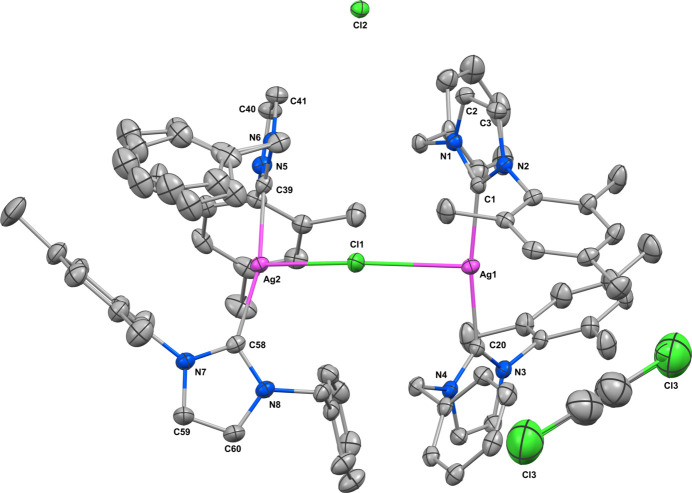
The solid-state structural analysis of the title compound reveals that the two molecules of bis(1-benzyl-3-mesitylimidazol-2-ylidene)silver are connected via a bridging chloride atom. The structure also reveals non-classical intermolecular hydrogen-bonding interactions involving the chloride counter-anion.
Keywords: crystal structure, N-heterocyclic carbene, silver chloride, bis(NHC) silver complex
Abstract
The title compound, [Ag2(C19H20N2)4]Cl·0.5C2H4Cl2, can be readily generated by treatment of (1-benzyl-3-(2,4,6-trimethylphenyl)imidazolium chloride with sodium bis(trimethylsilyl)amide followed by silver chloride. The molecular structure of the compound was confirmed using NMR spectroscopy and single-crystal X-ray diffraction analysis. The crystal structure of the title compound at 110 K has monoclinic (P21/c) symmetry. The represented silver compound is of interest with respect to antibacterial properties and the structure displays a series of weak intermolecular hydrogen-bonding interactions with the chloride counter-anion.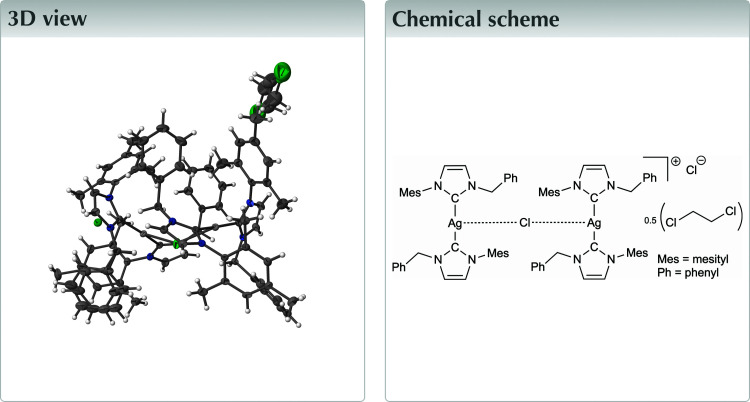
Structure description
Recent research has focused on discovering new and more effective silver-based antibacterial compounds. In 2004, Young’s group reported a variety of silver(I) complexes containing N-heterocyclic carbenes as a new class of antibiotics (Melaiye et al., 2004 ▸). N-heterocyclic carbenes (NHC) form strong M—Ccarbene bonds (Arduengo et al., 1991 ▸) that are far more stable than most phosphines due to their increased σ-donation, as well as π-back-donation from metal to carbene (Jafarpour et al., 1999 ▸; Herrmann & Köcher, 1997 ▸). The stability and versatility of these ligands allow them to serve as metal carriers for transition metals such as copper, gold, and silver in biological media (Medici et al., 2016 ▸). Subsequently, NHC-containing silver complexes have been targeted for the slow release of silver ions under biological conditions (Streciwilk et al., 2014 ▸; Karatas et al., 2016 ▸; Aher et al., 2014 ▸; A Patil et al., 2020 ▸). In relevance to this context, we prepared the title compound and studied its solid and solution-state structural features. The leading results pertaining to the title compound are presented below.
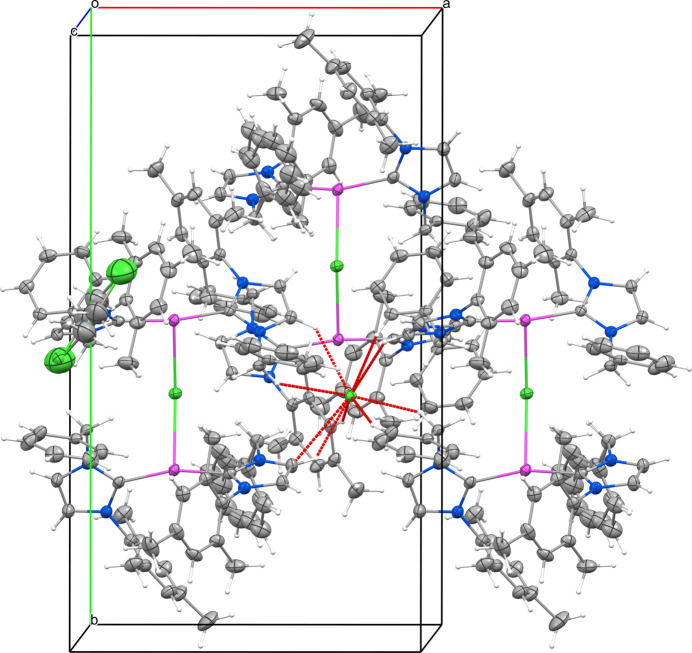
The title compound crystallizes in the monoclinic space group P21/c with four silver-carbene complex molecules and two 1,2-dichloroethane molecules in the unit cell. The molecular structure of the compound is presented in Fig. 1 ▸. The molecular geometry around the silver atom is linear, where two NHCs are attached to the silver atom with C20—Ag1—C1 and C39—Ag2—C58 bond angles of 170.55 (8) and 163.97 (8)°, respectively. However, a chloride anion bridges the two bis(NHC) silver units with an Ag2—Cl1—Ag1 bond angle of 148.90 (2)°. The observed Ag1—Cl1 and Ag2—Cl1 bond lengths are 2.8755 (6) Å and 2.8149 (6) Å. Subsequently, a T-shaped coordination environment is observed around the silver atom. The Ag—Ccarbene bond lengths Ag1—C1, Ag1—C20, Ag2—C39, Ag1—C58 are 2.099 (2), 2.098 (2), 2.098 (2) and 2.104 (2) Å. These parameters are well within the reported bond parameters for Ag—Ccarbene and Ccarbene—Ag—Ccarbene. The compound also engages in weak intermolecular C—H⋯Cl interactions. A pictorial representation of the non-classical hydrogen bonding and the bond parameters are presented in Fig. 2 ▸ and Table 1 ▸, respectively.
Figure 1.
The molecular structure of the title compound with solvate, the displacement ellipsoids drawn at the 50% probability level. Hydrogen atoms are omitted for clarity.
Figure 2.
Intermolecular C—H⋯·Cl interactions (dotted lines) in the title compound. Displacement ellipsoids are drawn at the 50% probability level.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯Cl2 | 0.95 | 2.80 | 3.573 (2) | 140 (1) |
| C8—H8⋯Cl2 | 0.95 | 2.81 | 3.669 (2) | 157 (1) |
| C13—H13A⋯Cl2 | 0.99 | 2.91 | 3.784 (2) | 147 (1) |
| C21—H21⋯Cl2 | 0.95 | 2.78 | 3.567 (2) | 141 (1) |
| C38—H38⋯Cl2 | 0.95 | 2.90 | 3.779 (2) | 155 (1) |
| C41—H41⋯Cl2 | 0.95 | 2.66 | 3.419 (2) | 137 (1) |
| C70—H70A⋯Cl2 | 0.99 | 2.74 | 3.634 (2) | 151 (1) |
The 1,2-dichloroethane solvate molecule is located on a crystallographic inversion center, and is disordered over two pseudo-mirror related moieties.
A CSD structure search for bis[(1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylidene)silver(I)] revealed no hits. However, a few mono-NHC and bis-NHC silver complexes bearing 1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylidene or similar NHCs have been reported. A few of the bis-NHC silver complexes include, bis-[1-benzyl-3-(4-methylphenyl)imidazol-2-ylidene]silver(I) hexafluoridophosphate (Huang & Qin, 2011 ▸), and mono-NHC silver complexes include di-μ-acetato-bis-[3-benzyl-1-(2,4,6-trimethylphenyl)imidazol-2-ylidene]silver(I)] (Jayaraman et al., 2019 ▸), (1-benzyl-3-mesitylimidazol-2-ylidene)chlorosilver(i) (Samantaray et al., 2011 ▸) and bis(μ2-bromo)bis(1-benzyl-3-mesityl-2,3-dihydro-1H-imidazol-2-ylidene)disilver (Ortiz et al., 2016 ▸). In scanning the literature, the CNHC—Ag, C—N and N—C—N bond lengths and CNHC—Ag—CNHC and N—CNHC—N bond angles are comparable to those of the title compound.
Synthesis and crystallization
All synthetic procedures were executed under a nitrogen atmosphere glove box (Inert Glove Box System). All glasswares were subjected to heat at 110°C for 12 h before use. The starting material 1-(benzyl)-3-(2,4,6-trimethylphenyl)imidazolium chloride was prepared according to literature procedures (Maishal et al., 2009 ▸). Solvents (CH2Cl2, Et2O, THF, and toluene) were dried with a solvent purification system (Inert Innovative Technology, Inc.), degassed using three consecutive freeze–pump–thaw cycles and stored over 4 Å molecular sieves in the glove box. The NMR solvents: CDCl3 (99.9%) was purchased from Acros Laboratories, dried over 4 Å molecular sieves and stored in the glove box prior to use. All other chemicals were purchased commercially and used as received. The 1H and 13C NMR spectra were recorded on a Bruker 300 MHz spectrometer. Spectra were referenced to the residual solvent as an internal standard, for 1H NMR: CDCl3, 7.26 p.p.m. and 13C NMR: CDCl3, 77.16 p.p.m.
