Abstract
Objective
This study aimed to evaluate the efficacy of autologous blood preparations, namely Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), and Concentrated Growth Factor (CGF), in maxillary sinus floor elevation surgery. The focus was on their impact on new bone formation, maxillary sinus floor height, and soft tissue healing.
Methods
A systematic search was conducted across PubMed/MEDLINE, Web of Science, Embase, and Scopus databases up to April 2024. This systematic review included both randomized clinical trials (RCTs) and controlled clinical trials (CCTs) that evaluated the efficacy of autologous blood preparations in maxillary sinus floor elevation surgery. The primary outcomes measured were the percentage of new bone formation, maxillary sinus floor height, and he percentage of soft tissue area. Data from the selected studies were extracted and analyzed to determine the impact of autologous blood preparations on these outcomes. The risk of bias was assessed using Cochrane’s risk of bias tool and ROBINS-I, and meta-analyses were performed using Review Manager 5.4 software to calculate effect sizes and integrate results from multiple studies.
Results
Among the 507 screened articles, 30 studies met the inclusion criteria. The results indicated that the application of PRP significantly increased new bone formation during maxillary sinus floor elevation surgery (primary outcome, MD = 4.40, CI = 0.37 to 8.44, P = 0.03), as well as improving maxillary sinus floor height elevation (secondary outcome, MD = 1.00, CI = 0.78 to 1.23, P < 0.00001). The absence of PRP during surgery had a statistically significant effect on the percentage of soft tissue area (secondary outcome, MD= -5.25, CI= -7.29 to 3.20, P < 0.00001). However, based on the research findings, PRF did not show significant effects on enhancing new bone formation, maxillary sinus floor height elevation, and promoting soft tissue regeneration.
Conclusions
PRP demonstrates efficacy in maxillary sinus floor elevation surgery by enhancing new bone formation and increasing sinus height. Further studies are needed to validate the outcomes of PRF and CGF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-04938-8.
Keywords: PRP, PRF, CGF, Maxillary sinus floor elevation surgery, Efficacy
Introduction
Bone resorption due to long-term tooth loss can significantly reduce bone volume, affecting the success rates of dental implant surgery, particularly in the maxillary region where anatomical barriers like maxillary sinus pneumatization further complicate the procedure [1, 2]. The maxillary bone must withstand high chewing forces, necessitating strong implant support. Studies have shown that the success rate of implants is influenced by their height and diameter, with longer or thicker implants having better outcomes [3, 4]. When bone conditions are inadequate, bone augmentation surgeries, such as maxillary sinus floor elevation, are essential to increase bone volume [5]. Therefore, when bone conditions at the implantation site are inadequate, bone augmentation surgery is initial to increase the load-bearing bone volume in that area.
Maxillary sinus floor elevation is a widely used technique to enhance bone height in the posterior maxillary region [6]. Initially, this procedure relied on autologous bone grafts from intraoral or extraoral donor sites [7, 8]. Over time, these grafts have been supplemented or replaced by various bone substitutes like allografts, xenografts, and alloplastic materials, either alone or in combination with growth factors [9–13]. Growth factors are crucial as they promote bone formation by initiating signal transduction cascades in osteogenic cells, enhancing osteogenesis [14].
In recent years, autologous blood concentrates, such as PRP, PRF, and CGF, have been proposed as sources of growth factors [15–17]. These concentrates release growth factors like PDGF, TGF-β, IGF, VEGF, bFGF, and HGF, which can enhance bone and soft tissue healing and regeneration [18, 19]. Due to their autologous origin, they avoid immune rejection and reduce infection risks, making them ideal for bone augmentation surgeries [20–22].
PRP, PRF, and CGF differ in their preparation and properties. PRP requires exogenous additives for polymerization, forming a gel-like structure that can hinder cytokine attachment and cell migration due to dense fibrin connections [23]. In contrast, PRF does not need anticoagulants or activators, resulting in a flexible meshwork structure that traps leukocytes and cytokines, releasing growth factors slowly over time [24, 25]. CGF, the latest generation, has a denser fibrin matrix rich in leukocytes and CD34 + cells, promoting endothelial cell migration and angiogenesis [26, 27].
The use of concentrated blood platelets in maxillary sinus floor elevation aims to enhance bone regeneration and soft tissue healing, offering a promising alternative to traditional graft materials [28, 29]. However, the effectiveness of these autologous preparations remains controversial, with some studies reporting significant benefits and others showing no advantage over conventional methods [30, 31]. This systematic review and meta-analysis aim to evaluate the efficacy of PRP, PRF, and CGF in maxillary sinus floor elevation surgery, focusing on their impact on new bone formation, maxillary sinus floor height, and soft tissue healing.
Materials and methods
This systematic review followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered in the International Prospective Register of Systematic Reviews with registration number CRD42024532696 [32]. The AMSTAR II tool was used to assess the quality of the review [33]. Ethical approval was not required for this study since it did not involve the use of human or animal subjects.
The formulation of the guiding question for this systematic review followed the PICO format, PICO Framework:
Population (P): Patients undergoing maxillary sinus floor elevation surgery.
Intervention (I): Use of concentrated blood platelets, including PRP, PRF, and CGF.
Comparison (C): Patients receiving other graft materials.
Outcomes (O): Primary Outcome: Percentage of new bone formation. Secondary Outcomes: Maxillary sinus floor height and the percentage of soft tissue area.
The specific question is: “In patients undergoing maxillary sinus floor elevation surgery (P), does use of concentrated blood platelets (PRP, PRF, CGF) (I) increase the percentage of new bone formation, maxillary sinus floor height, and the percentage of soft tissue area (O) compared to patients receiving other graft materials (C)?”
Search strategy
A comprehensive literature search was conducted across multiple databases, including PubMed/MEDLINE, Web of Science, Embase, and Scopus, to identify relevant articles published until April 2024. The selection of these databases was based on their reputation as prominent sources of pharmaceutical and biomedical literature, ensuring the inclusion of a wide range of relevant articles. The search strategy comprised several key components, such as concentrated blood platelets, Platelet-Rich Plasma, PRP, Platelet-Rich Fibrin, PRF, concentrated growth factor, CGF, maxillary sinus floor elevation, and sinus floor Augmentation. The specific search strategies employed for each database can be found in Table 1. In addition to the database search, a manual search of the reference lists of selected articles was conducted to supplement the study and ensure the inclusion of relevant literature. The search strategies were carefully designed to capture a comprehensive range of articles at the intersection of the key components mentioned.
Table 1.
Search strategy
| Database | Search Strategy | Search Data |
|---|---|---|
| PubMed |
#1 (maxillary sinus floor elevation OR “Sinus Floor Augmentation“[Mesh]) #2 (CGF OR concentrated growth factor OR “Platelet-Rich Fibrin“[Mesh] OR PRF OR “Platelet-Rich Plasma“[Mesh] OR PRP) #1 and #2 |
137 |
| Web of Science |
#1 maxillary sinus floor elevation OR Sinus Floor Augmentation (Topic) #2 concentrated growth factor OR CGF OR Platelet-Rich Fibrin OR PRF OR Platelet-Rich Plasma OR PRP (Topic) #1 and #2 |
440 |
| Scopus |
#1 TITLE-ABS-KEY (maxillary sinus floor elevation) OR TITLE-ABS-KEY (Sinus Floor Augmentation) #2 TITLE-ABS-KEY (concentrated growth factor) OR TITLE-ABS-KEY(CGF) OR TITLE-ABS-KEY (Platelet-Rich Fibrin) OR TITLE-ABS-KEY (PRF) OR TITLE-ABS-KEY (Platelet-Rich Plasma) OR TITLE-ABS-KEY (PRP) #1 and #2 |
218 |
| Embase | (‘maxillary sinus floor elevation’/exp OR ‘maxillary sinus floor elevation’ OR ((‘maxillary’/exp OR maxillary) AND (‘sinus’/exp OR sinus) AND floor AND (‘elevation’/exp OR elevation)) OR ‘sinus floor augmentation’/exp OR ‘sinus floor augmentation’) AND (cgf OR ‘concentrated growth factor’/exp OR ‘concentrated growth factor’ OR (concentrated AND (‘growth’/exp OR growth) AND factor) OR ‘platelet-rich fibrin’/exp OR ‘platelet-rich fibrin’ OR prf OR ‘platelet-rich plasma’/exp OR ‘platelet-rich plasma’ OR ‘prp’/exp OR prp) | 187 |
Eligibility criteria
In selecting publications, the following inclusion criteria were applied: (1) peer-reviewed research articles published in English. The majority of high-impact and widely cited journals in the fields of medicine and biomedical sciences publish in English, enhancing the accessibility and dissemination of research findings. (2) Randomized clinical trials (RCTs) and controlled clinical trials (CCTs) were considered appropriate study designs to assess the effectiveness of concentrated blood platelets; (3) maxillary sinus floor elevation surgery should have been implemented in the study.
The following criteria were used to exclude publications: (1) animal studies, review articles, abstracts, letters, editorials, correspondences, and case reports. (2) Studies that did not provide complete data on the percentage of new bone formation, alveolar ridge height, or soft tissue area. (3) Individuals receiving treatments other than maxillary sinus floor elevation surgery were also excluded.
Study selection and data collection process
The obtained articles from each search strategy were gathered, and duplicate entries were removed. Two authors independently evaluated the title and abstract of each article based on the eligibility criteria. Articles that were deemed ineligible by both authors were immediately excluded, while those considered ineligible by one author but eligible by the other were retained for full-text reading. Subsequently, the remaining studies were read in full by two investigators working together. The eligible studies were then selected, and data extraction was performed. Any discrepancies that arose were resolved through discussion and consensus among all authors.
