The title compound is an example for a chiral-at-metal complex, with the RuIII atom having an octahedral coordination environment by three bidentate ligands.
Keywords: crystal structure, absolute configuration, ruthenium, chiral-at-metal complex
Abstract
The title compound, [Ru(C12H14NO2)2(C12H8N2)]PF6 crystallizes in the tetragonal Sohnke space group P41212. The two bidentate chiral salicyloxazoline ligands and the phenanthroline co-ligand coordinate to the central RuIII atom through N,O and N,N atom pairs to form bite angles of 89.76 (15) and 79.0 (2)°, respectively. The octahedral coordination of the bidentate ligands leads to a propeller-like shape, which induces metal-centered chirality onto the complex, with a right-handed (Δ) absolute configuration [the Flack parameter value is −0.003 (14)]. Both the complex cation and the disordered PF6− counter-anion are located on twofold rotation axes. Apart from Coulombic forces, the crystal cohesion is ensured by non-classical C—H⋯O and C—H⋯F interactions.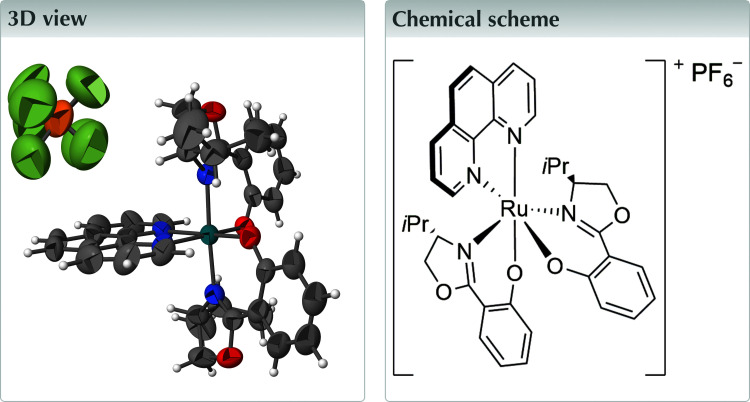
Structure description
The syntheses of optically pure metal complexes are usually costly and sophisticated, especially with the use of traditional methods for the resolution of racemic mixtures. A straightforward alternative strategy, therefore, requires the coordination of pure chiral auxiliary ligands tailored for the selective synthesis of diastereomers, which are easily converted to the corresponding enantiomerically pure complexes (Knof & von Zelewsky, 1999 ▸). Hayoz and co-workers were the first to report the diastereoselective synthesis of optically pure ruthenium polypyridyl complexes in the quest for generating compounds with metal-centered chirality, so-called chiral-at-metal complexes (Hayoz et al., 1993 ▸). Such metal-centered chirality refers to the type of chirality induced at a central metal atom as a result of an helical octahedral coordination around a metal in bis-chelate or tris-chelate systems. In this context, optically pure salicyloxazoline is often used as an auxiliary ligand to implement and control the absolute configuration at central metal atoms during ligand exchange. In this case, the absolute configurations at the central metal could either be right-handed or left-handed twist systems, which are symbolized by Δ and Λ stereochemical descriptors, respectively (Gong et al., 2010 ▸). The salicyloxazoline ligand is often used in this manner because of its reversible coordination upon acid protonation of its phenolate group while leaving the stereochemistry of the metal complex intact (Gong et al., 2009 ▸, 2010 ▸, 2013 ▸).
