot Medroxyprogesterone Acetate.” Journal of Feline Medicine and Surgery 7: 43–52. 10.1016/j.jfms.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, G. , Pugliese M., Pecchia F., et al. 2021. “Conservative Treatments for Feline Fibroadenomatous Changes of the Mammary Gland.” Open Veterinary Journal 11: 680–685. 10.5455/OVJ.2021.v11.i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayayo, S. L. , Bo S., and Pisu M. C.. 2018. “Mammary Fibroadenomatous Hyperplasia in a Male Cat.” Journal of Feline Medicine and Surgery Open Reports 4, no. 1. 10.1177/2055116918760155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan‐Carreira, R. 2013. “Feline Mammary Fibroepithelial Hyperplasia: A Clinical Approach.” In Insights From Veterinary Medicine, edited by Payan‐Carreira C. R., 292. London, UK: IntechOpen. [Google Scholar]
- Schmidt, P. M. , Chakraborty P. K., and Wildt D. E.. 1983. “Ovarian Activity, Circulating Hormones and Sexual Behavior in the Cat. II. Relationships During Pregnancy, Parturition, Lactation and the Postpartum Estrus.” Biology of Reproduction 28, no. 3: 657–671. 10.1095/biolreprod28.3.657. [DOI] [PubMed] [Google Scholar]
- Vasiu, I. , Dabrowski R., Wochnik M., Plusa A., and Tvarijonaviciute A.. 2023. “A Systematic Review of Mammary Gland Inflammations in Queens (Felis catus).” Animal Reproduction Science 256: 107318. 10.1016/j.anireprosci.2023.107318. [DOI] [PubMed] [Google Scholar]
- Vitasek, R. , and Dendisová H.. 2006. “Treatment of Feline Mammary Fibroepithelial Hyperplasia Following a Single Injection of Proligestone.” Acta Veterinaria Brno 75, no. 2: 295–297. 10.2754/avb200675020295. [DOI] [Google Scholar]
- Wehrend, A. , Hospes R., and Gruber A. D.. 2001. “Treatment of Feline Mammary Fibroadenomatous Hyperplasia With a Progesterone‐Antagonist.” Veterinary Record 148: 346–347. 10.1136/vr.148.11.346. [DOI] [PubMed] [Google Scholar]
- Weiss, D. J. and Wardrop, eds., 2011. Schalm's Veterinary Hematology. John Wiley & Sons. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