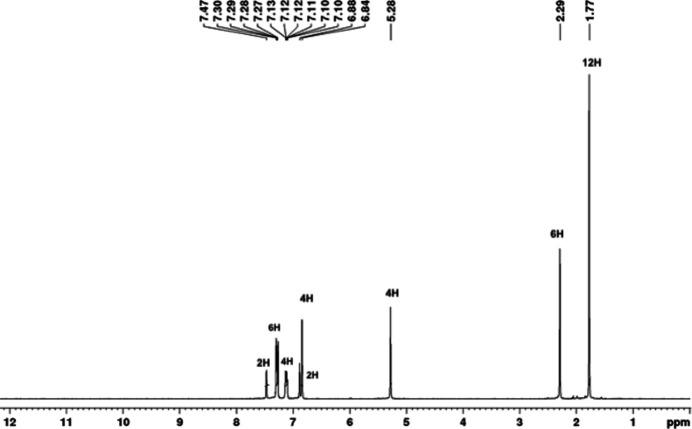
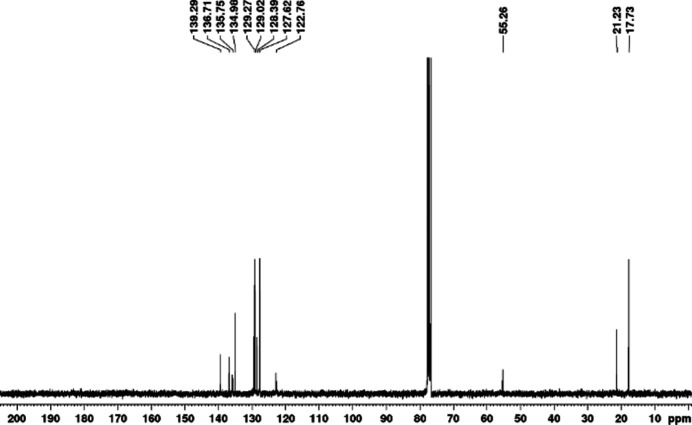
In a 10 ml vial equipped with a stir bar, 1-(benzyl)-3-(2,4,6-trimethylphenyl) imidazolium chloride (0.120 g, 0.384 mmol) and sodium bis(trimethylsilyl)amide (0.077 g, 0.422 mmol) were mixed in 2 ml of toluene. After 2 h, the yellow solution was filtered through a plug of celite into a vial containing AgCl (0.0247 g, 0.173 mmol) in 2 ml of toluene. The mixture was stirred for 24 h. The resulting solution was filtered through a plug of celite and dried under vacuum. The brown residue was dissolved in minimum amount (∼2 ml) of CH2Cl2 and the product was precipitated with 15 ml Et2O and further washed with 3 ×10 ml Et2O to produce a white solid. Yield: 0.75 g, 78%. 1H NMR spectroscopic analysis of the silver complex proved consistent with the molecular structure. The absence of the hydrogen atoms attached to the Ccarbene in the 1H NMR of complexes proves the formation of a silver–carbene bond. The proton NMR (CDCl3) spectrum shows mesityl H atoms (ortho-CH3 and para-CH3) at 1.77 and 2.29 p.p.m., respectively. The benzylic CH2 H atoms were observed at 5.28 p.p.m. The 6.88 and 7.47 p.p.m. signals correspond to the C2 and C3 imidazole H atoms. The two aromatic mesityl H atoms are represented by a singlet at 6.84 p.p.m.; the rest of the H atoms corresponding to the phenyl rings were observed between 7.30 p.p.m. and 7.12 p.p.m.. All signals corresponding to the carbon atoms were observed by 13C NMR spectroscopy. 1H NMR (δ, CDCl3, 300 MHz): δ 7.47 (m, 2H), 7.30–7.27 (m, 6H), 7.13–7.12 (m, 4H), 6.88 (s, 2H), 6.84 (s, 4H), 5.28 (s, 4H), 2.29 (s, 6H), 1.77 (s, 12H) (Fig. 3 ▸).13C NMR (δ, CDCl3, 75 MHz): δ 139.29, 136.71, 135.75, 134.98, 129.27, 129.02, 128.39, 127.62, 122.76, 55.26, 21.23, 17.73 (Fig. 4 ▸).
Figure 3.
1H NMR of the title compound in CDCl3
Figure 4.
13C NMR of the title compound in CDCl3
Colorless crystals of the title compound were obtained by diffusing diethyl ether into a saturated solution of 1,2-dichloroethane solution.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The title compound co-crystallizes with half a solvent molecule of 1,2-dichloromethane per asymmetric unit. The chlorine and carbon atoms of the solvent molecule are disordered (Cl3 and Cl3B; C77 and C77B). The positions of Cl3 and Cl3B as well as C77 and C77B are split into two. The C—Cl bond lengths of the solvate molecule were restrained to a target value of 1.77 (2) Å. The C52–C57 phenyl ring was refined as disordered. The geometry (bond lengths and angles) of the two disordered moieties were restrained to be similar to those of another better defined phenyl ring (C14–C19) using SAME and SADI restraints (with an e.s.d. of 0.02 Å). For all disordered atoms Uij components of ADPs closer to each other than 2.0 Å were restrained to be similar (with an e.s.d. of 0.01 Å2). Subject to these conditions, the solvate disorder refined to an occupancy ratio of 0.423 (16):0.577 (16), and that of the phenyl group to 0.446 (13) to 0.554 (13).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Ag2(C19H20N2)4]Cl·0.5C2H4Cl2 |
| M r | 1441.59 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 110 |
| a, b, c (Å) | 12.7605 (2), 22.4142 (4), 25.0233 (4) |
| β (°) | 93.055 (1) |
| V (Å3) | 7146.9 (2) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 5.79 |
| Crystal size (mm) | 0.25 × 0.21 × 0.2 |
| Data collection | |
| Diffractometer | Xcalibur, Sapphire3 |
| Absorption correction | Analytical (CrysAlis PRO; Rigaku OD, 2015 ▸) |
| Tmin, Tmax | 0.545, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 54034, 14039, 13203 |
| R int | 0.038 |
| (sin θ/λ)max (Å−1) | 0.618 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.037, 0.093, 1.06 |
| No. of reflections | 14039 |
| No. of parameters | 897 |
| No. of restraints | 281 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 2.12, −0.65 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624008617/zl4077sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624008617/zl4077Isup2.hkl
CCDC reference: 2380960
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors acknowledge support by funds from the Chemistry Department, Wright State University, College of Science and Mathematics. The authors would also like to acknowledge Dr Grossie, Wright State University, for help with the low-temperature data X-ray diffraction collection.
full crystallographic data
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Crystal data
| [Ag2(C19H20N2)4]Cl·0.5C2H4Cl2 | F(000) = 2980 |
| Mr = 1441.59 | Dx = 1.340 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54178 Å |
| a = 12.7605 (2) Å | Cell parameters from 9034 reflections |
| b = 22.4142 (4) Å | θ = 2.5–3.1° |
| c = 25.0233 (4) Å | µ = 5.79 mm−1 |
| β = 93.055 (1)° | T = 110 K |
| V = 7146.9 (2) Å3 | Block, colorless |
| Z = 4 | 0.25 × 0.21 × 0.2 mm |
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Data collection
| Xcalibur, Sapphire3 diffractometer | 13203 reflections with I > 2σ(I) |
| ω scans | Rint = 0.038 |
| Absorption correction: analytical (CrysAlisPro; Rigaku OD, 2015) | θmax = 72.2°, θmin = 3.5° |
| Tmin = 0.545, Tmax = 0.746 | h = −11→15 |
| 54034 measured reflections | k = −24→27 |
| 14039 independent reflections | l = −30→30 |
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.093 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0555P)2 + 3.6026P] where P = (Fo2 + 2Fc2)/3 |
| 14039 reflections | (Δ/σ)max = 0.002 |
| 897 parameters | Δρmax = 2.12 e Å−3 |
| 281 restraints | Δρmin = −0.65 e Å−3 |
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Aromatic (C—H) H atoms were added using a riding-model approximation with C—H bond lengths of 0.95 Å with Uiso (H) = 1.2 Ueq(CarH). Methyl (CH3) H atoms were treated as a rotating group and added using a riding-model approximation to the carbon atom to which they are attached. Methyl H atoms were fixed at a distance of 0.98 Å with Uiso (H) = 1.5 Ueq(CH3). Methylene (CH2) H atoms were added using riding-model approximations with a C—H bond distance of 0.99 Å. |
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Ag1 | 0.25650 (2) | 0.49164 (2) | 0.33797 (2) | 0.01912 (6) | |
| Ag2 | 0.26003 (2) | 0.73310 (2) | 0.37289 (2) | 0.01975 (6) | |
| Cl1 | 0.26361 (4) | 0.60834 (2) | 0.38575 (2) | 0.02881 (12) | |
| Cl2 | 0.76215 (4) | 0.61174 (2) | 0.38397 (2) | 0.02090 (10) | |
| N1 | 0.50114 (15) | 0.50958 (8) | 0.34948 (7) | 0.0197 (4) | |
| N2 | 0.45511 (14) | 0.42868 (8) | 0.38637 (7) | 0.0185 (3) | |
| N3 | 0.05469 (15) | 0.48589 (8) | 0.26312 (8) | 0.0211 (4) | |
| N4 | 0.01433 (15) | 0.51172 (8) | 0.34141 (7) | 0.0193 (4) | |
| N5 | 0.45631 (15) | 0.76470 (8) | 0.30943 (7) | 0.0205 (4) | |
| N6 | 0.50338 (15) | 0.71660 (9) | 0.37995 (7) | 0.0211 (4) | |
| N7 | 0.06974 (15) | 0.79873 (9) | 0.41778 (8) | 0.0225 (4) | |
| N8 | 0.01457 (15) | 0.72187 (9) | 0.37458 (8) | 0.0218 (4) | |