The complex cation of the title salt constitutes of two optically pure bidentate salicyloxazoline ligands and a phenanthroline co-ligand arranged within an octahedral coordination sphere around the central RuIII atom, which is located about a twofold rotation axis bisecting the phenantroline ligand (Fig. 1 ▸). This right-handed twist of the ligands leads to a Δ stereochemical configuration of the complex; the correctness of the absolute configuration is indicated by a Flack parameter (Parsons et al., 2013 ▸) value of −0.003 (14). The bite angles, 89.76 (15)°, for the salicyloxazoline ligands are comparable with reported values, e.g. 86.68° (Brunner et al., 1998 ▸), 88.29° (Davenport et al., 2004 ▸), 86.88° (Kelani et al., 2024 ▸), or 90.00 (Gong et al., 2010 ▸) while that for the phenanthroline ligand, 79.0 (2)°, is almost similar to that of 80.12° (Gong et al., 2010 ▸). The bond lengths of the RuIII atom with the ligating atoms of 1.974 (3), 2.079 (4) and 2.072 (4) Å to O1, N1(phenanthroline) and N2(salicyloxazoline) atoms, respectively, also agree well with reported values. The crystal packing (Fig. 2 ▸) includes the disordered PF6− counter-anion (located about a twofold rotation axis). Non-classical intermolecular interactions featuring C—H⋯O and C—H⋯F contacts (Table 1 ▸) are present.
Figure 1.
The molecular structure of the title compound drawn with displacement ellipsoids at the 50% probability level; hydrogen atoms and the PF6− counter-anion were removed for clarity. Non-labelled atoms are generated by a twofold rotation axis (symmetry operation: y, x, –z).
Figure 2.
Crystal packing arrangement of the title compound in a view along the c axis. Non-classical hydrogen-bonding interactions are indicated by dotted lines.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1⋯O1i | 0.95 | 2.59 | 3.102 (6) | 114 |
| C16—H16⋯O1i | 1.00 | 2.53 | 3.224 (6) | 126 |
| C17—H17A⋯F3ii | 0.98 | 2.52 | 3.464 (11) | 162 |
| C18—H18A⋯F2 | 0.98 | 2.48 | 3.357 (12) | 149 |
Symmetry codes: (i) 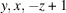 ; (ii)
; (ii)  .
.
Synthesis and crystallization
Dichlorido-bis(1,10-phenanthroline)ruthenium(II) (50.0 mg, 0.09 mmol, 1 eq) was added to (S)-isopropyl-2-(2-hydroxyphenyl)oxazoline (38.5 mg, 0.2 mmol, 2 eq) in ethanol in the presence of K2CO3 (26.0 mg, 0.2 mmol, 2 eq). The reaction mixture was refluxed for 6 h under continuous stirring after which it was cooled to room temperature and then concentrated in vacuo under reduced pressure. The crude product was purified by column chromatography with silica gel using a solvent system of CH2Cl2:CH3OH:CH3CN = 9.7:0.2:0.1 v:v:v) to obtain a purple crystalline compound. Yield, 31 mg (46%, 0.04 mmol).
Refinement
Details of the data collection, solution and refinement are given in Table 2 ▸. The disordered PF6− anion was treated as equally disordered around the twofold rotation axis and was kept stable with SADI, SIMU and DELU restraints in SHELXL (Sheldrick, 2015b ▸). The highest remaining maximum and minimum electron density are 1.32 and 0.76 Å away from F1A and Ru1, respectively.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Ru(C12H14NO2)2(C12H8N2)]PF6 |
| M r | 834.73 |
| Crystal system, space group | Tetragonal, P41212 |
| Temperature (K) | 173 |
| a, c (Å) | 15.3094 (13), 15.315 (2) |
| V (Å3) | 3589.5 (8) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.56 |
| Crystal size (mm) | 0.46 × 0.43 × 0.42 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| Tmin, Tmax | 0.638, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 53896, 4516, 3564 |
| R int | 0.067 |
| (sin θ/λ)max (Å−1) | 0.669 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.038, 0.107, 1.04 |
| No. of reflections | 4516 |
| No. of parameters | 245 |
| No. of restraints | 26 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.38, −0.41 |
| Absolute structure | Flack x determined using 1296 quotients [(I+)−(I−)]/[(I+)+(I−)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | −0.003 (14) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624008939/wm4221sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624008939/wm4221Isup3.hkl
CCDC reference: 2383614
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Dr B. Vatsha at the Department of Chemical Sciences, University of Johannesburg, for the opportunity provided towards the collection of the data.
