Abstract
Background
Streptococcus pyogenes (Group A Streptococcus, GAS) is a significant pathogen that causes diverse infections, ranging from pharyngitis to severe invasive diseases. Asymptomatic carriage in children is pivotal for transmission. The COVID-19 pandemic’s health measures, including mask wearing and enhanced hand hygiene, likely influenced GAS transmission dynamics. This study evaluated the impact of these precautions on the prevalence of asymptomatic pharyngeal GAS carriage among schoolchildren in the southern West Bank, Palestine.
Methods
This cross-sectional study was conducted in two phases: pre-COVID-19 (November 2019–January 2020) and post-COVID-19 (November 2023–April 2024). Throat swabs were collected from 701 children (345 pre-COVID-19, 356 post-COVID-19) via cluster sampling. The samples were tested with the ABON Strep A rapid test and confirmed by culture. Sociodemographic, health, and household data were also collected. The statistical analyses included descriptive statistics, chi-square tests, and binary logistic regression.
Results
The prevalence of asymptomatic pharyngeal GAS carriage declined from 15.7% pre-COVID-19 to 10.4% post-COVID-19 (p = 0.038). Significant reductions were observed among urban residents (23.5–10.1%, p = 0.003) and those from medium socioeconomic backgrounds (16.0–9.1%, p = 0.008). Compared with urban residents, rural residents had lower GAS carriage rates (adjusted OR = 0.505, p = 0.023). Carriage rates also decreased among children with frequent sore throats (17.6–7.3%, p = 0.007) and those using private wells (52.5–14.9%, p < 0.001). Higher BMI was a significant risk factor (adjusted OR = 17.68, p < 0.001), whereas frequent tooth brushing (adjusted OR = 0.055, p < 0.001) and hand washing (adjusted OR = 0.367, p < 0.001) were protective factors.
Conclusions
COVID-19-related health precautions were correlated with a significant reduction in asymptomatic GAS carriage among Palestinian children. These findings suggest that public health measures, such as mask wearing and hand hygiene, can influence the transmission of respiratory pathogens. Ongoing surveillance and targeted interventions are essential for managing GAS infections, particularly in resource-limited settings.
Keywords: Asymptomatic GAS carriage; COVID-19 precautions; Schoolchildren; Southern west bank, Palestine
Background
Streptococcus pyogenes, also known as Group A Streptococcus (GAS), is a common human pathogen responsible for a wide range of infections, from uncomplicated pharyngitis to life-threatening invasive diseases such as necrotizing fasciitis and streptococcal toxic shock syndrome [1, 2]. Severe GAS infections cause approximately 500,000 fatalities per year, creating a major global health burden, especially in resource-limited settings [3–5]. The morbidity and mortality linked to these infections highlight how crucial it is to understand the dynamics of GAS transmission, risk factors, and epidemiology.
GAS pharyngitis, often known as strep throat, is a frequent illness that mostly affects children [4, 6]. Differentiating it from viral pharyngitis, which is more prevalent and typically self-limiting, is crucial for accurate diagnosis [7]. Prompt antibiotic treatment of GAS pharyngitis is essential to prevent complications such as rheumatic fever and acute glomerulonephritis and to reduce transmission [8]. Asymptomatic GAS carriage, defined as the presence of GAS in the nasopharynx or oropharynx without signs of acute infection, is a significant reservoir for transmission and a known precursor to invasive disease [9, 10].
The southern West Bank region of Palestine faces unique healthcare challenges. High population density, limited healthcare infrastructure, and geographic barriers contribute to a higher incidence of infectious diseases, including GAS infections [11, 12]. The region’s socioeconomic conditions and environmental factors further complicate efforts to manage and control these infections. Previous studies have estimated that 5–15% of school children worldwide develop GAS pharyngitis annually [13, 14]. However, data on the prevalence and determinants of asymptomatic GAS carriage in this region remain sparse.
When the COVID-19 pandemic broke out, the West Bank region was among the many places in the world where public health initiatives were implemented to prevent the spread of the virus. These precautions, which included mask usage, hand washing, social distancing, and temporary school closures, were implemented [15]. The transmission dynamics of other infectious agents, such as GAS, have been altered by these measures in addition to the spread of SARS-CoV-2. Closing schools and imposing social distancing measures, for example, may have changed the exposure patterns of children, whereas mask use and better hand hygiene may have decreased the spread of respiratory diseases [16].
This cross-sectional study investigated the potential influence of COVID-19 public health measures on the asymptomatic carriage of Group A Streptococcus (GAS) in the pharynx of healthy schoolchildren residing in the southern West Bank, Palestine. The project originated prior to the COVID-19 pandemic, aiming to assess the baseline prevalence and risk factors associated with asymptomatic GAS carriage. However, the study was necessarily halted due to the emergence of COVID-19 in 2020. We have now resumed the study with the added objective of exploring whether the implemented public health measures have impacted GAS carriage rates. We will also continue to examine potential contributing factors such as socioeconomic status, household characteristics, and past medical history. This research has substantial public health implications. Understanding these mechanisms is critical for designing targeted strategies to prevent GAS infections. This project will provide vital information about GAS epidemiology in an area with specific healthcare problems, eventually guiding efforts to improve children’s health.
Materials and methods
Study design and setting
This study employed a two-stage cross-sectional design, encompassing the Hebron and Bethlehem governorates in the southern West Bank, Palestine. The first stage occurred between November 2019 and January 2020, whereas the second stage occurred from November 2023 to April 2024. In 2019, there were 770 schools in this region, with a total student population of 211,025 children. By 2023, the number of schools had increased to 807, and the total number of children had risen to 230,463 [17, 18].