Detailed information from the selected studies was extracted and compiled into a single document. This included the author(s), publication year, country, funding information, study of type, study settings, sample size, age, Male/Female ratio, platelet concentrate category, type of bone filler used, the number of sinuses, sinus lift complications, the number of implants, implant surgery, baseline information, follow-up time, intervention, Implant survival, the percentage of new bone, the percentage of soft tissue area, outcome measure, and main conclusions. The extracted data were cross-checked by reviewers, and any disagreement was resolved through discussion until a consensus was reached.
Risk of bias
The risk of bias for the included RCTs was assessed using the updated Cochrane Risk of Bias (ROB 2) tool, published in 2020 [34]. This tool evaluates the risk of bias across five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. To ensure a comprehensive evaluation of quality and potential biases in non-RCTs, the ROBINS-I tool was used. This tool assessed the risk of bias by examining domains related to pre-intervention, intervention, and post-intervention. Study assessments were categorized as follows: low risk of bias (≤ one moderate concern within the included domains, comparable), moderate risk of bias (≤ four moderate concerns, credible but not comparable), serious risk of bias (at least one serious concern or multiple moderate concerns, with significant design issues), and critical risk of bias (critical concerns or multiple serious concerns, unreliable) [35]. This rigorous evaluation provided a detailed understanding of the potential biases within each study while minimizing repetition.
Meta-analysis
The meta-analysis was conducted using Review Manager 5.4 software. The primary outcome variables included percentages of new bone formation determined through histologic and histomorphometric analyses of bone graft biopsies harvested prior to implant placement, as well as percentages of soft tissue area assessed by similar analyses of the biopsies. Additionally, maxillary sinus floor height was measured using radiographic imaging. For continuous outcomes, we used the mean difference (MD) when the same units were used across studies. Heterogeneity among the studies was assessed using the I² statistic. Heterogeneity was classified as low (I² < 25%), moderate (25% ≤ I² < 75%), or high (I² ≥ 75%). Based on the level of heterogeneity, either a fixed-effect model or a random-effects model was applied. A fixed-effect model was used when heterogeneity was low, assuming that the observed variability across studies was due to chance. When heterogeneity was moderate or high, a random-effects model was applied to account for the variability among study estimates [36].
We performed subgroup analyses based on the type of bone filler used in the included studies. This approach allowed us to evaluate whether the type of bone filler influenced the efficacy of autologous blood preparations (PRP, PRF, CGF) in maxillary sinus floor elevation surgery. We extracted data specific to each subgroup and conducted separate meta-analyses for each category.
Quality of evidence
The quality of evidence for the analyzed outcomes was assessed using the GRADE system [37]. The GRADE approach involves evaluating the risk of bias, inconsistency, indirectness, imprecision, and publication bias for each outcome. Two reviewers independently assessed the certainty of evidence, and any discrepancies were resolved through discussion and consensus. The evidence was categorized into four levels: high, moderate, low, and very low. The table of evidence quality was created using the GRADEpro software.
Results
Study selection
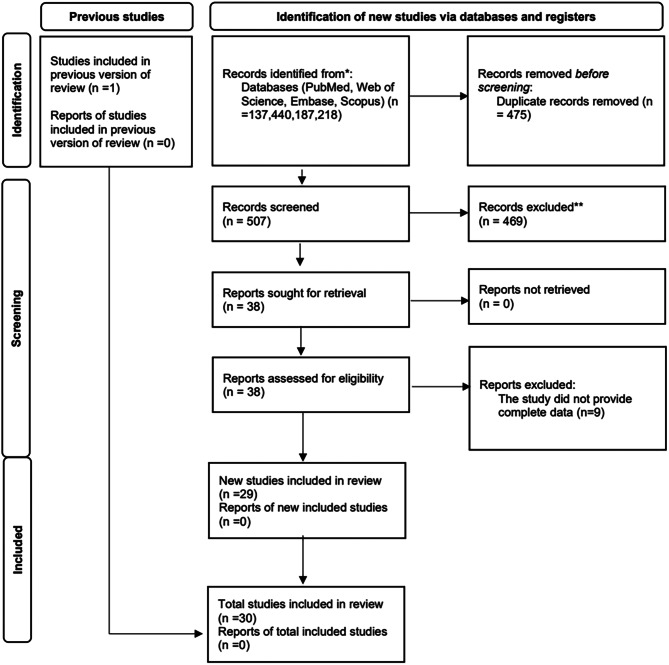
A meticulous electronic search retrieved a total of 982 papers from multiple databases: 137 from PubMed/MEDLINE, 440 from Web of Science, 187 from Embase, and 218 from Scopus. Following the removal of duplicates, 507 studies remained. Subsequent scrutiny of titles and abstracts led to the exclusion of 469 studies. A thorough evaluation of the full texts of the remaining studies resulted in the exclusion of 9 studies that did not meet the eligibility criteria. Ultimately, 30 studies were included in this systematic review, as illustrated in Fig. 1.
Fig. 1.
Flow chart of the literature search and results
The characteristics of the included literature
Table 2 presents a comprehensive description of key features within the analyzed studies. The included studies spanned from 2005 to 2023 and were conducted across various countries, with Turkey (6 studies), China (4 studies), and South Korea (2 studies) contributing the most articles. Other articles originated from countries such as Sweden, Spain, and Brazil. In terms of study design, 17 studies employed a RCT design, while 15 studies used a CCT design. Sample sizes ranged from 5 to 87, with 3 studies having sample sizes exceeding 50. Regarding the age of the study population, the majority of studies focused on individuals aged 40 to 60 years, with most studies maintaining a balanced gender ratio. Regarding platelet concentrate categories, PRF was the most commonly studied, followed by PRP, with comparatively fewer studies on CGF. The number of maxillary sinuses addressed ranged from 10 to 144 across the included studies. While most studies reported no postoperative complications or lacked relevant reports, 9 studies documented complications following sinus floor elevation procedures. The number of implants ranged from 11 to 286 across the included studies, with 4 studies reporting implant numbers exceeding 100. Surgical approaches included immediate, early, and delayed implantation. Most included samples presented with insufficient alveolar bone height. Follow-up times ranged from 5 to 6 months on average, with some studies extending beyond 12 months. Experimental interventions involved the addition or omission of platelet concentrates combined with other graft materials as variables. Postoperative implant survival rates mostly remained at 100%; however, some studies reported proportions of implant surgery failures. While most studies reported percentages of postoperative new bone formation and the percentages of new soft tissue area, some studies did not mention relevant data.
Table 2.
The characteristics of included studies
| Authors, year | Country | Funding information | Type of Study | Study settings | Sample size(n) | age | M/F ratio |
|---|---|---|---|---|---|---|---|
| Adali et al. [38] | Turkey | None declared | RCT | Ege University | 10 | 57 | 8 M/2F |
| Amam et al. [39] | Syria | None declared | RCT | Damascus University, | 9 | 45–70 | NA |
| Anitua et al. [40] | Spain | Basque and Spanish governments | CCT | Spain | 5 | NR | 2 M/3F |
| Batas et al. [41] | Greece | None declared | RCT | Aristotle University | C: 6 T: 6 | NR | NR |
| Bolukbasi et al. [42] | Turkey | None declared | CCT | Istanbul University | 25 |
T: 50.06 ± 12.15 C: 47.43 ± 8.2 |
10 M/15F |
| Bosshardt et al. [43] | Switzerland | None declared | CCT | University of Geneva | T: 5 C: 3 | 41–64 | 1 M/7F |
| Cabbar et al. [18] | Turkey | None declared | CCT | Yeditepe University | 10 | 53.7 ± 0.8 | 7 M/3F |
| Cho et al. [44] | Korea | Hanyang University | RCT |
Hanyang University Hospital and Apsun Dental Hospital |
40 | T: 46.8 C: 44.4 | 21 M/19F |
| Choukroun et al. [45] | France | None declared | CCT | France | 9 | NR | NR |
| Cömert et al. [46] | Turkey | Ataturk University | RCT | Ataturk University | 26 A: 9 B: 8 C: 9 |
A: 34.01 ± 9.59 B:35.48 ± 9.53 C: 31.51 ± 8.52 |
A:5 M/4F B:5 M/3F C:7 M/2F |
| de et al. [47] | Brazil | Coordenação de Aperfeiçoamento de Pessoal de Nível Superior | RCT | School of Dentistry at Araraquara | 24 | 54.08 ± 10.07 | 10 M/14F |
| Gassling et al. [48] | Germany | The author’s institution | RCT | University Hospital of Schleswig-Holstein | 6 | 61 | NA |