full crystallographic data
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Crystal data
| [Ru(C12H14NO2)2(C12H8N2)]PF6 | Dx = 1.545 Mg m−3 |
| Mr = 834.73 | Mo Kα radiation, λ = 0.71073 Å |
| Tetragonal, P41212 | Cell parameters from 8341 reflections |
| a = 15.3094 (13) Å | θ = 2.3–20.4° |
| c = 15.315 (2) Å | µ = 0.56 mm−1 |
| V = 3589.5 (8) Å3 | T = 173 K |
| Z = 4 | Cuboid, purple |
| F(000) = 1700 | 0.46 × 0.43 × 0.42 mm |
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Data collection
| Bruker APEXII CCD diffractometer | 4516 independent reflections |
| Radiation source: sealed-tube | 3564 reflections with I > 2σ(I) |
| Triumph monochromator | Rint = 0.067 |
| φ and ω scans | θmax = 28.4°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | h = −20→20 |
| Tmin = 0.638, Tmax = 0.746 | k = −20→20 |
| 53896 measured reflections | l = −20→20 |
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H-atom parameters constrained |
| wR(F2) = 0.107 | w = 1/[σ2(Fo2) + (0.0587P)2 + 0.7144P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 4516 reflections | Δρmax = 0.38 e Å−3 |
| 245 parameters | Δρmin = −0.41 e Å−3 |
| 26 restraints | Absolute structure: Flack x determined using 1296 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| Primary atom site location: structure-invariant direct methods | Absolute structure parameter: −0.003 (14) |
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.2882 (4) | 0.4278 (4) | 0.6429 (3) | 0.0615 (13) | |
| H1 | 0.237967 | 0.400278 | 0.666771 | 0.074* | |
| C2 | 0.3215 (4) | 0.5005 (4) | 0.6832 (4) | 0.0760 (18) | |
| H2 | 0.294934 | 0.521584 | 0.735125 | 0.091* | |
| C3 | 0.3917 (5) | 0.5426 (4) | 0.6499 (4) | 0.0828 (19) | |
| H3 | 0.412522 | 0.594417 | 0.676826 | 0.099* | |
| C4 | 0.4345 (4) | 0.5090 (4) | 0.5742 (4) | 0.0615 (13) | |
| C5 | 0.3979 (3) | 0.4353 (3) | 0.5381 (3) | 0.0463 (10) | |
| C6 | 0.5100 (4) | 0.5455 (4) | 0.5356 (4) | 0.0723 (16) | |
| H6 | 0.535851 | 0.595990 | 0.560765 | 0.087* | |
| C7 | 0.3015 (3) | 0.1129 (3) | 0.4108 (3) | 0.0539 (12) | |
| C8 | 0.2786 (4) | 0.0607 (4) | 0.3381 (3) | 0.0678 (14) | |
| H8 | 0.238849 | 0.082913 | 0.295931 | 0.081* | |
| C9 | 0.3130 (5) | −0.0217 (4) | 0.3273 (4) | 0.0815 (19) | |
| H9 | 0.295857 | −0.055716 | 0.278301 | 0.098* | |