Participants
The study included a total of 701 children divided into two time intervals. During the first interval, 345 children without symptoms of sore throat were sampled and tested. The study was put on hold in January 2020 due to the spread of the COVID-19 pandemic and resumed in late 2023, with a second stage in which 356 children without symptoms of sore throat were sampled. Schools and participants were selected via a cluster sampling method. Throat swab samples were collected from school-aged children (5–12 years old) during school hours across selected kindergartens and primary schools in cities, villages, and refugee camps. Written informed consent from parents or guardians was obtained before sampling. Although guardians were not present during collection, the process was supervised by school staff and research personnel.
Sample size calculation
The sample size for this study was initially calculated on the basis of an estimated 15% prevalence of asymptomatic GAS carriage in school-aged children from previous studies [13, 14]. Assuming a desired precision of ± 5% with a 95% confidence interval, the required sample size was determined to be 196 participants. To increase the statistical power and account for potential variability, we doubled this target beyond the calculated sample size, recruiting 345 participants before the COVID-19 pandemic. Following the onset of the pandemic and subsequent changes in study aims, we recalculated the sample size for the post-COVID-19 period and recruited a similar number of participants (356) to ensure that the study findings would remain robust and comparable.
The inclusion criteria for each sampling period were as follows: all students aged 5–12 years who were enrolled in selected classes at randomly chosen schools and present during the sampling period were eligible for the study. However, participation required signed written consent from their parents or legal guardians.
Exclusion criteria
To ensure a representative sample of asymptomatic carriers, we excluded children who presented with symptoms suggestive of upper respiratory tract infection (URTI) at enrollment and those with a history of tonsillectomy or recent antibiotic use within one week of sample collection. Additionally, children who were diagnosed with chronic medical conditions and who were older than 12 years of age were excluded from the study.
Information collection
All the participating children provided a detailed medical history, either directly or through their parents or guardians. This information included age, gender, school name and location, place of residence, province, and parental socioeconomic status. The medical history also included the frequency and severity of sore throat episodes over the past year and any current symptoms, such as fever, sore throat, difficulty swallowing, ear pain, or skin rash. The severity of sore throat episodes was assessed by parents or guardians on the basis of factors such as pain intensity, child behavior during illness, impact on daily activities, degree of temperature elevation, and level of difficulty swallowing. Additionally, data were collected on recent contacts with individuals experiencing sore throats. The inquiry further extended to family and environmental factors, such as the number of siblings, the number of rooms in the household, the water supply system, the number of people sharing the child’s room, and personal hygiene practices, including the frequency of tooth brushing and hand washing.
Sample collection and group a streptococcus (GAS) identification
Sterile cotton swabs and tongue depressors were used for primary throat swab collection from the participants. The research team adhered to rigorous hygiene protocols, utilizing personal protective equipment, including gloves, to mitigate the risk of cross-contamination. During the initial rapid testing phase, the ABON Strep A throat swab rapid test kit was utilized (ABON, lot number IST-502, Germany). Sample processing was performed according to the manufacturer’s instructions. According to the manufacturer’s kit leaflet, this test has a sensitivity of 94.4% (95% CI: 88.3-97.9%) and a specificity of 98.2% (95% CI: 96.3-99.3%). This rapid antigen detection test (RADT) serves as an indicator of asymptomatic Streptococcus carriage, which is consistent with the findings of Rystedt et al.. (2023), who reported 91% concordance between RADTs and culture results at the 21-day follow-up [19].
For samples yielding positive results in the rapid test, a second set of sterile swabs was collected via Cliniswab TS (APTACA Spa) with Amies gel transport medium. This medium complied with the CLSI standard M40-A2 according to the manufacturer’s instructions. The swabs were then transported to the microbiology laboratory within one hour at room temperature (18–25 °C). The throat swabs were subsequently inoculated onto commercially available 5% sheep blood agar plates and incubated for 24 h under optimal conditions (37 °C, 5–10% CO2). If no bacterial growth was detected in the culture despite a positive RDT, the result was classified as a false positive, and the case was considered negative. After the incubation period, suspected colonies exhibiting beta-hemolysis were identified, subcultured onto fresh 5% blood agar plates, and incubated for an additional 24 h. The hemolysis pattern was then evaluated in these subcultures. Plates displaying beta-hemolysis were subjected to Gram staining and catalase testing. If the bacteria were identified as gram-positive cocci arranged in chains and catalase negative, they were subcultured onto another 5% blood agar plate for bacitracin and trimethoprim-sulfamethoxazole (SXT) susceptibility testing. The presence of Streptococcus pyogenes (Group A Streptococcus; GAS) was confirmed in bacitracin-sensitive and SXT-resistant isolates.
Statistical analysis
Data analysis was performed via IBM SPSS version 27 and MedCalc® Statistical Software version 22.0. Descriptive statistics, including means and standard deviations, were employed to summarize quantitative variables, whereas frequencies and percentages were utilized to present categorical variables. The chi-square test was used to assess the existence of associations and to evaluate the statistical significance of the results. Additionally, binary logistic regression analysis was conducted to determine the effects of the independent variables. We quantified risk via odds ratios (ORs) with 95% confidence intervals. A p value less than or equal to 0.05 was considered to indicate statistical significance.
Ethical considerations
The study received ethical approval from the Research Ethics Committee (REC) of Al-Quds University, the institution responsible for ensuring adherence to high ethical standards. Written informed consent was obtained from parents or guardians, with verbal assent from the children. The REC’s mandatory review confirmed that the study met all ethical requirements, including benefiting participants, avoiding harm, and respecting diverse backgrounds.