| Jia et al. [49] | China | Peking University School and Hospital of Stomatology | RCT | Peking University Hospital of Stomatology | 23 | T: 51.1 ± 10.2 C: 49.1 ± 10.2 | 12 M/11F |
| Kanayama et al. [50] | Japan | None declared | CCT | Nagoya City University Hospital | 27 | 54.2 | 12 M/15F |
| Karagah et al. [51] | Iran | No external funding | RCT | Qazvin University of Medical Sciences | 10 | 48.3 ± 8.31 | 3 M/7F |
| Kassolis et al. [52] | USA | None declared | CCT | University of Maryland Dental School | 10 | NR | NR |
| Kiliç et al. [53] | Turkey | Atatürk University | RCT | Atatürk University | 18 |
T: 34.01 ± 9.59 C: 31.51 ± 8.52 |
T:5 M/4F C:7 M/2F |
| Lv et al. [54] | China | National Nature Science Foundation of China and Key Scientific and Technological Projects of Jilin Province, China | RCT | Stomatology Hospital of Jilin University | 40 | A: 51.00 B: 54.50 | 18 M/22F |
| Molemans et al. [55] | Belgium | None declared | CCT | Stomatology Hospital of Jilin University | 26 | 55 ± 10.1 | 14 M/12F |
| Nizam et al. [56] | Turkey | None declared | RCT | Ege University | 13 | 49.92 ± 10.37 | 9 M/4F |
| Olgun et al. [57] | Turkey | Kirikkale University | RCT | Kirikkale University | 18 | T: 51 ± 7.94 C: 53 ± 8.96 | 9 M/9F |
| Pichotano et al. [58] | Brazil | Geistlich Pharma AG; Neodent; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior | RCT | São Paulo State University | 12 | 54.17 ± 6.95 | 6 M/6F |
| Poeschl et al. [59] | Austria | None declared | CCT | Medical University of Vienna | 25 | 26–82 | NR |
| Raghoebar et al. [60] | Netherlands | None declared | CCT | University Hospital Groningen | 5 | 58.4 ± 1.9 | 2 M/3F |
| Taschieri et al. [61] | Italy | None declared | CCT | Dental Clinic of the IRCCS Istituto Ortopedico Galeazzi | T: 5 C: 5 | 48–71 | 2 M/4F |
| Tatullo et al. [62] | Italy | None declared | RCT | University of Bari, the Dental Clinic “Calabrodental” and the Institute of Research “Tecnologica” | 60 T: 30 C: 18 Split mouth: 12 | 43–62 | 12 M/48F |
| Thor et al. [63] | Sweden | None declared | CCT | Stockholm Söder Hospital | 19 | 58 | 2 M/17F |
| Thor et al. [31] | Sweden | None declared | CCT | Uppsala University Hospital | 11 | 33–72 | 1 M/10F |
| Torres et al. [64] | Spain | the Ministry of Science and Technology, the UCM Programme for Research Groups, and the Spanish Agency of International Cooperation | RCT | The dental clinic “Clinica Dental Alcala” | 87 | 52–78 | 40 M/47F |
| Zhang et al. [65] | China | None declared | RCT | Peking University | 11 |
T: 43.5 C: 46.2 |
8 M/2F |
| Authors, year | platelet concentrate category | type of bone filler used | the number of sinuses | Sinus Lift Complications | the number of implants | Implant Surgery | |
| Adali et al. [38] | CGF | allogenic bone material | 20 | T: 0 C: 0 | 20 | 6 months after sinus lifting | |
| Amam et al. [39] | A-PRF | CS (on one side) TCP (on the other side) | 18 | NR | NR | NR | |
| Anitua et al. [40] | PRGF | Bio-Oss | 10 | T: 0 C: 0 | NR | NR | |
| Batas et al. [41] | PRGF | Bio-Oss | 12 | T: 0 C: 0 | NR | NR | |
| Bolukbasi et al. [42] | PRF | bovine bone graft | T: 17 C: 15 | T: 0 C: 0 | T: 34 C: 32 | 6 months after sinus lifting | |
| Bosshardt et al. [43] | PRF | Synthetic nanocrystalline hydroxyapatite embedded in highly porous silica gel matrix | 12 T: 8 C: 4 | T:0 C:0 | 16 | 7–11 months after sinus lifting | |
| Cabbar et al. [18] | L-PRP | USB | 20 | T: 2 C: 2 | 28 | 6 months after sinus lifting | |
| Cho et al. [44] | PRF | None | 40 T: 20 C: 20 | T: 0 C: 0 | 45 | Immediate | |
| Choukroun et al. [45] | PRF | FDBA | T: 6 C: 3 | T: 1 C: 0 | NR | T: 4 months after sinus lifting C: 8 months after sinus lifting | |
| Cömert et al. [46] | P-PRP, PRF | β-TCP | NR | A: 1 B: 2 C: 2 | NR | 6 months after sinus lifting | |
| de et al. [47] | LPRF | DBBM | 24 | T: 0 C: 0 | 37 | 8 months after sinus lifting | |
| Gassling et al. [48] | PRP | Bio-Oss | T: 6 C: 6 | NR | 32 | 5 months after sinus lifting | |
| Jia et al. [49] | CGF | Bovine-derived xenograft | T: 10 C: 13 | NR | 38 | Immediate | |
| Kanayama et al. [50] | PRF | None | A: 20 B: 19 | T: 0 C: 0 | A: 20 B: 19 | Immediate | |
| Karagah et al. [51] | PRF | FDBA | 20 | NR | 20 | Immediate | |
| Kassolis et al. [52] | PRP | FDBA | 20 | NR | NR | NR | |
| Kiliç et al. [53] | PRP | β-TCP | 18 T: 9 C: 9 | 3 | NR | 6 months after sinus lifting | |
| Lv et al. [54] | PRF | DBBM | NR | NR | 57 | Immediate | |
| Molemans et al. [55] | LPRF | None | 28 | NR | 29 | Immediate | |
| Nizam et al. [56] | L-PRF | DBBM | T: 13 C: 13 | T: 0 C: 0 | T: 30 C: 28 | 6 months after sinus lifting | |
| Olgun et al. [57] | T-PRF | Allografts | T: 10 C: 8 | T: 0 C: 0 | 37 | T: 4 months after sinus lifting C: 6 months after sinus lifting | |
| Pichotano et al. [58] | L-PRF | DBBM | T: 12 C: 12 | T: 0 C: 0 | T: 19 C: 19 | T: 4 months after sinus lifting C: 8 months after sinus lifting | |
| Poeschl et al. [59] | PRP | collected bone and algae-derived hydroxyapatite | 31 T: 18 C: 13 | 1 | 42 | 6–9 months after sinus lifting | |
| Raghoebar et al. [60] | PRP | autologous bone graft from the iliac crest | 10 | 1 | 30 | 3 months after sinus lifting | |
| Taschieri et al. [61] | P-PRP | Bio-Oss | 10 | NR | NR | 6 months after sinus lifting | |
| Tatullo et al. [62] | PRF | DBBM | 72 | T: 0 C: 0 | 240 | Early: 20 Intermediate: 20 Late: 20 | |
| Thor et al. [63] | PRP | autologous bone graft from the iliac crest | 38 | NR | 152 | 6 months after sinus lifting | |
| Thor et al. [31] | PRP | autologous bone graft from the iliac crest | 22 | NR | NR | 3 months after sinus lifting | |
| Torres et al. [64] | P-PRP | Bio-Oss | 144 T: 74 C: 70 | T: 3 C: 2 | 286 | deferred in 57 sinuses and immediate in 87 | |
| Zhang et al. [65] | PRF | Bio-Oss | 11 T: 6 C: 5 | NR | T:6 C:5 | 6 months after sinus lifting | |
| Authors, year | Baseline information | Follow-up time(m) | Intervention | Implant survival | the percentage of new bone | soft-tissue area | |
| Adali et al. [38] | Residual bone height: about 1–3 mm | 6 | T: allograft + CGF C: allograft | 100% | T: 36.41% C: 35.49% | NR | |
| Amam et al. [39] |
Mean bone height values (mm): CS + A-PRF: 3.55 ± 2.13 TCP + A-PRF: 3.86 ± 1.73 CS/TCP + A-PRF: 3.71 ± 1.89 |
6 | CS + A-PRF (on one side) TCP + A-PRF (on the other side) | NR | NR | NR | |
| Anitua et al. [40] | Residual bone height: 1–3 mm | 5 | T: Bio-Oss + PRGF C: Bio-Oss | NR | T: 24.9 ± 4.94 C: 8.3 ± 0.14 | NR | |
| Batas et al. [41] | Residual bone height < 3 mm | 6 | T: Bio-Oss + PRGF C: Bio-Oss | NR | T: 35.6 ± 8.26 C: 37.8 ± 3.15 | T: 34.69 ± 5.86 C: 34.88 ± 5.81 | |
| Bolukbasi et al. [42] | Residual crest height < 5 mm | 6 |
T: bovine bone graft + PRF C: resorbable collagen membrane + bovine bone graft |
100% | T: 35.0 ± 8.60 C: 32.97 ± 9.71 | T: 30.63 ± 7.53 C: 33.94 ± 9.15 | |
| Bosshardt et al. [43] | The vertical height of the residual ridge: <4 mm | 7–11 |
T: Synthetic nanocrystalline hydroxyapatite embedded in highly porous silica gel matrix + PRF C: Synthetic nanocrystalline hydroxyapatite embedded in highly porous silica gel matrix + collagen membrane |
NA | T: 28.6 ± 6.9 C: 28.7 ± 5.4 |
T: 45.7 ± 9.3 C: 45.8 ± 3.2 |
|
| Cabbar et al. [18] | The mean height of the residual alveolar crest(mm): T: 4.7 ± 1.3 C: 5.6 ± 1.4 | 12-15.2 | T: USB + L-PRP C: USB | 92.9% | T: 16.0 ± 3.8 C: 15.8 ± 4.8 |
T: 57.8 ± 4.4 C: 59.9 ± 7.5 |
|
| Cho et al. [44] | RABH (mm): T: 7.2 ± 1.1 C: 6.4 ± 1.1 | 6; 12 | T: PRF C: saline | 100% | NR | NR | |
| Choukroun et al. [45] | NR | 6/8 |
T: FDBA + PRF C: FDBA |
NR |
T after 4 months: 20.95 C after 8 months: 20.31 |
NR | |
| Cömert et al. [46] | Residual bone crest height: 7 mm or lesser | 6 |
A: P-PRP + β-TCP B: PRF + β-TCP C: β-TCP |
NR | A: 34.83 ± 10.1 B: 32.03 ± 6.3 C: 33.40 ± 10.4 | A: 36.19 ± 13.9 B: 35.31 ± 10.8 C: 36.21 ± 10.6 | |