| C10 | 0.3710 (5) | −0.0552 (4) | 0.3853 (5) | 0.0852 (19) | |
| H10 | 0.393287 | −0.112595 | 0.377536 | 0.102* | |
| C11 | 0.3975 (4) | −0.0054 (4) | 0.4556 (5) | 0.0759 (17) | |
| H11 | 0.439237 | −0.028411 | 0.495301 | 0.091* | |
| C12 | 0.3633 (4) | 0.0795 (3) | 0.4695 (3) | 0.0592 (12) | |
| C13 | 0.3904 (3) | 0.1261 (4) | 0.5476 (3) | 0.0553 (12) | |
| C14 | 0.4704 (4) | 0.1478 (5) | 0.6678 (4) | 0.0838 (19) | |
| H14A | 0.529714 | 0.171713 | 0.658200 | 0.101* | |
| H14B | 0.468526 | 0.119533 | 0.725909 | 0.101* | |
| C15 | 0.4011 (3) | 0.2208 (4) | 0.6615 (3) | 0.0618 (13) | |
| H15 | 0.430056 | 0.279348 | 0.662139 | 0.074* | |
| C16 | 0.3308 (4) | 0.2163 (4) | 0.7321 (3) | 0.0730 (15) | |
| H16 | 0.285475 | 0.260943 | 0.717068 | 0.088* | |
| C17 | 0.2847 (5) | 0.1280 (5) | 0.7371 (4) | 0.102 (2) | |
| H17A | 0.240548 | 0.129560 | 0.783417 | 0.152* | |
| H17B | 0.256291 | 0.115575 | 0.681064 | 0.152* | |
| H17C | 0.327529 | 0.082258 | 0.749904 | 0.152* | |
| C18 | 0.3698 (6) | 0.2424 (7) | 0.8209 (4) | 0.122 (3) | |
| H18A | 0.398851 | 0.299210 | 0.815570 | 0.183* | |
| H18B | 0.323026 | 0.246367 | 0.864392 | 0.183* | |
| H18C | 0.412379 | 0.198299 | 0.839249 | 0.183* | |
| N1 | 0.3248 (2) | 0.3946 (3) | 0.5709 (2) | 0.0483 (9) | |
| N2 | 0.3638 (3) | 0.2026 (3) | 0.5728 (2) | 0.0523 (9) | |
| O1 | 0.2614 (2) | 0.1896 (2) | 0.4175 (2) | 0.0570 (9) | |
| O2 | 0.4479 (3) | 0.0854 (3) | 0.5994 (3) | 0.0767 (11) | |
| F1A | 0.6349 (16) | 0.2780 (8) | 0.7374 (14) | 0.203 (8) | 0.5 |
| F1B | 0.6026 (17) | 0.3041 (13) | 0.8114 (17) | 0.250 (11) | 0.5 |
| F2 | 0.5257 (5) | 0.3866 (6) | 0.7660 (8) | 0.247 (5) | |
| F3 | 0.6459 (7) | 0.4151 (7) | 0.8395 (6) | 0.241 (4) | |
| P1 | 0.6246 (2) | 0.3754 (2) | 0.750000 | 0.1405 (16) | |
| Ru1 | 0.28566 (2) | 0.28566 (2) | 0.500000 | 0.04461 (15) |
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.049 (3) | 0.084 (4) | 0.051 (2) | 0.001 (3) | 0.006 (2) | −0.025 (2) |
| C2 | 0.064 (3) | 0.093 (4) | 0.072 (3) | 0.001 (3) | 0.007 (3) | −0.046 (3) |
| C3 | 0.096 (5) | 0.072 (4) | 0.080 (4) | 0.000 (3) | −0.015 (4) | −0.036 (3) |
| C4 | 0.060 (3) | 0.062 (3) | 0.063 (3) | −0.004 (2) | −0.009 (3) | −0.013 (3) |
| C5 | 0.046 (2) | 0.048 (2) | 0.046 (2) | 0.0082 (19) | −0.0020 (19) | −0.0070 (19) |
| C6 | 0.067 (4) | 0.060 (3) | 0.090 (4) | −0.017 (3) | −0.005 (3) | −0.006 (3) |