Results
Asymptomatic pharyngeal carriage of group a streptococcus
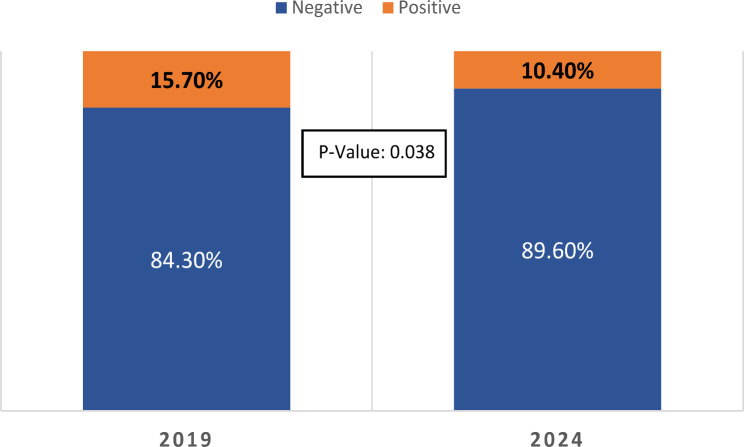
The study examined 701 children from the southwest Bank region of Palestine, with data collected in 2019 and 2024. A notable decrease in the prevalence of asymptomatic GAS carriage among these children was observed between the two time points. In 2019, 15.7% (54/345) of the children were asymptomatic carriers of GAS, with a 95% confidence interval (CI) of 12.1–19.8%. By 2024, this prevalence had dropped to 10.4% (37/356), with a 95% CI of 7.5–13.9% (Fig. 1). Statistical analysis indicated that this reduction in the prevalence of asymptomatic GAS carriage was significant (p = 0.038).
Fig. 1.
Prevalence of Group A Streptococcus Asymptomatic Carriage among Palestinian Children (2019 vs. 2024)
Sociodemographic characteristics of the study participants and GAS carriage
Table 1 provides an overview of the demographic and socioeconomic characteristics of the study participants from the 2019 and 2024 intervals. The gender distribution remained stable across both periods, with males and females comprising approximately equal proportions. There was a modest, nonsignificant increase in the proportion of participants under 7 years of age and those residing in urban areas. The distribution of participants across governorates and socioeconomic statuses remained consistent, with Hebron and the medium socioeconomic category remaining predominant.
Table 1.
Demographic data, socioeconomic information, and GAS pharyngeal carriage data (2019 vs. 2024)
| 2019 | 2024 | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Category | Negative (n %) | Positive (n %) | Total (345) % | Negative (n %) | Positive (n %) | Total (356) % | ||
| Sex | Male | 136 (84.5%) | 25 (15.5%) | 161 (46.7%) | 154 (89.5%) | 18 (10.5%) | 172 (48.3%) | 0.169 |
| Female | 155 (84.2%) | 29 (15.8%) | 184 (53.3%) | 165 (89.7%) | 19 (10.3%) | 184 (51.7%) | 0.122 | |
| Age Groups | < 7 | 108 (83.1%) | 22 (16.9%) | 130 (37.7%) | 130 (87.8%) | 18 (12.2%) | 148 (41.6%) | 0.259 |
| 8–9 | 116 (85.3%) | 20 (14.7%) | 136 (39.4%) | 101 (89.4%) | 12 (10.6%) | 113 (31.7%) | 0.337 | |
| 9+ | 67 (84.8%) | 12 (15.2%) | 79 (22.9%) | 88 (92.6%) | 7 (7.4%) | 95 (26.7%) | 0.1 | |
| Place of Residence | City | 91 (76.5%) | 28 (23.5%) | 119 (34.5%) | 133 (89.9%) | 15 (10.1%) | 148 (41.6%) | 0.003 |
| Village | 162 (88.0%) | 22 (12.0%) | 184 (53.3%) | 147 (93.6%) | 10 (6.4%) | 157 (44.1%) | 0.078 | |
| Camp | 38 (90.5%) | 4 (9.5%) | 42 (12.2%) | 39 (76.5%) | 12 (23.5%) | 51 (14.3%) | 0.075 | |
| Governorate | Hebron | 202 (86.3%) | 32 (13.7%) | 234 (67.8%) | 228 (89.8%) | 26 (10.2%) | 254 (71.3%) | 0.241 |
| Bethlehem | 89 (80.2%) | 22 (19.8%) | 111 (32.2%) | 91 (89.2%) | 11 (10.8%) | 102 (28.7%) | 0.069 | |
| Family Socioeconomic Status | Low | 10 (83.3%) | 2 (16.7%) | 12 (3.5%) | 15 (83.3%) | 3 (16.7%) | 18 (5.1%) | 1 |
| Medium | 268 (84.0%) | 51 (16.0%) | 319 (92.5%) | 300 (90.9%) | 30 (9.1%) | 330 (92.7%) | 0.008 | |
| High | 13 (92.9%) | 1 (7.1%) | 14 (4.1%) | 4 (50.0%) | 4 (50.0%) | 8 (2.2%) | - | |
With respect to GAS carriage, a notable decline was observed in pharyngeal carriage rates across both genders: from 15.5% in males and 15.8% in females in 2019 to 10.5% and 10.3% in 2024, respectively. Urban residents experienced a significant reduction in carriage rates, from 23.5 to 10.1% (p = 0.003). Similarly, individuals from medium socioeconomic backgrounds experienced a substantial decrease in carriage rates, from 16.0% in 2019 to 9.1% (p = 0.008).
Health, household characteristics, and GAS carriage
Table 2 reveals a significant decline in GAS carriage among participants with two to three instances of sore throat, decreasing from 17.6% in 2019 to 7.3% in 2024 (p = 0.007). Severe sore throat cases also demonstrated a marked reduction in GAS carriage, from 17.5 to 4.5% (p = 0.018). Children from families relying on private wells experienced a significant decrease in GAS carriage, from 52.5 to 14.9% (p < 0.001). Additionally, residents living in independent houses showed a substantial reduction in GAS positivity, from 22.7 to 7.9% (p < 0.001). Households with six or more members presented a decrease in GAS carriage from 17.0 to 9.3% (p = 0.018).
Table 2.