| C1 | 0.41603 (17) | 0.47505 (10) | 0.35709 (8) | 0.0187 (4) | |
| C2 | 0.59158 (18) | 0.48536 (10) | 0.37375 (9) | 0.0224 (4) | |
| H2 | 0.660426 | 0.501508 | 0.373856 | 0.027* | |
| C3 | 0.56224 (17) | 0.43432 (11) | 0.39716 (9) | 0.0224 (4) | |
| H3 | 0.606450 | 0.407418 | 0.417178 | 0.027* | |
| C4 | 0.39110 (17) | 0.38217 (10) | 0.40749 (8) | 0.0187 (4) | |
| C5 | 0.37793 (19) | 0.32912 (11) | 0.37902 (9) | 0.0249 (5) | |
| C6 | 0.3192 (2) | 0.28405 (11) | 0.40114 (10) | 0.0276 (5) | |
| H6 | 0.310208 | 0.247313 | 0.382528 | 0.033* | |
| C7 | 0.27347 (18) | 0.29150 (11) | 0.44979 (9) | 0.0249 (5) | |
| C8 | 0.28657 (17) | 0.34547 (11) | 0.47676 (8) | 0.0229 (4) | |
| H8 | 0.254234 | 0.351120 | 0.509711 | 0.027* | |
| C9 | 0.34635 (17) | 0.39148 (10) | 0.45631 (8) | 0.0197 (4) | |
| C10 | 0.4275 (3) | 0.32017 (13) | 0.32639 (11) | 0.0388 (6) | |
| H10A | 0.393562 | 0.346391 | 0.299338 | 0.058* | |
| H10B | 0.418929 | 0.278518 | 0.315097 | 0.058* | |
| H10C | 0.502468 | 0.329787 | 0.330465 | 0.058* | |
| C11 | 0.2123 (2) | 0.24108 (13) | 0.47354 (11) | 0.0361 (6) | |
| H11A | 0.212994 | 0.245736 | 0.512502 | 0.054* | |
| H11B | 0.244511 | 0.202866 | 0.464800 | 0.054* | |
| H11C | 0.139633 | 0.241978 | 0.458708 | 0.054* | |
| C12 | 0.3636 (2) | 0.44866 (12) | 0.48676 (10) | 0.0316 (5) | |
| H12A | 0.438467 | 0.453327 | 0.496626 | 0.047* | |
| H12B | 0.323910 | 0.447659 | 0.519230 | 0.047* | |
| H12C | 0.339534 | 0.482312 | 0.464308 | 0.047* | |
| C13 | 0.49867 (18) | 0.56636 (9) | 0.32015 (9) | 0.0216 (4) | |
| H13A | 0.550170 | 0.594040 | 0.337756 | 0.026* | |
| H13B | 0.428173 | 0.584412 | 0.322128 | 0.026* | |
| C14 | 0.52324 (19) | 0.55953 (9) | 0.26197 (9) | 0.0226 (4) | |
| C15 | 0.6231 (2) | 0.57206 (12) | 0.24564 (11) | 0.0322 (5) | |
| H15 | 0.676933 | 0.583082 | 0.271447 | 0.039* | |
| C16 | 0.6453 (3) | 0.56864 (14) | 0.19173 (12) | 0.0432 (7) | |
| H16 | 0.714000 | 0.577032 | 0.180893 | 0.052* | |
| C17 | 0.5665 (3) | 0.55291 (13) | 0.15408 (11) | 0.0439 (7) | |
| H17 | 0.580819 | 0.551430 | 0.117231 | 0.053* | |
| C18 | 0.4673 (3) | 0.53941 (13) | 0.16999 (11) | 0.0397 (6) | |
| H18 | 0.413780 | 0.528103 | 0.144145 | 0.048* | |
| C19 | 0.4457 (2) | 0.54233 (12) | 0.22396 (10) | 0.0308 (5) | |
| H19 | 0.377575 | 0.532520 | 0.234804 | 0.037* | |
| C20 | 0.09670 (18) | 0.49400 (9) | 0.31352 (9) | 0.0196 (4) | |
| C21 | −0.07723 (18) | 0.51556 (10) | 0.30951 (9) | 0.0235 (4) | |
| H21 | −0.144391 | 0.527346 | 0.320335 | 0.028* | |
| C22 | −0.0520 (2) | 0.49915 (11) | 0.25977 (10) | 0.0258 (5) | |
| H22 | −0.098055 | 0.497096 | 0.228714 | 0.031* | |
| C23 | 0.11352 (18) | 0.46358 (10) | 0.21996 (9) | 0.0226 (4) | |
| C24 | 0.1439 (2) | 0.50266 (11) | 0.18046 (10) | 0.0288 (5) | |
| C25 | 0.2013 (2) | 0.47913 (13) | 0.13931 (11) | 0.0349 (6) | |
| H25 | 0.221595 | 0.504744 | 0.111437 | 0.042* | |
| C26 | 0.2293 (2) | 0.41914 (14) | 0.13811 (11) | 0.0353 (6) | |
| C27 | 0.1974 (2) | 0.38204 (12) | 0.17792 (11) | 0.0335 (5) | |
| H27 | 0.216216 | 0.341048 | 0.177229 | 0.040* | |
| C28 | 0.1380 (2) | 0.40301 (11) | 0.21938 (10) | 0.0278 (5) | |
| C29 | 0.1175 (3) | 0.56839 (13) | 0.18214 (13) | 0.0449 (7) | |
| H29A | 0.041118 | 0.573424 | 0.180233 | 0.067* | |
| H29B | 0.147185 | 0.588618 | 0.151690 | 0.067* | |
| H29C | 0.147133 | 0.585707 | 0.215589 | 0.067* | |
| C30 | 0.2943 (3) | 0.39469 (16) | 0.09405 (13) | 0.0491 (8) | |
| H30A | 0.263823 | 0.407707 | 0.059197 | 0.074* | |
| H30B | 0.294579 | 0.351007 | 0.095605 | 0.074* | |
| H30C | 0.366370 | 0.409572 | 0.098869 | 0.074* | |
| C31 | 0.1033 (3) | 0.36087 (12) | 0.26152 (13) | 0.0417 (7) | |
| H31A | 0.144739 | 0.367921 | 0.295015 | 0.062* | |
| H31B | 0.113539 | 0.319673 | 0.249783 | 0.062* | |
| H31C | 0.028789 | 0.367497 | 0.267293 | 0.062* | |
| C32 | 0.01990 (18) | 0.52358 (10) | 0.39944 (8) | 0.0210 (4) | |
| H32A | −0.021671 | 0.559746 | 0.406547 | 0.025* | |
| H32B | 0.093721 | 0.531671 | 0.411422 | 0.025* | |
| C33 | −0.02075 (18) | 0.47215 (10) | 0.43157 (8) | 0.0205 (4) | |
| C34 | 0.03972 (19) | 0.42105 (11) | 0.44112 (10) | 0.0286 (5) | |
| H34 | 0.105805 | 0.417284 | 0.425668 | 0.034* | |
| C35 | 0.0037 (2) | 0.37566 (11) | 0.47314 (11) | 0.0333 (5) | |
| H35 | 0.045391 | 0.341042 | 0.479654 | 0.040* | |
| C36 | −0.0929 (2) | 0.38064 (11) | 0.49565 (9) | 0.0300 (5) | |
| H36 | −0.116953 | 0.349696 | 0.517853 | 0.036* | |
| C37 | −0.1542 (2) | 0.43094 (11) | 0.48568 (9) | 0.0261 (5) | |
| H37 | −0.220860 | 0.434232 | 0.500615 | 0.031* | |
| C38 | −0.11791 (19) | 0.47658 (10) | 0.45372 (8) | 0.0222 (4) | |
| H38 | −0.159971 | 0.511025 | 0.447036 | 0.027* | |
| C39 | 0.41754 (18) | 0.73962 (9) | 0.35341 (9) | 0.0197 (4) | |
| C40 | 0.56418 (19) | 0.75694 (12) | 0.30829 (10) | 0.0260 (5) | |
| H40 | 0.608404 | 0.770335 | 0.281377 | 0.031* | |
| C41 | 0.59329 (19) | 0.72658 (11) | 0.35306 (10) | 0.0251 (5) | |
| H41 | 0.662423 | 0.714382 | 0.364024 | 0.030* | |
| C42 | 0.38975 (17) | 0.79130 (10) | 0.26771 (8) | 0.0208 (4) | |
| C43 | 0.3758 (2) | 0.85258 (11) | 0.26738 (9) | 0.0254 (5) | |
| C44 | 0.3079 (2) | 0.87696 (11) | 0.22738 (10) | 0.0299 (5) | |
| H44 | 0.296964 | 0.918877 | 0.226316 | 0.036* | |
| C45 | 0.2562 (2) | 0.84111 (12) | 0.18926 (9) | 0.0302 (5) | |
| C46 | 0.2743 (2) | 0.77998 (12) | 0.19039 (9) | 0.0278 (5) | |
| H46 | 0.240336 | 0.755401 | 0.163841 | 0.033* | |
| C47 | 0.34106 (18) | 0.75395 (11) | 0.22945 (9) | 0.0232 (4) | |
| C48 | 0.4312 (3) | 0.89177 (12) | 0.30863 (11) | 0.0382 (6) | |
| H48A | 0.507127 | 0.889639 | 0.304648 | 0.057* | |
| H48B | 0.415216 | 0.878140 | 0.344499 | 0.057* | |
| H48C | 0.407498 | 0.933070 | 0.303623 | 0.057* | |
| C49 | 0.1799 (3) | 0.86776 (16) | 0.14784 (12) | 0.0445 (7) | |
| H49A | 0.178161 | 0.843244 | 0.115376 | 0.067* | |
| H49B | 0.202271 | 0.908328 | 0.139285 | 0.067* | |
| H49C | 0.109750 | 0.869045 | 0.161915 | 0.067* | |
| C50 | 0.3594 (2) | 0.68754 (11) | 0.23057 (11) | 0.0329 (5) | |
| H50A | 0.331122 | 0.670652 | 0.262933 | 0.049* | |
| H50B | 0.434881 | 0.679496 | 0.230600 | 0.049* | |
| H50C | 0.324084 | 0.669276 | 0.198900 | 0.049* | |
| C51 | 0.49911 (19) | 0.68416 (11) | 0.43059 (9) | 0.0263 (5) | |
| H51A | 0.566747 | 0.663624 | 0.438797 | 0.032* | 0.446 (13) |
| H51B | 0.443045 | 0.653621 | 0.427603 | 0.032* | 0.446 (13) |
| H51C | 0.568487 | 0.665685 | 0.439051 | 0.032* | 0.554 (13) |
| H51D | 0.447191 | 0.651509 | 0.425834 | 0.032* | 0.554 (13) |
| C52A | 0.477 (2) | 0.7281 (8) | 0.4758 (8) | 0.0306 (19) | 0.446 (13) |
| C53A | 0.4191 (10) | 0.7132 (6) | 0.5196 (5) | 0.043 (2) | 0.446 (13) |
| H53A | 0.385766 | 0.675254 | 0.519121 | 0.052* | 0.446 (13) |
| C54A | 0.4068 (10) | 0.7489 (7) | 0.5632 (4) | 0.048 (2) | 0.446 (13) |
| H54A | 0.364780 | 0.736519 | 0.591374 | 0.058* | 0.446 (13) |
| C55A | 0.4569 (10) | 0.8035 (5) | 0.5651 (4) | 0.049 (2) | 0.446 (13) |