| C7 | 0.063 (3) | 0.050 (3) | 0.049 (2) | −0.013 (2) | 0.009 (2) | −0.0008 (19) |
| C8 | 0.076 (4) | 0.068 (3) | 0.060 (3) | −0.015 (3) | 0.009 (3) | −0.015 (3) |
| C9 | 0.098 (5) | 0.065 (4) | 0.081 (4) | −0.015 (3) | 0.020 (4) | −0.025 (3) |
| C10 | 0.091 (5) | 0.059 (4) | 0.106 (5) | −0.001 (3) | 0.021 (4) | −0.018 (4) |
| C11 | 0.069 (4) | 0.060 (3) | 0.099 (4) | 0.001 (3) | 0.020 (3) | −0.003 (3) |
| C12 | 0.063 (3) | 0.056 (3) | 0.060 (3) | −0.006 (2) | 0.013 (2) | 0.005 (2) |
| C13 | 0.055 (3) | 0.057 (3) | 0.054 (3) | −0.001 (2) | 0.004 (2) | 0.004 (2) |
| C14 | 0.078 (4) | 0.108 (5) | 0.066 (3) | 0.011 (4) | −0.018 (3) | 0.017 (4) |
| C15 | 0.063 (3) | 0.073 (3) | 0.049 (2) | −0.003 (3) | −0.012 (2) | 0.005 (3) |
| C16 | 0.076 (3) | 0.098 (4) | 0.044 (3) | −0.001 (4) | −0.003 (2) | 0.001 (3) |
| C17 | 0.110 (5) | 0.124 (6) | 0.071 (4) | −0.029 (5) | 0.013 (4) | 0.019 (4) |
| C18 | 0.125 (6) | 0.195 (10) | 0.047 (3) | −0.025 (6) | −0.005 (4) | −0.020 (4) |
| N1 | 0.042 (2) | 0.061 (2) | 0.0424 (18) | 0.0039 (17) | −0.0001 (16) | −0.0117 (17) |
| N2 | 0.054 (2) | 0.064 (3) | 0.0390 (16) | −0.0075 (19) | −0.0008 (16) | 0.0044 (19) |
| O1 | 0.071 (2) | 0.0503 (19) | 0.0500 (17) | −0.0074 (16) | −0.0063 (16) | −0.0046 (14) |
| O2 | 0.082 (3) | 0.072 (3) | 0.075 (3) | 0.009 (2) | −0.015 (2) | 0.012 (2) |
| F1A | 0.29 (2) | 0.091 (7) | 0.225 (17) | 0.020 (10) | −0.067 (18) | −0.003 (11) |
| F1B | 0.24 (2) | 0.145 (16) | 0.36 (3) | −0.025 (16) | 0.01 (2) | 0.080 (16) |
| F2 | 0.149 (6) | 0.235 (9) | 0.357 (14) | −0.025 (6) | −0.022 (8) | −0.047 (11) |
| F3 | 0.268 (10) | 0.262 (11) | 0.194 (8) | −0.019 (9) | −0.078 (8) | −0.046 (8) |
| P1 | 0.1223 (17) | 0.1223 (17) | 0.177 (4) | −0.025 (2) | −0.051 (2) | −0.051 (2) |
| Ru1 | 0.04954 (19) | 0.04954 (19) | 0.0347 (2) | −0.0046 (2) | 0.00213 (16) | −0.00213 (16) |
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Geometric parameters (Å, º)
| C1—N1 | 1.337 (5) | C13—N2 | 1.299 (7) |
| C1—C2 | 1.370 (8) | C13—O2 | 1.338 (6) |
| C1—H1 | 0.9500 | C14—O2 | 1.458 (7) |
| C2—C3 | 1.353 (9) | C14—C15 | 1.544 (8) |
| C2—H2 | 0.9500 | C14—H14A | 0.9900 |
| C3—C4 | 1.426 (8) | C14—H14B | 0.9900 |
| C3—H3 | 0.9500 | C15—N2 | 1.499 (6) |
| C4—C5 | 1.375 (7) | C15—C16 | 1.528 (7) |
| C4—C6 | 1.414 (8) | C15—H15 | 1.0000 |