Health, Household Information and GAS Pharyngeal Carriage (2019 vs. 2024)
| 2019 | 2024 | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Category | Negative (n %) | Positive (n %) | Total (345) % | Negative (n %) | Positive (n %) | Total (356) % | ||
| Sore Throat Frequency Over Past Year | Never | 48 (85.7%) | 8 (14.3%) | 56 (16.2%) | 51 (85.0%) | 9 (15.0%) | 60 (16.9%) | 0.913 |
| Once | 105 (88.2%) | 14 (11.8%) | 119 (34.5%) | 105 (88.2%) | 14 (11.8%) | 119 (33.4%) | 1 | |
| Two to three times | 126 (82.4%) | 27 (17.6%) | 153 (44.3%) | 139 (92.7%) | 11 (7.3%) | 150 (42.1%) | 0.007 | |
| More than three times | 12 (70.6%) | 5 (29.4%) | 17 (4.9%) | 24 (88.9%) | 3 (11.1%) | 27 (7.6%) | 0.125 | |
| Sore Throat Severity | Mild | 45 (77.6%) | 13 (22.4%) | 58 (20.1%) | 59 (88.1%) | 8 (11.9%) | 67 (22.6%) | 0.118 |
| Moderate | 151 (86.8%) | 23 (13.2%) | 174 (60.2%) | 145 (89.5%) | 17 (10.5%) | 162 (54.7%) | 0.441 | |
| Severe | 47 (82.5%) | 10 (17.5%) | 57 (19.7%) | 64 (95.5%) | 3 (4.5%) | 67 (22.6%) | 0.018 | |
| Drinking Water Source | Municipal water system | 247 (92.5%) | 20 (7.5%) | 267 (77.4%) | 204 (91.9%) | 18 (8.1%) | 222 (62.4%) | 0.8 |
| Private well | 29 (47.5%) | 32 (52.5%) | 61 (17.7%) | 97 (85.1%) | 17 (14.9%) | 114 (32.0%) | < 0.001 | |
| Bottled mineral water from the market | 15 (88.2%) | 2 (11.8%) | 17 (4.9%) | 18 (90.0%) | 2 (10.0%) | 20 (5.6%) | 0.863 | |
| Type of House | Apartment | 215 (87.0%) | 32 (13.0%) | 247 (71.6%) | 152 (87.4%) | 22 (12.6%) | 174 (49.0%) | 0.925 |
| House | 75 (77.3%) | 22 (22.7%) | 97 (28.1%) | 164 (92.1%) | 14 (7.9%) | 178 (50.1%) | < 0.001 | |
| Other | 1 (100.0%) | 0 (0.0%) | 1 (0.3%) | 2 (66.7%) | 1 (33.3%) | 3 (0.8%) | - | |
| Contact with Individuals Experiencing Sore Throat | Yes | 28 (93.3%) | 2 (6.7%) | 30 (8.7%) | 19 (95.0%) | 1 (5.0%) | 20 (5.6%) | 0.808 |
| No | 250 (83.6%) | 49 (16.4%) | 299 (86.7%) | 277 (88.5%) | 36 (11.5%) | 313 (87.9%) | 0.081 | |
| Do not Know | 13 (81.3%) | 3 (18.8%) | 16 (4.6%) | 23 (100.0%) | 0 (0.0%) | 23 (6.5%) | 0.031 | |
| BMI Categories | < 18.50 | 45 (100.0%) | 0 (0.0%) | 45 (16.1%) | 46 (93.9%) | 3 (6.1%) | 49 (14.0%) | 0.092 |
| 18.50–24.99 | 174 (87.4%) | 25 (12.6%) | 199 (71.1%) | 260 (92.5%) | 21 (7.5%) | 281 (80.1%) | 0.062 | |
| 25.00–29.99 | 9 (31.0%) | 20 (69.0%) | 29 (10.4%) | 8 (42.1%) | 11 (57.9%) | 19 (5.4%) | 0.433 | |
| 30.00–34.99 | 4 (57.1%) | 3 (42.9%) | 7 (2.5%) | 0 (0.0%) | 2 (100.0%) | 2 (0.6%) | - | |
| Number of Siblings | < 3 | 107 (89.2%) | 13 (10.8%) | 120 (34.8%) | 118 (89.4%) | 14 (10.6%) | 132 (37.1%) | 0.954 |
| 3–5 | 142 (82.6%) | 30 (17.4%) | 172 (49.9%) | 173 (90.6%) | 18 (9.4%) | 191 (53.7%) | 0.024 | |
| 6+ | 42 (79.2%) | 11 (20.8%) | 53 (15.4%) | 28 (84.8%) | 5 (15.2%) | 33 (9.3%) | 0.516 | |
| People in Household | < 3 | 3 (75.0%) | 1 (25.0%) | 4 (1.2%) | 2 (66.7%) | 1 (33.3%) | 3 (0.8%) | - |
| 3–5 | 97 (87.4%) | 14 (12.6%) | 111 (32.2%) | 123 (88.5%) | 16 (11.5%) | 139 (39.0%) | 0.79 | |
| 6+ | 191 (83.0%) | 39 (17.0%) | 230 (66.7%) | 194 (90.7%) | 20 (9.3%) | 214 (60.1%) | 0.018 | |
| Rooms in House | < 3 | 29 (78.4%) | 8 (21.6%) | 37 (10.7%) | 44 (78.6%) | 12 (21.4%) | 56 (15.7%) | 0.982 |
| 3–5 | 234 (83.9%) | 45 (16.1%) | 279 (80.9%) | 249 (90.9%) | 25 (9.1%) | 274 (77.0%) | 0.013 | |
| 6+ | 28 (96.6%) | 1 (3.4%) | 29 (8.4%) | 26 (100.0%) | 0 (0.0%) | 26 (7.3%) | - | |
| Number of other people sleeping in your room | 0 | 41 (83.7%) | 8 (16.3%) | 49 (14.2%) | 82 (93.2%) | 6 (6.8%) | 88 (24.7%) | 0.078 |
| 1 | 152 (88.9%) | 19 (11.1%) | 171 (49.6%) | 131 (93.6%) | 9 (6.4%) | 140 (39.3%) | 0.151 | |
| 2+ | 98 (78.4%) | 27 (21.6%) | 125 (36.2%) | 106 (82.8%) | 22 (17.2%) | 128 (36.0%) | 0.375 | |
Bivariate and multivariate analysis
Demographic and household factors
Bivariate analysis indicated that factors such as higher BMI (25.00–29.99) and living in a city were significantly associated with increased odds of GAS carriage. Children with a BMI of 25.00–29.99 had substantially greater odds of GAS carriage (crude OR = 17.2, 95% CI: 8.85–33.46, p < 0.001; adjusted OR = 17.68, 95% CI: 9.03–34.61, p < 0.001) (Table 3). Compared with living in a city, living in a village was associated with lower odds of GAS carriage (adjusted OR = 0.505, 95% CI: 0.280–0.909, p = 0.023).