| H55A | 0.451417 | 0.828630 | 0.595344 | 0.059* | 0.446 (13) |
| C56A | 0.5153 (10) | 0.8216 (5) | 0.5228 (4) | 0.046 (2) | 0.446 (13) |
| H56A | 0.550281 | 0.858996 | 0.524217 | 0.055* | 0.446 (13) |
| C57A | 0.5227 (16) | 0.7848 (6) | 0.4783 (5) | 0.034 (2) | 0.446 (13) |
| H57A | 0.559839 | 0.798675 | 0.448817 | 0.041* | 0.446 (13) |
| C52B | 0.4708 (18) | 0.7213 (6) | 0.4769 (6) | 0.0305 (16) | 0.554 (13) |
| C53B | 0.3990 (8) | 0.6978 (4) | 0.5104 (3) | 0.0369 (16) | 0.554 (13) |
| H53B | 0.366095 | 0.660689 | 0.502215 | 0.044* | 0.554 (13) |
| C54B | 0.3752 (8) | 0.7288 (4) | 0.5560 (3) | 0.0461 (17) | 0.554 (13) |
| H54B | 0.327336 | 0.712149 | 0.579657 | 0.055* | 0.554 (13) |
| C55B | 0.4197 (8) | 0.7834 (5) | 0.5675 (3) | 0.0460 (18) | 0.554 (13) |
| H55B | 0.404442 | 0.803825 | 0.599372 | 0.055* | 0.554 (13) |
| C56B | 0.4870 (8) | 0.8083 (4) | 0.5322 (4) | 0.0446 (18) | 0.554 (13) |
| H56B | 0.514633 | 0.847130 | 0.538734 | 0.054* | 0.554 (13) |
| C57B | 0.5144 (13) | 0.7767 (6) | 0.4874 (5) | 0.0384 (19) | 0.554 (13) |
| H57B | 0.562989 | 0.793219 | 0.463944 | 0.046* | 0.554 (13) |
| C58 | 0.10313 (17) | 0.75233 (10) | 0.38836 (9) | 0.0210 (4) | |
| C59 | −0.03804 (19) | 0.79686 (12) | 0.42260 (11) | 0.0294 (5) | |
| H59 | −0.079278 | 0.824304 | 0.441382 | 0.035* | |
| C60 | −0.07233 (19) | 0.74830 (12) | 0.39529 (11) | 0.0291 (5) | |
| H60 | −0.142830 | 0.734764 | 0.391062 | 0.035* | |
| C61 | 0.13917 (18) | 0.84400 (11) | 0.43993 (10) | 0.0236 (4) | |
| C62 | 0.1780 (2) | 0.88649 (11) | 0.40505 (10) | 0.0275 (5) | |
| C63 | 0.2491 (2) | 0.92812 (12) | 0.42635 (11) | 0.0336 (5) | |
| H63 | 0.276448 | 0.957406 | 0.403441 | 0.040* | |
| C64 | 0.2815 (2) | 0.92812 (13) | 0.48039 (12) | 0.0360 (6) | |
| C65 | 0.2385 (2) | 0.88693 (13) | 0.51405 (10) | 0.0354 (6) | |
| H65 | 0.257943 | 0.887981 | 0.551219 | 0.042* | |
| C66 | 0.1669 (2) | 0.84359 (12) | 0.49453 (10) | 0.0283 (5) | |
| C67 | 0.1434 (2) | 0.88746 (12) | 0.34665 (11) | 0.0364 (6) | |
| H67A | 0.165768 | 0.850568 | 0.329602 | 0.055* | |
| H67B | 0.175026 | 0.921770 | 0.329369 | 0.055* | |
| H67C | 0.066737 | 0.890663 | 0.342947 | 0.055* | |
| C68 | 0.3635 (3) | 0.97202 (18) | 0.50161 (16) | 0.0563 (9) | |
| H68A | 0.369911 | 0.969524 | 0.540751 | 0.084* | |
| H68B | 0.342517 | 1.012538 | 0.490904 | 0.084* | |
| H68C | 0.431151 | 0.962457 | 0.486940 | 0.084* | |
| C69 | 0.1239 (3) | 0.79815 (15) | 0.53190 (11) | 0.0406 (6) | |
| H69A | 0.047369 | 0.802099 | 0.531808 | 0.061* | |
| H69B | 0.154248 | 0.804813 | 0.568210 | 0.061* | |
| H69C | 0.141986 | 0.757970 | 0.520017 | 0.061* | |
| C70 | 0.00879 (18) | 0.66948 (10) | 0.33935 (9) | 0.0236 (4) | |
| H70A | −0.041346 | 0.640322 | 0.353202 | 0.028* | |
| H70B | 0.078601 | 0.650183 | 0.339519 | 0.028* | |
| C71 | −0.02584 (19) | 0.68623 (10) | 0.28266 (9) | 0.0235 (4) | |
| C72 | −0.1306 (2) | 0.67892 (12) | 0.26496 (11) | 0.0337 (5) | |
| H72 | −0.179734 | 0.663474 | 0.288622 | 0.040* | |
| C73 | −0.1635 (2) | 0.69413 (14) | 0.21289 (12) | 0.0392 (6) | |
| H73 | −0.235068 | 0.689455 | 0.201207 | 0.047* | |
| C74 | −0.0922 (3) | 0.71609 (13) | 0.17798 (11) | 0.0375 (6) | |
| H74 | −0.114459 | 0.725929 | 0.142237 | 0.045* | |
| C75 | 0.0119 (2) | 0.72364 (14) | 0.19547 (11) | 0.0377 (6) | |
| H75 | 0.060788 | 0.739088 | 0.171711 | 0.045* | |
| C76 | 0.0448 (2) | 0.70883 (12) | 0.24728 (11) | 0.0312 (5) | |
| H76 | 0.116333 | 0.714105 | 0.258851 | 0.037* | |
| Cl3 | 0.0888 (3) | 0.4284 (2) | −0.0300 (3) | 0.0605 (14) | 0.423 (16) |
| C77 | 0.0185 (11) | 0.4734 (6) | 0.0150 (5) | 0.079 (3) | 0.423 (16) |
| H77A | −0.041789 | 0.450984 | 0.028195 | 0.095* | 0.423 (16) |
| H77B | 0.065000 | 0.485270 | 0.046163 | 0.095* | 0.423 (16) |
| Cl3B | 0.0857 (6) | 0.4297 (3) | −0.0383 (4) | 0.123 (3) | 0.577 (16) |
| C77B | 0.0149 (8) | 0.4967 (4) | −0.0277 (4) | 0.075 (3) | 0.577 (16) |
| H77C | 0.058576 | 0.531196 | −0.037272 | 0.090* | 0.577 (16) |
| H77D | −0.049328 | 0.497044 | −0.051768 | 0.090* | 0.577 (16) |
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.01787 (9) | 0.02148 (9) | 0.01785 (8) | −0.00119 (5) | −0.00058 (6) | 0.00167 (5) |
| Ag2 | 0.01887 (9) | 0.02164 (9) | 0.01876 (8) | −0.00295 (5) | 0.00135 (6) | 0.00269 (5) |
| Cl1 | 0.0251 (3) | 0.0186 (2) | 0.0431 (3) | −0.00207 (19) | 0.0057 (2) | −0.0027 (2) |
| Cl2 | 0.0202 (2) | 0.0215 (2) | 0.0210 (2) | −0.00032 (17) | 0.00176 (17) | 0.00138 (18) |
| N1 | 0.0206 (9) | 0.0200 (9) | 0.0186 (8) | −0.0013 (7) | 0.0021 (7) | −0.0013 (7) |
| N2 | 0.0192 (8) | 0.0203 (8) | 0.0163 (8) | −0.0014 (7) | 0.0026 (6) | −0.0014 (6) |
| N3 | 0.0221 (9) | 0.0216 (9) | 0.0193 (9) | −0.0009 (7) | −0.0013 (7) | −0.0043 (7) |
| N4 | 0.0207 (9) | 0.0189 (9) | 0.0180 (8) | −0.0017 (7) | −0.0018 (7) | −0.0020 (6) |
| N5 | 0.0218 (9) | 0.0230 (9) | 0.0167 (8) | −0.0009 (7) | −0.0003 (7) | 0.0050 (7) |
| N6 | 0.0218 (9) | 0.0224 (9) | 0.0186 (8) | −0.0018 (7) | −0.0015 (7) | 0.0042 (7) |
| N7 | 0.0208 (9) | 0.0227 (9) | 0.0239 (9) | −0.0029 (7) | 0.0016 (7) | 0.0014 (7) |
| N8 | 0.0207 (9) | 0.0217 (9) | 0.0231 (9) | −0.0038 (7) | 0.0006 (7) | 0.0023 (7) |
| C1 | 0.0194 (10) | 0.0196 (9) | 0.0173 (9) | −0.0007 (8) | 0.0018 (7) | −0.0020 (8) |
| C2 | 0.0193 (10) | 0.0262 (11) | 0.0216 (10) | −0.0019 (8) | 0.0015 (8) | −0.0010 (8) |
| C3 | 0.0196 (10) | 0.0264 (11) | 0.0212 (10) | 0.0015 (8) | 0.0011 (8) | 0.0019 (8) |
| C4 | 0.0185 (9) | 0.0223 (10) | 0.0151 (9) | −0.0022 (8) | −0.0002 (7) | 0.0024 (8) |
| C5 | 0.0288 (11) | 0.0280 (12) | 0.0182 (10) | −0.0054 (9) | 0.0045 (8) | −0.0031 (8) |
| C6 | 0.0326 (12) | 0.0275 (11) | 0.0229 (11) | −0.0087 (10) | 0.0027 (9) | −0.0045 (9) |
| C7 | 0.0242 (11) | 0.0288 (12) | 0.0218 (10) | −0.0055 (9) | 0.0013 (8) | 0.0062 (9) |
| C8 | 0.0216 (10) | 0.0331 (12) | 0.0140 (9) | 0.0025 (9) | 0.0021 (8) | 0.0041 (8) |
| C9 | 0.0197 (10) | 0.0239 (10) | 0.0155 (9) | 0.0024 (8) | −0.0006 (7) | 0.0014 (8) |
| C10 | 0.0556 (17) | 0.0373 (14) | 0.0249 (12) | −0.0169 (13) | 0.0163 (12) | −0.0115 (10) |
| C11 | 0.0389 (14) | 0.0371 (14) | 0.0328 (13) | −0.0119 (11) | 0.0071 (11) | 0.0077 (11) |
| C12 | 0.0427 (14) | 0.0291 (12) | 0.0238 (11) | −0.0005 (11) | 0.0105 (10) | −0.0060 (9) |
| C13 | 0.0251 (10) | 0.0159 (9) | 0.0241 (10) | −0.0002 (8) | 0.0040 (8) | 0.0002 (8) |
| C14 | 0.0310 (11) | 0.0143 (9) | 0.0227 (10) | 0.0016 (8) | 0.0026 (9) | 0.0019 (8) |
| C15 | 0.0365 (13) | 0.0322 (13) | 0.0283 (12) | −0.0079 (10) | 0.0061 (10) | −0.0003 (10) |
| C16 | 0.0563 (18) | 0.0401 (15) | 0.0350 (14) | −0.0101 (13) | 0.0198 (13) | 0.0008 (11) |
| C17 | 0.079 (2) | 0.0317 (14) | 0.0220 (12) | 0.0025 (14) | 0.0092 (13) | 0.0018 (10) |
| C18 | 0.0587 (18) | 0.0338 (14) | 0.0253 (12) | 0.0068 (12) | −0.0084 (12) | −0.0040 (10) |
| C19 | 0.0348 (13) | 0.0272 (12) | 0.0299 (12) | 0.0045 (10) | −0.0032 (10) | −0.0007 (9) |
| C20 | 0.0221 (10) | 0.0179 (9) | 0.0186 (10) | −0.0012 (8) | −0.0008 (8) | −0.0007 (7) |