| C5—N1 | 1.376 (6) | C16—C17 | 1.527 (10) |
| C5—C5i | 1.421 (8) | C16—C18 | 1.538 (8) |
| C6—C6i | 1.335 (12) | C16—H16 | 1.0000 |
| C6—H6 | 0.9500 | C17—H17A | 0.9800 |
| C7—O1 | 1.329 (6) | C17—H17B | 0.9800 |
| C7—C12 | 1.402 (7) | C17—H17C | 0.9800 |
| C7—C8 | 1.414 (7) | C18—H18A | 0.9800 |
| C8—C9 | 1.377 (9) | C18—H18B | 0.9800 |
| C8—H8 | 0.9500 | C18—H18C | 0.9800 |
| C9—C10 | 1.356 (10) | N1—Ru1 | 2.079 (4) |
| C9—H9 | 0.9500 | N2—Ru1 | 2.072 (4) |
| C10—C11 | 1.380 (9) | O1—Ru1 | 1.974 (3) |
| C10—H10 | 0.9500 | F1A—P1 | 1.512 (12) |
| C11—C12 | 1.418 (8) | F1B—P1 | 1.479 (14) |
| C11—H11 | 0.9500 | F2—P1 | 1.544 (8) |
| C12—C13 | 1.453 (7) | F3—P1 | 1.535 (8) |
| N1—C1—C2 | 121.6 (5) | H17A—C17—H17B | 109.5 |
| N1—C1—H1 | 119.2 | C16—C17—H17C | 109.5 |
| C2—C1—H1 | 119.2 | H17A—C17—H17C | 109.5 |
| C3—C2—C1 | 120.8 (5) | H17B—C17—H17C | 109.5 |
| C3—C2—H2 | 119.6 | C16—C18—H18A | 109.5 |
| C1—C2—H2 | 119.6 | C16—C18—H18B | 109.5 |
| C2—C3—C4 | 120.0 (5) | H18A—C18—H18B | 109.5 |
| C2—C3—H3 | 120.0 | C16—C18—H18C | 109.5 |
| C4—C3—H3 | 120.0 | H18A—C18—H18C | 109.5 |
| C5—C4—C6 | 119.3 (5) | H18B—C18—H18C | 109.5 |
| C5—C4—C3 | 115.8 (5) | C1—N1—C5 | 118.0 (4) |
| C6—C4—C3 | 124.9 (5) | C1—N1—Ru1 | 127.9 (4) |
| C4—C5—N1 | 123.8 (4) | C5—N1—Ru1 | 114.1 (3) |
| C4—C5—C5i | 119.8 (3) | C13—N2—C15 | 108.5 (4) |
| N1—C5—C5i | 116.4 (2) | C13—N2—Ru1 | 125.1 (3) |
| C6i—C6—C4 | 120.9 (3) | C15—N2—Ru1 | 126.4 (4) |
| C6i—C6—H6 | 119.5 | C7—O1—Ru1 | 128.4 (3) |
| C4—C6—H6 | 119.5 | C13—O2—C14 | 106.1 (4) |
| O1—C7—C12 | 125.7 (4) | F1B—P1—F1Bii | 138 (2) |
| O1—C7—C8 | 116.5 (5) | F1B—P1—F1Aii | 93.8 (14) |
| C12—C7—C8 | 117.8 (5) | F1Bii—P1—F1Aii | 51.5 (10) |
| C9—C8—C7 | 121.1 (6) | F1A—P1—F1Aii | 79.1 (18) |
| C9—C8—H8 | 119.4 | F1B—P1—F3 | 76.9 (11) |
| C7—C8—H8 | 119.4 | F1Bii—P1—F3 | 108.7 (11) |
| C10—C9—C8 | 121.2 (6) | F1A—P1—F3 | 118.9 (9) |
| C10—C9—H9 | 119.4 | F1Aii—P1—F3 | 73.6 (8) |
| C8—C9—H9 | 119.4 | F1B—P1—F3ii | 108.7 (11) |
| C9—C10—C11 | 119.6 (6) | F1Bii—P1—F3ii | 76.9 (11) |
| C9—C10—H10 | 120.2 | F1A—P1—F3ii | 73.6 (8) |
| C11—C10—H10 | 120.2 | F1Aii—P1—F3ii | 118.9 (9) |
| C10—C11—C12 | 121.0 (7) | F3—P1—F3ii | 165.1 (9) |
| C10—C11—H11 | 119.5 | F1B—P1—F2 | 75.9 (10) |
| C12—C11—H11 | 119.5 | F1Bii—P1—F2 | 143.1 (12) |