Table 3.
Bivariate and multivariate analyses of demographic and household factors associated with the prevalence of GAS
| Variable | Category | Crude Odds Ratio (OR) | 95% CI for OR | P Value | Adjusted OR* | 95% CI for Adjusted OR | P Value |
|---|---|---|---|---|---|---|---|
| Sex | Male (reference) | 1 | - | - | 1 | - | - |
| Female | 0.959 | 0.651–1.573 | 0.879 | 1.04 | 0.626–1.73 | 0.879 | |
| BMI | < 18.5 | 0.311 | 0.095–1.02 | 0.054 | 0.31 | 0.095–1.03 | 0.055 |
| 18.5-24.99 (reference) | 1 | - | - | 1 | - | - | |
| 25-29.99 | 17.2 | 8.85–33.46 | < 0.001 | 17.68 | 9.03–34.61 | < 0.001 | |
| 30+ | 11.79 | 3.06–45.46 | 0.003 | 12.71 | 3.24–49.85 | < 0.001 | |
| Age | 5–6 Years (reference) | 1 | - | - | 1 | - | - |
| 7–8 Years | 0.626 | 0.389–1.008 | 0.054 | 1.215 | 0.685–2.155 | 0.506 | |
| 9–12 Years | 0.49 | 0.276–0.868 | 0.015 | 1.261 | 0.635–2.506 | 0.507 | |
| Number of Siblings | < 3 (reference) | 1 | - | - | 1 | - | - |
| 3–5 | 1.27 | 0.769–2.097 | 0.351 | 1.104 | 0.627–1.945 | 0.731 | |
| 6+ | 1.905 | 0.971–3.737 | 0.061 | 1.758 | 0.804–3.843 | 0.157 | |
| Number of people living in your home | < 3 (reference) | 1 | - | - | 1 | - | - |
| 3–5 | 0.341 | 0.063–1.836 | 0.21 | 1.007 | 0.117–8.673 | 0.995 | |
| 6+ | 0.383 | 0.073–2.02 | 0.258 | 0.863 | 0.102–7.276 | 0.892 | |
| Number of rooms in your home | < 3 (reference) | 1 | - | - | 1 | - | - |
| 3–5 | 0.529 | 0.304–0.921 | 0.024 | 0.585 | 0.31–1.103 | 0.097 | |
| 6+ | 0.068 | 0.009–0.519 | 0.01 | 0 | 0–0 | 0.997 | |
| Number of other people sleeping in your room | 0 (reference) | 1 | - | - | 1 | - | - |
| 1 | 0.869 | 0.442–1.708 | 0.684 | 0.553 | 0.259–1.178 | 0.125 | |
| 2+ | 2.11 | 1.119–3.981 | 0.021 | 1.572 | 0.778–3.173 | 0.207 | |
| Place of Residence | City (reference) | 1 | - | - | 1 | - | - |
| Village | 0.539 | 0.331–0.880 | 0.013 | 0.505 | 0.280–0.909 | 0.023 | |
| Camp | 1.082 | 0.577–2.032 | 0.805 | 1.31 | 0.653–2.628 | 0.448 | |
| Governorate | Hebron (reference) | 1 | - | - | 1 | - | - |
| Bethlehem | 1.359 | 0.857–2.156 | 0.192 | 1.303 | 0.763–2.551 | 0.333 |
*: adjusted for age and BMI group
Hygiene practices and environmental and behavioral factors
Table 4 shows that frequent tooth brushing was associated with lower odds of GAS carriage. Brushing teeth once or twice daily significantly reduced the odds of GAS carriage (adjusted OR for brushing twice daily = 0.055, 95% CI: 0.019–0.157, p < 0.001). Children who reported always washing their hands with soap and water also had lower odds of GAS carriage (adjusted OR = 0.367, 95% CI: 0.220–0.613, p < 0.001). Additionally, while several environmental and behavioral factors were examined, only the source of drinking water was significantly associated with GAS carriage. The odds of GAS carriage were significantly greater for children who drank from private wells than for those who drank from municipal water (adjusted OR = 3.989, 95% CI: 2.32–6.858, p < 0.001).
Table 4.
