Abstract
Background:
Multiple sclerosis is a chronic immune-mediated disease of the central nervous system affecting nearly 3 million people worldwide. Although much progress has been made in the understanding and treatment of MS, cures remain elusive.
Objectives:
To accelerate the development of cures for MS by updating the Pathways to Cures Research Roadmap based on a contemporary understanding of disease. The refined Roadmap will help to promote research in scientific areas with great potential to reveal insights leading to cures and inspire greater coordination of global resources.
Methods:
Refinements to the Roadmap were achieved during a Global Summit that included close to 200 academic and industry scientists, health care providers, policy makers, funders, and people with MS from 15 countries.
Results:
The refined Roadmap describes three pathways that target opportunities for generating scientific insights leading to cures. Recommendations for accelerating research progress include, lowering barriers for global data sharing, enhancing collaboration and coordination among research supporters, committing to sustained funding, considering implications for implementation, engaging PwMS and committing to diversity, equity, and inclusion in the global MS movement.
Conclusion:
The refined roadmap provides a strategic framework for tackling the complexities of MS and advancing prevention strategies, effective treatments, and cures.
Keywords: Multiple sclerosis, strategy, funding, advocacy
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated disease of the central nervous system (CNS) affecting nearly 3 million people worldwide that can cause significant disability. 1 Although much progress has been made in understanding the pathogenesis of MS and there are many effective treatments for relapsing disease, strategies that prevent incident cases do not yet exist, there is a paucity of therapies that slow disability progression or restore function, and cures for MS remain elusive. The Pathways to Cures Research Roadmap (Roadmap) was established several years ago with input from scientific experts, health care providers, and people with MS (PwMS) and has been endorsed by 31 leading global MS advocacy and professional organizations. 2 The goals of the Roadmap are to (1) promote research in scientific areas with great potential to reveal insights leading to cures, and (2) inspire greater coordination of global resources that accelerate scientific progress.
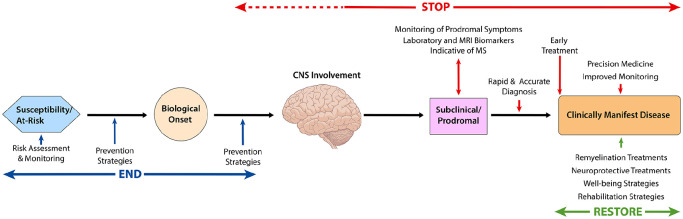
There are three distinct but overlapping Pathways described in the Roadmap: (1) The Stop pathway is focused on achieving a state of no new disease activity or CNS injury, (2) the Restore pathway aims to reverse symptoms and recover neurological function, and (3) the End pathway strives to prevent incident cases of MS. The opportunities for achieving cures in the context of the current understanding of the natural history and biological basis of MS are illustrated in Figure 1.
Figure 1.
Opportunities for Pathways to Cures Interventions.
The Roadmap is a living document and significant advances in the understanding and treatment of MS have been achieved since it was first established. These new insights and refinements were discussed during a recent Global Summit that included nearly 200 academic and industry scientists, health care providers, policy makers, funders, and PwMS from 15 countries. This report contains an updated Roadmap that considers recent scientific advances and provides recommendations which could accelerate progress toward cures through enhanced global collaboration.
Stop pathway
Objective 1
The first objective of the Stop pathway is early detection and treatment. Progress toward this objective includes ongoing refinements to the MS diagnostic criteria that allow for earlier and more accurate diagnosis.3,4 New insights are revealing early features of MS that could identify people at the very beginning of disease. 5 For example, radiologically isolated syndrome (RIS) is a condition wherein lesions characteristic of MS are detected by magnetic resonance imaging (MRI) that often precede the onset of clinical disease by several years. 6 Two recent studies have reported that treatment with disease-modifying therapies (DMTs) can delay and, in some cases, prevent the onset of clinical MS in people with RIS.7,8 Prodromal features of MS have also been recognized, representing another opportunity to identify individuals who are starting to develop MS. 9 These and other research advances have created optimism that MS can be detected and treated at its very earliest stages with greatly improved outcomes.
Research priorities
Refine the MS diagnostic criteria to facilitate ever earlier and more accurate diagnosis and treatment.
Improve the RIS criteria to enhance its predictive value.
Establish clinical, imaging, fluid, genetic, and immune biomarkers that allow for the sensitive and specific detection of the pre-clinical and prodromal stages of disease in diverse populations.