| de et al. [47] | Residual height of the alveolar ridge: less than or equal to 4 mm | 8 | T: LPRF and DBBM C: DBBM | 100% | T: 46.56 ± 12.25 C: 32.34 ± 9.49 | T: 17.76 ± 12.03 C: 28.29 ± 10.07 | |
| Gassling et al. [48] | RABH < 5 mm | 5 |
PRP (on one side) collagen (on the other side) |
NR | T: 17.0 C: 17.2 | NR | |
| Jia et al. [49] | RABH (mm): T: 3.58 ± 1.49 C: 4.12 ± 1.61 | 12 | T: CGF + bone window repositioning C: CGF | 100% | NR | NR | |
| Kanayama et al. [50] | Residual bone height(mm): A: 2.85 ± 1.13 B: 2.68 ± 1.20 | 12 |
A: PRF + sandblasted acid-etched implant B: PRF + hydroxyapatite implant |
100% | NR | NR | |
| Karagah et al. [51] | ISQ: FDBA: 53.25 ± 2.268 PRF: 53.00 ± 2.384 | 2; 4; 6 | PRF (on one side) FDBA (on the other side) | NR | NR | NR | |
| Kassolis et al. [52] |
Residual bone height: T: 5.2 ± 2.2 C: 5.0 ± 3.2 mm |
2 | T: FDBA + PRP C: FDBA + membrane | NR | T: 33.3 ± 1.3 C: 26.5 ± 6.8 | NR | |
| Kiliç et al. [53] | Residual bone crest height: T: 2.70 ± 2.57 C: 4.88 ± 2.37 | 6 |
T: β-TCP + PRP C: β-TCP |
NR | NR | NR | |
| Lv et al. [54] | Residual bone height: 2–6 mm | 18 |
A: PRF + lapless endoscope-assisted osteotome SFE B: deproteinised bovine bone matrix + lateral SFE |
A:96.15% B:100% | NR | NR | |
| Molemans et al. [55] |
Residual bone height (mm): A: 6.1 ± 1.6 B: 4.6 ± 2.0 |
6 |
A: transalveolar SFE + LPRF B: lateral SFE + LPRF |
93.10% | NR | NR | |
| Nizam et al. [56] |
Residual bone height (mm): T: 2.45 ± 0.79 C: 2.53 ± 0.61 |
6 | T: DBBM + L-PRF C: DBBM | 100% | T: 21.4 ± 8.8 C: 21.2 ± 5.6 | T: 52.7 ± 12.5 C: 45.9 ± 8.4 | |
| Olgun et al. [57] | Residual crest height of < 5 mm | 6 | T: T-PRF C: Allografts | NR | T: 16.6 ± 1.1 C: 17.3 ± 2.5 | NR | |
| Pichotano et al. [58] | Residual bone height < 4 mm | 4 for the test group and 8 for the control group | T: DBBM + L-PRF C: DBBM | 100% | T: 44.6 ± 13.9 C: 30.0 ± 8.4 | T: 26.6 ± 11.1 C: 30.6 ± 12.5 | |
| Poeschl et al. [59] | Residual bone height < 5 mm | 7 | T: Algae-derived HA + PRP C: Algae-derived HA | 96.4% | T: 29.0 ± 13.2 C: 22.3 ± 12.3 | NR | |
| Raghoebar et al. [60] | The mean vertical height of the alveolar bone(mm): 1–3 | 3 | PRP (left or right sinus) | NR | Non-PRP side: 41.1 ± 8.3 PRP side: 38.4 ± 11.3 | NR | |
| Taschieri et al. [61] |
Residual Bone Height(mm): T: 2.80 ± 1.04 C: 2.40 ± 1.08 Vital Bone (%): T: 30.70 ± 7.89 C: 22.72 ± 9.21 |
6 | T: Bio-Oss + P-PRP C: Bio-Oss | 100% |
T: 30.70 ± 7.89 C: 22.72 ± 9.21 |
NR | |
| Tatullo et al. [62] | The residual ridge: < 5 mm | 3–5 | T: DBBM + PRF C: DBBM | 100% | NR | NR | |
| Thor et al. [63] |
The mean marginal bone level(mm): T: 1.3 ± 1.9 C: 1.5 ± 1.7 |
18 |
T: particulated bone mixed + PRP C: onlay block grafts |
98.7% T:100% C:97.4% | NR | NR | |
| Thor et al. [31] | NR | NR | T: Autogenous bone + PRP C: Autogenous bone | NR | T:14 ± 7 C:13 ± 6 | NR | |
| Torres et al. [64] | Residual crest height < 7 mm | 6 | T: Bio-Oss + PRP C: Bio-Oss | 97.50% | T: 30 ± 6 C: 20 ± 4 |
T: 22 ± 9 C: 28 ± 4 |
|
| Zhang et al. [65] | Residual crest height < 5 mm | 6 |
T: Bio-Oss and PRF C: Bio-Oss |
NR | T: 18.4 ± 5.6 C: 12.9 ± 5.3 | NR |
CS: calcium sulfate; A-PRF: advanced platelet-rich fibrin; TCP: tricalcium phosphate; CGF: concentrated growth factor; P-PRP: pure platelet-rich plasma; PRGF: platelet-rich growth factor; DBB: deproteinized bovine bone; PRF: Platelet-rich fibrin; USB: Unilab Surgibone; RABH: residual alveolar bone height; β-TCP: beta-tricalcium phosphate; LPRF: leukocyte- and platelet-rich fibrin; DBBM: deproteinized bovine bone mineral; PRP: platelet-rich plasma; PRF: Platelet-rich fibrin; FDBA: freeze-dried bone allograft; ISQ: implant stability quotient; SFE: Sinus floor elevation; T-PRF: titanium-prepared platelet rich fibrin; L-PRF: leukocyte and platelet-rich fibrin
The main conclusions drawn from the included literature
Table 3 provides a range of findings on the efficacy of autologous blood preparations in sinus floor elevation surgery. The primary outcomes of interest in these studies were the percentage of new bone formation, maxillary sinus floor height, and the percentage of soft tissue area.
Table 3.
The main findings
| Authors, year | outcome measure | Main finding |
|---|---|---|
| Adali et al. [38] |
New bone formation (%): T: 36.41% C: 35.49% Residual graft particles (%): T: 5.10% C: 5.80% |
Using CGF with allografts supports the stabilization of gained vertical bone height after sinus augmentation, but further research is needed to determine the accelerating effects of CGF on new bone formation. |
| Amam et al. [39] |
The mean bone gain(mm): CS + A-PRF: 7.96 ± 2.78 TCP + A-PRF: 7.53 ± 1.15 Mean bone height values (mm): CS + A-PRF: 11.51 ±2.44 TCP + A-PRF: 11.39 ± 0.93 CS/TCP + A-PRF: 11.45 ± 1.79 |
Using CS or TCP mixed with A-PRF was beneficial and safe in the two-stage maxillary sinus lifting procedure. |
| Anitua et al. [40] | New bone formation (%): T: 24.9 ± 4.94 C: 8.3 ± 0.14 | PRGF may present a role in reducing tissue inflammation after surgery, increasing new bone formation and promoting the vascularization of bone tissue. |
| Batas et al. [41] | Relative volumes of bone formation(mm): T: 35.6 ± 8.26 C: 37.8 ± 3.15 | PRGF as adjunct to DBB for MSFA, except from improved handling during the operation, does not appear to enhance nor interfere with bone formation inside the human sinus 6 months after MSFA, compared with the use of DBB alone. |
| Bolukbasi et al. [42] |
Means percentages of new bone formation (%): T: 35.0 ± 8.60 C: 32.97 ± 9.71 Means percentages of biomaterial remnants (%): T: 33.05 ± 6.29 C: 33.79 ± 8.57 |
PRF and bovine bone graft material combination may be an alternative treatment option to the routinely used bovine bone graft material and collagen membrane combination. |
| Bosshardt et al. [43] | Residual graft material (%): T: 25.7 ± 8.8 C: 25.5 ± 7.6 | The nanoporoushydroxyapatite-silicagelused for sinus floor elevation in humans is osteoconductive supports new bone formation comparable to most other bone substitute materials. |
| Cabbar et al. [18] | Residual graft: T: 23.6 ± 5.9 C: 21.9% ± 6.6% | The combination of USB and PRP does not have any effect on new bone formation and implant stabilization. |
| Cho et al. [44] |
The increases in residual alveolar bone height(mm): 6 m: T:3.0 ± 0.8 C:3.4 ± 0.7 12 m: T:2.6 ± 1.1 C:1.7 ± 1.0 |
The feasibility of hydraulic transrectal sinus lifting without bone graft was confirmed and PRF might be a better filler to support the elevated sinus membrane. |
| Choukroun et al. [45] | Non vital bone (%): T after 4 months: 9.41% C after 8 months: 10.93% | Sinus floor augmentation with FDBA and PRF leads to a reduction of healing time prior to implant placement. |
| Cömert et al. [46] |
Means percentages of new bone (%): A: 34.83 ± 10.12 B: 32.03 ± 6.34 C: 33.40 ± 10.43 Means percentages of graft particle (%): A: 28.98 ± 7.94 B: 32.66 ± 7.46 C: 30.39 ± 10.29 |
Adding P-PRP or PRF to β-TCP graft substitute was not beneficial on new bone formation and regeneration, and P-PRP plus β-TCP or PRF plus β-TCP is not superior to β-TCP alone. |
| de et al. [47] |
Bone neoformation (%): T: 46.56 ± 12.25 C: 32.34 ± 9.49 Percentage of residual graft (%): T: 7.01 ± 8.49 C: 12.58 ± 9.19 |
The use of L-PRF might be an interesting alternative to use in combination with DBBM for augment the maxillary sinuses allowing the installation of appropriate length implants in shorter period of time. |
| Gassling et al. [48] |
Mean vital bone formation (%): collagen side: 17.2 PRP side: 17.0 The mean of residual bone-substitute (%): collagen side: 17.3 PRP side: 15.9 |
Within the limits of the study the coverage of the lateral sinus window with two different absorbable membranes has been shown to result in a similar amount of vital bone formation and residual bone-substitute. |
| Jia et al. [49] |
Alveolar bone height(mm): T: 13.32 ± 1.86 C: 10.92 ± 1.51 Intra-sinus bone augmentation(mm): T: 9.74 ± 2.20 C: 6.86 ± 2.40 |