Bivariate and multivariate analyses of environmental and behavioral factors associated with the prevalence of GAS
| Variable | Category | Crude Odds Ratio (OR) | 95% CI for OR | P Value | Adjusted OR* | 95% CI for Adjusted OR | P Value |
|---|---|---|---|---|---|---|---|
| Family Socioeconomic Status | Low (reference) | 1 | - | - | 1 | - | - |
| Medium | 0.713 | 0.265–1.915 | 0.502 | 0.8 | 0.251–2.551 | 0.707 | |
| High | 1.471 | 0.368–5.87 | 0.585 | 2.293 | 0.47–11.188 | 0.305 | |
| Frequency of Sore Throat in the Past Year | Never (reference) | 1 | - | - | 1 | - | - |
| Once | 0.776 | 0.406–1.485 | 0.444 | 0.685 | 0.334–1.404 | 0.302 | |
| Two to three times | 0.835 | 0.451–1.547 | 0.567 | 0.808 | 0.408–1.6 | 0.541 | |
| More than three times | 1.294 | 0.514–3.257 | 0.584 | 1.584 | 0.541–4.639 | 0.402 | |
| Severity of Sore Throats | Mild (reference) | 1 | - | - | 1 | - | - |
| Moderate | 0.669 | 0.377–1.188 | 0.17 | 0.765 | 0.401–1.46 | 0.417 | |
| Severe | 0.58 | 0.276–1.218 | 0.15 | 0.53 | 0.222–1.27 | 0.155 | |
| Source of Drinking Water | Municipal water system (reference) | 1 | - | - | 1 | - | - |
| Private well | 4.615 | 2.892–7.366 | < 0.001 | 3.989 | 2.32–6.858 | < 0.001 | |
| Bottled mineral water | 1.439 | 0.484–4.275 | 0.513 | 1.648 | 0.453–5.998 | 0.449 | |
| Type of Residence | Apartment (reference) | 1 | - | - | 1 | - | - |
| House | 1.024 | 0.651–1.609 | 0.919 | 1.179 | 0.703–1.978 | 0.533 | |
| Other | 2.265 | 0.231–22.173 | 0.482 | 1.525 | 0.123–18.842 | 0.742 | |
| Washing Hands Regularly with Soap and Water | Sometimes (reference) | 1 | - | - | 1 | - | |
| Always | 0.326 | 0.208–0.513 | < 0.001 | 0.367 | 0.220–0.613 | < 0.001 | |
| Frequency of Brushing Teeth | 0 (reference) | 1 | - | - | 1 | - | - |
| 1 | 0.188 | 0.113–0.311 | < 0.001 | 0.219 | 0.123–0.391 | < 0.001 | |
| 2 | 0.08 | 0.036–0.18 | < 0.001 | 0.055 | 0.019–0.157 | < 0.001 | |
| 3 | 0.24 | 0.052–1.112 | 0.068 | 0.274 | 0.054–1.384 | 0.117 | |
| Close Contact with Someone with Sore Throat | Yes (reference) | 1 | - | - | 1 | - | - |
| No | 2.527 | 0.769–8.301 | 0.127 | 3.037 | 0.578–15.971 | 0.19 | |
| Do not Know | 1.306 | 0.249–6.854 | 0.753 | 1.37 | 0.177–10.633 | 0.763 |
*: adjusted for age and BMI group
Discussion
This pioneering study provides a comprehensive assessment of Group A Streptococcus (GAS) carriage among Palestinian children, offering novel insights into its epidemiology within this population. The findings establish a crucial baseline for future research and public health interventions, significantly contributing to the understanding of the potential burden of GAS-related diseases in the Palestinian context.
Effects of COVID-19 health prevention on asymptomatic GAS carriage
The implementation of COVID-19 health precautions significantly reduced the prevalence of asymptomatic GAS carriage among Palestinian children, from 15.7% in 2019 to 10.4% in 2024 (p = 0.038). This reduction is likely attributed to pandemic measures that disrupted GAS transmission pathways, including increased hand hygiene, mask wearing, and social distancing. Enhanced hand hygiene practices, in particular, were crucial and aligned with previous research demonstrating their effectiveness in reducing the transmission of respiratory and other infectious diseases [16, 20].
Sociodemographic and household characteristics and geographic disparities
Changes in sociodemographic and household characteristics were also found to be significant factors influencing GAS carriage [21]. This study revealed significant changes in living environments and water sources that influence GAS carriage rates. The proportion of children residing in urban areas increased from 34.5% in 2019 to 41.6% in 2024. Concurrently, the use of private wells as a primary source for storing and drinking water rose significantly from 17.7 to 32.0% (p < 0.001). These private wells typically serve individual households and collect rainwater during the winter. In other seasons, they store municipal or truck-delivered water. This practice potentially introduces contamination risks due to inadequate treatment or storage conditions [22, 23].
The analysis revealed that children residing in rural areas had a lower risk of GAS carriage than their urban counterparts did (adjusted OR for living in a village = 0.505, 95% CI: 0.280–0.909, p = 0.023). This finding suggests that factors inherent to urban living may contribute to an increased risk of GAS carriage. Such factors could include increased population density, overcrowding in schools and public spaces, or greater exposure to diverse bacterial strains through frequent social interactions [2, 24].
Interestingly, while there was an overall decrease in GAS carriage among children from families using private wells (from 52.5% in 2019 to 14.9% in 2024, p < 0.001), these children still presented a fourfold greater risk compared to those with municipal water access (adjusted OR = 3.989, 95% CI: 2.32–6.858, p < 0.001). This suggests a potential disparity in water quality between private wells and municipal systems, which may contribute to the heightened risk of infections [25]. The increased reliance on private wells due to chronic municipal water shortages, particularly in water-scarce regions such as the Palestinian territories, highlights the need to investigate potential contaminants or water treatment practices that might impact the prevalence of infections. Although this study did not include water quality testing for Streptococci, the observed association warrants further investigation into these factors.
Hygiene practices and BMI
Improved hygiene behaviors were strongly associated with a lower likelihood of GAS carriage. Children who consistently washed their hands with soap and water had significantly lower odds of carrying GAS (OR = 0.367, 95% CI: 0.184–0.730, p < 0.001). Similarly, daily tooth brushing significantly reduced the odds of carriage (OR for brushing twice = 0.055, 95% CI: 0.019–0.157, p < 0.001). These findings underscore the importance of personal and oral hygiene in controlling respiratory infections [4, 26].
A higher body mass index (BMI) was significantly associated with increased odds of GAS carriage. Compared with their normal-weight peers, children with a BMI between 25.00 and 29.99 had markedly greater odds of GAS carriage (adjusted OR = 17.68, 95% CI: 9.03–34.61, p < 0.001). Similarly, those with a BMI of 30 or higher presented increased odds (adjusted OR = 12.71, 95% CI: 3.24–49.85, p < 0.001). This association may be attributed to factors such as compromised immune function, increased skin surface area, and potential skin folds that create favorable environments for bacterial colonization [4, 27, 28]. Addressing childhood obesity through preventive measures and interventions could reduce GAS carriage.