| C21 | 0.0219 (11) | 0.0250 (11) | 0.0234 (11) | 0.0014 (8) | −0.0019 (8) | −0.0046 (8) |
| C22 | 0.0249 (11) | 0.0279 (11) | 0.0239 (11) | 0.0025 (9) | −0.0061 (9) | −0.0059 (9) |
| C23 | 0.0255 (11) | 0.0236 (11) | 0.0185 (10) | −0.0013 (8) | −0.0008 (8) | −0.0066 (8) |
| C24 | 0.0364 (13) | 0.0247 (12) | 0.0255 (11) | −0.0018 (10) | 0.0028 (10) | −0.0032 (9) |
| C25 | 0.0439 (15) | 0.0361 (13) | 0.0255 (12) | −0.0027 (12) | 0.0087 (11) | −0.0026 (10) |
| C26 | 0.0382 (14) | 0.0398 (14) | 0.0281 (12) | 0.0025 (11) | 0.0053 (10) | −0.0125 (11) |
| C27 | 0.0399 (14) | 0.0252 (12) | 0.0355 (13) | 0.0042 (10) | 0.0030 (11) | −0.0112 (10) |
| C28 | 0.0325 (12) | 0.0234 (11) | 0.0274 (11) | 0.0000 (9) | 0.0010 (9) | −0.0035 (9) |
| C29 | 0.066 (2) | 0.0253 (13) | 0.0454 (16) | 0.0043 (13) | 0.0186 (14) | 0.0058 (11) |
| C30 | 0.059 (2) | 0.0523 (18) | 0.0375 (15) | 0.0052 (15) | 0.0176 (14) | −0.0151 (13) |
| C31 | 0.0599 (19) | 0.0229 (12) | 0.0432 (15) | 0.0011 (12) | 0.0132 (13) | 0.0017 (11) |
| C32 | 0.0252 (10) | 0.0219 (10) | 0.0159 (9) | −0.0031 (8) | 0.0002 (8) | −0.0030 (8) |
| C33 | 0.0249 (10) | 0.0194 (10) | 0.0167 (9) | −0.0018 (8) | −0.0035 (8) | −0.0031 (8) |
| C34 | 0.0254 (11) | 0.0271 (12) | 0.0332 (12) | 0.0028 (9) | 0.0001 (9) | 0.0001 (9) |
| C35 | 0.0389 (14) | 0.0229 (12) | 0.0373 (13) | 0.0044 (10) | −0.0066 (11) | 0.0042 (10) |
| C36 | 0.0450 (14) | 0.0243 (11) | 0.0201 (10) | −0.0066 (10) | −0.0043 (9) | 0.0030 (8) |
| C37 | 0.0315 (12) | 0.0289 (12) | 0.0180 (10) | −0.0058 (9) | 0.0012 (8) | −0.0027 (8) |
| C38 | 0.0277 (11) | 0.0220 (10) | 0.0164 (9) | 0.0007 (9) | −0.0026 (8) | −0.0024 (8) |
| C39 | 0.0233 (10) | 0.0179 (9) | 0.0177 (9) | −0.0026 (8) | 0.0000 (8) | 0.0027 (7) |
| C40 | 0.0223 (11) | 0.0337 (12) | 0.0225 (10) | 0.0006 (9) | 0.0043 (8) | 0.0063 (9) |
| C41 | 0.0204 (10) | 0.0299 (12) | 0.0250 (11) | 0.0014 (9) | 0.0012 (9) | 0.0042 (9) |
| C42 | 0.0218 (10) | 0.0237 (11) | 0.0170 (9) | 0.0027 (8) | 0.0027 (8) | 0.0063 (8) |
| C43 | 0.0325 (12) | 0.0228 (11) | 0.0214 (10) | 0.0008 (9) | 0.0069 (9) | 0.0044 (8) |
| C44 | 0.0409 (14) | 0.0239 (11) | 0.0257 (11) | 0.0110 (10) | 0.0103 (10) | 0.0107 (9) |
| C45 | 0.0311 (12) | 0.0389 (13) | 0.0211 (10) | 0.0111 (10) | 0.0066 (9) | 0.0138 (9) |
| C46 | 0.0289 (12) | 0.0366 (13) | 0.0178 (10) | 0.0018 (10) | 0.0010 (9) | 0.0039 (9) |
| C47 | 0.0237 (11) | 0.0263 (11) | 0.0197 (10) | 0.0005 (9) | 0.0027 (8) | 0.0028 (8) |
| C48 | 0.0562 (17) | 0.0247 (12) | 0.0334 (13) | −0.0009 (12) | −0.0009 (12) | −0.0019 (10) |
| C49 | 0.0427 (16) | 0.0572 (19) | 0.0333 (13) | 0.0170 (14) | 0.0003 (12) | 0.0190 (13) |
| C50 | 0.0403 (14) | 0.0250 (12) | 0.0326 (12) | 0.0009 (10) | −0.0048 (10) | −0.0004 (9) |
| C51 | 0.0278 (11) | 0.0291 (11) | 0.0215 (10) | −0.0017 (9) | −0.0034 (8) | 0.0104 (9) |
| C52A | 0.035 (4) | 0.040 (4) | 0.017 (3) | 0.011 (3) | 0.003 (3) | 0.013 (3) |
| C53A | 0.054 (4) | 0.045 (4) | 0.030 (3) | 0.009 (3) | 0.005 (3) | 0.011 (3) |
| C54A | 0.064 (4) | 0.051 (5) | 0.031 (3) | 0.003 (4) | 0.013 (3) | 0.009 (4) |
| C55A | 0.067 (4) | 0.048 (4) | 0.033 (3) | 0.011 (3) | 0.008 (3) | 0.004 (3) |
| C56A | 0.060 (4) | 0.047 (4) | 0.030 (4) | 0.007 (3) | 0.005 (3) | −0.001 (3) |
| C57A | 0.045 (4) | 0.037 (4) | 0.020 (4) | 0.005 (3) | −0.002 (3) | 0.002 (3) |
| C52B | 0.034 (3) | 0.035 (3) | 0.023 (3) | 0.009 (3) | −0.005 (2) | 0.012 (3) |
| C53B | 0.050 (3) | 0.039 (4) | 0.023 (3) | 0.008 (3) | 0.009 (2) | 0.012 (2) |
| C54B | 0.066 (4) | 0.042 (4) | 0.032 (3) | 0.011 (3) | 0.016 (3) | 0.009 (3) |
| C55B | 0.065 (4) | 0.048 (4) | 0.026 (2) | 0.011 (3) | 0.013 (3) | 0.003 (3) |
| C56B | 0.058 (4) | 0.049 (4) | 0.028 (3) | 0.010 (3) | 0.009 (3) | −0.001 (3) |
| C57B | 0.045 (3) | 0.045 (4) | 0.025 (4) | 0.006 (3) | 0.002 (3) | 0.003 (3) |
| C58 | 0.0210 (10) | 0.0219 (10) | 0.0199 (10) | −0.0034 (8) | −0.0002 (8) | 0.0052 (8) |
| C59 | 0.0227 (11) | 0.0292 (12) | 0.0367 (13) | −0.0021 (9) | 0.0056 (9) | −0.0040 (10) |
| C60 | 0.0191 (11) | 0.0318 (12) | 0.0364 (13) | −0.0036 (9) | 0.0018 (9) | −0.0033 (10) |
| C61 | 0.0207 (10) | 0.0233 (11) | 0.0269 (11) | −0.0033 (8) | 0.0008 (8) | −0.0006 (9) |
| C62 | 0.0298 (12) | 0.0219 (11) | 0.0311 (12) | −0.0008 (9) | 0.0045 (9) | 0.0023 (9) |
| C63 | 0.0377 (13) | 0.0260 (12) | 0.0377 (13) | −0.0094 (10) | 0.0077 (11) | 0.0004 (10) |
| C64 | 0.0344 (13) | 0.0350 (13) | 0.0386 (14) | −0.0116 (11) | 0.0009 (11) | −0.0089 (11) |
| C65 | 0.0385 (14) | 0.0420 (15) | 0.0255 (12) | −0.0070 (11) | −0.0012 (10) | −0.0051 (11) |
| C66 | 0.0304 (12) | 0.0298 (12) | 0.0248 (11) | −0.0041 (10) | 0.0024 (9) | −0.0003 (9) |
| C67 | 0.0476 (16) | 0.0310 (13) | 0.0304 (13) | −0.0071 (11) | −0.0003 (11) | 0.0086 (10) |
| C68 | 0.059 (2) | 0.055 (2) | 0.0547 (19) | −0.0323 (17) | 0.0014 (16) | −0.0141 (16) |
| C69 | 0.0488 (16) | 0.0467 (16) | 0.0263 (12) | −0.0120 (13) | 0.0010 (11) | 0.0054 (11) |
| C70 | 0.0249 (11) | 0.0182 (10) | 0.0274 (11) | −0.0038 (8) | −0.0019 (8) | 0.0008 (8) |
| C71 | 0.0277 (11) | 0.0162 (10) | 0.0265 (11) | −0.0009 (8) | −0.0009 (9) | −0.0006 (8) |
| C72 | 0.0336 (13) | 0.0342 (13) | 0.0326 (13) | −0.0116 (10) | −0.0044 (10) | 0.0083 (10) |
| C73 | 0.0380 (14) | 0.0444 (15) | 0.0339 (13) | −0.0128 (12) | −0.0113 (11) | 0.0071 (12) |
| C74 | 0.0492 (16) | 0.0386 (14) | 0.0241 (11) | −0.0001 (12) | −0.0034 (11) | 0.0009 (10) |
| C75 | 0.0403 (15) | 0.0445 (15) | 0.0291 (13) | 0.0027 (12) | 0.0108 (11) | 0.0019 (11) |
| C76 | 0.0282 (12) | 0.0347 (13) | 0.0310 (12) | 0.0021 (10) | 0.0042 (10) | −0.0018 (10) |
| Cl3 | 0.0393 (18) | 0.0433 (19) | 0.099 (3) | −0.0002 (12) | 0.0088 (14) | 0.0211 (18) |
| C77 | 0.081 (6) | 0.067 (6) | 0.089 (6) | 0.011 (5) | 0.006 (5) | 0.011 (5) |
| Cl3B | 0.136 (5) | 0.096 (4) | 0.137 (4) | −0.012 (3) | 0.015 (3) | −0.016 (3) |
| C77B | 0.070 (5) | 0.066 (5) | 0.089 (5) | 0.010 (4) | 0.001 (4) | 0.028 (4) |
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Geometric parameters (Å, º)
| Ag1—Cl1 | 2.8755 (6) | C36—C37 | 1.388 (4) |
| Ag1—C1 | 2.099 (2) | C37—H37 | 0.9500 |
| Ag1—C20 | 2.098 (2) | C37—C38 | 1.393 (3) |
| Ag2—Cl1 | 2.8149 (6) | C38—H38 | 0.9500 |
| Ag2—C39 | 2.098 (2) | C40—H40 | 0.9500 |
| Ag2—C58 | 2.104 (2) | C40—C41 | 1.346 (3) |
| N1—C1 | 1.355 (3) | C41—H41 | 0.9500 |
| N1—C2 | 1.386 (3) | C42—C43 | 1.385 (3) |
| N1—C13 | 1.469 (3) | C42—C47 | 1.393 (3) |
| N2—C1 | 1.351 (3) | C43—C44 | 1.400 (4) |
| N2—C3 | 1.385 (3) | C43—C48 | 1.503 (4) |
| N2—C4 | 1.442 (3) | C44—H44 | 0.9500 |
| N3—C20 | 1.356 (3) | C44—C45 | 1.387 (4) |
| N3—C22 | 1.392 (3) | C45—C46 | 1.390 (4) |
| N3—C23 | 1.437 (3) | C45—C49 | 1.507 (3) |
| N4—C20 | 1.352 (3) | C46—H46 | 0.9500 |
| N4—C21 | 1.382 (3) | C46—C47 | 1.390 (3) |
| N4—C32 | 1.474 (3) | C47—C50 | 1.507 (3) |
| N5—C39 | 1.353 (3) | C48—H48A | 0.9800 |
| N5—C40 | 1.389 (3) | C48—H48B | 0.9800 |
| N5—C42 | 1.440 (3) | C48—H48C | 0.9800 |