| C7—C12—C11 | 119.1 (5) | F1A—P1—F2 | 103.4 (10) |
| C7—C12—C13 | 122.9 (5) | F1Aii—P1—F2 | 163.5 (9) |
| C11—C12—C13 | 117.9 (5) | F3—P1—F2 | 91.3 (7) |
| N2—C13—O2 | 116.7 (4) | F3ii—P1—F2 | 77.1 (5) |
| N2—C13—C12 | 126.7 (5) | F1B—P1—F2ii | 143.1 (12) |
| O2—C13—C12 | 116.6 (5) | F1Bii—P1—F2ii | 75.9 (10) |
| O2—C14—C15 | 105.4 (4) | F1A—P1—F2ii | 163.5 (9) |
| O2—C14—H14A | 110.7 | F1Aii—P1—F2ii | 103.4 (10) |
| C15—C14—H14A | 110.7 | F3—P1—F2ii | 77.1 (5) |
| O2—C14—H14B | 110.7 | F3ii—P1—F2ii | 91.3 (7) |
| C15—C14—H14B | 110.7 | F2—P1—F2ii | 78.9 (8) |
| H14A—C14—H14B | 108.8 | O1—Ru1—O1i | 97.4 (2) |
| N2—C15—C16 | 111.3 (4) | O1—Ru1—N2 | 89.76 (15) |
| N2—C15—C14 | 100.6 (4) | O1i—Ru1—N2 | 88.30 (15) |
| C16—C15—C14 | 114.1 (5) | O1—Ru1—N2i | 88.30 (15) |
| N2—C15—H15 | 110.2 | O1i—Ru1—N2i | 89.77 (15) |
| C16—C15—H15 | 110.2 | N2—Ru1—N2i | 177.1 (2) |
| C14—C15—H15 | 110.2 | O1—Ru1—N1 | 170.53 (15) |
| C17—C16—C15 | 113.6 (5) | O1i—Ru1—N1 | 91.80 (15) |
| C17—C16—C18 | 111.4 (5) | N2—Ru1—N1 | 92.57 (16) |
| C15—C16—C18 | 109.9 (5) | N2i—Ru1—N1 | 89.70 (15) |
| C17—C16—H16 | 107.2 | O1—Ru1—N1i | 91.80 (15) |
| C15—C16—H16 | 107.2 | O1i—Ru1—N1i | 170.53 (15) |
| C18—C16—H16 | 107.2 | N2—Ru1—N1i | 89.70 (15) |
| C16—C17—H17A | 109.5 | N2i—Ru1—N1i | 92.56 (16) |
| C16—C17—H17B | 109.5 | N1—Ru1—N1i | 79.0 (2) |
| N1—C1—C2—C3 | 1.6 (10) | O2—C14—C15—N2 | −15.9 (6) |
| C1—C2—C3—C4 | −2.9 (11) | O2—C14—C15—C16 | 103.4 (5) |
| C2—C3—C4—C5 | 2.0 (9) | N2—C15—C16—C17 | 58.9 (7) |
| C2—C3—C4—C6 | −177.6 (7) | C14—C15—C16—C17 | −54.2 (7) |
| C6—C4—C5—N1 | 179.7 (5) | N2—C15—C16—C18 | −175.5 (6) |
| C3—C4—C5—N1 | 0.1 (8) | C14—C15—C16—C18 | 71.4 (8) |
| C6—C4—C5—C5i | −1.3 (9) | C2—C1—N1—C5 | 0.5 (8) |
| C3—C4—C5—C5i | 179.1 (6) | C2—C1—N1—Ru1 | 179.8 (5) |
| C5—C4—C6—C6i | 0.8 (11) | C4—C5—N1—C1 | −1.3 (7) |
| C3—C4—C6—C6i | −179.6 (7) | C5i—C5—N1—C1 | 179.6 (5) |
| O1—C7—C8—C9 | −176.8 (5) | C4—C5—N1—Ru1 | 179.2 (4) |
| C12—C7—C8—C9 | 2.8 (8) | C5i—C5—N1—Ru1 | 0.2 (6) |
| C7—C8—C9—C10 | −1.0 (10) | O2—C13—N2—C15 | −5.2 (6) |
| C8—C9—C10—C11 | −1.2 (10) | C12—C13—N2—C15 | 173.8 (5) |
| C9—C10—C11—C12 | 1.6 (10) | O2—C13—N2—Ru1 | 173.3 (3) |
| O1—C7—C12—C11 | 177.1 (5) | C12—C13—N2—Ru1 | −7.7 (7) |
| C8—C7—C12—C11 | −2.4 (7) | C16—C15—N2—C13 | −108.2 (5) |