Lateral sinus floor elevation with bone window repositioning may result in higher bone augmentation after 1 year than the traditional approach. |
| Kanayama et al. [50] |
Endo sinus bone gains around the implant (mm): A: 4.38 ± 1.67 B: 4.00 ± 1.63 |
Platelet-rich fibrin promoted end sinus bone gain when used as the grafting material in the crestal approach to sinus floor elevation. |
| Karagah et al. [51] |
ISQ: 2 m: FDBA: 56.50 ± 3.171 PRF: 59.95 ± 3.284 4 m: FDBA: 60.75 ± 2.573 PRF: 67.55 ± 1.791 6 m: FDBA: 62.65 ± 2.110 PRF: 69.85 ± 2.059 |
Within the limitations of this study, PRF yielded superior results compared with FDBA regarding the stability of one-stage dental implants. |
| Kassolis et al. [52] |
Residual graft particles (%): T: 21.2 ± 8.3 C: 37.0 ± 15.7 Alveolar bone height(mm): T: 13.9 ± 2.1 C: 13.2 ± 1.3 |
FDBA and PRP enhances the rate of formation of bone compared with FDBA and membrane, when used in sub antral sinus augmentation. |
| Kiliç et al. [53] | Vertical bone height gain(mm): T: 13.19 ± 3.32 C: 11.59 ± 3.02 | Both grafting materials produced sufficient vertical bone height gain for safe implant placement. |
| Lv et al. [54] |
Peri-implant residual bone height immediately post-surgery(mm): A: 3.35 ± 0.79 B: 2.92 ± 0.63 The marginal bone loss(mm): A: 0.60 ± 0.25 B: 0.69 ± 0.35 |
Within the limitations of this study, PESS was associated with lower postoperative morbidity and was more tolerable than LSFE. |
| Molemans et al. [55] |
The mean vertical bone gain (mm): A: 3.4 ± 1.2 B: 5.4 ± 1.5 |
L-PRF as a sole graft material during simultaneous SFE and implant placement proved to be a practical, safe, and economical sub sinus graft material, resulting in natural bone formation. |
| Nizam et al. [56] |
Augmented bone height (mm): T: 13.60 ± 1.09 C: 13.53 ± 1.20 The percentage of newly formed bone (%): T: 21.38 ± 8.78 C: 21.25 ± 5.59 The percentage of residual bone graft (%): T: 25.95 ± 9.54 C: 32.79 ± 5.89 |
The addition of L-PRF in DBBM did not improve the amount of regenerated bone or the amount of the graft integrated into the newly formed bone under histological and histomorphometry evaluation. |
| Olgun et al. [57] |
ISQ: T: 68.50 ± 8.87 C: 66.37 ± 8.31 Newly formed bone (%): T: 16.58 ± 1.05 C: 17.28 ± 2.53 |
The use of T-PRF alone in sinus-lifting operations has successful clinical and histomorphometry results. |
| Pichotano et al. [58] |
Newly formed bone (%): T: 44.58 ± 13.90 C: 30.02 ± 8.42 Residual graft material (%): T: 3.59 ± 4.22 C: 13.75 ± 9.99 |
The addition of L-PRF to the DBBM into the maxillary sinus allowed early implant placement with increased new bone formation than DBBM alone after 8 months of healing. |
| Poeschl et al. [59] | Biomaterial area (%): T: 20.1 ± 13.0 C: 20.3%±12.9 | Better overall resorption of algae-derived hydroxyapatite AlgOss/C Graft/Algipore and increased new bone formation when PRP was used, especially in the apical region. |
| Raghoebar et al. [60] |
The relative area occupied with bone in the first premolar (P1) and first molar (M1) region (%; mean SD): non-PRP side: 41.1 ± 8.3 PRP side: 38.4 ± 11.3 |
In this study, no beneficial effect of PRP on wound healing and bone remodeling was observed. |
| Taschieri et al. [61] | The mean percentage of vital bone: T: 22.72 ± 9.21 C: 30.70 ± 7.89 | The adjunct of pure platelet-rich plasma to deproteinized bovine bone mineral may enhance vital bone formation in the first 6 months after sinus floor augmentation. |
| Tatullo et al. [62] |
Medullary spaces: Early protocol: Early protocol T: 70.2 C: 68.4 Intermediate protocol T: 70.0 C: 68.2 Late protocol: T: 61.4 C: 58.2 Osteoid borders: Early protocol: T: 7.01 C: 5.12 Intermediate protocol: T: 3.84 C: 3.12 Late protocol: T: 3.5 C: 2.9 Trabecular bone: Early protocol: T: 22.8 C: 26.4 Intermediate protocol: T:26.2 C: 28.7 Late protocol: T: 37.1 C: 38.9 |
The use of PRF and piezosurgery reduced the healing time, compared to the 150 days described in literature, favoring optimal bone regeneration. |
| Thor et al. [63] |
The mean marginal bone level after 1 year in function(mm): T: 1.8 ± 1.1 C: 2.0 ± 0.9 |
A high implant survival rate and stable marginal bone conditions can be achieved after 1 year of loading in the maxilla following autogenous bone grafting whether or not PRP is used. |
| Thor et al. [31] | Bone-to-implant contact: T: 17 ± 13 C: 20 ± 15 | PRP has a rather low regenerative capacity but may influence the early phase of bone healing. |
| Torres et al. [64] | The height of the augmented bone(mm): T: 10.4 ± 0.7 C: 9.4 ± 0.7 | PRP can improve the osteoconductive properties of ABB by increasing the volume of new bone formed. |
| Zhang et al. [65] |
Means percentages of new bone formation (%): T: 18.35 ± 5.62 C: 12.95 ± 5.33 Means percentages of biomaterial remnants (%): T: 19.16 ± 6.89 C: 28.54 ± 12.01 |
Neither an advantage nor disadvantage of the application of PRF in combination with deproteinized bovine bone mineral in sinus augmentation after a healing period of 6 months. |
MSFA: maxillary sinus floor augmentation
Studies involving PRP demonstrated a significantly higher rate of new bone formation in the treatment groups compared to controls, indicating that PRP helps reduce tissue inflammation and promote bone tissue vascularization [18, 31, 46, 52, 59, 61, 63]. Several studies indicated that the percentage of new bone formation was higher in the experimental groups using PRF compared to control groups, suggesting that PRF aids in stabilizing the vertical bone height achieved. Additionally, PRF combined with other grafting materials may represent a superior treatment option [42, 47, 56, 58, 65]. However, some studies reported contradictory findings, suggesting that the use of autologous blood preparations may have little impact on the percentage of new bone formation [46, 57, 60].
Research indicated that the increase in maxillary sinus floor height was greater in the experimental groups than in the control groups, implying that autologous blood preparations might serve as better fillers for supporting the elevated sinus membrane [53, 56, 64]. Furthermore, one study found that after one year of treatment with lateral window sinus floor elevation, the bone gain in the treatment groups was greater than in the control groups, further supporting the efficacy of autologous blood concentrates [49].
The percentage of soft tissue area was measured in the same tissue sections as the percentage of new bone, based on histological and histomorphometric analysis of bone graft biopsies collected prior to implantation. Multiple studies observed that autologous blood concentrates had a minimal effect on soft tissue generation, with the percentage of soft tissue area being lower in the experimental groups compared to the control groups [42, 43, 46, 47, 58]. This suggests that autologous blood concentrates may predominantly promote new bone regeneration.
Risk of bias
Figure 2A offers an overview of the quality assessment conducted on the included RCTs. A total of 17 RCTs were evaluated using the RoB 2 tool. The assessment revealed that 6 studies were classified as having a low risk of bias, 11 studies had some unclear bias primarily due to unclear bias related to the blinding of participants and personnel, and none were judged to be at a high risk of bias. Figure 2B shows the quality assessment for the 13 included CCTs. Of these, 5 studies were assessed as having a low risk of bias, while 8 studies were categorized as having a moderate risk of bias.
Fig. 2.
Risk of bias
Meta-analysis
Through analysis of the included studies, we excluded those that did not provide data on the percentage of new bone, maxillary sinus floor height, or soft tissue area. Ultimately, we conducted meta-analyses on the effects of PRP and PRF on maxillary sinus floor elevation surgery outcomes. However, relevant indicators were not provided in studies on CGF, precluding meta-analysis.