| N6—C39 | 1.353 (3) | C49—H49A | 0.9800 |
| N6—C41 | 1.379 (3) | C49—H49B | 0.9800 |
| N6—C51 | 1.465 (3) | C49—H49C | 0.9800 |
| N7—C58 | 1.356 (3) | C50—H50A | 0.9800 |
| N7—C59 | 1.388 (3) | C50—H50B | 0.9800 |
| N7—C61 | 1.439 (3) | C50—H50C | 0.9800 |
| N8—C58 | 1.349 (3) | C51—H51A | 0.9900 |
| N8—C60 | 1.382 (3) | C51—H51B | 0.9900 |
| N8—C70 | 1.468 (3) | C51—H51C | 0.9900 |
| C2—H2 | 0.9500 | C51—H51D | 0.9900 |
| C2—C3 | 1.348 (3) | C51—C52A | 1.538 (10) |
| C3—H3 | 0.9500 | C51—C52B | 1.487 (8) |
| C4—C5 | 1.392 (3) | C52A—C53A | 1.394 (11) |
| C4—C9 | 1.391 (3) | C52A—C57A | 1.399 (11) |
| C5—C6 | 1.390 (3) | C53A—H53A | 0.9500 |
| C5—C10 | 1.505 (3) | C53A—C54A | 1.371 (12) |
| C6—H6 | 0.9500 | C54A—H54A | 0.9500 |
| C6—C7 | 1.388 (3) | C54A—C55A | 1.379 (12) |
| C7—C8 | 1.391 (4) | C55A—H55A | 0.9500 |
| C7—C11 | 1.513 (3) | C55A—C56A | 1.387 (11) |
| C8—H8 | 0.9500 | C56A—H56A | 0.9500 |
| C8—C9 | 1.396 (3) | C56A—C57A | 1.392 (11) |
| C9—C12 | 1.501 (3) | C57A—H57A | 0.9500 |
| C10—H10A | 0.9800 | C52B—C53B | 1.378 (10) |
| C10—H10B | 0.9800 | C52B—C57B | 1.382 (10) |
| C10—H10C | 0.9800 | C53B—H53B | 0.9500 |
| C11—H11A | 0.9800 | C53B—C54B | 1.383 (9) |
| C11—H11B | 0.9800 | C54B—H54B | 0.9500 |
| C11—H11C | 0.9800 | C54B—C55B | 1.372 (10) |
| C12—H12A | 0.9800 | C55B—H55B | 0.9500 |
| C12—H12B | 0.9800 | C55B—C56B | 1.381 (10) |
| C12—H12C | 0.9800 | C56B—H56B | 0.9500 |
| C13—H13A | 0.9900 | C56B—C57B | 1.387 (9) |
| C13—H13B | 0.9900 | C57B—H57B | 0.9500 |
| C13—C14 | 1.513 (3) | C59—H59 | 0.9500 |
| C14—C15 | 1.387 (4) | C59—C60 | 1.346 (4) |
| C14—C19 | 1.390 (4) | C60—H60 | 0.9500 |
| C15—H15 | 0.9500 | C61—C62 | 1.400 (3) |
| C15—C16 | 1.395 (4) | C61—C66 | 1.393 (3) |
| C16—H16 | 0.9500 | C62—C63 | 1.388 (4) |
| C16—C17 | 1.386 (5) | C62—C67 | 1.504 (4) |
| C17—H17 | 0.9500 | C63—H63 | 0.9500 |
| C17—C18 | 1.381 (5) | C63—C64 | 1.393 (4) |
| C18—H18 | 0.9500 | C64—C65 | 1.382 (4) |
| C18—C19 | 1.394 (4) | C64—C68 | 1.512 (4) |
| C19—H19 | 0.9500 | C65—H65 | 0.9500 |
| C21—H21 | 0.9500 | C65—C66 | 1.404 (4) |
| C21—C22 | 1.353 (3) | C66—C69 | 1.506 (4) |
| C22—H22 | 0.9500 | C67—H67A | 0.9800 |
| C23—C24 | 1.391 (4) | C67—H67B | 0.9800 |
| C23—C28 | 1.393 (3) | C67—H67C | 0.9800 |
| C24—C25 | 1.398 (4) | C68—H68A | 0.9800 |
| C24—C29 | 1.512 (4) | C68—H68B | 0.9800 |
| C25—H25 | 0.9500 | C68—H68C | 0.9800 |
| C25—C26 | 1.392 (4) | C69—H69A | 0.9800 |
| C26—C27 | 1.376 (4) | C69—H69B | 0.9800 |
| C26—C30 | 1.517 (4) | C69—H69C | 0.9800 |
| C27—H27 | 0.9500 | C70—H70A | 0.9900 |
| C27—C28 | 1.398 (4) | C70—H70B | 0.9900 |
| C28—C31 | 1.500 (4) | C70—C71 | 1.511 (3) |
| C29—H29A | 0.9800 | C71—C72 | 1.396 (4) |
| C29—H29B | 0.9800 | C71—C76 | 1.392 (4) |
| C29—H29C | 0.9800 | C72—H72 | 0.9500 |
| C30—H30A | 0.9800 | C72—C73 | 1.390 (4) |
| C30—H30B | 0.9800 | C73—H73 | 0.9500 |
| C30—H30C | 0.9800 | C73—C74 | 1.385 (4) |
| C31—H31A | 0.9800 | C74—H74 | 0.9500 |
| C31—H31B | 0.9800 | C74—C75 | 1.386 (5) |
| C31—H31C | 0.9800 | C75—H75 | 0.9500 |
| C32—H32A | 0.9900 | C75—C76 | 1.382 (4) |
| C32—H32B | 0.9900 | C76—H76 | 0.9500 |
| C32—C33 | 1.513 (3) | Cl3—C77 | 1.790 (14) |
| C33—C34 | 1.395 (3) | C77—C77i | 1.47 (3) |
| C33—C38 | 1.388 (3) | C77—H77A | 0.9900 |
| C34—H34 | 0.9500 | C77—H77B | 0.9900 |
| C34—C35 | 1.388 (4) | Cl3B—C77B | 1.780 (11) |
| C35—H35 | 0.9500 | C77B—C77Bi | 1.47 (2) |
| C35—C36 | 1.386 (4) | C77B—H77C | 0.9900 |
| C36—H36 | 0.9500 | C77B—H77D | 0.9900 |
| C1—Ag1—Cl1 | 93.30 (6) | N5—C40—H40 | 127.0 |
| C20—Ag1—Cl1 | 96.14 (6) | C41—C40—N5 | 106.0 (2) |
| C20—Ag1—C1 | 170.55 (8) | C41—C40—H40 | 127.0 |
| C39—Ag2—Cl1 | 94.92 (6) | N6—C41—H41 | 126.6 |
| C39—Ag2—C58 | 163.97 (8) | C40—C41—N6 | 106.8 (2) |
| C58—Ag2—Cl1 | 101.09 (6) | C40—C41—H41 | 126.6 |
| Ag2—Cl1—Ag1 | 148.90 (2) | C43—C42—N5 | 119.1 (2) |
| C1—N1—C2 | 111.69 (19) | C43—C42—C47 | 122.6 (2) |
| C1—N1—C13 | 124.68 (19) | C47—C42—N5 | 118.3 (2) |
| C2—N1—C13 | 123.62 (19) | C42—C43—C44 | 117.8 (2) |
| C1—N2—C3 | 111.56 (18) | C42—C43—C48 | 121.3 (2) |
| C1—N2—C4 | 123.70 (18) | C44—C43—C48 | 120.9 (2) |
| C3—N2—C4 | 124.53 (19) | C43—C44—H44 | 119.4 |
| C20—N3—C22 | 111.36 (19) | C45—C44—C43 | 121.3 (2) |
| C20—N3—C23 | 123.3 (2) | C45—C44—H44 | 119.4 |
| C22—N3—C23 | 125.28 (19) | C44—C45—C46 | 119.0 (2) |
| C20—N4—C21 | 112.12 (19) | C44—C45—C49 | 120.6 (3) |
| C20—N4—C32 | 124.30 (19) | C46—C45—C49 | 120.4 (3) |
| C21—N4—C32 | 123.54 (19) | C45—C46—H46 | 119.2 |
| C39—N5—C40 | 111.75 (19) | C45—C46—C47 | 121.6 (2) |
| C39—N5—C42 | 122.33 (19) | C47—C46—H46 | 119.2 |
| C40—N5—C42 | 125.72 (19) | C42—C47—C50 | 121.2 (2) |
| C39—N6—C41 | 111.82 (19) | C46—C47—C42 | 117.7 (2) |
| C39—N6—C51 | 123.2 (2) | C46—C47—C50 | 121.1 (2) |
| C41—N6—C51 | 125.0 (2) | C43—C48—H48A | 109.5 |
| C58—N7—C59 | 111.4 (2) | C43—C48—H48B | 109.5 |
| C58—N7—C61 | 123.06 (19) | C43—C48—H48C | 109.5 |
| C59—N7—C61 | 125.5 (2) | H48A—C48—H48B | 109.5 |
| C58—N8—C60 | 111.4 (2) | H48A—C48—H48C | 109.5 |
| C58—N8—C70 | 125.0 (2) | H48B—C48—H48C | 109.5 |
| C60—N8—C70 | 123.5 (2) | C45—C49—H49A | 109.5 |
| N1—C1—Ag1 | 129.90 (16) | C45—C49—H49B | 109.5 |
| N2—C1—Ag1 | 125.80 (16) | C45—C49—H49C | 109.5 |
| N2—C1—N1 | 103.89 (18) | H49A—C49—H49B | 109.5 |
| N1—C2—H2 | 126.9 | H49A—C49—H49C | 109.5 |
| C3—C2—N1 | 106.2 (2) | H49B—C49—H49C | 109.5 |
| C3—C2—H2 | 126.9 | C47—C50—H50A | 109.5 |
| N2—C3—H3 | 126.7 | C47—C50—H50B | 109.5 |
| C2—C3—N2 | 106.7 (2) | C47—C50—H50C | 109.5 |
| C2—C3—H3 | 126.7 | H50A—C50—H50B | 109.5 |
| C5—C4—N2 | 119.04 (19) | H50A—C50—H50C | 109.5 |
| C9—C4—N2 | 118.7 (2) | H50B—C50—H50C | 109.5 |
| C9—C4—C5 | 122.2 (2) | N6—C51—H51A | 109.8 |
| C4—C5—C10 | 121.1 (2) | N6—C51—H51B | 109.8 |
| C6—C5—C4 | 118.0 (2) | N6—C51—H51C | 108.6 |
| C6—C5—C10 | 120.8 (2) | N6—C51—H51D | 108.6 |
| C5—C6—H6 | 119.2 | N6—C51—C52A | 109.6 (9) |
| C7—C6—C5 | 121.5 (2) | N6—C51—C52B | 114.7 (8) |
| C7—C6—H6 | 119.2 | H51A—C51—H51B | 108.2 |
| C6—C7—C8 | 119.0 (2) | H51C—C51—H51D | 107.6 |
| C6—C7—C11 | 120.3 (2) | C52A—C51—H51A | 109.8 |
| C8—C7—C11 | 120.7 (2) | C52A—C51—H51B | 109.8 |
| C7—C8—H8 | 119.4 | C52B—C51—H51C | 108.6 |
| C7—C8—C9 | 121.2 (2) | C52B—C51—H51D | 108.6 |
| C9—C8—H8 | 119.4 | C53A—C52A—C51 | 123.3 (10) |
| C4—C9—C8 | 118.0 (2) | C53A—C52A—C57A | 114.9 (9) |
| C4—C9—C12 | 121.2 (2) | C57A—C52A—C51 | 121.5 (10) |
| C8—C9—C12 | 120.8 (2) | C52A—C53A—H53A | 117.5 |
| C5—C10—H10A | 109.5 | C54A—C53A—C52A | 125.0 (10) |
| C5—C10—H10B | 109.5 | C54A—C53A—H53A | 117.5 |
| C5—C10—H10C | 109.5 | C53A—C54A—H54A | 120.9 |
| H10A—C10—H10B | 109.5 | C53A—C54A—C55A | 118.2 (9) |
| H10A—C10—H10C | 109.5 | C55A—C54A—H54A | 120.9 |
| H10B—C10—H10C | 109.5 | C54A—C55A—H55A | 120.0 |
| C7—C11—H11A | 109.5 | C54A—C55A—C56A | 120.0 (8) |
| C7—C11—H11B | 109.5 | C56A—C55A—H55A | 120.0 |
| C7—C11—H11C | 109.5 | C55A—C56A—H56A | 120.0 |