| O1—C7—C12—C13 | 1.0 (8) | C14—C15—N2—C13 | 13.0 (6) |
| C8—C7—C12—C13 | −178.5 (5) | C16—C15—N2—Ru1 | 73.3 (6) |
| C10—C11—C12—C7 | 0.3 (8) | C14—C15—N2—Ru1 | −165.4 (4) |
| C10—C11—C12—C13 | 176.5 (5) | C12—C7—O1—Ru1 | 8.7 (7) |
| C7—C12—C13—N2 | −1.2 (8) | C8—C7—O1—Ru1 | −171.7 (3) |
| C11—C12—C13—N2 | −177.3 (5) | N2—C13—O2—C14 | −5.9 (6) |
| C7—C12—C13—O2 | 177.9 (5) | C12—C13—O2—C14 | 175.0 (5) |
| C11—C12—C13—O2 | 1.7 (7) | C15—C14—O2—C13 | 13.9 (6) |
Symmetry codes: (i) y, x, −z+1; (ii) −y+1, −x+1, −z+3/2.
Δ-Bis[(S)-2-(4-isopropyl-4,5-dihydrooxazol-2-yl)phenolato-κ2N,O1](1,10-phenanthroline-κ2N,N')ruthenium(III) hexafluoridophosphate . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1···O1i | 0.95 | 2.59 | 3.102 (6) | 114 |
| C16—H16···O1i | 1.00 | 2.53 | 3.224 (6) | 126 |
| C17—H17A···F3iii | 0.98 | 2.52 | 3.464 (11) | 162 |
| C18—H18A···F2 | 0.98 | 2.48 | 3.357 (12) | 149 |
Symmetry codes: (i) y, x, −z+1; (iii) x−1/2, −y+1/2, −z+7/4.
References
- Bruker (2010). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Brunner, H., Nuber, B. & Prommesberger, M. (1998). Tetrahedron Asymmetry, 9, 3223–3229.
- Davenport, A. J., Davies, D. L., Fawcett, J. & Russell, D. R. (2004). Dalton Trans.9, 1481–1492. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst.45, 849–854.
- Gong, L., Mulcahy, S. P., Devarajan, D., Harms, K., Frenking, G. & Meggers, E. (2010). Inorg. Chem.49, 7692–7699. [DOI] [PubMed]
- Gong, L., Mulcahy, S. P., Harms, K. & Meggers, E. (2009). J. Am. Chem. Soc.131, 9602–9603. [DOI] [PubMed]
- Gong, L., Wenzel, M. & Meggers, E. (2013). Acc. Chem. Res.46, 2635–2644. [DOI] [PubMed]
- Hayoz, P., von Zelewsky, A. & Stoeckli-Evans, H. (1993). J. Am. Chem. Soc.115, 5111–5114.
- Kelani, M. T., Muller, A. & Lammertsma, K. (2024). IUCrData, 9, x240720. [DOI] [PMC free article] [PubMed]
- Knof, U. & von Zelewsky, A. (1999). Angew. Chem. Int. Ed.38, 302–322. [DOI] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst.48, 3–10. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst.53, 226–235. [DOI] [PMC free article] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624008939/wm4221sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624008939/wm4221Isup3.hkl
CCDC reference: 2383614
Additional supporting information: crystallographic information; 3D view; checkCIF report