Comparison with similar studies
The observed reduction in GAS carriage aligns with reports of decreased respiratory infections associated with pandemic control measures [29–31]. For example, a Taiwanese study documented significant declines in common respiratory illnesses, including streptococcal pharyngitis, during the COVID-19 pandemic, which was attributed to intensified public health interventions [32]. Similarly, research from several European countries revealed lower rates of respiratory infections in primary care settings, further suggesting broader benefits of COVID-19 prevention for infectious disease control [33, 34]. However, maintaining consistent public health measures presents distinct challenges in conflict settings such as Palestine. A study by Marou et al.. (2024) on healthcare in conflict zones emphasized the difficulties in ensuring reliable water quality and consistent public health practices, which can have variable effects on infectious disease transmission [35].
Limitations and future directions
While this study provides valuable insights, it has limitations. The cross-sectional nature prevents us from making causal inferences regarding the observed associations. Additionally, the absence of water quality testing for Streptococci limits our ability to definitively establish causality between the use of private wells and GAS carriage.
This study was conducted in winter, a season linked to increased respiratory infections and potential changes in pathogen prevalence. While our findings offer insights into GAS carriage during this period, seasonal factors such as temperature, indoor crowding, and immune responses may influence these rates. Future studies across different seasons are needed to fully understand the epidemiology of GAS.
Conclusion
This study demonstrated a significant reduction in asymptomatic GAS carriage among Palestinian children following the implementation of COVID-19 health precautions. This finding suggested that improved hygiene practices and pandemic-related measures, despite potential counteracting factors such as reliance on private wells, had a net positive impact on GAS prevalence. These results underscore the importance of sustained public health efforts and personal hygiene in curbing the spread of infectious diseases. It is essential for public health strategies to maintain and promote hygiene practices beyond the pandemic to mitigate the burden of GAS and other infectious diseases. This study provides a strong foundation for understanding GAS carriage in Palestinian children and offers important insights for developing targeted interventions in the region.
Acknowledgements
We gratefully acknowledge the Al-Quds University Health Research Ethical Review Committee for providing ethical clearance for this research. We also extend our sincere thanks to the study participants and everyone who, in any way, supported the completion of this project. In the preparation of this manuscript, AI-assisted language tools were employed to ensure the text is free from language errors and to enhance the overall clarity and readability of the document. These tools were used to check grammar, spelling, and syntax while maintaining the integrity and originality of the authors’ work.
Abbreviations
- OR
Odds ratio
- BMI
Body mass index
- GAS
Group a streptococcus
Author contributions
I.G. initiated the study in 2019 and collected data at that time. All authors collected data in 2024. I.G. and Y.G. processed and analyzed the data. All authors contributed to the conceptualization, study design, execution, data acquisition, and interpretation. Each author participated in drafting, revising, or critically reviewing the article. All authors approved the final version for publication and consented to the journal selected for submission.
Funding
The authors self-funded the financial costs associated with collecting the research data.
Data availability
The researchers are willing to provide the data sets generated and/or analyzed as part of this study to interested parties upon reasonable request made to the corresponding author.
Declarations
Ethics approval and consent to participate
This research received ethical approval from the Al-Quds University Research Ethics Committee and adhered to the principles of the Declaration of Helsinki. The data collectors and/or the investigator thoroughly explained the study’s purpose and the significance of participation to each child’s parent/guardian. Written informed consent was obtained from all parents/guardians, and assent was obtained from the children themselves before the study began. Participation was entirely voluntary.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pallon J, Roost M, Sundqvist M, Hedin K. The aetiology of pharyngotonsillitis in primary health care: a prospective observational study. BMC Infect Dis. 2021;21(1):971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayiga I, Okello E, Lwabi P, Ndeezi G. Prevalence of group a streptococcus pharyngeal carriage and clinical manifestations in school children aged 5–15 yrs in Wakiso District, Uganda. BMC Infect Dis. 2017;17(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Increased incidence of scarlet fever and invasive Group. A Streptococcus infection - multi-country. [https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429]
- 4.Avire NJ, Whiley H, Ross K. A review of Streptococcus pyogenes: Public Health risk factors, Prevention and Control. Pathogens 2021, 10(2). [DOI] [PMC free article] [PubMed]
- 5.Armitage EP, de Crombrugghe G, Keeley AJ, Senghore E, Camara FE, Jammeh M, Bittaye A, Ceesay H, Ceesay I, Samateh B, et al. Streptococcus pyogenes carriage and infection within households in the Gambia: a longitudinal cohort study. Lancet Microbe. 2024;5(7):679–88. [DOI] [PubMed] [Google Scholar]
- 6.Robinson JL. Paediatrics: how to manage pharyngitis in an era of increasing antimicrobial resistance. Drugs Context 2021, 10. [DOI] [PMC free article] [PubMed]
- 7.Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. 2018;12(3):e0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for treatment of sore throat in children and adults. Cochrane Database Syst Rev. 2021;12(12):CD000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijaya D, Sathish J, Janakiram K. The prevalence of group a streptococci carriers among asymptomatic school children. J Clin Diagn Res. 2013;7(3):446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin J. The Carrier State of Streptococcus pyogenes. In: Streptococcus pyogenes: Basic Biology to Clinical Manifestations. 2nd edn. Edited by Ferretti JJ, Stevens DL, Fischetti VA. Oklahoma City (OK); 2022. [PubMed]
- 11.Organization WH. Health conditions in the occupied Palestinian territory, including east Jerusalem, and in the occupied Syrian Golan. In.; 2023.