| H11A—C11—H11B | 109.5 | C55A—C56A—C57A | 119.9 (9) |
| H11A—C11—H11C | 109.5 | C57A—C56A—H56A | 120.0 |
| H11B—C11—H11C | 109.5 | C52A—C57A—H57A | 119.1 |
| C9—C12—H12A | 109.5 | C56A—C57A—C52A | 121.8 (10) |
| C9—C12—H12B | 109.5 | C56A—C57A—H57A | 119.1 |
| C9—C12—H12C | 109.5 | C53B—C52B—C51 | 117.2 (8) |
| H12A—C12—H12B | 109.5 | C53B—C52B—C57B | 120.1 (7) |
| H12A—C12—H12C | 109.5 | C57B—C52B—C51 | 122.6 (9) |
| H12B—C12—H12C | 109.5 | C52B—C53B—H53B | 120.3 |
| N1—C13—H13A | 108.9 | C52B—C53B—C54B | 119.5 (7) |
| N1—C13—H13B | 108.9 | C54B—C53B—H53B | 120.3 |
| N1—C13—C14 | 113.15 (18) | C53B—C54B—H54B | 119.6 |
| H13A—C13—H13B | 107.8 | C55B—C54B—C53B | 120.9 (7) |
| C14—C13—H13A | 108.9 | C55B—C54B—H54B | 119.6 |
| C14—C13—H13B | 108.9 | C54B—C55B—H55B | 120.3 |
| C15—C14—C13 | 120.2 (2) | C54B—C55B—C56B | 119.4 (6) |
| C15—C14—C19 | 119.1 (2) | C56B—C55B—H55B | 120.3 |
| C19—C14—C13 | 120.7 (2) | C55B—C56B—H56B | 119.9 |
| C14—C15—H15 | 119.6 | C55B—C56B—C57B | 120.2 (8) |
| C14—C15—C16 | 120.7 (3) | C57B—C56B—H56B | 119.9 |
| C16—C15—H15 | 119.6 | C52B—C57B—C56B | 119.7 (9) |
| C15—C16—H16 | 120.2 | C52B—C57B—H57B | 120.1 |
| C17—C16—C15 | 119.6 (3) | C56B—C57B—H57B | 120.1 |
| C17—C16—H16 | 120.2 | N7—C58—Ag2 | 125.92 (16) |
| C16—C17—H17 | 119.9 | N8—C58—Ag2 | 129.91 (18) |
| C18—C17—C16 | 120.2 (3) | N8—C58—N7 | 104.11 (19) |
| C18—C17—H17 | 119.9 | N7—C59—H59 | 127.0 |
| C17—C18—H18 | 120.0 | C60—C59—N7 | 106.1 (2) |
| C17—C18—C19 | 120.1 (3) | C60—C59—H59 | 127.0 |
| C19—C18—H18 | 120.0 | N8—C60—H60 | 126.5 |
| C14—C19—C18 | 120.3 (3) | C59—C60—N8 | 106.9 (2) |
| C14—C19—H19 | 119.8 | C59—C60—H60 | 126.5 |
| C18—C19—H19 | 119.8 | C62—C61—N7 | 118.1 (2) |
| N3—C20—Ag1 | 126.71 (17) | C66—C61—N7 | 119.5 (2) |
| N4—C20—Ag1 | 128.83 (16) | C66—C61—C62 | 122.4 (2) |
| N4—C20—N3 | 103.88 (19) | C61—C62—C67 | 121.3 (2) |
| N4—C21—H21 | 126.9 | C63—C62—C61 | 117.6 (2) |
| C22—C21—N4 | 106.2 (2) | C63—C62—C67 | 121.0 (2) |
| C22—C21—H21 | 126.9 | C62—C63—H63 | 119.1 |
| N3—C22—H22 | 126.8 | C62—C63—C64 | 121.8 (2) |
| C21—C22—N3 | 106.4 (2) | C64—C63—H63 | 119.1 |
| C21—C22—H22 | 126.8 | C63—C64—C68 | 120.3 (3) |
| C24—C23—N3 | 119.4 (2) | C65—C64—C63 | 119.0 (2) |
| C24—C23—C28 | 122.3 (2) | C65—C64—C68 | 120.7 (3) |
| C28—C23—N3 | 118.2 (2) | C64—C65—H65 | 119.3 |
| C23—C24—C25 | 117.6 (2) | C64—C65—C66 | 121.5 (2) |
| C23—C24—C29 | 121.5 (2) | C66—C65—H65 | 119.3 |
| C25—C24—C29 | 120.9 (3) | C61—C66—C65 | 117.6 (2) |
| C24—C25—H25 | 119.1 | C61—C66—C69 | 122.1 (2) |
| C26—C25—C24 | 121.7 (3) | C65—C66—C69 | 120.3 (2) |
| C26—C25—H25 | 119.1 | C62—C67—H67A | 109.5 |
| C25—C26—C30 | 121.1 (3) | C62—C67—H67B | 109.5 |
| C27—C26—C25 | 118.8 (2) | C62—C67—H67C | 109.5 |
| C27—C26—C30 | 120.2 (3) | H67A—C67—H67B | 109.5 |
| C26—C27—H27 | 119.1 | H67A—C67—H67C | 109.5 |
| C26—C27—C28 | 121.8 (2) | H67B—C67—H67C | 109.5 |
| C28—C27—H27 | 119.1 | C64—C68—H68A | 109.5 |
| C23—C28—C27 | 117.8 (2) | C64—C68—H68B | 109.5 |
| C23—C28—C31 | 122.1 (2) | C64—C68—H68C | 109.5 |
| C27—C28—C31 | 120.1 (2) | H68A—C68—H68B | 109.5 |
| C24—C29—H29A | 109.5 | H68A—C68—H68C | 109.5 |
| C24—C29—H29B | 109.5 | H68B—C68—H68C | 109.5 |
| C24—C29—H29C | 109.5 | C66—C69—H69A | 109.5 |
| H29A—C29—H29B | 109.5 | C66—C69—H69B | 109.5 |
| H29A—C29—H29C | 109.5 | C66—C69—H69C | 109.5 |
| H29B—C29—H29C | 109.5 | H69A—C69—H69B | 109.5 |
| C26—C30—H30A | 109.5 | H69A—C69—H69C | 109.5 |
| C26—C30—H30B | 109.5 | H69B—C69—H69C | 109.5 |
| C26—C30—H30C | 109.5 | N8—C70—H70A | 109.3 |
| H30A—C30—H30B | 109.5 | N8—C70—H70B | 109.3 |
| H30A—C30—H30C | 109.5 | N8—C70—C71 | 111.58 (18) |
| H30B—C30—H30C | 109.5 | H70A—C70—H70B | 108.0 |
| C28—C31—H31A | 109.5 | C71—C70—H70A | 109.3 |
| C28—C31—H31B | 109.5 | C71—C70—H70B | 109.3 |
| C28—C31—H31C | 109.5 | C72—C71—C70 | 119.7 (2) |
| H31A—C31—H31B | 109.5 | C76—C71—C70 | 121.4 (2) |
| H31A—C31—H31C | 109.5 | C76—C71—C72 | 118.9 (2) |
| H31B—C31—H31C | 109.5 | C71—C72—H72 | 119.8 |
| N4—C32—H32A | 109.0 | C73—C72—C71 | 120.3 (3) |
| N4—C32—H32B | 109.0 | C73—C72—H72 | 119.8 |
| N4—C32—C33 | 112.72 (18) | C72—C73—H73 | 119.9 |
| H32A—C32—H32B | 107.8 | C74—C73—C72 | 120.2 (3) |
| C33—C32—H32A | 109.0 | C74—C73—H73 | 119.9 |
| C33—C32—H32B | 109.0 | C73—C74—H74 | 120.2 |
| C34—C33—C32 | 121.0 (2) | C73—C74—C75 | 119.7 (3) |
| C38—C33—C32 | 119.8 (2) | C75—C74—H74 | 120.2 |
| C38—C33—C34 | 119.2 (2) | C74—C75—H75 | 119.8 |
| C33—C34—H34 | 119.9 | C76—C75—C74 | 120.4 (3) |
| C35—C34—C33 | 120.2 (2) | C76—C75—H75 | 119.8 |
| C35—C34—H34 | 119.9 | C71—C76—H76 | 119.7 |
| C34—C35—H35 | 119.9 | C75—C76—C71 | 120.6 (3) |
| C36—C35—C34 | 120.3 (2) | C75—C76—H76 | 119.7 |
| C36—C35—H35 | 119.9 | Cl3—C77—H77A | 110.3 |
| C35—C36—H36 | 120.1 | Cl3—C77—H77B | 110.3 |
| C35—C36—C37 | 119.8 (2) | C77i—C77—Cl3 | 107.0 (12) |
| C37—C36—H36 | 120.1 | C77i—C77—H77A | 110.3 |
| C36—C37—H37 | 120.1 | C77i—C77—H77B | 110.3 |
| C36—C37—C38 | 119.9 (2) | H77A—C77—H77B | 108.6 |
| C38—C37—H37 | 120.1 | Cl3B—C77B—H77C | 109.1 |
| C33—C38—C37 | 120.5 (2) | Cl3B—C77B—H77D | 109.1 |
| C33—C38—H38 | 119.7 | C77Bi—C77B—Cl3B | 112.6 (9) |
| C37—C38—H38 | 119.7 | C77Bi—C77B—H77C | 109.1 |
| N5—C39—Ag2 | 127.91 (16) | C77Bi—C77B—H77D | 109.1 |
| N6—C39—Ag2 | 128.31 (16) | H77C—C77B—H77D | 107.8 |
| N6—C39—N5 | 103.66 (19) | ||
| N1—C2—C3—N2 | 0.2 (2) | C32—N4—C20—N3 | 177.14 (19) |
| N1—C13—C14—C15 | −99.3 (3) | C32—N4—C21—C22 | −177.4 (2) |
| N1—C13—C14—C19 | 82.5 (3) | C32—C33—C34—C35 | 176.4 (2) |
| N2—C4—C5—C6 | 177.6 (2) | C32—C33—C38—C37 | −176.72 (19) |
| N2—C4—C5—C10 | −1.2 (4) | C33—C34—C35—C36 | 0.3 (4) |
| N2—C4—C9—C8 | −178.58 (19) | C34—C33—C38—C37 | 0.8 (3) |
| N2—C4—C9—C12 | 0.1 (3) | C34—C35—C36—C37 | 0.7 (4) |
| N3—C23—C24—C25 | 179.8 (2) | C35—C36—C37—C38 | −0.9 (4) |
| N3—C23—C24—C29 | 0.7 (4) | C36—C37—C38—C33 | 0.2 (3) |
| N3—C23—C28—C27 | −178.5 (2) | C38—C33—C34—C35 | −1.0 (4) |
| N3—C23—C28—C31 | 1.1 (4) | C39—N5—C40—C41 | −0.5 (3) |
| N4—C21—C22—N3 | −0.1 (3) | C39—N5—C42—C43 | 98.1 (3) |
| N4—C32—C33—C34 | 76.8 (3) | C39—N5—C42—C47 | −81.3 (3) |
| N4—C32—C33—C38 | −105.8 (2) | C39—N6—C41—C40 | 0.2 (3) |
| N5—C40—C41—N6 | 0.2 (3) | C39—N6—C51—C52A | −72.1 (12) |
| N5—C42—C43—C44 | −177.9 (2) | C39—N6—C51—C52B | −68.1 (10) |
| N5—C42—C43—C48 | 1.9 (3) | C40—N5—C39—Ag2 | −175.70 (17) |
| N5—C42—C47—C46 | 178.0 (2) | C40—N5—C39—N6 | 0.6 (3) |
| N5—C42—C47—C50 | −1.6 (3) | C40—N5—C42—C43 | −87.4 (3) |
| N6—C51—C52A—C53A | 147 (2) | C40—N5—C42—C47 | 93.2 (3) |
| N6—C51—C52A—C57A | −39 (3) | C41—N6—C39—Ag2 | 175.78 (17) |
| N6—C51—C52B—C53B | 135.9 (14) | C41—N6—C39—N5 | −0.5 (3) |
| N6—C51—C52B—C57B | −45 (2) | C41—N6—C51—C52A | 109.2 (12) |
| N7—C59—C60—N8 | 0.0 (3) | C41—N6—C51—C52B | 113.2 (10) |
| N7—C61—C62—C63 | −176.8 (2) | C42—N5—C39—Ag2 | −0.5 (3) |
| N7—C61—C62—C67 | 3.8 (4) | C42—N5—C39—N6 | 175.8 (2) |
| N7—C61—C66—C65 | 177.2 (2) | C42—N5—C40—C41 | −175.5 (2) |
