Abstract
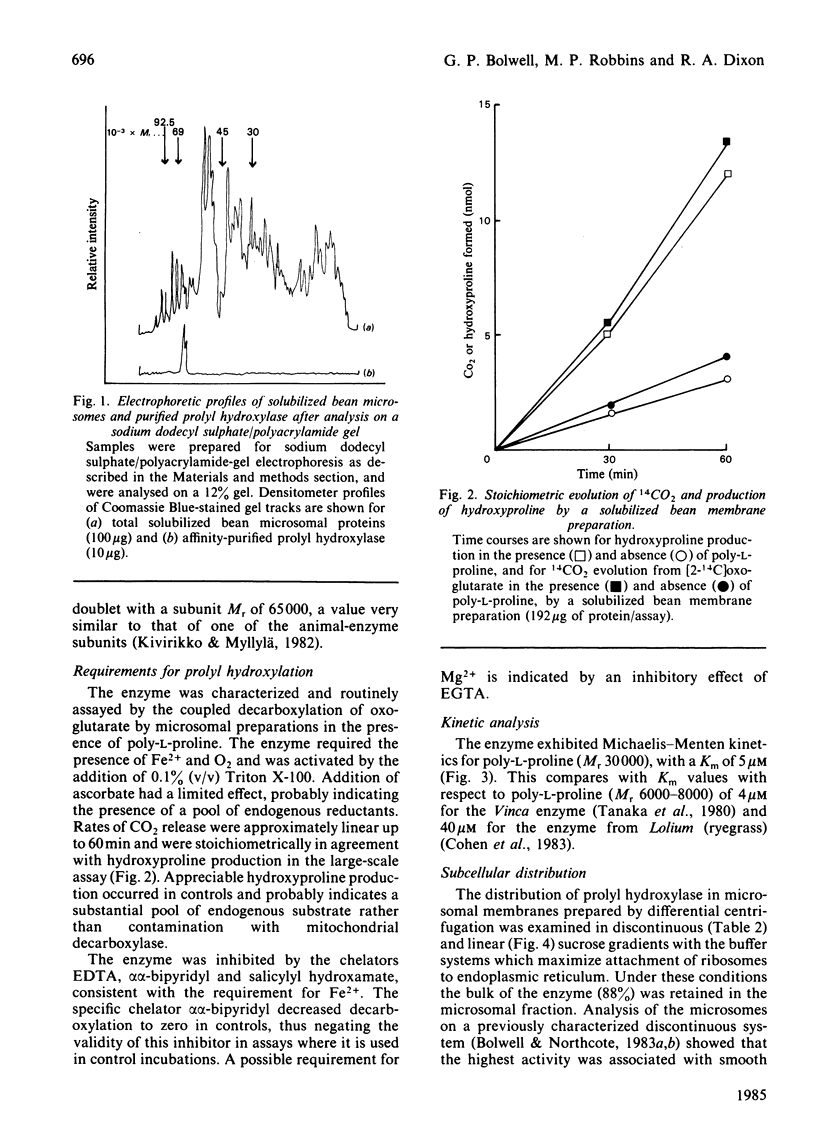
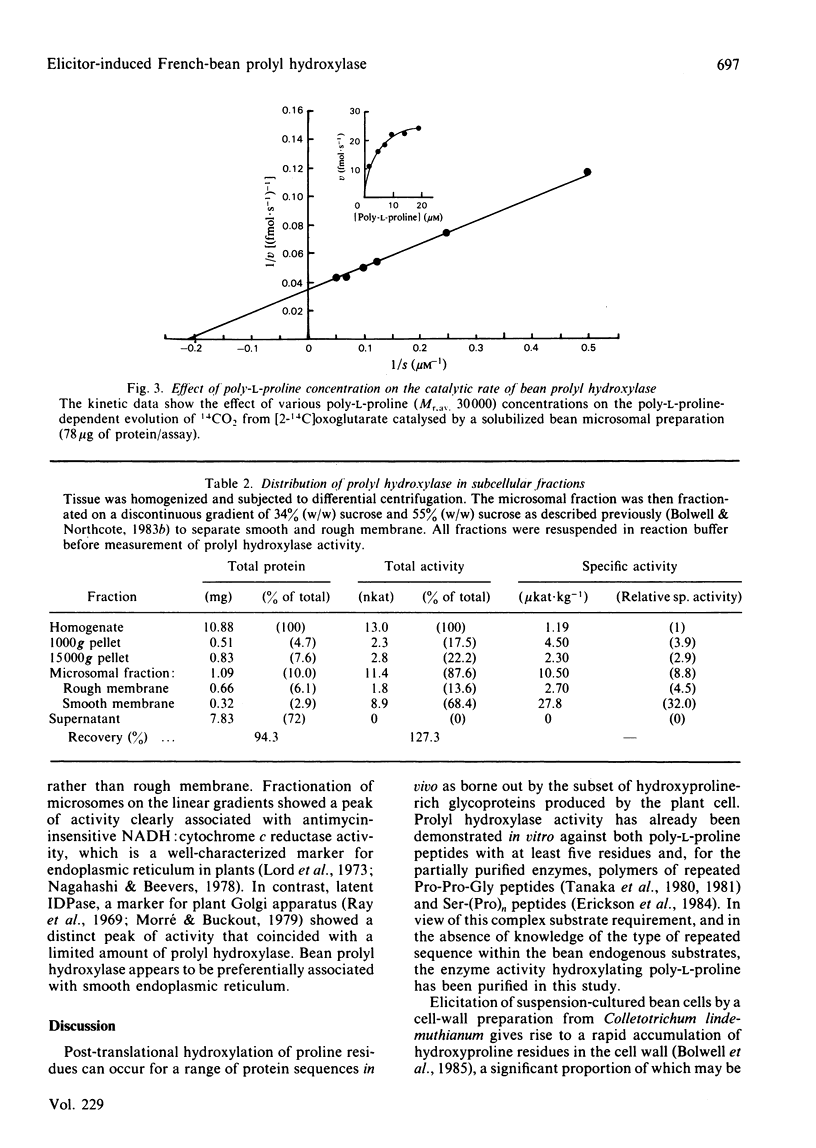
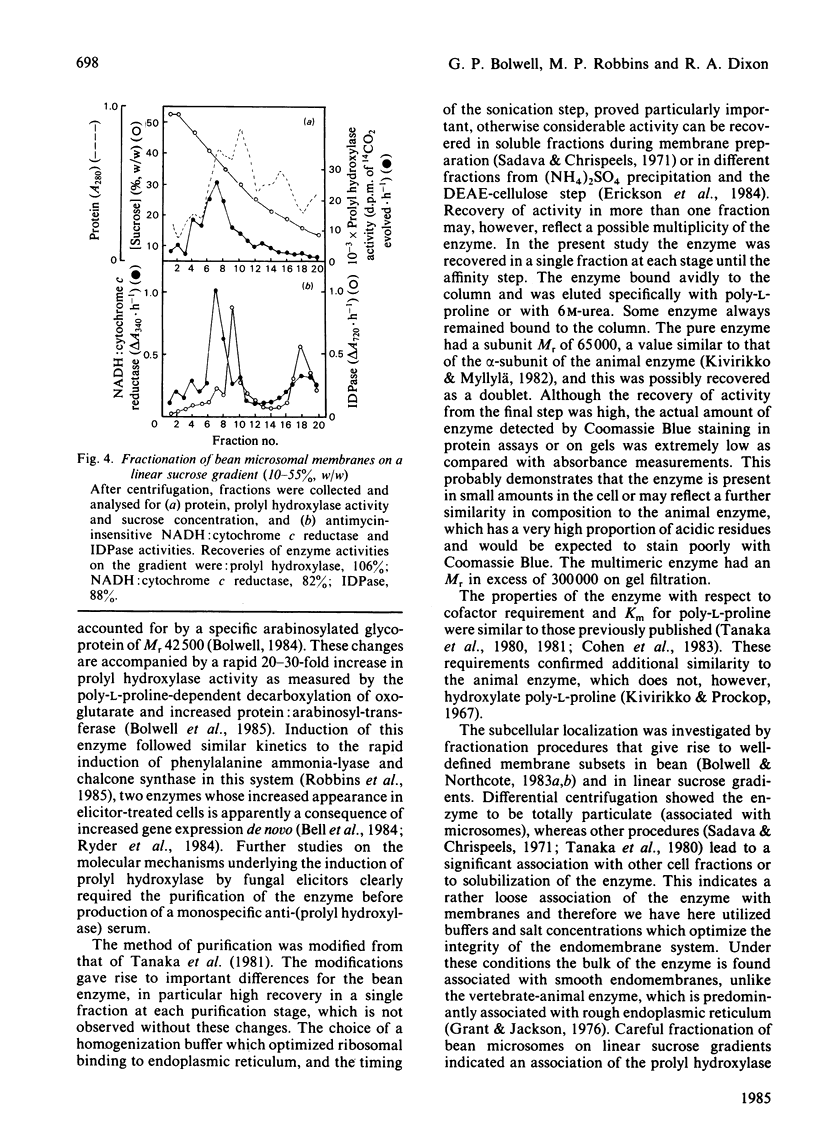
The enzyme prolyl hydroxylase (proline: 2-oxoglutarate dioxygenase, EC 1.14.11.12), induced in suspension-cultured cells of Phaseolus vulgaris L. (French bean) by treatment with an elicitor preparation from the phytopathogenic fungus Colletotrichum lindemuthianum, has been investigated. The enzyme, which catalyses the hydroxylation of poly-L-proline with the stoichiometric decarboxylation of 2-oxoglutarate, has been shown to be localized mainly in smooth endoplasmic reticulum. After solubilization from microsomal membranes, the hydroxylase was purified by ion-exchange chromatography and affinity chromatography on poly-L-proline-Sepharose 4B. The subunit Mr, as assessed by sodium dodecyl sulphate/poly-acrylamide-gel electrophoresis, was 65 000, the subunit apparently being recovered as a doublet: the subunits associate under non-denaturing conditions to give at least a tetramer. The bean hydroxylase has kinetic properties and cofactor requirements similar to those previously reported for the enzyme from other plants. Elicitor treatment of suspension-cultured bean cells leads to a rapid induction of prolyl hydroxylase activity concomitant with induction of a protein: arabinosyl-transferase and increased levels of an arabinosylated hydroxyproline-rich protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P. Differential patterns of arabinosylation by membranes of suspension-cultured cells of Phaseolus vulgaris (French bean) after subculture or elicitation. Biochem J. 1984 Sep 1;222(2):427–435. doi: 10.1042/bj2220427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Arabinan synthase and xylan synthase activities of Phaseolus vulgaris. Subcellular localization and possible mechanism of action. Biochem J. 1983 Feb 15;210(2):497–507. doi: 10.1042/bj2100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Induction by growth factors of polysaccharide synthases in bean cell suspension cultures. Biochem J. 1983 Feb 15;210(2):509–515. doi: 10.1042/bj2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. B., Schibeci A., Fincher G. B. Biosynthesis of Arabinogalactan-Protein in Lolium multiflorum (Ryegrass) Endosperm Cells : III. Subcellular Distribution of Prolyl Hydroxylase. Plant Physiol. 1983 Jul;72(3):754–758. doi: 10.1104/pp.72.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. E., Jackson D. S. The biosynthesis of procollagen. Essays Biochem. 1976;12:77–113. [PubMed] [Google Scholar]
- Karr A. L. Isolation of an enzyme system which will catalyze the glycosylation of extensin. Plant Physiol. 1972 Aug;50(2):275–282. doi: 10.1104/pp.50.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Kishida Y., Sakakibara S., Prockop D. J. Hydroxylation of (X-Pro-Gly)n by protocollagen proline hydroxylase. Effect of chain length, helical conformation and amino acid sequence in the substrate. Biochim Biophys Acta. 1972 Jul 21;271(2):347–356. doi: 10.1016/0005-2795(72)90209-7. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. J., Northcote D. H. The location of arabinosyl:hydroxyproline transferase in the membrane system of potato tissue culture cells. Biochem J. 1981 Jun 1;195(3):661–667. doi: 10.1042/bj1950661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Cramer C. L., Bell J. N., Robbins M. P., Dixon R. A., Lamb C. J. Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5724–5728. doi: 10.1073/pnas.81.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline biosynthesis in plant cells. Peptidyl proline hydroxylase from carrot disks. Biochim Biophys Acta. 1971 Feb 10;227(2):278–287. doi: 10.1016/0005-2744(71)90060-x. [DOI] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Sato K., Uchida T. Plant prolyl hydroxylase recognizes poly(L-proline) II helix. J Biol Chem. 1981 Nov 25;256(22):11397–11400. [PubMed] [Google Scholar]
- Tanaka M., Shibata H., Uchida T. A new prolyl hydroxylase acting on poly-L-proline, from suspension cultured cells of Vinca rosea. Biochim Biophys Acta. 1980 Dec 4;616(2):188–198. doi: 10.1016/0005-2744(80)90137-0. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]