- 12.Najjar S, Sultan HO, Falana HH, Ata RO, Manasrah MA, Dreidi M, Abukhalil AD, Naseef H. Assessment of adherence to guidelines for testing and treatment of pharyngitis among children in Palestine: a retrospective review study. Germs. 2023;13(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abd El-Ghany SM, Abdelmaksoud AA, Saber SM, Abd El Hamid DH. Group a beta-hemolytic streptococcal pharyngitis and carriage rate among Egyptian children: a case-control study. Ann Saudi Med. 2015;35(5):377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Othman AM, Assayaghi RM, Al-Shami HZ, Saif-Ali R. Asymptomatic carriage of Streptococcus pyogenes among school children in Sana’a City, Yemen. BMC Res Notes. 2019;12(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlKhaldi M, Kaloti R, Shella D, Al Basuoni A, Meghari H. Health system’s response to the COVID-19 pandemic in conflict settings: policy reflections from Palestine. Glob Public Health. 2020;15(8):1244–56. [DOI] [PubMed] [Google Scholar]
- 16.Lee HH, Lin SH. Effects of COVID-19 Prevention measures on other common infections, Taiwan. Emerg Infect Dis. 2020;26(10):2509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Distribution of Schools in Palestine by Supervising Authority., Region and Governorate, for Scholastic Years 2011/2012–2022/2023 [https://www.pcbs.gov.ps/Portals/_Rainbow/Documents/Schools_en.html]
- 18.Distribution of Basic Stage Students in Schools in Palestine by Supervising Authority, Region and Governorate and sex for Scholastic Years 2011/2012–2022/2023. [https://www.pcbs.gov.ps/Portals/_Rainbow/Documents/Basic_Students_en.html]
- 19.Rystedt K, Hedin K, Tyrstrup M, Skoog-Stahlgren G, Edlund C, Giske CG, Gunnarsson R, Sundvall PD. Agreement between rapid antigen detection test and culture for group a streptococcus in patients recently treated for pharyngotonsillitis - a prospective observational study in primary care. Scand J Prim Health Care. 2023;41(1):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, van Driel ML, Nair S, Jones MA, Thorning S, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;2011(7):CD006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmadhany A, Advani N, Djer MM, Handryastuti S, Safari D. Prevalence and predicting factors of Group A beta-hemolytic Streptococcus carrier state in primary schoolchildren. Ann Pediatr Cardiol. 2021;14(4):471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zohud A, Alam L. A review of Groundwater Contamination in West Bank, Palestine: quality, sources, risks, and management. Water 2022, 14(21).
- 23.WorldBank. Securing water for development in West Bank and Gaza. In. Washington. DC.; 2018.
- 24.Steer AC, Jenney AW, Kado J, Good MF, Batzloff M, Magor G, Ritika R, Mulholland KE, Carapetis JR. Prospective surveillance of streptococcal sore throat in a tropical country. Pediatr Infect Dis J. 2009;28(6):477–82. [DOI] [PubMed] [Google Scholar]
- 25.Woolf AD, Stierman BD, Barnett ED, Byron LG, Council On Environmental H, Climate C. Committee on infectious D: drinking Water from private Wells and risks to Children. Pediatrics 2023, 151(2). [DOI] [PubMed]
- 26.DeMuri GP, Wald ER. The Group A streptococcal Carrier State Reviewed: still an Enigma. J Pediatr Infect Dis Soc. 2014;3(4):336–42. [DOI] [PubMed] [Google Scholar]
- 27.Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37(3):333–40. [DOI] [PubMed] [Google Scholar]
- 28.Kaspersen KA, Pedersen OB, Petersen MS, Hjalgrim H, Rostgaard K, Moller BK, Juul-Sorensen C, Kotze S, Dinh KM, Erikstrup LT, et al. Obesity and risk of infection: results from the Danish blood Donor Study. Epidemiology. 2015;26(4):580–9. [DOI] [PubMed] [Google Scholar]
- 29.Zuo Z, Yang C, Ye F, Wang M, Wu J, Tao C, Xun Y, Li Z, Liu S, Huang J, et al. Trends in respiratory diseases before and after the COVID-19 pandemic in China from 2010 to 2021. BMC Public Health. 2023;23(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen JL, Benigno M, Malhotra D, Khan F, Angulo FJ, Hammond J, Swerdlow DL, Reimbaeva M, Emir B, McLaughlin JM. Pandemic-related declines in hospitalization for non-COVID-19-related illness in the United States from January through July 2020. PLoS ONE. 2022;17(1):e0262347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadambari S, Goldacre R, Morris E, Goldacre MJ, Pollard AJ. Indirect effects of the covid-19 pandemic on childhood infection in England: population based observational study. BMJ. 2022;376:e067519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang MC, Su YT, Chen PH, Tsai CC, Lin TI, Wu JR. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front Cell Infect Microbiol. 2023;13:1200617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanat M, Hoste ME, Gobat NH, Anastasaki M, Bohmer F, Chlabicz S, Colliers A, Farrell K, Hollerbach S, Karkana MN et al. Patients’ and clinicians’ perspectives on the primary care consultations for acute respiratory infections during the first wave of the COVID-19 pandemic: an eight-country qualitative study in Europe. BJGP Open 2022, 6(2). [DOI] [PMC free article] [PubMed]
- 34.van der Velden AW, Bax EA, Bongard E, Munck Aabenhus R, Anastasaki M, Anthierens S, Balan A, Bohmer F, Bruno P, Chlabicz S, et al. Primary care for patients with respiratory tract infection before and early on in the COVID-19 pandemic: an observational study in 16 European countries. BMJ Open. 2021;11(7):e049257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marou V, Vardavas CI, Aslanoglou K, Nikitara K, Plyta Z, Leonardi-Bee J, Atkins K, Condell O, Lamb F, Suk JE. The impact of conflict on infectious disease: a systematic literature review. Confl Health. 2024;18(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The researchers are willing to provide the data sets generated and/or analyzed as part of this study to interested parties upon reasonable request made to the corresponding author.