| N7—C61—C66—C69 | −1.9 (4) | C42—C43—C44—C45 | 0.0 (4) |
| N8—C70—C71—C72 | −98.4 (3) | C43—C42—C47—C46 | −1.4 (3) |
| N8—C70—C71—C76 | 81.7 (3) | C43—C42—C47—C50 | 179.1 (2) |
| C1—N1—C2—C3 | 0.0 (3) | C43—C44—C45—C46 | −1.4 (4) |
| C1—N1—C13—C14 | −94.7 (3) | C43—C44—C45—C49 | 177.5 (2) |
| C1—N2—C3—C2 | −0.4 (3) | C44—C45—C46—C47 | 1.5 (4) |
| C1—N2—C4—C5 | 96.2 (3) | C45—C46—C47—C42 | −0.1 (4) |
| C1—N2—C4—C9 | −85.1 (3) | C45—C46—C47—C50 | 179.4 (2) |
| C2—N1—C1—Ag1 | 172.57 (16) | C47—C42—C43—C44 | 1.5 (3) |
| C2—N1—C1—N2 | −0.3 (2) | C47—C42—C43—C48 | −178.7 (2) |
| C2—N1—C13—C14 | 86.6 (3) | C48—C43—C44—C45 | −179.8 (2) |
| C3—N2—C1—Ag1 | −172.80 (15) | C49—C45—C46—C47 | −177.4 (2) |
| C3—N2—C1—N1 | 0.4 (2) | C51—N6—C39—Ag2 | −3.1 (3) |
| C3—N2—C4—C5 | −89.5 (3) | C51—N6—C39—N5 | −179.4 (2) |
| C3—N2—C4—C9 | 89.2 (3) | C51—N6—C41—C40 | 179.1 (2) |
| C4—N2—C1—Ag1 | 2.1 (3) | C51—C52A—C53A—C54A | 173.0 (18) |
| C4—N2—C1—N1 | 175.35 (18) | C51—C52A—C57A—C56A | −171 (2) |
| C4—N2—C3—C2 | −175.3 (2) | C51—C52B—C53B—C54B | 175.8 (13) |
| C4—C5—C6—C7 | 0.9 (4) | C51—C52B—C57B—C56B | −178.0 (17) |
| C5—C4—C9—C8 | 0.1 (3) | C52A—C53A—C54A—C55A | −2 (2) |
| C5—C4—C9—C12 | 178.8 (2) | C53A—C52A—C57A—C56A | 3 (3) |
| C5—C6—C7—C8 | 0.3 (4) | C53A—C54A—C55A—C56A | 1.9 (16) |
| C5—C6—C7—C11 | −178.5 (2) | C54A—C55A—C56A—C57A | 0.5 (17) |
| C6—C7—C8—C9 | −1.3 (4) | C55A—C56A—C57A—C52A | −3 (2) |
| C7—C8—C9—C4 | 1.1 (3) | C57A—C52A—C53A—C54A | −1 (3) |
| C7—C8—C9—C12 | −177.6 (2) | C52B—C53B—C54B—C55B | 2.0 (16) |
| C9—C4—C5—C6 | −1.0 (4) | C53B—C52B—C57B—C56B | 1 (3) |
| C9—C4—C5—C10 | −179.8 (2) | C53B—C54B—C55B—C56B | 1.8 (12) |
| C10—C5—C6—C7 | 179.6 (3) | C54B—C55B—C56B—C57B | −4.1 (13) |
| C11—C7—C8—C9 | 177.4 (2) | C55B—C56B—C57B—C52B | 2.6 (19) |
| C13—N1—C1—Ag1 | −6.3 (3) | C57B—C52B—C53B—C54B | −3 (2) |
| C13—N1—C1—N2 | −179.10 (18) | C58—N7—C59—C60 | −0.3 (3) |
| C13—N1—C2—C3 | 178.86 (19) | C58—N7—C61—C62 | 73.9 (3) |
| C13—C14—C15—C16 | −177.0 (3) | C58—N7—C61—C66 | −105.0 (3) |
| C13—C14—C19—C18 | 176.4 (2) | C58—N8—C60—C59 | 0.4 (3) |
| C14—C15—C16—C17 | 0.4 (5) | C58—N8—C70—C71 | −97.7 (3) |
| C15—C14—C19—C18 | −1.8 (4) | C59—N7—C58—Ag2 | −176.80 (17) |
| C15—C16—C17—C18 | −1.5 (5) | C59—N7—C58—N8 | 0.5 (3) |
| C16—C17—C18—C19 | 0.9 (4) | C59—N7—C61—C62 | −105.1 (3) |
| C17—C18—C19—C14 | 0.8 (4) | C59—N7—C61—C66 | 76.1 (3) |
| C19—C14—C15—C16 | 1.2 (4) | C60—N8—C58—Ag2 | 176.64 (17) |
| C20—N3—C22—C21 | −0.4 (3) | C60—N8—C58—N7 | −0.5 (3) |
| C20—N3—C23—C24 | −105.6 (3) | C60—N8—C70—C71 | 78.2 (3) |
| C20—N3—C23—C28 | 74.5 (3) | C61—N7—C58—Ag2 | 4.1 (3) |
| C20—N4—C21—C22 | 0.6 (3) | C61—N7—C58—N8 | −178.6 (2) |
| C20—N4—C32—C33 | −99.9 (2) | C61—N7—C59—C60 | 178.8 (2) |
| C21—N4—C20—Ag1 | 170.81 (16) | C61—C62—C63—C64 | 0.1 (4) |
| C21—N4—C20—N3 | −0.8 (2) | C62—C61—C66—C65 | −1.6 (4) |
| C21—N4—C32—C33 | 77.8 (3) | C62—C61—C66—C69 | 179.3 (3) |
| C22—N3—C20—Ag1 | −171.10 (16) | C62—C63—C64—C65 | −2.5 (5) |
| C22—N3—C20—N4 | 0.7 (2) | C62—C63—C64—C68 | 176.6 (3) |
| C22—N3—C23—C24 | 77.7 (3) | C63—C64—C65—C66 | 2.9 (5) |
| C22—N3—C23—C28 | −102.3 (3) | C64—C65—C66—C61 | −0.9 (4) |
| C23—N3—C20—Ag1 | 11.7 (3) | C64—C65—C66—C69 | 178.2 (3) |
| C23—N3—C20—N4 | −176.4 (2) | C66—C61—C62—C63 | 2.0 (4) |
| C23—N3—C22—C21 | 176.7 (2) | C66—C61—C62—C67 | −177.4 (3) |
| C23—C24—C25—C26 | −1.4 (4) | C67—C62—C63—C64 | 179.5 (3) |
| C24—C23—C28—C27 | 1.5 (4) | C68—C64—C65—C66 | −176.2 (3) |
| C24—C23—C28—C31 | −178.8 (3) | C70—N8—C58—Ag2 | −6.9 (3) |
| C24—C25—C26—C27 | 1.6 (4) | C70—N8—C58—N7 | 175.89 (19) |
| C24—C25—C26—C30 | −178.2 (3) | C70—N8—C60—C59 | −176.1 (2) |
| C25—C26—C27—C28 | −0.1 (4) | C70—C71—C72—C73 | −180.0 (3) |
| C26—C27—C28—C23 | −1.4 (4) | C70—C71—C76—C75 | 179.7 (2) |
| C26—C27—C28—C31 | 179.0 (3) | C71—C72—C73—C74 | 0.7 (5) |
| C28—C23—C24—C25 | −0.2 (4) | C72—C71—C76—C75 | −0.1 (4) |
| C28—C23—C24—C29 | −179.4 (3) | C72—C73—C74—C75 | −0.9 (5) |
| C29—C24—C25—C26 | 177.8 (3) | C73—C74—C75—C76 | 0.7 (5) |
| C30—C26—C27—C28 | 179.6 (3) | C74—C75—C76—C71 | −0.1 (4) |
| C32—N4—C20—Ag1 | −11.3 (3) | C76—C71—C72—C73 | −0.1 (4) |
Symmetry code: (i) −x, −y+1, −z.
µ-Chlorido-bis{[1-benzyl-3-(2,4,6-trimethylphenyl)imidazol-2-ylideneκC]silver(I)} chloride 1,2-dichloroethane hemisolvate . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···Cl2 | 0.95 | 2.80 | 3.573 (2) | 140 (1) |
| C8—H8···Cl2 | 0.95 | 2.81 | 3.669 (2) | 157 (1) |
| C13—H13A···Cl2 | 0.99 | 2.91 | 3.784 (2) | 147 (1) |
| C21—H21···Cl2 | 0.95 | 2.78 | 3.567 (2) | 141 (1) |
| C38—H38···Cl2 | 0.95 | 2.90 | 3.779 (2) | 155 (1) |
| C41—H41···Cl2 | 0.95 | 2.66 | 3.419 (2) | 137 (1) |
| C70—H70A···Cl2 | 0.99 | 2.74 | 3.634 (2) | 151 (1) |
References
- Aher, S. B., Muskawar, P. N., Thenmozhi, K. & Bhagat, P. R. (2014). Eur. J. Med. Chem.81, 408–419. [DOI] [PubMed]
- A Patil, S. P., Hoagland, A. A., Patil, S. & Bugarin, A. (2020). Future Med. Chem.12, 2239–2275. [DOI] [PubMed]
- Arduengo, A. J., Harlow, R. L. & Kline, M. (1991). J. Am. Chem. Soc.113, 361–363.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Herrmann, W. A. & Köcher, C. (1997). Angew. Chem. Int. Ed. Engl.36, 2162–2187.
- Huang, K. & Qin, D.-B. (2011). Acta Cryst. E67, m1116. [DOI] [PMC free article] [PubMed]
- Jafarpour, L., Schanz, H. Jr, Stevens, E. D. & Nolan, S. P. (1999). Organometallics, 18, 5416–5419.
- Jayaraman, S., Castillo Guel, R. A., Malek, K. & Arumugam, K. (2019). IUCrData, 4, x191003. [DOI] [PMC free article] [PubMed]
- Karataş, M. O., Olgundeniz, B., Günal, S., Özdemir, I., Alıcı, B. & Çetinkaya, E. (2016). Bioorg. Med. Chem.24, 643–650. [DOI] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst.53, 226–235. [DOI] [PMC free article] [PubMed]
- Maishal, T. K., Basset, J.-M., Boualleg, M., Copéret, C., Veyre, L. & Thieuleux, C. (2009). Dalton Trans. pp. 6956–6959. [DOI] [PubMed]
- Medici, S., Peana, M., Crisponi, G., Nurchi, V. M., Lachowicz, J. I., Remelli, M. & Zoroddu, M. A. (2016). Coord. Chem. Rev.327–328, 349–359.
- Melaiye, A., Simons, R. S., Milsted, A., Pingitore, F., Wesdemiotis, C., Tessier, C. A. & Youngs, W. J. (2004). J. Med. Chem.47, 973–977. [DOI] [PubMed]
- Ortiz, A. M., Sánchez-Méndez, A., de Jesús, E., Flores, J. C., Gómez-Sal, P. & Mendicuti, F. (2016). Inorg. Chem.55, 1304–1314. [DOI] [PubMed]
- Rigaku OD (2015). Rigaku Journal, 31, 27–28.
- Samantaray, M. K., Dash, C., Shaikh, M. M., Pang, K., Butcher, R. J. & Ghosh, P. (2011). Inorg. Chem.50, 1840–1848. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Streciwilk, W., Cassidy, J., Hackenberg, F., Müller-Bunz, H., Paradisi, F. & Tacke, M. (2014). J. Organomet. Chem.749, 88–99.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624008617/zl4077sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624008617/zl4077Isup2.hkl
CCDC reference: 2380960
Additional supporting information: crystallographic information; 3D view; checkCIF report