Objective 2
Tailoring DMTs to the dominant pathological feature in an individual is the second objective of the Stop pathway. Progress has been made in defining the biological basis for the different clinical presentations of MS, and these insights are contributing to a refined classification of disease subtypes. An international work group has begun updating the MS classification based on the underlying pathophysiology that is well supported by the MS community.10–15
Blood biomarkers that identify different MS trajectories have been reported, possibly enabling a match between a PwMS and their optimal treatment.16–18 Machine learning algorithms have been applied to brain MRI scans that identified specific disease subtypes. 19 In addition, genetic studies have identified a locus associated with more rapid clinical progression, which may reveal targets leading to the development of DMTs for progressive subtypes of MS. 20 These and other recent advancements, are moving us toward a more personalized approach to treatment of MS.
Research priorities
Combine insights from genetic signatures of disease subtypes with multimodal immune phenotyping to decipher the underlying pathophysiology of MS disease subtypes.
Explore MRI-based subtyping using machine learning to better understand underlying disease mechanisms and to help predict prognosis and treatment response.
Develop effective DMTs for progressive MS.
Restore pathway
Objective 1
The first objective of the Restore pathway is to develop strategies to repair myelin, restore axonal function, preserve neurons, and reestablish neural circuits. Currently, the most achievable regenerative process is the restoration of myelin. 21 In pre-clinical models, remyelination has been shown to reestablish saltatory conduction, promote functional recovery, and protect axons from degeneration. 21
A leading approach for enhancing remyelination in MS is to promote the expansion, recruitment to lesions, and differentiation of resident oligodendrocyte precursor cells (OPCs) into myelin producing cells. Several agents that regulate receptors, cell metabolism, as well as signaling pathways that promote OPC differentiation have shown promise in pre-clinical models and some are being tested in MS.22–25 Modest improvements in outcomes such as visual evoked potential latency, low contrast visual acuity, and MRI measures such as magnetization transfer ratio, myelin water fraction, and diffusion tensor imaging have been reported. 22 Increasing the pool of OPCs through transplantation of progenitor cells into the CNS is another strategy being tested to promote remyelination, and a recent report of human fetal neural precursor cell transplantation in PwMS supports this approach. 26
For myelin repair strategies to be effective, it is imperative that the tissue microenvironment be permissive. Damaging inflammatory factors must be subdued and inhibitors of repair neutralized for optimal remyelination. Inhibitors including LINGO-1, myelin debris, and several extracellular matrix molecules have been identified, and in some cases pharmacological agents that mitigate these factors have been developed.27–29 In addition, drugs that promote phagocytic activity within lesions and expedite debris removal have been proposed to promote a “repair friendly” environment. 30
Research priorities
Engineer better animal models of remyelination with higher translational value that takes into account the inflammatory nature of MS lesions.
Combine remyelinating approaches with different and complementary mechanisms of action.
Achieve a better understanding of regional remyelination given that gray matter lesions remyelinate better than white matter lesions. 31
Develop fluid biomarkers that inform on remyelination, along with continued improvement in quantifiable imaging measures for CNS remyelination.
Objective 2
The second objective of the Restore Pathway is to develop prehabilitation and rehabilitation strategies that leverage the plasticity of the CNS to achieve preventive, restorative, and compensatory approaches for maintaining or improving function. Physical activity and exercise are cornerstone interventions in MS with documented benefits for a wide array of symptoms. 32 Emerging evidence suggests that increased levels of physical activity and/or structured exercise may have a disease-modifying potential in MS and may even lower the risk of disease.33,34 Recently, interventions deployed early in the disease course (prehabilitation), aiming to prevent loss of function rather than restoring lost abilities, have been explored. 35
Cognitive rehabilitation is another promising area with documented improvement in several cognitive domains. 36 Cognitive rehabilitation can also improve quality of life, suggesting that memory rehabilitation is beneficial and meaningful to PwMS. 37 Whereas physical activity and exercise have the potential to build a physical reserve, it is still debated whether this is also possible with cognitive rehabilitation. The ability of cognitive and exercise interventions in combination to ameliorate cognitive dysfunction in progressive MS have been recently explored—albeit with mixed results. 38 Dietary interventions, balance training, and sleep interventions have also shown promise and are currently being investigated.35,39–43
Research priorities
Enhance the methodological quality of MS rehabilitation studies.
Prioritize testing of strategies deployed early in the disease course that prevent function loss.
Test complex interventions combining several approaches and/or alongside pharmacological treatment.
Develop a better understanding of the “active ingredient” of non-pharmacological interventions and develop a deeper understanding of the underlying mechanisms leading to functional recovery.
Achieve a better understanding of dose-response relationships and how different subgroups of patients may respond to treatment.
