Abstract
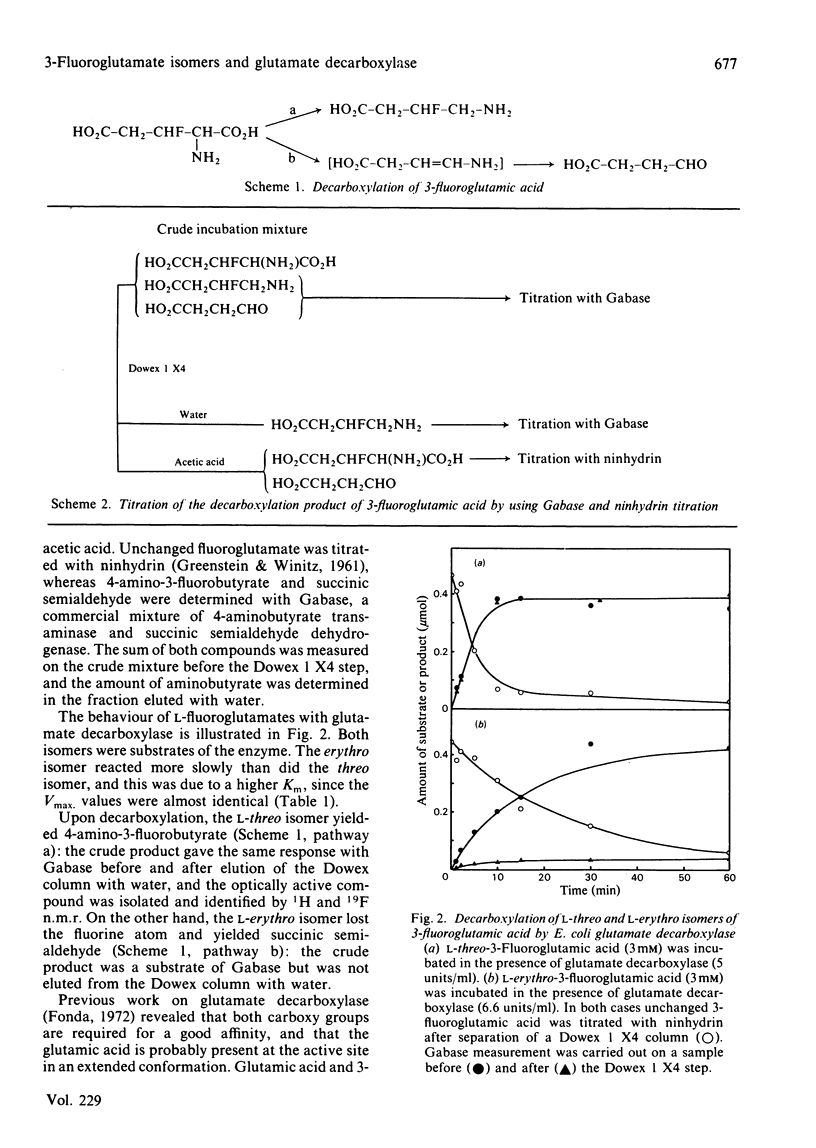
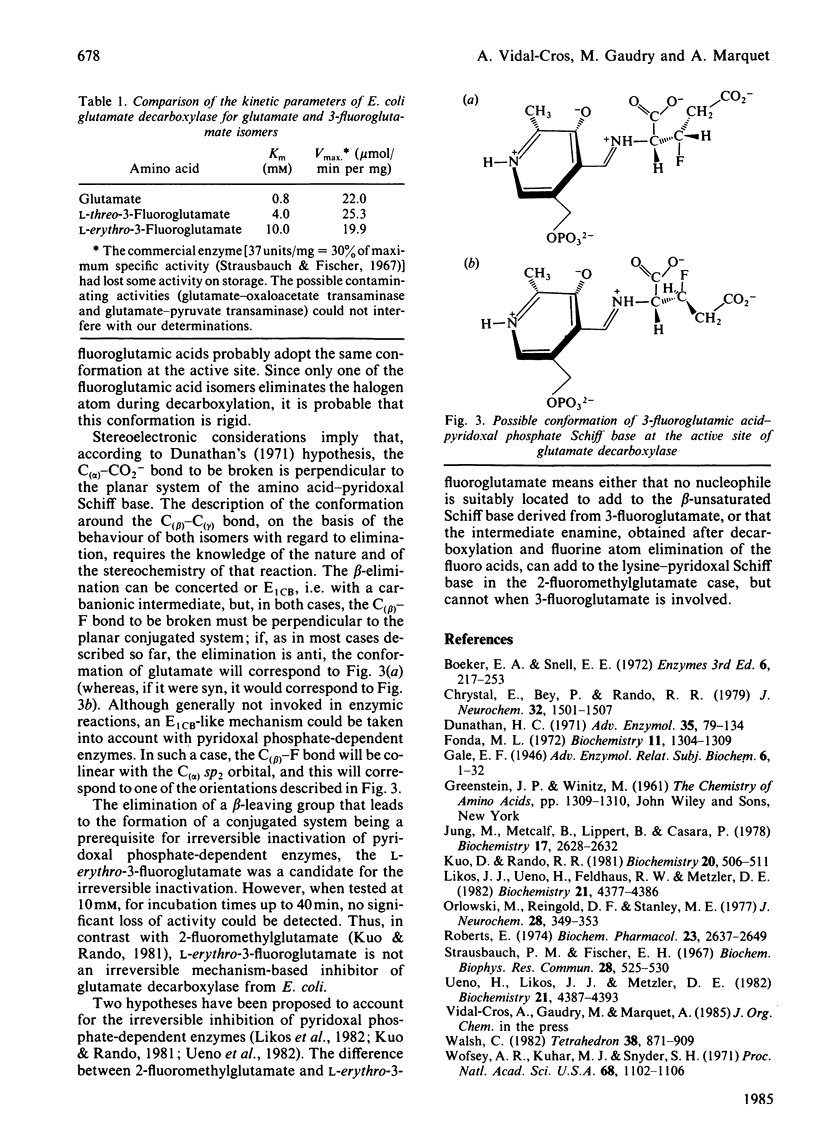
L-threo-3-Fluoroglutamate and L-erythro-3-fluoroglutamate were tested with glutamate decarboxylase from Escherichia coli. Both isomers were substrates: the threo isomer was decarboxylated into optically active 4-amino-3-fluorobutyrate, whereas the erythro isomer lost the fluorine atom during the reaction, yielding succinic semialdehyde after hydrolysis of the unstable intermediate enamine. The difference between the two isomers demonstrates that the glutamic acid-pyridoxal phosphate Schiff base is present at the active site under a rigid conformation. Furthermore, although the erythro isomer lost the fluorine atom, yielding a reactive aminoacrylic acid in the active site, no irreversible inactivation of E. coli glutamate decarboxylase was observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrystal E., Bey P., Rando R. R. The irreversible inhibition of brain L-glutamate-1-decarboxylase by (2RS,3E)-2-methyl-3,4-didehydroglutamic acid. J Neurochem. 1979 May;32(5):1501–1507. doi: 10.1111/j.1471-4159.1979.tb11091.x. [DOI] [PubMed] [Google Scholar]
- Dunathan H. C. Stereochemical aspects of pyridoxal phosphate catalysis. Adv Enzymol Relat Areas Mol Biol. 1971;35:79–134. doi: 10.1002/9780470122808.ch3. [DOI] [PubMed] [Google Scholar]
- Fonda M. L. Glutamate decarboxylase. Substrate specificity and inhibition by carboxylic acids. Biochemistry. 1972 Mar 28;11(7):1304–1309. doi: 10.1021/bi00757a029. [DOI] [PubMed] [Google Scholar]
- Jung M. J., Metcalf B. W., Lippert B., Casara P. Mechanism of the stereospectific irreversible inhibition of bacterial glutamic acid decarboxylase by (R)-(--)-4-aminohex-5-ynoic acid, an analogue of 4-aminobutyric acid. Biochemistry. 1978 Jun 27;17(13):2628–2632. doi: 10.1021/bi00606a026. [DOI] [PubMed] [Google Scholar]