PRP
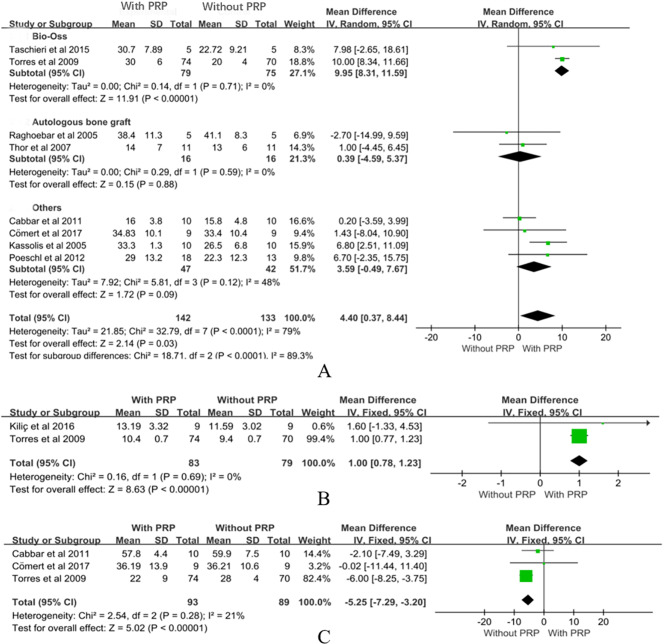
Meta-analysis of the impact of PRP on the percentage of new bone formation included 8 studies [18, 46, 52, 59–61, 63, 64], involving 142 surgical patients in the experimental group and 133 in the control group, demonstrating the positive effect of PRP on new bone generation in maxillary sinus floor elevation surgery. The overall analysis indicated that the use of PRP increased new bone formation by 4.4% points (MD = 4.40, CI = 0.37 to 8.44, P = 0.03, Fig. 3A). However, significant heterogeneity was observed in the analysis (P < 0.00001, I2 = 79%). Studies using Bio-Oss as bone fillers demonstrated a significant increase in new bone formation with PRP compared to controls. The meta-analysis revealed a mean difference of 9.95% (CI: 8.31 to 11.59, P < 0.00001) for PRP [61, 64]. PRP combined with autologous bone graft did not show a statistically significant effect on new bone formation (MD = 0.39, CI: -4.59 to 5.37, P = 0.88) [60, 63]. The use of other materials with PRP did not result in a significant increase in new bone formation (MD = 3.59, CI: -0.49 to 7.67, P = 0.09) [18, 46, 52, 59].
Fig. 3.
Meta-analysis results of PRP
Regarding the influence of PRP on maxillary sinus floor height, 2 studies were included in the meta-analysis [53, 64], encompassing 83 patients in the experimental group and 79 patients in the control group. The results demonstrated that the application of PRP in maxillary sinus floor elevation surgery significantly increased maxillary sinus floor height. The overall analysis revealed a significant increase of 1 mm in height with the use of PRP (MD = 1.00, CI = 0.78 to 1.23, P < 0.00001, Fig. 3B). Additionally, no heterogeneity was observed among the studies (P = 0.69, I²=0%).
Meta-analysis of PRP’s impact on the percentage of soft tissue area included 3 studies [18, 46, 64], involving 93 patients in the experimental group and 89 patients in the control group. The results indicated that the absence of PRP during surgery had a statistically significant effect on the percentage of soft tissue area (MD= -5.25, CI= -7.29 to 3.20, P < 0.00001, Fig. 3C), suggesting that a larger percentage of new bone was present in the same tissue section. Therefore, PRP may be more conducive to promoting new bone formation.
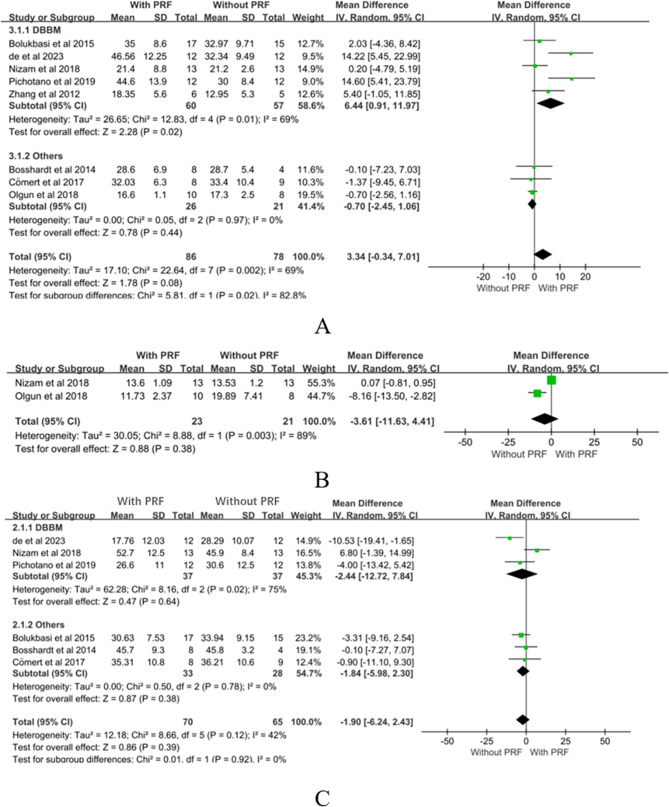
PRF
An analysis of PRF’s impact on the percentage of new bone formation included 8 studies [42, 43, 46, 47, 56–58, 65], involving 86 surgical patients in the experimental group and 78 surgical patients in the control group. The findings demonstrated a positive trend of PRF on new bone generation during maxillary sinus floor elevation surgery. However, the overall analysis did not reveal a statistically significant difference (MD = 3.34, CI= -0.34 to 7.01, P = 0.08, Fig. 4A), with moderate heterogeneity observed across the studies (P = 0.002, I²=69%). Studies using deproteinized bovine bone mineral (DBBM) as bone fillers demonstrated a significant increase in new bone formation with PRF compared to controls. The meta-analysis revealed a mean difference of 6.44% (CI: 0.91 to 11.97, P = 0.02) for PRF [42, 47, 56, 58, 65]. When combined with other materials, PRF did not show a statistically significant effect on new bone formation (MD=-0.70, CI: -2.45 to 1.06, P = 0.44) [43, 46, 57].
Fig. 4.
Meta-analysis results of PRF
To evaluate the impact of PRF on maxillary sinus floor height, we included 2 studies [56, 57], comprising a total of 44 patients in both the experimental and control groups. The results indicated that the application of PRF during maxillary sinus floor elevation surgery did not demonstrate a significant advantage in increasing maxillary sinus floor height (MD= -3.61, CI= -11.63 to 4.41, P = 0.38, Fig. 4B), and significant heterogeneity was observed between the groups (P = 0.38, I²=89%).
Examining the impact of PRF on the percentage of soft tissue area involved 6 studies [42, 43, 46, 47, 56, 58], with a total of 70 patients in the experimental group and 65 patients in the control group. The results indicated that intraoperative use of PRF did not significantly influence the percentage of soft tissue healing area (MD= -1.90, CI= -6.24 to 2.43, P = 0.39, Fig. 4C), with a moderate level of heterogeneity observed (P = 0.12, I²= 42%). Studies using DBBM as bone fillers showed no significant increase in the percentage of soft tissue area with PRF compared to controls. The meta-analysis revealed a mean difference of -2.44% (CI: -12.72 to 7.84, P = 0.64) for PRF [47, 56, 58]. When combined with other materials, PRF had varying impacts on soft tissue healing, with no statistically significant effect observed for PRF (MD=-1.84, CI: -5.98 to 2.30, P = 0.38) [42, 43, 46].
Quality of evidence
According to the GRADE criteria, the evidence for PRP in promoting new bone formation is rated as low quality, primarily due to serious risk of bias and substantial inconsistency across studies. For PRP’s effect on maxillary sinus height augmentation, the evidence quality is assessed as moderate, as 1 out of 2 studies showed potential bias. The evidence for PRP’s impact on soft tissue area formation is also rated as moderate quality, with 2 out of 3 studies demonstrating potential bias (Supplementary table S1).
The evidence for PRF’s effect on the percentage of new bone formation is rated as low quality, due to potential bias in 5 out of 8 studies and a high level of inconsistency in the results. For PRF’s effect on maxillary sinus height augmentation, the evidence is of very low quality, affected by small sample sizes and serious bias. In terms of PRF’s effect on soft tissue area formation, although there was no significant inconsistency, indirectness, or imprecision, 3 out of 6 studies showed potential bias, leading to a moderate quality rating for the evidence (Supplementary table S2).
Discussion
Maxillary sinus floor elevation surgery is a commonly utilized bone augmentation technique in oral implant surgery, aimed at addressing insufficient bone volume in the posterior maxillary region, rendering it unsuitable for implant placement [66]. In recent years, autologous blood products have gained increasing traction as a method to promote bone regeneration and hasten the healing process in oral implant surgery [67]. These products, obtained through various centrifugation techniques, yield different categories of platelet concentrates such as PRP, PRF, and CGF, which function by releasing growth factors to facilitate tissue regeneration [20]. This systematic review and meta-analysis assess the efficacy of autologous blood products in maxillary sinus floor elevation surgery, particularly their effects on new bone formation, maxillary sinus floor height, and the percent of soft tissue area.
Our findings indicate that PRP significantly enhances new bone formation and increases maxillary sinus floor height in maxillary sinus floor elevation surgery. Specifically, the use of PRP was associated with an increase in new bone formation by 4.4% points and a 1 mm increase in maxillary sinus floor height. These results highlight the potential of PRP in improving surgical outcomes in maxillary sinus floor elevation surgery [40, 41]. However, the evidence for the impact of PRP on soft tissue healing remains inconsistent, necessitating further investigation through high-quality randomized controlled trials. The results for PRF, on the other hand, were less conclusive. Although there was a positive trend in promoting new bone formation, the overall effect did not reach statistical significance [18, 42, 43]. Furthermore, PRF did not demonstrate significant advantages in increasing maxillary sinus floor height or enhancing soft tissue healing. This suggests that while PRF may have some beneficial effects, it is not as effective as PRP in the context of maxillary sinus floor elevation surgery. The lack of sufficient data on CGF precluded a meta-analysis, highlighting a gap in the current literature. Future research should focus on evaluating the efficacy of CGF, as well as comparing it with PRP and PRF, to determine the most effective autologous blood product for maxillary sinus floor elevation surgery.