End pathway
Objective 1
The first objective of this pathway is to develop strategies for prevention of incident cases of MS in the general population by limiting or preventing exposure to modifiable risk factors. There has been considerable progress made in identifying risk factors associated with the onset of MS. Both genetic and epidemiological studies of large-scale cohorts or national health care systems data have returned robust and well validated associations. 44 These observations have prioritized different mechanisms potentially related to the onset of MS, including metabolic factors such as obesity in adolescence, environmental exposures that influence vitamin D levels, pollutants such as those found in cigarette smoke, and exposure to Epstein Barr Virus (EBV).45–49 While causation has yet to be determined for any of these risk factors, strategies that minimize or eliminate exposures to modifiable risks factors (e.g. neutralizing EBV vaccination) represent promising areas of research to explore.
Genetic studies have provided additional insights from the more than 234 MS susceptibility variants identified. 44 Most of these variants appear to influence the expression of genes in peripheral immune cells and up to half of them also influence susceptibility to other autoimmune diseases, suggesting that the earliest molecular events leading to MS involve peripheral immune dysregulation and a propensity for autoimmunity. 50 It is currently unknown how these features of immune dysfunction, driven by MS risk variants, may relate to the environmental exposures and life experiences associated with MS risk.
Research priorities
Understand better the modifiable risk factors, genetic determinants, and the interactions contributing to MS etiology in European and non-European populations.
Increase knowledge of early life exposures (including prenatal and perinatal) that contribute to the development of pediatric MS.
Promote collaboration with public health and other patient advocacy organizations with a common interest in preventing MS in the design and implementation of prevention trials.
Objective 2
The second objective of the End pathway is to prevent MS in people with an elevated risk for developing disease. Studies of first-degree relatives of individuals with rheumatoid arthritis and type 1 diabetes have shown that biomarkers and genetic factors can predict disease risk and they are being used to enable prevention trials.51,52 While the ability to predict the risk for MS has not yet been validated, these studies offer examples for the design of MS prevention approaches.
Designing MS prevention studies in high-risk individuals inspires a key semantic question of “what is MS?” While formal diagnostic criteria for MS exist, they are not helpful for prevention studies because the CNS is already damaged by the time MS is diagnosed. Since RIS and clinically isolated syndrome (CIS) also respond to MS DMTs, we can conceptualize MS starting once the CNS is involved. Therefore, for high-risk prevention studies, individuals in this pre-clinical stage of disease (prior to engagement of the CNS and any radiological evidence of MS and/or prodromal symptoms) will need to be identified. Candidate biomarkers which can identify people at high risk for MS have been reported, but their relevance for identifying people in the pre-clinical stage of disease is still unclear.53–56
Key factors needed to develop therapeutic strategies for prevention of MS in the high risk population are coming into focus, including (1) guidance from other diseases on how to approach prevention studies, (2) genetic tools to identify family members at higher risk of MS, (3) biomarkers of immune dysfunction and CNS damage that may indicate pre-clinical stages of disease, and (4) molecular mechanisms that are influenced by susceptibility variants that could provide therapeutic targets.
Research priorities
Optimize approaches to communicate the magnitude of an individual’s risk of MS in a manner that protects privacy and minimizes adverse outcomes.
Map the pre-clinical stages of the disease so that informative biomarkers and timely targeting of interventions can be realized.
Encourage studies with agents that target a specific immune perturbation along with deep longitudinal characterization of participants.
Recommendations for accelerating progress
Lower barriers to data sharing
The direction of travel for each Pathway is clear, but continued progress toward the development of MS cures will require statistical power enabled by large data sets and multi-center/multi-national collaboration. Collaborative efforts are often delayed or halted due to overly complicated and cumbersome policies and regulations governing data sharing such as (1) regulatory limits on data sharing based on concerns for participant confidentiality and privacy, (2) lack of infrastructure and/or resources to support data sharing, (3) technical considerations (e.g. data security/interoperability/governance and database architecture), (4) regulatory constraints on sharing data that do not align with the wishes of most PwMS, and (5) psychological, social, and motivational challenges.57,58 Advocating for changes in policy that overcome these obstacles would undoubtedly accelerate progress.