- Kuo D., Rando R. R. Irreversible inhibition of glutamate decarboxylase by alpha-(fluoromethyl)glutamic acid. Biochemistry. 1981 Feb 3;20(3):506–511. doi: 10.1021/bi00506a010. [DOI] [PubMed] [Google Scholar]
- Likos J. J., Ueno H., Feldhaus R. W., Metzler D. E. A novel reaction of the coenzyme of glutamate decarboxylase with L-serine O-sulfate. Biochemistry. 1982 Aug 31;21(18):4377–4386. doi: 10.1021/bi00261a029. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Reingold D. F., Stanley M. E. D-and L-stereoisomers of allylglycine: convulsive action and inhibition of brain L-glutamate decarboxylase. J Neurochem. 1977 Feb;28(2):349–353. doi: 10.1111/j.1471-4159.1977.tb07754.x. [DOI] [PubMed] [Google Scholar]
- Roberts E. Gamma-aminobutyric acid and nervous system function--a perspective. Biochem Pharmacol. 1974 Oct 1;23(19):2637–2649. doi: 10.1016/0006-2952(74)90033-1. [DOI] [PubMed] [Google Scholar]
- Strausbauch P. H., Fischer E. H., Cunningham C., Hager L. P. Crystallization and properties of glutamate decarboxylase from Escherichia coli strain w+. Biochem Biophys Res Commun. 1967 Aug 23;28(4):525–530. doi: 10.1016/0006-291x(67)90345-2. [DOI] [PubMed] [Google Scholar]
- Ueno H., Likos J. J., Metzler D. E. Chemistry of the inactivation of cytosolic aspartate aminotransferase by serine O-sulfate. Biochemistry. 1982 Aug 31;21(18):4387–4393. doi: 10.1021/bi00261a030. [DOI] [PubMed] [Google Scholar]
- Wofsey A. R., Kuhar M. J., Snyder S. H. A unique synaptosomal fraction, which accumulates glutamic and aspartic acids, in brain tissue. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1102–1106. doi: 10.1073/pnas.68.6.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]