Drawing upon existing research, previous systematic reviews have explored the applications of concentrated platelet growth factors in various aspects of dental medicine, such as in the healing of extraction sites [68], periodontal bone defects [69], and peri-implantitis [70]. Comparative analysis has led to the conclusion that the effectiveness of these growth factors varies across different regions of the oral cavity. Additionally, one study has systematically reviewed the application of blood-concentrated growth factors in sinus floor elevation surgery, concluding that while further research is needed, the use of autologous platelet concentrates can enhance bone height, surgical site thickness, vascularization, and postoperative healing, particularly in cases requiring significant sinus elevation [67].
In summary, autologous blood products, particularly PRP, have shown promising effects in promoting new bone formation and increasing maxillary sinus floor height following sinus floor elevation surgery. These findings suggest a potential treatment strategy for enhancing surgical outcomes and accelerating patient recovery. However, when interpreting the results of this meta-analysis, it is important to consider that the quality of evidence, as evaluated by the GRADE system, was generally low to moderate. While PRP demonstrated positive outcomes, significant heterogeneity and risk of bias across studies limit the strength of these conclusions. The evidence supporting PRF was also of low quality due to inconsistent findings and small sample sizes. Furthermore, the limited data on CGF precludes definitive conclusions regarding its efficacy. To better inform clinical practice, future research should focus on addressing these limitations, including improving study design and increasing sample sizes, to provide more robust evidence.
Given that different platelet concentrates exhibit varying biological activities due to differences in preparation methods, components, and growth factor release properties, future research should aim to compare the clinical applications of these various concentrates and their long-term impact on surgical success rates. Additionally, it is crucial to consider the potential interaction between the percentage of new bone formation and the soft tissue area when measured on the same tissue slice, as this could introduce biases in the results. Such interactions might lead to an overestimation or underestimation of the efficacy of autologous blood products. To mitigate this risk, future studies should consider using separate tissue slices or advanced imaging techniques to independently evaluate these parameters. This approach will help in accurately assessing the effects of PRP, PRF, and CGF on bone and soft tissue healing. Moreover, the type of bone filler used in maxillary sinus floor elevation surgery significantly influences the interpretation of results. Different bone fillers possess unique properties that affect their effectiveness when combined with autologous blood preparations such as PRP, PRF, and CGF. Understanding these nuances through comprehensive research will enhance our knowledge of the mechanisms of action of autologous blood products in oral implant surgery. This, in turn, will enable clinicians to develop more precise and effective treatment strategies. When interpreting the results of this meta-analysis, it is essential to acknowledge its limitations. The number of studies included was relatively small, which impacts the robustness of the findings. Consequently, further exploration and validation in well-designed clinical trials are necessary to strengthen these findings.
Conclusions
The findings of this review and meta-analysis underscore the effectiveness of PRP in maxillary sinus floor elevation surgery, particularly in promoting new bone formation and increasing maxillary sinus floor height. This provides crucial evidence to enhance surgical outcomes and expedite patient recovery in clinical settings. However, given the inconsistent evidence regarding the efficacy of PRF and CGF, as well as the heterogeneity observed among studies, further high-quality randomized controlled trials are warranted to thoroughly investigate the specific clinical utility and mechanisms of action of different types of autologous blood products. Furthermore, future research efforts should focus on comparing the biological activity of various platelet concentrates to optimize treatment strategies for oral implant surgery and further improve patient outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
P.Q. and X.Z. established the research plans. R.C., H.X. and Z.J. conducted the data analysis. J.L. and P.Q. wrote the main manuscript. J.L. revised the manuscript. All authors have approved the final version of the manuscript.
Funding
The Chinese Yang Fan Special Fund, with grant number 23YF1450600.
Data availability
Data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Piaopiao Qiu and Xuehan Zhang Contributed equally.
References
- 1.Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38(8):613–6. [PubMed] [Google Scholar]
- 2.Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 1994;15(2):152–62. [PubMed] [Google Scholar]
- 3.Emmerich D, Att W, Stappert C. Sinus floor elevation using osteotomes: a systematic review and meta-analysis. J Periodontol. 2005;76(8):1237–51. 10.1902/jop.2005.76.8.1237. [DOI] [PubMed] [Google Scholar]
- 4.Shalabi MM, Manders P, Mulder J, et al. A meta-analysis of clinical studies to estimate the 4.5-year survival rate of implants placed with the osteotome technique. Int J Oral Maxillofac Implants. 2007;22(1):110–6. [PubMed] [Google Scholar]
- 5.Sorní M, Guarinós J, García O, et al. Implant rehabilitation of the atrophic upper jaw: a review of the literature since 1999. Med Oral Patol Oral Cir Bucal. 2005;10(Suppl 1):E45–56. Published 2005 Apr 1. [PubMed] [Google Scholar]
- 6.Fugazzotto PA, Vlassis J. Long-term success of sinus augmentation using various surgical approaches and grafting materials. Int J Oral Maxillofac Implants. 1998;13(1):52–8. [PubMed] [Google Scholar]
- 7.Wood RM, Moore DL. Grafting of the maxillary sinus with intraorally harvested autogenous bone prior to implant placement. Int J Oral Maxillofac Implants. 1988;3(3):209–14. [PubMed] [Google Scholar]
- 8.Raghoebar GM, Brouwer TJ, Reintsema H, et al. Augmentation of the maxillary sinus floor with autogenous bone for the placement of endosseous implants: a preliminary report. J Oral Maxillofac Surg. 1993;51(11):1198–205. 10.1016/s0278-2391(10)80288-5. [DOI] [PubMed] [Google Scholar]
- 9.Hallman M, Sennerby L, Lundgren S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int J Oral Maxillofac Implants. 2002;17(5):635–43. [PubMed] [Google Scholar]
- 10.Stavropoulos A, Karring T, Kostopoulos L. Fully vs. partially rough implants in maxillary sinus floor augmentation: a randomized-controlled clinical trial. Clin Oral Implants Res. 2007;18(1):95–102. 10.1111/j.1600-0501.2006.01289.x. [DOI] [PubMed] [Google Scholar]
- 11.Stavropoulos A, Sima C, Sima A et al. Histological evaluation of healing after transalveolar maxillary sinus augmentation with bioglass and autogenous bone. Clin Oral Implants Res. 2012;23(1):125–131. 10.1111/j.1600-0501.2011.02161. x. [DOI] [PubMed]
- 12.Stavropoulos A, Becker J, Capsius B, et al. Histological evaluation of maxillary sinus floor augmentation with recombinant human growth and differentiation factor-5-coated β-tricalcium phosphate: results of a multicenter randomized clinical trial. J Clin Periodontol. 2011;38(10):966–74. 10.1111/j.1600-051X.2011.01754.x. [DOI] [PubMed] [Google Scholar]
- 13.Khairy NM, Shendy EE, Askar NA, et al. Effect of platelet rich plasma on bone regeneration in maxillary sinus augmentation (randomized clinical trial). Int J Oral Maxillofac Surg. 2013;42(2):249–55. 10.1016/j.ijom.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Shu DY, Hutcheon AEK, Zieske JD et al. Epidermal Growth Factor Stimulates Transforming Growth Factor-Beta Receptor Type II Expression In Corneal Epithelial Cells. Sci Rep. 2019;9(1):8079. Published 2019 May 30. 10.1038/s41598-019-42969-2 [DOI] [PMC free article] [PubMed]
- 15.Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 16.Lucarelli E, Beccheroni A, Donati D, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24(18):3095–100. 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 17.Leitner GC, Gruber R, Neumüller J et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91(2):135–139. 10.1111/j.1423-0410.2006.00815. x. [DOI] [PubMed]
- 18.Cabbar F, Güler N, Kürkcü M, et al. The effect of bovine bone graft with or without platelet-rich plasma on maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2011;69(10):2537–47. 10.1016/j.joms.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13(7):1231–56. 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez-Aristizabal RF, López C, Álvarez ME, et al. Long-term cytokine and growth factor release from equine platelet-rich fibrin clots obtained with two different centrifugation protocols. Cytokine. 2017;97:149–55. 10.1016/j.cyto.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Anitua E, Pelacho B, Prado R, et al. Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodeling after severe Hind limb ischemia. J Control Release. 2015;202:31–9. 10.1016/j.jconrel.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Lei L, Yu Y, Han J, et al. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J Periodontol. 2020;91(4):462–72. 10.1002/JPER.19-0290. [DOI] [PubMed] [Google Scholar]
- 23.Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. 10.1111/j.1749-6632. 2001.tb03491. x. [DOI] [PubMed] [Google Scholar]
- 24.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–44. 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353–60. 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 26.Rodella LF, Favero G, Boninsegna R, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74(8):772–7. 10.1002/jemt.20968. [DOI] [PubMed] [Google Scholar]
- 27.Calabriso N, Stanca E, Rochira A et al. Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components. Pharmaceutics. 2021;13(5):635. Published 2021 Apr 29. 10.3390/pharmaceutics13050635 [DOI] [PMC free article] [PubMed]