Challenges to international collaboration also impede research progress. Guidelines for data sharing differ at the national and international levels and there are no consistently applied international templates for data transfer, storage, and consent that help to overcome these diverse legal and regulatory rules. Regional and national imperatives for local data analysis that prevent a more centralized approach, also impedes progress. Likewise, different countries use different definitions for anonymized, coded, and identifiable patient information, which leads to different requirements for formal consent. Fortunately, efforts such as the FAIR Guiding Principles for data sharing and Global MS Data Alliance, point the way to lowering some of these barriers.59–61
Promote collaboration
A strategic and global collaborative approach for supporting research that reduces duplication and leads to major new insights and cures for MS is urgently needed (Figure 2). These global collaborative efforts will require effective leadership, a clear focus, a consensus on priorities, and agreement on equitable rules of engagement. Funders should support collaborative projects when it adds value and when it cannot be done by a single research group. Keys to a successful global collaboration framework include (1) a clear governance structure, (2) a plan for prioritizing joint initiatives, and (3) a way to balance funding of longer-term initiatives while allowing for innovation and investigator-initiated ideas. Defined milestones and clear metrics to measure progress will also be essential for success. Efforts such as the International Progressive MS Alliance point the way to achieving this goal.62–65
Figure 2.
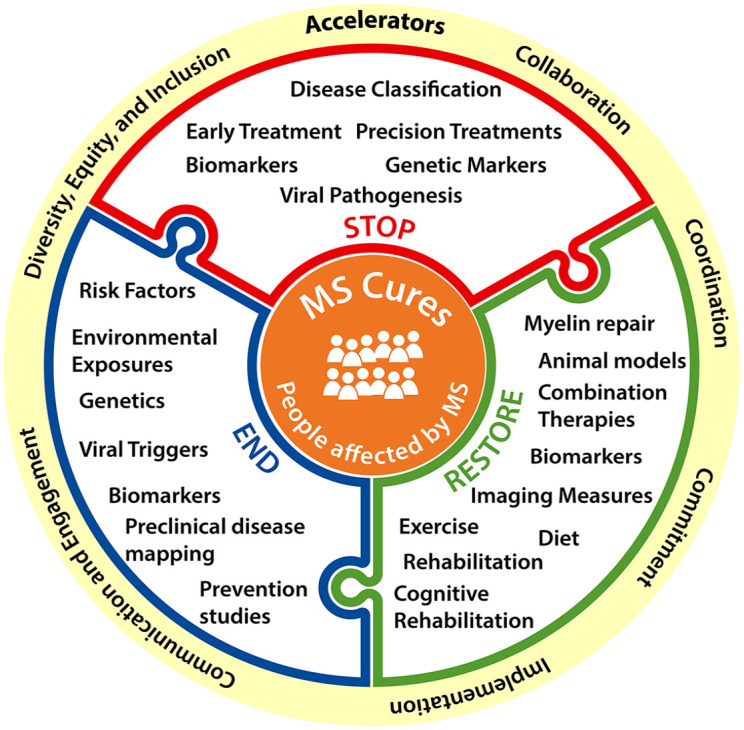
Recommendations for accelerating Pathways to Cures.
Enhance coordination
Resources available globally to fund MS research are finite, thus better coordination of research programs by funders has the potential to enhance their collective impact by applying investments strategically. Establishment of global research priorities and coordination among MS research funders will help ensure that resources are focused on opportunities with the greatest potential to generate insights leading to new prevention approaches, treatments, and cures. Coordination can help reduce redundancies and can also leverage grant making infrastructure to reduce the administrative expense. Enhanced coordination of research resources like biobanks (e.g. tissue, fluid, DNA) is an example of an opportunity that would have a major impact. Global collaborative efforts like the International Progressive MS Alliance and the Patient Reported Outcome for Multiple Sclerosis (PROMS) have proven that global MS funders can work together to coordinate research investments and accelerate progress.65–67 These efforts have set the stage for broader global coordination of research investments.
Provide sustained funding
The global MS community must be committed to sustained research funding. The uncertainty caused by sporadic funding disincentivizes researchers from pursuing science advancing Pathways to Cures. Furthermore, a lack of long-term funding commitments can result in projects terminating before they yield results, which wastes resources and delays progress. One possible solution would be for global funders to pool resources creating a shared fund that would make awards only when sufficient resources were available for the life of the project.
Consider implementation
As funding of more sophisticated and expensive science advances, it will be important to consider how the results will be disseminated and the outcomes implemented. It would be unfortunate if commercialized therapies, diagnostic tools, and advanced imaging modalities, are for financial, technical, or logistical reasons, inaccessible to PwMS. Engagement of policy makers and payers at very early stages of development will be necessary to assure access to PwMS in a timely manner. Funders should consider deploying resources to understand and overcome access challenges during the early stages of the projects they support.
Engage people affected by MS
At the center of any powerful collaboration is the individual who stands to benefit most from research progress—PwMS. The global MS movement must engage PwMS at every level of research. Meaningful engagement has the potential to enhance the quality, relevance, and impact of sponsored research. For example, involvement of PwMS in the design of clinical trials enables the development of therapies that are acceptable and feasible, potentially enhancing uptake and adherence once approved. Opportunities exist for engaging PwMS more deeply in research and design and potentially as advocates for overcoming some of the barriers to research progress like data sharing.68,69 In addition, communicating the outcomes and impacts of research investments to the public is critical for acquiring and retaining the support of the global MS movement. Funders should anticipate the communication needs of their supporters and develop plans to address these needs at the very earliest stages of major funding initiatives.
The generalizability of research breakthroughs is threatened by a lack of diversity in MS research and clinical care.70,71 The MS movement must do a better job of diversifying the MS research and clinical workforce, perhaps through the development of fellowships and/or early career awards targeting underrepresented scholars. In addition, researchers and funders should engage people from underrepresented groups in the design, implementation, and participation in both fundamental and clinical research studies. Better engagement will enhance the quality of the results, reveal important effectiveness and safety information, and potentially help discover population-specific differences in the natural history of disease and response to therapy, thereby assuring that better treatments and cures are developed for everyone living with MS.
Conclusion
Scientific breakthroughs leading to MS cures will require strategic investments in research priorities and enhanced global collaboration among all stakeholders in the MS movement. The refined Roadmap provides a foundation for a dialogue among MS research funders to enhance collaboration and coordination of investments focused on accelerating progress toward cures. A recent declaration by the major global MS advocacy organizations to focus resources on research areas with high potential to accelerate progress has created excitement and optimism that MS cures are within our reach.
Acknowledgments
Thanks to Anne Helme, Paola Zaratin, Jorge Correale, and Kathy Smith for their service on the Pathways to Cures Global Summit Organizing Committee. Thanks to Shawna Golden and Kate Daniels for their assistance with the Global Summit. Thanks also to Cathy Carlson and Susan Atlas for writing support.
Footnotes
Data availability statement: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BFB has as no relevant conflicts. BLB serves as a consultant to Novartis, UCB, Roche, and Sanofi for work unrelated to the present manuscript. BLB receives funding for research grants from the NIH and National MS Society. CCW has no relevant conflicts. TC has received travel support from Sanofi. UD has no relevant conflicts. PLD has received research support from Biogen and Merck Serono. AKP has no relevant conflicts. VWY is funded by research grants from MS Canada, the Canadian Institutes of Health Research, USA Department of Defense Multiple Sclerosis Research Program, Genentech and Novartis. He has received speaker honoraria from Biogen, EMD Serono, Novartis, Roche, Sanofi-Genzyme and Teva Canada. He is the recipient of unrestricted educational grants from Biogen, EMD Serono, Novartis, Roche, Sanofi-Genzyme and Teva Canada to support educational activities of the Alberta MS Network, which he directs. ENB has no relevant conflicts. AJT is Co-Chair, UCL-Eisai Steering Committee drug discovery collaboration (paid to institution), Member, National MS Society (USA) Scientific Advisory Committee (receive support for travel), Clinical Trials Committee, Progressive MS Alliance (receive support for travel), Board member, European Charcot Foundation (receive support for travel), Editor in Chief, Multiple Sclerosis Journal (receiving honorarium from SAGE Publishers), Editorial Board Member, The Lancet Neurology (receiving free subscription).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Activities supporting the development of the Roadmap were funded by the National Multiple Sclerosis Society (USA).
ORCID iDs: Bruce F Bebo  https://orcid.org/0000-0003-0941-9169
https://orcid.org/0000-0003-0941-9169
Timothy Coetzee  https://orcid.org/0000-0002-3031-7549
https://orcid.org/0000-0002-3031-7549
Ulrik Dalgas  https://orcid.org/0000-0003-4132-2789
https://orcid.org/0000-0003-4132-2789
Phillip L De Jager  https://orcid.org/0000-0002-8057-2505
https://orcid.org/0000-0002-8057-2505
Alan J Thompson  https://orcid.org/0000-0002-4333-8496
https://orcid.org/0000-0002-4333-8496
Contributor Information
Bruce F Bebo, Jr, National Multiple Sclerosis Society, New York, NY, USA.
Brenda L Banwell, Division of Child Neurology, Children’s Hospital of Philadelphia, Departments of Neurology and Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Caroline C Whitacre, National Multiple Sclerosis Society, New York, NY, USA.
Timothy Coetzee, National Multiple Sclerosis Society, New York, NY, USA.
Ulrik Dalgas, Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark.
Phillip L De Jager, Center for Translational and Computational Neuroimmunology, Department of Neurology, Columbia University, New York, NY, USA.
Anne-Katrin Proebstel, Research Center for Clinical Neuroimmunology and Neuroscience, Departments of Neurology, Biomedicine, and Clinical Research, University Hospital Basel, Basel, Switzerland.
V Wee Yong, Department of Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada.
Etty N Benveniste, Department of Cell, Developmental and Integrative Biology, The University of Alabama at Birmingham, Birmingham, AL, USA.
Alan J Thompson, Queen Square Multiple Sclerosis Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, NIHR University College London Hospitals Biomedical Research Centre, Faculty of Brain Sciences, University College London, London, UK.
References
- 1. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler 2020; 26(14): 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bebo BF, Jr, Allegretta M, Landsman D, et al. Pathways to cures for multiple sclerosis: A research roadmap. Mult Scler 2022; 28: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 4. Jakimovski D, Kavak KS, Zakalik K, et al. Improvement in time to multiple sclerosis diagnosis: 25-year retrospective analysis from New York State MS Consortium (NYSMSC). Mult Scler 2023; 29: 753–756. [DOI] [PubMed] [Google Scholar]
- 5. Zamecnik CR, Sowa GM, Abdelhak A, et al. An autoantibody signature predictive for multiple sclerosis. Nat Med 2024; 30: 1300–1308. [DOI] [PubMed] [Google Scholar]
- 6. Lebrun-Frénay C, Kantarci O, Siva A, et al. Radiologically isolated syndrome: 10-year risk estimate of a clinical event. Ann Neurol 2020; 88(2): 407–417. [DOI] [PubMed] [Google Scholar]
- 7. Okuda DT, Kantarci O, Lebrun-Frénay C, et al. Dimethyl fumarate delays multiple sclerosis in radiologically isolated syndrome. Ann Neurol 2023; 93(3): 604–614. [DOI] [PubMed] [Google Scholar]
- 8. Lebrun-Frénay C, Siva A, Sormani MP, et al. Teriflunomide and time to clinical multiple sclerosis in patients with radiologically isolated syndrome: The TERIS randomized clinical trial. JAMA Neurol 2023; 80: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marrie RA, Allegretta M, Barcellos LF, et al. From the prodromal stage of multiple sclerosis to disease prevention. Nat Rev Neurol 2022; 18(9): 559–572. [DOI] [PubMed] [Google Scholar]
- 10. Kuhlmann T, Moccia M, Coetzee T, et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol 2023; 22(1): 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krieger S, Cook K, Hersh CM. Understanding multiple sclerosis as a disease spectrum: Above and below the clinical threshold. Curr Opin Neurol 2024; 37: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson AJ, Moccia M, Amato MP, et al. Do the current MS clinical course descriptors need to change and if so how? A survey of the MS community. Mult Scler 2023; 29(11–12): 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitt D, Lo CH, Gauthier SA, et al. Toward precision phenotyping of multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2022; 9(6): e200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellucci G, Buscarinu MC, Reniè R, et al. Disentangling multiple sclerosis phenotypes through Mendelian disorders: A network approach. Mult Scler 2024; 30(3): 325–335. [DOI] [PubMed] [Google Scholar]
- 15. Gross CC, Schulte-Mecklenbeck A, Steinberg OV, et al. Multiple sclerosis endophenotypes identified by high-dimensional blood signatures are associated with distinct disease trajectories. Sci Transl Med 2024; 16: eade8560. [DOI] [PubMed] [Google Scholar]
- 16. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kapoor R, Smith KE, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology 2020; 95: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol 2022; 21(3): 246–257. [DOI] [PubMed] [Google Scholar]
- 19. Eshaghi A, Young AL, Wijeratne PA, et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat Commun 2021; 12: 2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Multiple Sclerosis Genetics Consortium and MultipleMS Consortium. Locus for severity implicates CNS resilience in progression of multiple sclerosis. Nature 2023; 619(7969): 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franklin RJM, ffrench-Constant C. Regenerating CNS myelin—From mechanisms to experimental medicines. Nat Rev Neurosci 2017; 18: 753–769. [DOI] [PubMed] [Google Scholar]
- 22. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017; 390: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 23. Neumann B, Baror R, Zhao C, et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 2019; 25: 473.e8–485.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown JWL, Cunniffe NG, Prados F, et al. Safety and efficacy of bexarotene in patients with relapsing-remitting multiple sclerosis (CCMR One): A randomised, double-blind, placebo-controlled, parallel-group, phase 2a study. Lancet Neurol 2021; 20(9): 709–720. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Liu WQ, Hosseinpour Z, et al. Feasibility study to assess lesion repair in relapsing-remitting multiple sclerosis: A randomized controlled pilot clinical trial of domperidone add-on treatment. Mult Scler Relat Disord 2024; 85: 105525. [DOI] [PubMed] [Google Scholar]
- 26. Genchi A, Brambilla E, Sangalli F, et al. Neural stem cell transplantation in patients with progressive multiple sclerosis: An open-label, phase 1 study. Nat Med 2023; 29(1): 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cadavid D, Mellion M, Hupperts R, et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2019; 18(9): 845–856. [DOI] [PubMed] [Google Scholar]
- 28. Gharagozloo M, Bannon R, Calabresi PA. Breaking the barriers to remyelination in multiple sclerosis. Curr Opin Pharmacol 2022; 63: 102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghorbani S, Jelinek E, Jain R, et al. Versican promotes T helper 17 cytotoxic inflammation and impedes oligodendrocyte precursor cell remyelination. Nat Commun 2022; 13: 2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rawji KS, Young AMH, Ghosh T, et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol 2020; 139: 893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strijbis EMM, Kooi EJ, Van der Valk P, et al. Cortical remyelination is heterogeneous in multiple sclerosis. J Neuropathol Exp Neurol 2017; 76: 390–401. [DOI] [PubMed] [Google Scholar]
- 32. Motl RW, Sandroff BM, Kwakkel G, et al. Exercise in patients with multiple sclerosis. Lancet Neurol 2017; 16: 848–856. [DOI] [PubMed] [Google Scholar]
- 33. Dalgas U, Langeskov-Christensen M, Stenager E, et al. Exercise as medicine in multiple sclerosis-time for a paradigm shift: Preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep 2019; 19: 88. [DOI] [PubMed] [Google Scholar]
- 34. Wesnes K, Myhr KM, Riise T, et al. Physical activity is associated with a decreased multiple sclerosis risk: The EnvIMS study. Mult Scler 2018; 24(2): 150–157. [DOI] [PubMed] [Google Scholar]
- 35. Riemenschneider M, Hvid LG, Ringgaard S, et al. Investigating the potential disease-modifying and neuroprotective efficacy of exercise therapy early in the disease course of multiple sclerosis: The Early Multiple Sclerosis Exercise Study (EMSES). Mult Scler 2022; 28(10): 1620–1629. [DOI] [PubMed] [Google Scholar]
- 36. DeLuca J, Chiaravalloti ND, Sandroff BM. Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat Rev Neurol 2020; 16(6): 319–332. [DOI] [PubMed] [Google Scholar]
- 37. Chiaravalloti ND, Moore NB, DeLuca J. The efficacy of the modified Story Memory Technique in progressive MS. Mult Scler 2020; 26: 354–362. [DOI] [PubMed] [Google Scholar]
- 38. Feinstein A, Amato MP, Brichetto G, et al. Cognitive rehabilitation and aerobic exercise for cognitive impairment in people with progressive multiple sclerosis (CogEx): A randomised, blinded, sham-controlled trial. Lancet Neurol 2023; 22: 912–924. [DOI] [PubMed] [Google Scholar]
- 39. Katz Sand I, Benn EKT, Fabian M, et al. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: A pilot study. Mult Scler Relat Disord 2019; 36: 101403. [DOI] [PubMed] [Google Scholar]
- 40. Wahls TL, Titcomb TJ, Bisht B, et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin. Epub ahead of print 31 July 2021. DOI: 10.1177/20552173211035399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amato MP. “Brain reserve” and “cognitive reserve” should always be taken into account when studying neurodegeneration—Commentary. Mult Scler 2018; 24(5): 577–578. [DOI] [PubMed] [Google Scholar]
- 42. Corrini C, Gervasoni E, Perini G, et al. Mobility and balance rehabilitation in multiple sclerosis: A systematic review and dose-response meta-analysis. Mult Scler Relat Disord 2023; 69: 104424. [DOI] [PubMed] [Google Scholar]
- 43. Morsali S, Sabahi Z, Kakaei J, et al. Clinical efficacy and safety of melatonin supplementation in multiple sclerosis: A systematic review. Inflammopharmacology 2023; 31(5): 2213–2220. [DOI] [PubMed] [Google Scholar]
- 44. International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019; 365: eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Munger KL, Ascherio A. Prevention and treatment of MS: Studying the effects of vitamin D. Mult Scler 2011; 17(12): 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006; 296: 2832–2838. [DOI] [PubMed] [Google Scholar]
- 47. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375: 296–301. [DOI] [PubMed] [Google Scholar]
- 48. Wingerchuk DM. Smoking: Effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord 2012; 5(1): 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mokry LE, Ross S, Timpson NJ, et al. Obesity and multiple sclerosis: A Mendelian randomization study. PLoS Med 2016; 13: e1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patsopoulos NA, Bayer Pharma MS. Genetics Working Group; Steering Committees of Studies Evaluating IFNβ-1b and a CCR1-Antagonist, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol 2011; 70: 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deane KD. Rheumatoid arthritis: Prediction of future clinically-apparent disease, and prevention. Curr Opin Rheumatol 2024; 36: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Russell WE, Bundy BN, Anderson MS, et al. Abatacept for delay of type 1 diabetes progression in stage 1 relatives at risk: A randomized, double-masked, controlled trial. Diabetes Care 2023; 46: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lebrun-Frenay C, Kantarci O, Siva A, et al. Radiologically isolated syndrome. Lancet Neurol 2023; 22: 1075–1086. [DOI] [PubMed] [Google Scholar]
- 54. Tintore M, Cobo-Calvo A, Carbonell P, et al. Effect of changes in MS diagnostic criteria over 25 years on time to treatment and prognosis in patients with clinically isolated syndrome. Neurology 2021; 97: e1641–e1652. [DOI] [PubMed] [Google Scholar]
- 55. Xia Z, Steele SU, Bakshi A, et al. Assessment of early evidence of multiple sclerosis in a prospective study of asymptomatic high-risk family members. JAMA Neurol 2017; 74: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xia Z, White CC, Owen EK, et al. Genes and Environment in Multiple Sclerosis project: A platform to investigate multiple sclerosis risk. Ann Neurol 2016; 79(2): 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. DuBois JM, Mozersky J, Parsons M, et al. Exchanging words: Engaging the challenges of sharing qualitative research data. Proc Natl Acad Sci U S A 2023; 120: e2206981120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shilo S, Rossman H, Segal E. Axes of a revolution: Challenges and promises of big data in healthcare. Nat Med 2020; 26(1): 29–38. [DOI] [PubMed] [Google Scholar]
- 59. Peeters LM, Parciak T, Kalra D, et al. Multiple Sclerosis Data Alliance—A global multi-stakeholder collaboration to scale-up real world data research. Mult Scler Relat Disord 2021; 47: 102634. [DOI] [PubMed] [Google Scholar]
- 60. Pirmani A, De Brouwer E, Geys L, et al. The journey of data within a global data sharing initiative: A federated 3-layer data analysis pipeline to scale up multiple sclerosis research. JMIR Med Inform 2023; 11: e48030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016; 3: 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zaratin P, Comi G, Coetzee T, et al. Progressive MS Alliance industry forum: Maximizing collective impact to enable drug development. Trends Pharmacol Sci 2016; 37(10): 808–810. [DOI] [PubMed] [Google Scholar]
- 63. Zackowski KM, Freeman J, Brichetto G, et al. Prioritizing progressive MS rehabilitation research: A call from the International Progressive MS Alliance. Mult Scler 2021; 27(7): 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dangond F, Donnelly A, Hohlfeld R, et al. Facing the urgency of therapies for progressive MS—A progressive MS Alliance proposal. Nat Rev Neurol 2021; 17(3): 185–192. [DOI] [PubMed] [Google Scholar]
- 65. Thompson AJ, Carroll W, Ciccarelli O, et al. Charting a global research strategy for progressive MS-An international progressive MS Alliance proposal. Mult Scler 2022; 28(1): 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zaratin P, Vermersch P, Amato MP, et al. The agenda of the global patient reported outcomes for multiple sclerosis (PROMS) initiative: Progresses and open questions. Mult Scler Relat Disord 2022; 61: 103757. [DOI] [PubMed] [Google Scholar]
- 67. Bebo BF, Jr, Fox RJ, Lee K, et al. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler 2018; 24: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gray E, Amjad A, Robertson J, et al. Enhancing involvement of people with multiple sclerosis in clinical trial design. Mult Scler 2023; 29(9): 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zaratin P, Bertorello D, Guglielmino R, et al. The MULTI-ACT model: The path forward for participatory and anticipatory governance in health research and care. Health Res Policy Syst 2022; 20: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Amezcua L, Rivera VM, Vazquez TC, et al. Health disparities, inequities, and social determinants of health in multiple sclerosis and related disorders in the US: A review. JAMA Neurol 2021; 78: 1515–1524. [DOI] [PubMed] [Google Scholar]
- 71. Marrie RA, Chataway J, Bierer BE, et al. Enhancing diversity of clinical trial populations in multiple sclerosis. Mult Scler 2023; 29(9): 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]