- 28.Magesh DP, Kumaravelu C, Maheshwari GU. Efficacy of PRP in the Reconstruction of Mandibular Segmental defects using iliac bone grafts. J Maxillofac Oral Surg. 2013;12(2):160–7. 10.1007/s12663-012-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakimi M, Grassmann JP, Betsch M et al. The composite of bone marrow concentrate and PRP as an alternative to autologous bone grafting. PLoS One. 2014;9(6): e100143. Published 2014 Jun 20. 10.1371/journal.pone.0100143 [DOI] [PMC free article] [PubMed]
- 30.Badr M, Coulthard P, Alissa R, et al. The efficacy of platelet-rich plasma in grafted maxillae. A randomised clinical trial. Eur J Oral Implantol. 2010;3(3):233–44. [PubMed] [Google Scholar]
- 31.Thor A, Franke-Stenport V, Johansson CB, et al. Early bone formation in human bone grafts treated with platelet-rich plasma: preliminary histomorphometric results. Int J Oral Maxillofac Surg. 2007;36(12):1164–71. 10.1016/j.ijom.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. 10.1136/bmj.n71. Published 2021 Mar 29. [DOI] [PMC free article] [PubMed]
- 33.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358. 10.1136/bmj.j4008. Published 2017. [DOI] [PMC free article] [PubMed]
- 34.Minozzi S, Cinquini M, Gianola S, et al. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37–44. 10.1016/j.jclinepi.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355. 10.1136/bmj.i4919. Published 2016 Oct 12. [DOI] [PMC free article] [PubMed]
- 36.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Adalı E, Yüce MO, Günbay T, et al. Does concentrated growth factor used with allografts in Maxillary Sinus Lifting have adjunctive benefits? J Oral Maxillofac Surg. 2021;79(1):98–108. 10.1016/j.joms.2020.07.217. [DOI] [PubMed] [Google Scholar]
- 39.Amam MA, Abdo A, Alnour A, et al. Comparison of calcium sulfate and tricalcium phosphate in bone grafting after sinus lifting for dental implantation: a randomized controlled trial. Dent Med Probl. 2023;60(2):239–46. 10.17219/dmp/151983. [DOI] [PubMed] [Google Scholar]
- 40.Anitua E, Prado R, Orive G. Bilateral sinus elevation evaluating plasma rich in growth factors technology: a report of five cases. Clin Implant Dent Relat Res. 2012;14(1):51–60. 10.1111/j.1708-8208.2009.00233. x. [DOI] [PubMed]
- 41.Batas L, Tsalikis L, Stavropoulos A. PRGF as adjunct to DBB in maxillary sinus floor augmentation: histological results of a pilot split-mouth study. Int J Implant Dent. 2019;5(1):14. Published 2019 Apr 1. 10.1186/s40729-019-0166-6 [DOI] [PMC free article] [PubMed]
- 42.Bolukbasi N, Ersanlı S, Keklikoglu N, et al. Sinus augmentation with platelet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J Oral Implantol. 2015;41(5):586–95. 10.1563/AAID-JOI-D-13-00129. [DOI] [PubMed] [Google Scholar]
- 43.Bosshardt DD, Bornstein MM, Carrel JP, et al. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: histologic and histomorphometric results. Int J Periodontics Restor Dent. 2014;34(2):259–67. 10.11607/prd.1419. [DOI] [PubMed] [Google Scholar]
- 44.Cho YS, Hwang KG, Jun SH, et al. Radiologic comparative analysis between saline and platelet-rich fibrin filling after hydraulic transcrestal sinus lifting without adjunctive bone graft: a randomized controlled trial. Clin Oral Implants Res. 2020;31(11):1087–93. 10.1111/clr.13655. [DOI] [PubMed] [Google Scholar]
- 45.Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299–303. 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Cömert Kılıç S, Güngörmüş M, Parlak SN. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: a randomized clinical trial. Clin Implant Dent Relat Res. 2017;19(5):959–67. 10.1111/cid.12522. [DOI] [PubMed] [Google Scholar]
- 47.de Almeida Malzoni CM, Pichotano EC, Freitas de Paula LG, et al. Combination of leukocyte and platelet-rich fibrin and demineralized bovine bone graft enhanced bone formation and healing after maxillary sinus augmentation: a randomized clinical trial. Clin Oral Investig. 2023;27(9):5485–98. 10.1007/s00784-023-05167-z. [DOI] [PubMed] [Google Scholar]
- 48.Gassling V, Purcz N, Braesen JH, et al. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: a preliminary study. J Craniomaxillofac Surg. 2013;41(1):76–82. 10.1016/j.jcms.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Jia PY, Li WJ, Tang YM, et al. Radiographic outcomes of lateral sinus floor elevation with and without bone window repositioning: one-year results of a randomized controlled trial. Int J Oral Maxillofac Surg. 2023;52(2):255–63. 10.1016/j.ijom.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Kanayama T, Horii K, Senga Y, et al. Crestal Approach to Sinus Floor Elevation for Atrophic Maxilla Using Platelet-Rich fibrin as the only grafting material: a 1-Year prospective study. Implant Dent. 2016;25(1):32–8. 10.1097/ID.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 51.Karagah A, Tabrizi R, Mohammadhosseinzade P, et al. Effect of Sinus Floor Augmentation with platelet-rich fibrin Versus Allogeneic Bone Graft on Stability of one-stage Dental implants: a Split-Mouth Randomized Clinical Trial. Int J Environ Res Public Health. 2022;19(15):9569. 10.3390/ijerph19159569. Published 2022 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kassolis JD, Reynolds MA. Evaluation of the adjunctive benefits of platelet-rich plasma in subantral sinus augmentation. J Craniofac Surg. 2005;16(2):280–7. 10.1097/00001665-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Kiliç SC, Güngörmüş M. Cone Beam Computed Tomography Assessment of Maxillary Sinus Floor Augmentation using Beta-tricalcium phosphate alone or in combination with platelet-rich plasma: a Randomized Clinical Trial. Int J Oral Maxillofac Implants. 2016;31(6):1367–75. 10.11607/jomi.5205. [DOI] [PubMed] [Google Scholar]
- 54.Lv H, Sun X, Wang J, et al. Flapless osteotome-mediated sinus floor elevation using platelet-rich fibrin versus lateral approach using deproteinised bovine bone mineral for residual bone height of 2–6 mm: a randomised trial. Clin Oral Implants Res. 2022;33(7):700–12. 10.1111/clr.13934. [DOI] [PubMed] [Google Scholar]
- 55.Molemans B, Cortellini S, Jacobs R, et al. Simultaneous sinus floor elevation and implant placement using leukocyte- and platelet-rich fibrin as a sole graft material. Int J Oral Maxillofac Implants. 2019;34(5):1195–201. 10.11607/jomi.7371. [DOI] [PubMed] [Google Scholar]
- 56.Nizam N, Eren G, Akcalı A, et al. Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: a split-mouth histological and histomorphometric study. Clin Oral Implants Res. 2018;29(1):67–75. 10.1111/clr.13044. [DOI] [PubMed] [Google Scholar]
- 57.Olgun E, Ozkan SY, Atmaca HT, et al. Comparison of the clinical, radiographic, and histological effects of titanium-prepared platelet rich fibrin to allograft materials in sinus-lifting procedures. J Investig Clin Dent. 2018;9(4):e12347. 10.1111/jicd.12347. [DOI] [PubMed] [Google Scholar]
- 58.Pichotano EC, de Molon RS, de Souza RV, et al. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: a randomized clinical trial. Clin Implant Dent Relat Res. 2019;21(2):253–62. 10.1111/cid.12713. [DOI] [PubMed] [Google Scholar]
- 59.Poeschl PW, Ziya-Ghazvini F, Schicho K, et al. Application of platelet-rich plasma for enhanced bone regeneration in grafted sinus. J Oral Maxillofac Surg. 2012;70(3):657–64. 10.1016/j.joms.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 60.Raghoebar GM, Schortinghuis J, Liem RS et al. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16(3):349–356. 10.1111/j.1600-0501.2005.01115. x. [DOI] [PubMed]
- 61.Taschieri S, Testori T, Corbella S, et al. Platelet-Rich plasma and deproteinized bovine bone matrix in Maxillary Sinus lift surgery: a Split-Mouth histomorphometric evaluation. Implant Dent. 2015;24(5):592–7. 10.1097/ID.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 62.Tatullo M, Marrelli M, Cassetta M, et al. Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9(10):872–80. 10.7150/ijms.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thor A, Wannfors K, Sennerby L, et al. Reconstruction of the severely resorbed maxilla with autogenous bone, platelet-rich plasma, and implants: 1-year results of a controlled prospective 5-year study. Clin Implant Dent Relat Res. 2005;7(4):209–20. 10.1111/j.1708-8208.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 64.Torres J, Tamimi F, Martinez PP, et al. Effect of platelet-rich plasma on sinus lifting: a randomized-controlled clinical trial. J Clin Periodontol. 2009;36(8):677–87. 10.1111/j.1600-051X.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Tangl S, Huber CD, et al. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40(4):321–8. 10.1016/j.jcms.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 66.Bornstein MM, Chappuis V, von Arx T et al. Performance of dental implants after staged sinus floor elevation procedures: 5-year results of a prospective study in partially edentulous patients. Clin Oral Implants Res. 2008;19(10):1034–1043. 10.1111/j.1600-0501.2008.01573. x. [DOI] [PubMed]
- 67.Anitua E, Sánchez M, Orive G, et al. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28(31):4551–60. 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 68.Al-Badran A, Bierbaum S, Wolf-Brandstetter C. Does the choice of Preparation Protocol for platelet-rich Fibrin have consequences for Healing and Alveolar Ridge Preservation after tooth extraction? A Meta-analysis. J Oral Maxillofac Surg. 2023;81(5):602–21. 10.1016/j.joms.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Yao M, Hu J, Jiang L, et al. Efficacy of concentrated growth factor combined with grafting materials vs. grafting materials alone for the treatment of periodontal intrabony defects: a systematic review and meta-analysis. Ann Transl Med. 2023;11(4):184. 10.21037/atm-23-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castro F, Bouzidi AS, Fernandes JCH, et al. Bone tissue regeneration in peri-implantitis: a systematic review of randomized clinical trials. Saudi Dent J. 2023;35(6):589–601. 10.1016/j.sdentj.2023.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript.