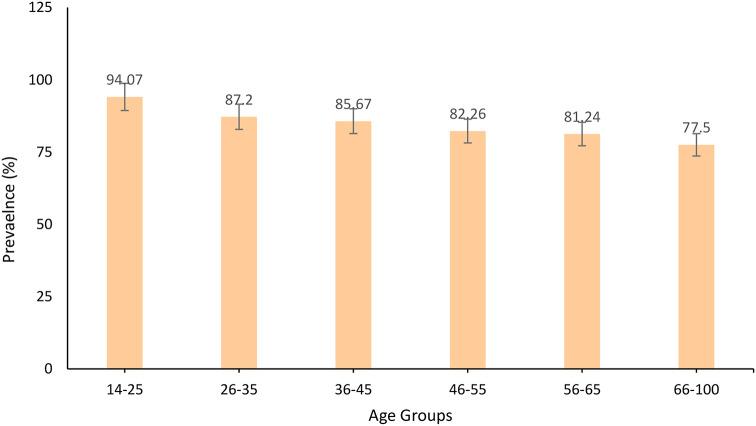Abstract
Background
Communities living along the shoreline and on the islands of Lake Victoria in northwestern Tanzania remain endemic for schistosomiasis and suffer from the life-threatening morbidities associated with the disease. Nevertheless, the control measures particularly the mass drug administration do not cover the adult population. The current project on Ukerewe island aims to close this gap by involving adult community members in the control program. Here we report the baseline results of S. mansoni infection and associated hepatosplenic morbidities and factors before implementing the project activities.
Methods
A cross-sectional analytical study was conducted with 4,043 participants aged ≥ 18 years living in 20 villages on Ukerewe island, northwestern Tanzania. Individual stool and urine samples were collected and examined using the Kato-Katz (KK) technique and point-of-care circulating cathodic antigen testing(POC-CCA) to identify S. mansoni eggs and antigens, respectively. All study participants underwent ultrasound evaluation of S. mansoni hepatosplenic morbidities using the Niamey protocol. Rapid diagnostic tests were used to diagnose HIV infection, hepatitis C and chronic hepatitis B. A questionnaire was used to collect demographic data and reported clinical symptoms of study participants.
Results
A total of 4,043 participants took part in the study, of which 49.7% (n = 2,009) and 50.3% (n = 2,035) were male and female, respectively. The overall prevalence of S. mansoni infection was 30.4% (95%CI:29.0-31.9%) and 84.7% (95%CI:83.3–85.9%), respectively, based on the KK technique and the POC-CCA test. The geometrical mean eggs per gram of faeces (GMepg) was 105.3 (95%CI:98.7-112.3% GMepg) with 53.9%, 32.4% and 13.7% of the participants having mild, had moderate and severe intensity of infection. The prevalence of hepatitis C, HIV, and hepatitis B was 0.4%, 2.2% and 4.7%, with 0.2%, 2.2% and 5.4% of the infected individuals coexisting with S. mansoni infection. The prevalence of splenomegaly, periportal fibrosis, hepatomegaly, and portal vein dilatation was 40.5%(95%CI: 38.8–42.1%), 48.1%(95%CI:64.4–49.7%), 66.2%(95%CI:4.6–67.7%) and 67.7%(95%CI:66.2–69.2%), with their prevalence varying depending on the demographic information and infection status of the participants. Other detectable ultrasound-related morbidities included ascites (1.7%), collateral veins (18.3%) and gall bladder wall thickness (40.4%). Age groups, gender, reported clinical characteristics, reported non-use of the drug praziquantel, liver imaging pattern, and place of residence remained independently associated with hepatosplenic morbidities.
Conclusion
The current study setting is endemic for S. mansoni infection and the population has a high prevalence of the disease associated hepatosplenic morbidities characterized by hepatomegaly, splenomegaly, ascites, gall bladder wall thickening, periportal fibrosis and portal vein dilatation. Several demographic, clinical and epidemiological circumstances remained independently associated with S. mansoni infection and associated morbidities. These findings call for integrative intervention efforts, starting with whole community MDA that includes all out of schools community members.
Keywords: Schistosoma mansoni, Hepatosplenomegaly, Risk factors, Adult, Ukerewe, Tanzania
Background
Schistosomiasis remains endemic in Tanzania and the country is the second country after Nigeria with the highest number of schistosomiasis cases [1, 2]. The country is endemic for parasites Schistosoma mansoni and Schistosoma haematobium [2]). The current study focused on S. mansoni, the causative agent of the intestinal form of schistosomiasis, which is highly endemic in the northwestern regions of Tanzania, particularly the regions bordering the Lake Victoria shoreline [3, 4]. Communities living along the shoreline of Lake Victoria bears the highest burden of the disease and its associated morbidities [4–8]. Previous studies have found a high prevalence of hepatomegaly, splenomegaly and periportal fibrosis in school aged children and the adult population [4, 5, 9, 10]. These are chronic manifestations of an S. mansoni infection that, if left untreated lead to the development of portal hypertension, oesophageal varices, portal-systematic venous shunts (collateral veins) and haematemesis [11, 12]. In northwestern Tanzania, these S. mansoni morbidities which are detected using an ultrasound are recorded during communities’ surveys [9, 13] and in hospital settings [14]). In the hospital settings, these morbidities are associated with high mortalities in the adult population [14].
The control of schistosomiasis follows the recommendations of the World Health Organization [15]. Currently, the common control measure is mass preventive chemotherapy with praziquantel drugs, with the main target group being the school aged children (SAC), who receive medication within the school environment [15]. Repeated mass preventive chemotherapy regimens against schistosomiasis targeting SAC over the past 15 years have resulted in a decrease in the prevalence and intensity of the infection [16, 17] but have not lead to the elimination of schistosomiasis as a public health problem [16]. Among the challenges that are currently facing the control programme, is the lack of inclusion of the adult population and children under five years of age. The untreated population maintain the transmission of the parasite within the communities and serves as a source of re-infection to the treated population. Furthermore, untreated adult, or community members outside of the school setting live with an untreated infection for many years, resulting in high morbidity and mortality [5, 18]. For example, in a tertiary hospital in northwestern Tanzania, 50% of adult cases with upper gastrointestinal bleeding are caused by chronic S. mansoni morbidities [14] and 25% of these cases die within six months of diagnosis [14]. In light of these challenges and in response to the sustainable Development Goals, that call for “ leaving no one behind,” the WHO has published a new guideline to combat schistosomiasis. They call for the inclusion of all age groups (≥ 2 years) [15], especially in areas where the prevalence of schistosomiasis is > 10% [15]. In order to reach the stage of elimination, the WHO recommendation emphasizes the need to integrate other intervention measures such as the provision of clean water, the use of improved sanitation facilities and improved hygiene in endemic communities [15]. This should be accompanied by the implementation of snail control strategies to reduce transmission [19].
Planning and implementation of schistosomiasis interventions requires understanding of the disease ecology by identifying areas with remaining sustained transmission, categorizing and characterizing these areas as recommended by WHO and finally, deciding on the type of interventions to implement [15]. Furthermore, for future monitoring of the impact of the intervention on the disease prevalence and associated morbidities, it is important to collect baseline information to serve as measurement indicators during the process monitoring and impact assessment. In that context, here we present the baseline characteristics of the adult (≥ 18 years) community members associated with S. mansoni infection, associated morbidities, and their associated factors in 20 villages of Ukerewe district, north-western Tanzania before the implementation of the five-years community-based mass preventive chemotherapy project. It combines adult treatment of the adult individuals, test-and-treat campaigns, public health education and capacity building of the healthcare workers, health facilities and affected communities to expand the provision of praziquantel drug to remote populations.
Methods
Study area
The project activities are being implemented at Ukerewe district, in the Mwanza region of northwestern Tanzania. Ukerewe District is an island in the Lake Victoria located at 2.0299° S and 33.0339° E. The districts have a tropical climate with temperatures between 17.0 °C and 27.0 °C, and the average annual rainfall is 1,090 mm. Rainfall is bimodal and is characterised by a short rainy season between September and January and a long rain season between March and June.
In total, the district consists of 38 islands, of which 15 islands have permanent residents and 23 are fishing camps. Ukara and Nansio are the biggest islands. The district consists of a total of 76 villages, 54 sub-villages and 25 wards spread across four divisions, including Mumbuga, Mumlambo, Ilangala and Ukara. The district has a district hospital located at Nansio (capital of the district), four (4) health centres and 32 dispensaries located on various islands in Lake Victoria (district health report). According to 2022 census, the district had a population of 387,815 [20]. The main economic activities of the inhabitants are fishing, subsistence agriculture (cultivating casava, corn, banana, sweet potatoes, and various fruits), livestock farming and small business.
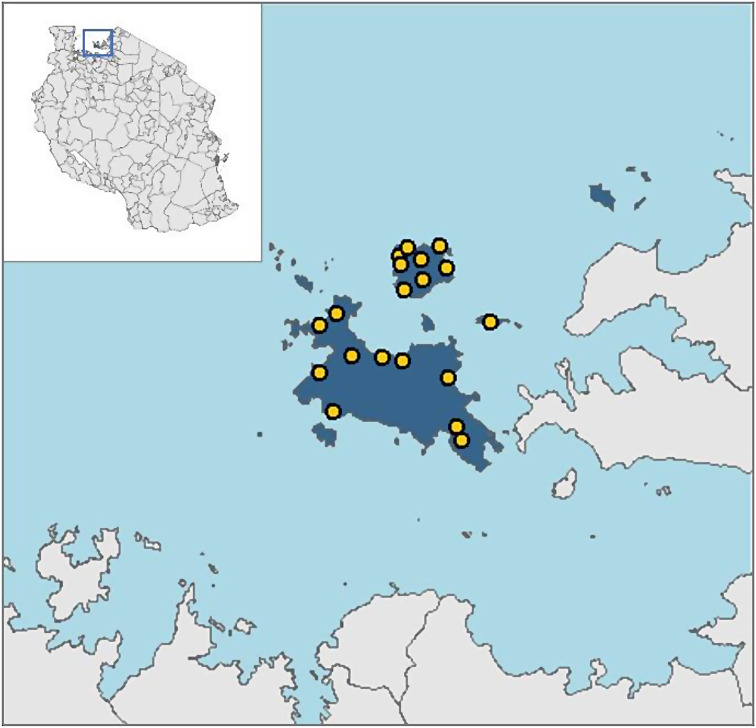
Intestinal schistosomiasis is highly endemic in the district and the prevalence is high both in children and the adult population [6, 7, 18]. Hepatosplenomegaly and associated sequalae are common in school aged children and the adult population [9]. To date, the control of S. mansoni infection in the study area has included mass chemotherapy with praziquantel drug, primarily, focused on school children to reduce the long-term morbidities associated with the infection. Adult individuals and non-school going children were not included. In contrast, the current study covers the adult population, approximately 55,000 adult in 20 villages. Project activities are implemented in 20 villages (Fig. 1) of the 76 villages, selected based on hospital data and result of previous studies [9, 21], which suggested that these villages were highly endemic for intestinal schistosomiasis and needed to be prioritized for interventions. The villages included in the study are Bugorola, Bugula, Bukiko, Bukindo, Bukondo, Bukungu, Busumba, Bwisya, Chibasi, Chifule, Gallu, Kamea, Kome, Kweru, Muriti, Muritilima, Musozi, Nebuye, Nyamanga and Nyang’ombe (Fig. 1).
Fig. 1.
Location of Ukerewe district and the 20 villages involved in the current study
Implemented intervention against intestinal schistosomiasis
A five-year community-based health care project is being implemented in 20 villages, in which the following measures are being taken:
-
(i)
Capacity building of health workers with a focus on the detection of schistosomiasis cases, treatment, and referral of cases of severe hepatosplenic disease associated with hematemesis to nearest health facilities.
-
(ii)
Improving early detection of patients with chronic manifestations of intestinal schistosomiasis at the community level through training of community healthcare workers/community drug distributors and targeted community and referral to health facilities.
-
(iii)
Improve community awareness of intestinal schistosomiasis (disease epidemiology, risk factors, modes of transmission, signs, and symptoms), preventive measures and behaviours involving various community stakeholders such as beach management units, village and sub-village administration, community healthcare workers and community drug distributors, owners of fishing camps and cooperatives and various economic groups in the targeted communities.
-
(iv)
Treatment of chronic diseases caused by intestinal schistosomiasis - establishment of an ultrasound and endoscopy department at the district hospital for the diagnosis and treatment of oesophageal varices and other hepatosplenic complications (ascites, haematemesis, portal hypertension, etc.) and capacity building of healthcare workers in the district hospital through knowledge transfer within primary and tertiary hospitals.
-
(v)
Annual community-based mass drug administration, preceded by community awareness rising, test-and-treat campaigns, and training of community drug distributors.
-
(vi)
Supporting healthcare facilities to ensure regular availability and distribution of praziquantel to community members.
Study design, inclusion, and exclusion criteria
This was a cross-sectional analytical study conducted among randomly selected community members in their households. Before starting the study, a population census was conducted to determine the population size of the targeted villages. This allowed participants to be randomly selected from each study village.
Participants were included in the study if they (i) were permanent residents of the target villages of the study and had lived in the village for at least two years, (ii) were ≥ 18 years old, both male and female, (iii) agreed to provide written consent, (iv) participants agreed to provide individual stool and urine samples for testing for S. mansoni infections, and (v) had not received treatment with the drug praziquantel in the past six months. On the other hand, participants were excluded from the study if they (i) suffered from a clinical illness requiring treatment in health facilities (ii) refused/declined to complete all study procedures and (iii) reported using praziquantel in the past six months.
Sample size and sampling procedures
A single proportion sample size formula for cross-sectional studies was used to calculate the sample size [22]. With a confidence interval of 95%, with the proportion of infected people from the previous study of 0.86 and a tolerable error of 1.1%, a total of 3,823 people had to be included in the study. With a refusal rate to participate of 5%, a total of 191 participants were added. The total sample size required was 4,014. However, a total of 4,043 participants agreed to participate in the study and were enrolled. For the study, 200 participants per villages were recruited and enrolled from 100 randomly selected households of each village.
Data collections
Questionnaire
Each participants was administered a structured questionnaire from the previous study in Tanzania [23] to collect demographic information (gender, age, village, and sub-village of residence). In addition, the questionnaire collected information on signs and symptoms of intestinal schistosomiasis including history of blood in faeces, history of abdominal pain, history of vomiting blood, current vomiting blood, abdominal distension, and the previous use of praziquantel.
Parasitological examination of Schistosoma mansoni infection
A single stool sample was collected in a labelled container. Duplicate thick Kato Katz (KK) smears were prepared from each sample after mixing the stool sample to obtained a homogenous mixture [24]. The thick KK smears were prepared using a 41.7 mg template and were examined for the presence of S. mansoni eggs after 24 h of clearance using a light microscope in the Parasitology laboratory of the Catholic University of Health and Allied Sciences, Mwanza, Tanzania. To adhered to quality assurance procedures, 20% of the positive and negative KK slides were re-examined by a third laboratory technicians who was not involved in the initial examination the slides.
Examination of Schistosoma mansoni related circulating Cathodic Antigen (CCA) using a point of care test
A single urine sample was collected from a randomly selected sub-sample of the study participants (73% of the total study participants) and tested for the presence of circulating cathodic antigens (CCA) using rapid point-of-care circulating cathodic (POC-CCA) test (http://www.rapid-diagnostic.com/). Trace results were considered positive. The use of the rapid test was preceded by refresher training of the laboratory technicians involved in our previous field work with the same diagnostic test. The team using the POC-CCA test to diagnose Schistosoma infection was not involved in the preparation or examination of the KK thick smears.
Examination of hepatitis-C and hepatitis B viral infections
A fingerpick blood sample was collected from each participant and examined for the presence of hepatitis C antibodies in whole blood using a rapid qualitative test (ACON Laboratories, Inc., San Diego, CA) [25]. The same fingerpick blood sample was used to detect chronic hepatitis B virus infection using a rapid test for hepatitis B surface antigen (HBsAg) test [25].
Examination of human immunodeficiency Virus-1 infection
A blood sample from fingerprick was used to diagnose HIV infection. According to the Tanzanian national algorithm, two types of rapid HIV antibody rapid tests were used: the SD Bioline and Uni-GOLD (Trinity Biotech PLV, Bray, Ireland). All participants were counselled before and after HIV testing according to Tanzanian guidelines [26].
Ultrasonographical examination for presence of S. Mansoni hepatosplenic morbidities
Height of each study participant was measured with a stadiometer (height was recorded in centimetre) and each of them was clinically examined for liver and spleen consistency before the ultrasonographical examination following established procedures described elsewhere [13]. A pair of ultrasonographers with experience in the Niamey protocol [13] examined all study participants.
To ensure quality, a sub-population of the study participants were examined by both Ultrasonographers and in case of any difference, the study participants were re-examined by both. All identified hepatosplenic morbidities (spleen size, peripheral portal vein branches (PPBs), periportal fibrosis, liver texture patterns, thickness of the peripheral portal vein branches) detected were classified according to the Niamey Protocol [13]. Periportal fibrosis was qualitatively classified based on the liver imaging patterns (A, B, C, D, E and F) [13], with liver imaging pattern A and B being classified as normal.
The size of the left liver lobe was measured along the right para-sternal line and that of the spleen was measured along the craniocaudal axis of it from the left flank side. The portal vein diameter (PVD) was measured at a halfway between the confluence of the splenic vein and the superior mesenteric veins and their bifurcation within the liver [13]. A portal vein diameter of 2 to ≤ 4 SD was considered dilated and > 4 SD was considered significantly marked dilated, as described in the Niamey protocol [27].
Data analysis and management
Data were entered using Excel and transferred to Stata Version 17 (StataCorp, statistical software, College Station, TX: StataCorp LP. Texas, USA). All continuous variables were summarized using mean, median and their standard deviations or interquartile ranges depending on data distributions. S. mansoni eggs counts were obtained from the two slides and the mean was multiplied by 24 to obtained eggs per gram of faeces. S. mansoni geometrical mean eggs per gram of faeces (GMepg) was calculated using the non-logarithmically transformed means. The comparison of mean S. mansoni egg counts were done using either student t-test (for two groups) or ANOVA (for more than two groups). Intensity of infection was classified according to WHO criteria in which individuals with 1–99 epg, 100–399 epg and ≥ 400 epg had low, moderate, and heavy intensity of infection [15]. Hepatosplenic morbidities were analysed according to the Niamey protocol after adjustment to the height of the reference population [13]. The same protocol was used to define cut-off points between normal and enlarged spleen, left liver lobe and dilatation of the portal vein diameter [13]). Generalized Linear Model (GLM) was used to investigate which risk factors were independently associated with S. mansoni infections, periportal fibrosis, left liver lobe and portal vein dilatation (PVD). At bivariate analysis, explanatory variables with P-value < 0.2 were transferred to multivariate analysis. The Prevalence ratio with their 95%CI were generated and presented in the final tables. For all analysis, a P-value of < 0.05 was considered significant.
Treatment
All participants received praziquantel drug (40 mg/kgBWT) regardless of their infection status. The medication was administered under direct observation at the study site within the village. After treatment, participants were asked to remain at the treatment site for one hour to observe any side effects of the medication and to be monitored by the project team.
Results
Demographic characteristics of the study participants
A total of 4,043 participants were enrolled in the study, of which 50.3% (n = 2,034) were female and 49.7% (n = 2,009) were male. The overall mean age of the participants was 45.3 ± 16.7 years. At enrolment, participants reported various symptoms and signs as presented in Table 1. In relation to sex, blood in feaces (P < 0.004), ever received PZQ (P < 0.001) and ever vomited blood (P < 0.004) were reported more frequently among males’ participants. There was no significant difference in relation to age for the following reported symptoms: (i) diarrhoea (χ2(5) = 1.4938, P = 0.9), (ii) blood in stool in the last two weeks (χ2(5) = 1.7392, P = 0.9) and (iii) abdominal pain (χ2(5) = 7.9583, P = 0.2). Having ever vomited blood was reported more frequently in the age group 36–45 years (1.6%), 46–55 years (3%) and 56–65 years (2.1%) (Exact = 0.1), currently vomiting blood (Exact = 0.9) and ever had abdominal distension problems (χ2(5) = 5.1942, P = 0.4). In the older age groups ≥ 36 years, the proportion of individuals who had ever taken PZQ treatment was higher compared to the younger age groups (χ2(1) = 164.2949, P = 0.001).
Table 1.
Reported symptoms and signs associated with S. mansoni among study participants
| Variable | Response | Sex | Statistical test | |
|---|---|---|---|---|
| Male | Female | |||
| Reported diarrhoea in the past two weeks | No | 1,388 (69.1%) | 1,415 (69.5%) |
χ2 = 0.0937 P = 0.8 |
| Yes | 621 (30.9%) | 620 (30.5%) | ||
| Blood in faeces | No | 1664 (82.8%) | 1,756 (86.3%) |
χ2 = 9.3724 P = 0.002 |
| Yes | 345 (17.2%) | 279 (13.7%) | ||
| Abdominal pain | No | 435 (21.7%) | 240 (11.8%) |
χ2 = 70.6663 P = 0.001 |
| Yes | 1,574 (78.3% | 1,795 (88.2%) | ||
| Ever vomited blood | No | 1961 (97.6%) | 2011 (98.8%) |
χ2 = 8.4626 P = 0.004 |
| Yes | 48 (2.4% | 24 (1.2%) | ||
| Currently vomiting blood | No | 1,998 (99.5%) | 2030 (99.7%) |
χ2 = 2.3402 P = 0.1 |
| Yes | 11 (0.5%) | 5 (0.3%) | ||
| Ever had abdominal distension | No | 1,922 (95.7)% | 1,961 (96.4%) |
χ2 = 1.2743 P = 0.3 |
| Yes | 87 (4.3%) | 74 (3.6%) | ||
| Ever received praziquantel (not in the past six month) | No | 894 (44.5%) | 1,284(63.1%) |
χ2 = 139.9255 P = 0.001 |
| Yes | 1,115 (55.5%) | 752 (36.9%) | ||
Prevalence and intensity of Schistosoma mansoni infection
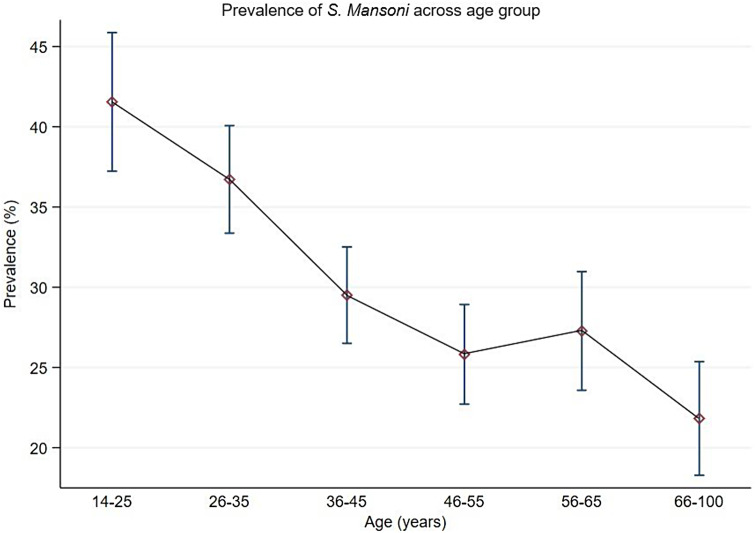
The overall prevalence of S. mansoni infection based on Kato-Katz-Technique was 30.4% (95%CI:29.0-31.9%), with male participants having a higher prevalence than female participants (32.2% versus 28%, χ2(1) = 11.2005, P = 0.001). The youngest age groups 18–25 years and 26–35 years had the highest prevalence compared to the older age groups ≥ 36 years (Table 2). Figure 2 shows the prevalence of S. mansoni categorised by age groups.
Table 2.
Prevalence of Schistosoma mansoni based on Kato Katz technique categorised by age and sex
| Variable | N | Schistosoma mansoni infection | Statistical test | |
|---|---|---|---|---|
| Positive | Negative | |||
| Sex | ||||
| Female | 2,034 | 570 (28%) | 1465 (71.9%) |
χ2(1) = 11.2005 P = 0.001 |
| Male | 2,009 | 660 (32.9% | 1349 (67.2%) | |
| Age groups (in years) | ||||
| 18–25 | 503 | 209 (41.6%) | 294 (58.4%) |
χ2(5) = 73.4663 P = 0.001 |
| 26–35 | 798 | 293 (36.7%) | 505 (63.3%) | |
| 36–45 | 888 | 262 (29.5%) | 626 (70.5%) | |
| 46–55 | 767 | 198 (25.8%) | 569 (74.2%) | |
| 56–65 | 561 | 153 (27.3%) | 408 (72.7%) | |
| ≥ 66 | 527 | 115 (21.8%) | 412 (78.2%) | |
Fig. 2.
Prevalence of Schistosoma mansoni infection categorized by age based on Kato Katz technique among adult community members on Ukerewe district
The geometrical mean of eggs per gram of faeces (GMepg) was 105.3 (95%CI:98.7-112.3 GMepg), with males having the highest GMepg value of 113.8 (95%CI:104.0–124.4 GMepg) while the GMepg value of females was 96.3 (87.8-105.6 GMepg) (t = 3.6076, P = 0.001). There was a significant difference in the intensity of infection between the age groups, with the youngest age groups 18–25 years (126.6–95%CI:100.3-131.9 GMepg) and 25–35 years (115.04-95%CI:100.3-131.9 GMepg) having the highest intensity of infection (F = 9.61, P = 0.001). In relation to infection intensity categories, 53.9%, 32.4% and 13.7% had light, moderate and heavy intensity of infection, respectively. No significant difference was observed in all infection categories (χ2(2) = 6.2142, P = 0.045) in relation to sex. The youngest age groups 18–25 years and 26–35 years had a higher proportion of people with heavy infection intensity (19% and 16.4%) than other age groups (χ2(10) = 21;2487, P = 0.02).
A limited number of POC-CCA tests were available, both stool and urine samples could therefore only be analysed from a random sample of participants (n = 2,945). Overall, the prevalence of Schistosoma based on the POC-CCA test was 84.7% (95%CI:83.3–85.9%), with no significant difference between male and female participants (84.9% versus 84.4%, χ2(1) = 0.1387, P = 0.7). The youngest age groups (Figs. 3), 18–26 years (94.1%) and 26–35 years (87.2%) had the highest prevalences compared to the oldest age groups (χ2(5) = 48.1767, P = 0.001. Of the 2494/2945 (84.7%) who had POC-CCA positive results, 803/2,995 (27.3%) had S. mansoni egg positive slides.
Fig. 3.
Prevalence of Schistosoma mansoni infection categorized by age and sex based on Point-of-Care Circulating Cathodic Antigen Test among adult community members on Ukerewe district
Based on KK-technique, factors associated with S. mansoni infections were being male (aPR = 1.2,95%CI:1.0-1.1, P = 0.001), age groups, 18–25 years (aPR = 1.2,95%CI:1.1–1.3, P < 0.001), 26–35 years (aPR = 1.2,95%CI:1.1–1.2, P < 0.001), 36–45 years (aPR = 1.1,95%CI:1.0-1.1, P < 0.001), 46–55 years (aPR = 1.1,95%CI:1.0-1.1,P < 0.04) and 56–65 years (aPR = 1.1,95%CI:1.0-1.1, P < 0.03), reported blood in stool (aPR = 1.1,95%CI:1.0-1.1, P < 0.002), no history of PZQ use (aPR = 1.1,95%CI:1.0-1.1, P < 0.001) and village of residence. Table 3 present risk factors associated with S. mansoni infection among the adult population.
Table 3.
Factors associated with S. mansoni infections among adult individuals in 20 selected villages of Ukerewe district
| Variable | PR | 95%CI | P-values | aPR | 95%CI | P-values |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1 | 1 | ||||
| Male | 1.0 | 1.0–1.1 | 0.001 | 1.2 | 1.0–1.1 | 0.001 |
| Age groups | ||||||
| 14–25 | 1.2 | 1.2–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| 26–35 | 1.2 | 1.1–1.2 | 0.001 | 1.2 | 1.1–1.2 | 0.001 |
| 36–45 | 1.1 | 1.0–1.3 | 0.002 | 1.1 | 1.0–1.1 | 0.001 |
| 46–55 | 1.0 | 0.9–1.1 | 0.1 | 1.1 | 1.0–1.1 | 0.04 |
| 56–65 | 1.1 | 1.0–1.1 | 0.04 | 1.1 | 1.0–1.1 | 0.03 |
| ≥ 66 | 1 | |||||
| Reported diarrhoea in the past two weeks | ||||||
| No | 1 | 1 | ||||
| yes | 0.9 | 0.9–1.1 | 0.2 | 0.9 | 0.9–1.1 | 0.04 |
| Reported blood in faeces | ||||||
| No | 1 | 1 | ||||
| Yes | 1.1 | 1.0–1.1 | 0.001 | 1.1 | 1.0–1.1 | 0.002 |
| Reported abdominal pain | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.9–1.0 | 0.004 | 0.9 | 0.9–1.0 | 0.03 |
| Reported history of blood vomiting | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.9–1.1 | 0.3 | --- | ---- | --- |
| Currently vomiting blood | ||||||
| No | 1 | |||||
| Yes | 0.8 | 0.7–1.1 | 0.3 | --- | --- | --- |
| History of abdominal distension | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.9–1.0 | 0.4 | --- | --- | --- |
| History of praziquantel use | ||||||
| No | 1.1 | 1.0–1.1 | 0.001 | 1.1 | 1.0–1.1 | 0.001 |
| Yes | 1 | 1 | ||||
| Hepatitis C | ||||||
| No | 1 | |||||
| Yes | 0.8 | 0.7–1.1 | 0.3 | ---- | ---- | ---- |
| Hepatitis B | ||||||
| No | 1 | |||||
| Yes | 1.1 | 0.9–1.1 | 0.3 | ---- | ---- | ---- |
| HIV infection | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.8–1.0 | 0.3 | ---- | ---- | ----- |
| Village of residence | ||||||
| Nebuye | 1 | 1 | ||||
| Bugorola | 1.1 | 0.9–1.1 | 0.2 | 1.0 | 0.9–1.1 | 0.3 |
| Bugula | 1.2 | 1.1–1.3 | 0.01 | 1.2 | 1.1–1.3 | 0.01 |
| Bukiko | 1.1 | 1.0–1.2 | 0.03 | 1.1 | 1.0–1.2 | 0.02 |
| Bukindo | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Bukondo | 1.0 | 0.9–1.1 | 0.2 | 1.1 | 0.9–1.2 | 0.1 |
| Bukungu | 1.4 | 1.2–1.5 | 0.001 | 1.4 | 1.2–1.5 | 0.001 |
| Busumba | 1.1 | 1.0–1.2 | 0.03 | 1.1 | 1.0–1.2 | 0.04 |
| Bwisya | 1.1 | 0.9–1.2 | 0.06 | 1.1 | 1.0–1.2 | 0.04 |
| Chibasi | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Chifule | 1.3 | 1.2–1.5 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Galu | 1.1 | 1.0–1.3 | 0.003 | 1.1 | 1.1–1.3 | 0.002 |
| Kamea | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.002 |
| Kome | 1.3 | 1.2–1.4 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Kweru | 1.3 | 1.2–1.4 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Muriti | 1.1 | 0.9–1.2 | 0.1 | 1.1 | 0.9–1.2 | 0.1 |
| Murutilima | 1.1 | 1.1–1.2 | 0.1 | 1.1 | 0.9–1.2 | 0.2 |
| Musozi | 1.3 | 1.2–1.4 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Nyang’ombe | 1.2 | 0.9–1.2 | 0.2 | 1.1 | 0.9–1.2 | 0.2 |
| Nyamanga | 1.3 | 1.2–1.4 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
Co-infection of S. mansoni with Hepatitis C, B and HIV-1
A total of 3,939 participants had complete results for hepatitis C antibodies, 3, 933 for hepatitis B surface antigen and 3,895 for HIV-1 antibodies. Overall, the prevalences of hepatitis C antibodies, HIV and hepatitis B surface antigen were 0.4%, 2.2% and 4.7%. In general, 0.3%, 2.2% and 5.4% of the participants were co-infected with hepatitis C and S. mansoni, HIV and S.mansoni and hepatitis B and S. mansoni. A possible triple infection with hepatitis B, C and S. mansoni infections was observed in one participant whereas triple infection with HIV, hepatitis B and S. mansoni infection was observed in two participants.
Schistosoma mansoni related hepatosplenic morbidities and their associated factors
Complete data for abdominal ultrasound data were available for 3,630 participants from 20 villages.
Periportal fibrosis and its associated factors
A total of 3,447 participants had complete data for hepatosplenomegaly. The overall prevalence of periportal fibrosis (PPF) was 48.1% (1,657/3,447,95%CI:64.4–49.7%) and 47.3% of them had a detectable active S. mansoni infection as indicated by S. mansoni egg positive slides. Male participants had a higher prevalence of PPF than female participants (55.8%,95%CI:56.1–60.7% versus 37.3%,95%CI:34.9–39.6%, χ2(1) = 154.0461, P = 0.001). The older age group (≥ 36 years) had the highest prevalence of PPF compared with the youngest age group < 36 years (χ2(2) = 21.4302, P = 0.001). For all the participants who had PPF, had liver image pattern C and Cb (15.2%), D (7.8%), E and F (2.9%).
Factors associated with PPF are presented in Table 4. At univariate analysis, increased liver image pattern was associated with increased risk of having PPF. At multivariable analysis, being male (aPR = 1.2, 95%CI:1.1–12,P < 0.001), older age groups, 36–45 years (aPR = 1.2,95%CI:1.0-1.2,P < 0.02), 46–55 years (aPR = 1.1,95%CI:1.1–1.2,P < 0.001), 56–65 years (aPR = 1.2,95%CI:1.0-1.1,P < 0.02) and ≥ 66 years (aPR = 1.2,95%CI:1.1–1.2,P < 0.001), reported ever vomited blood (aPR = 1.1,95%CI:1.0-1.3,P < 0.03), reported abdominal distension (aPR = 1.1,95%CI:1.1–1.3,P < 0.001) and villages of residences were associated with increased risk of having PPF.
Table 4.
Risk factors associated with periportal fibrosis among adult participants on Ukerewe Island
| Variable | cPR | 95%CI | P-value | aPR | 95%CI | P-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1 | |||||
| Male | 1.2 | 1.2–1.3 | 0.001 | 1.2 | 1.1–1.2 | 0.001 |
| Age groups in years | ||||||
| 15–25 | 1 | 1 | ||||
| 26–35 | 1.1 | 1.0–1.2 | 0.002 | 1.1 | 1.0 -1.1 | 0.2 |
| 36–45 | 1.1 | 1.1–1.2 | 0.001 | 1.2 | 1.1–1.2 | 0.02 |
| 46–55 | 1.1 | 1.1–1.2 | 0.001 | 1.1 | 1.1–1.2 | 0.001 |
| 56–65 | 1.1 | 1.0–1.2 | 0.002 | 1.2 | 1.0-1.1 | 0.02 |
| ≥ 66 | 1.1 | 1.1–1.2 | 0.001 | 1.2 | 1.1–1.2 | 0.001 |
| Reported ever vomited blood | ||||||
| No | 1 | |||||
| Yes | 1.2 | 1.1–1.4 | 0.004 | 1.1 | 1.0–1.3 | 0.03 |
| HIV serostatus | ||||||
| Negative | 1 | |||||
| Positive | 0.9 | 0.8–1.1 | 0.8 | -- | -- | -- |
| Hepatitis C | ||||||
| Negative | 1 | |||||
| Positive | 1.1 | 0.8–1.3 | 0.6 | -- | -- | -- |
| Hepatitis B | ||||||
| Negative | 1 | |||||
| Positive | 0.9 | 0.9–1.1 | 0.5 | -- | -- | -- |
| Reported current vomiting blood | ||||||
| No | 1 | |||||
| Yes | 1.2 | 0.9–1.6 | 0.1 | - | - | - |
| Reported abdominal distension | ||||||
| No | 1 | |||||
| Yes | 1.2 | 1.1–1.3 | 0.001 | 1.9 | 1.1–1.3 | 0.001 |
| Liver image pattern | ||||||
| A | 1 | |||||
| B | 1.2 | 1.0–1.3 | 0.01 | -- | -- | -- |
| C (Cb) | 1.5 | 1.4–1.6 | 0.001 | -- | -- | -- |
| D(Dc, Db, DcB) | 1.5 | 1.4–1.5 | 0.001 | -- | -- | -- |
| E(Ec, Eb, Ecb) | 1.7 | 1.6–1.8 | 0.001 | -- | -- | -- |
| F (Fc) | 1.7 | 1.6–1.8 | 0.001 | -- | -- | -- |
| Intensity of S. mansoni infection | ||||||
| Low | 1 | |||||
| Moderate | 1.0 | 0.9–1.1 | 0.2 | -- | -- | -- |
| Heavy | 1.0 | 0.9–1.1 | 0.5 | -- | -- | -- |
| S. mansoni infection | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.9–1.0 | 0.5 | -- | -- | -- |
| Villages of residence | ||||||
| Muriti | 1 | |||||
| Bugorola | 1.4 | 1.3–1.6 | 0.001 | 1.4 | 1.3–1.5 | 0.001 |
| Bugula | 1.1 | 0.9–1.2 | 0.2 | 1.1 | 0.9–1.3 | 0.2 |
| Bukiko | 1.5 | 1.3–1.6 | 0.001 | 1.5 | 1.3–1.6 | 0.001 |
| Bukindo | 1.3 | 1.2–1.5 | 0.001 | 1.3 | 1.2 1.5 | 0.001 |
| Bukondo | 1.1 | 0.9–1.2 | 0.1 | 1.0 | 0.9–1.2 | 0.4 |
| Bukungu | 1.1 | 1.0–1.3 | 0.01 | 1.1 | 0.9–1.2 | 0.1 |
| Busumba | 1.5 | 1.4–1.7 | 0.001 | 1.5 | 1.3–1.7 | 0.001 |
| Bwisya | 1.5 | 1.3–1.7 | 0.001 | 1.5 | 1.3–1.7 | 0.001 |
| Chibasi | 1.3 | 1.2–1.5 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Chifule | 1.5 | 1.3–1.7 | 0.001 | 1.5 | 1.3–1.6 | 0.001 |
| Gallu | 1.1 | 0.9–1.2 | 0.2 | 1.1 | 0.9–1.2 | 0.2 |
| Kamea | 1.4 | 1.2–1.4 | 0.001 | 1.4 | 1.2–1.5 | 0.001 |
| Kome | 1.3 | 1.2 1.4 | 0.001 | 1.3 | 1.1–1.4 | 0.001 |
| Kweru | 1.1 | 1.0–1.2 | 0.04 | 1.1 | 0.9–1.2 | 0.1 |
| Murutilima | 1.1 | 1.3–1.7 | 0.001 | 1.5 | 1.3–1.6 | 0.001 |
| Musozi | 1.2 | 1.1–1.3 | 0.002 | 1.2 | 1.0–1.3 | 0.01 |
| Nebuye | 1.1 | 1.0–1.3 | 0.01 | 1.1 | 1.0–1.3 | 0.02 |
| Nyamanga | 1.3 | 1.1–1.4 | 0.001 | 1.3 | 1.1–1.4 | 0.001 |
| Nyang’ombe | 1.3 | 1.2–1.5 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
Prevalence of splenomegaly
The overall prevalence of splenomegaly was 40.5% (95%CI: 38.8–42.1%) with significant difference observed between sex. Male participants had a higher prevalence than female participants (45.7% versus 35.1%, χ2(1) = 41.9573, P = 0.001). There was no significant difference in prevalence of splenomegaly between age groups (χ2(5) = 1.7894, P = 0.9). Of those with splenomegaly, 57.9% (n = 851) and 42.1% (n = 618) had enlarged and severely enlarged spleens.
Left liver lobe hepatomegaly and its associated factors
The overall prevalence of hepatomegaly (enlargement of the left liver lobe) was 66.2% (95%CI:4.6–67.7%). Female participants recorded a higher prevalence of hepatomegaly than male participants (70.4% versus 62.1%, χ2(4) = 28.2256, P = 0.001). By age groups, the older age groups ≥ 36 years recorded a higher prevalence of hepatomegaly then the youngest age groups (χ2(5) = 28.6018, P = 0.001). Of those who had hepatomegaly, 66.8% and 33.2% had enlarged and severely enlarged left liver.
Table 5 illustrates factors which remained independently associated with left liver lobe hepatomegaly were being female (aPR = 1.1,95%CI:1.0-1.1, P < 0.001), age groups, reported history of non-use of praziquantel drug (aPR = 1.1,95%CI:1.0-1.1, P < 0.002), village of residences and liver image pattern (C-D).
Table 5.
Factors associated with left liver lobe among adult participants in 20 selected villages of Ukerewe district
| Variable | cPR | 95%CI | P-value | aPR | 95%CI | P-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1.1 | 1.0–1.1 | 0.001 | 1.1 | 1.0–1.1 | 0.001 |
| Male | 1 | 1 | ||||
| Age groups (in years) | ||||||
| 14–25 | 1 | 1 | ||||
| 26–35 | 0.9 | 0.9–1.0 | 0.6 | 0.9 | 0.9–1.0 | 0.6 |
| 36–45 | 1.1 | 1.0–1.1 | 0.02 | 1.1 | 1.0–1.1 | 0.02 |
| 46–55 | 1.1 | 0.9–1.1 | 0.1 | 1.1 | 1.0–1.1 | 0.02 |
| 56–65 | 1.1 | 1.1–1.2 | 0.001 | 1.1 | 1.1–1.2 | 0.01 |
| ≥ 66 | 1.1 | 1.0–1.1 | 0.04 | 1.1 | 0.9–1.1 | 0.05 |
| History of blood vomiting | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.8–1.1 | 0.3 | --- | --- | --- |
| History of blood in stool | ||||||
| No | 1 | |||||
| Yes | 1.0 | 0.9–1.1 | 0.3 | --- | --- | --- |
| History abdominal pain | ||||||
| No | 1 | |||||
| Yes | 1.0 | 1.0–1.1 | 0.4 | --- | ---- | --- |
| History of abdominal distension | ||||||
| No | 1 | 1 | ||||
| Yes | 1.1 | 0.9–1.1 | 0.2 | 1.0 | 0.9–1.1 | 0.8 |
| History of praziquantel treatment | ||||||
| No | 1.0 | 1.0–1.1 | 0.01 | 1.1 | 1.0–1.1 | 0.002 |
| Yes | 1 | |||||
| HIV serostatus | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.8–1.2 | 0.9 | --- | --- | --- |
| Hepatitis C | ||||||
| No | 1 | |||||
| Yes | 1.1 | 0.8–1.4 | 0.5 | --- | --- | --- |
| Hepatitis B | ||||||
| No | 1 | |||||
| Yes | 1.0 | 0.9–1.1 | 0.9 | --- | --- | --- |
| S. mansoni infection status | ||||||
| No | 1 | 1 | ||||
| Yes | 1.0 | 0.9–1.1 | 0.2 | 1.0 | 0.9–1.1 | 0.2 |
| Intensity of S. mansoni infection | ||||||
| Low | 1 | |||||
| Moderate | 1.0 | 0.9–1.1 | 0.7 | --- | --- | --- |
| Heavy | 1.1 | 0.9–1.1 | 0.2 | --- | --- | --- |
| Village of residence | ||||||
| Muriti | 1 | 1 | ||||
| Bugorola | 1.1 | 0.9–1.2 | 0.08 | 1.1 | 0.9–1.2 | 0.2 |
| Bugula | 1.0 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Bukiko | 1.4 | 1.3–1.5 | 0.001 | 1.4 | 1.2–1.5 | 0.001 |
| Bukindo | 1.3 | 1.2–1.4 | 0.001 | 1.3 | 1.1–1.4 | 0.001 |
| Bukondo | 1.3 | 1.1–1.4 | 0.001 | 1.3 | 1.1–1.4 | 0.001 |
| Bukungu | 1.3 | 1.2–1.4 | 0.001 | 1.3 | 1.1–1.4 | 0.001 |
| Busumba | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Bwisya | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Chibasi | 1.3 | 1.1–1.4 | 0.001 | 1.3 | 1.1–1.4 | 0.001 |
| Chifule | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.002 |
| Gallu | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.003 |
| Kamea | 1.3 | 1.3–1.6 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Kome | 1.4 | 1.3–1.6 | 0.001 | 1.3 | 1.2–1.5 | 0.001 |
| Kweru | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Murutilima | 1.2 | 1.0–1.3 | 0.004 | 1.2 | 1.0–1.3 | 0.01 |
| Musozi | 1.4 | 1.3–1.5 | 0.001 | 1.4 | 1.2–1.5 | 0.001 |
| Nebuye | 1.2 | 1.1–1.4 | 0.001 | 1.2 | 1.1–1.4 | 0.001 |
| Nyamanga | 1.4 | 1.3–1.6 | 0.001 | 1.3 | 1.2–1.5 | 0.001 |
| Nyang’ombe | 1.2 | 1.1–1.4 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Liver image pattern | ||||||
| A and B | 1 | 1 | ||||
| C | 1.1 | 1.0–1.1 | 0.02 | 1.1 | 1.0–1.1 | 0.03 |
| D | 1.0 | 1.0–1.1 | 0.04 | 1.1 | 1.0–1.1 | 0.002 |
| E | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| F | 1.1 | 1.0–1.2 | 0.02 | 1.1 | 1.0–1.2 | 0.01 |
Portal vein dilatation and its associated factors
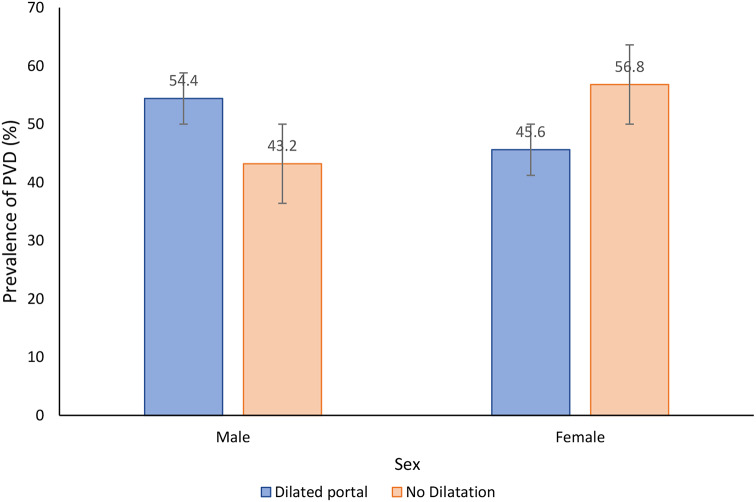
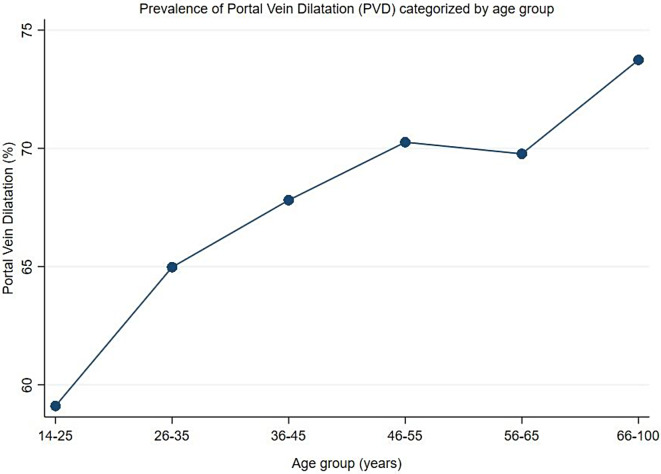
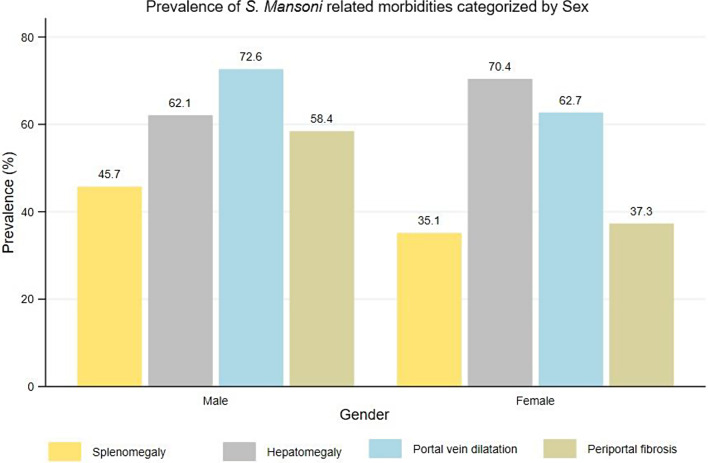
The overall prevalence of portal vein dilatation (PVD) was 67.7% (95%CI: 66.2–69.2%). Male participants had a higher prevalence of PVD than female participants (72.6% versus 62.7%,χ2(1) = 40.2610, P = 0.001) (Fig. 4). In relation to age groups, the older age groups (> 40 years), had the highest prevalence of PVD and the prevalence was observed to increase with increasing age (χ2(5) = 28.4994, P = 0.001) (Fig. 5). Of the participants with PVD, 51.2% and 48.8% had a dilated and severely dilated portal vein.
Fig. 4.
Prevalence of portal vein dilatation categorised by sex among adult population on Ukerewe island, north-western Tanzania
Fig. 5.
Prevalence of Portal Vein Dilatation categorized by age groups, the prevalence of PVD increased with increase in age
Factors associated with portal vein dilatation included age groups, 46–55 years (aPR = 1.1,95%CI:1.0-1.1, P < 0.03), 56–65 years (aPR = 1.1, 95%CI:1.0-1.1, P < 0.02) and (aPR = 1.1,95%CI:1.0-1.2, P < 0.001), as well as liver image pattern C (aPR = 1.5,95%CI:1.4–1.5, P < 0.001), pattern D (aPR = 1.4,95%CI:1.4–1.5, P < 0.001), pattern E (aPR = 1.6,95%CI:1.5–1.7, P < 0.001) and pattern F (aPR = 1.6,95%CI:1.5–1.8, P < 0.001). Other factors are shown in Table 6.
Table 6.
Factors associated with portal vein dilatation among adult participants from 20 villages of Ukerewe district, north-western Tanzania
| Variable | cPR | 95%CI | P-value | aPR | 95%CI | P-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1 | 1 | ||||
| Male | 1.1 | 1.0–1.1 | 0.001 | 1.0 | 0.9–1.0 | 0.2 |
| Age groups (in years) | ||||||
| 14–25 | 1 | 1 | ||||
| 26–35 | 1.1 | 1.0–1.1 | 0.04 | 1.0 | 0.9–1.1 | 0.3 |
| 36–45 | 1.1 | 1.0–1.2 | 0.002 | 1.0 | 0.9–1.1 | 0.1 |
| 46–55 | 1.1 | 1.1–1.2 | 0.001 | 1.1 | 1.0–1.1 | 0.03 |
| 56–65 | 1.1 | 1.0–1.2 | 0.001 | 1.1 | 1.0–1.1 | 0.02 |
| ≥ 66 | 1.2 | 1.1–1.2 | 0.001 | 1.1 | 1.0–1.2 | 0.001 |
| History of blood vomiting | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.8–1.1 | 0.3 | --- | --- | --- |
| History of blood in stool | ||||||
| No | 1 | 1 | ||||
| Yes | 1.2 | 1.0–1.3 | 0.01 | 1.1 | 0.9–1.2 | 0.2 |
| History abdominal pain | ||||||
| No | 1 | |||||
| Yes | 1.0 | 1.0–1.1 | 0.4 | --- | ---- | --- |
| History of abdominal distension | ||||||
| No | 1 | 1 | ||||
| Yes | 1.2 | 1.0–1.2 | 0.002 | 1.0 | 0.9–1.1 | 0.4 |
| History of praziquantel treatment | ||||||
| No | 1.0 | 1.0–1.1 | 0.01 | 1.1 | 1.0–1.1 | 0.002 |
| Yes | 1 | |||||
| HIV serostatus | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.8–1.1 | 0.8 | --- | --- | --- |
| Hepatitis C | ||||||
| No | 1 | |||||
| Yes | 1.1 | 0.8–1.3 | 0.6 | --- | --- | --- |
| Hepatitis B | ||||||
| No | 1 | |||||
| Yes | 0.9 | 0.9–1.1 | 0.5 | --- | --- | --- |
| S. mansoni infection status | ||||||
| No | 1 | |||||
| Yes | 1.0 | 0.9–1.0 | 0.4 | --- | --- | --- |
| Intensity of S. mansoni infection | ||||||
| Low | 1 | |||||
| Moderate | 1.0 | 0.9–1.1 | 0.4 | --- | --- | --- |
| Heavy | 0.9 | 0.9–1.1 | 0.9 | --- | --- | --- |
| Village of residence | ||||||
| Bugula | 1 | 1 | ||||
| Bugorola | 1.3 | 1.2–1.5 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Muriti | 1.1 | 0.9–1.2 | 0.1 | 1.1 | 1.0–1.2 | 0.01 |
| Bukiko | 1.3 | 1.3–1.5 | 0.001 | 1.2 | 1.2–1.3 | 0.001 |
| Bukindo | 1.3 | 1.2–1.4 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Bukondo | 1.1 | 0.9–1.2 | 0.2 | 1.1 | 0.9–1.2 | 0.1 |
| Bukungu | 1.2 | 1.1–1.3 | 0.001 | 1.1 | 0.9–1.2 | 0.2 |
| Busumba | 1.4 | 1.2–1.5 | 0.001 | 1.1 | 1.1–1.3 | 0.001 |
| Bwisya | 1.4 | 1.2–1.5 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Chibasi | 1.2 | 1.1–1.4 | 0.001 | 1.1 | 1.0–1.2 | 0.01 |
| Chifule | 1.4 | 1.2–1.5 | 0.001 | 1.2 | 1.1–1.4 | 0.001 |
| Gallu | 1.0 | 0.9–1.1 | 0.9 | 1.0 | 0.9–1.1 | 0.9 |
| Kamea | 1.2 | 1.1–1.4 | 0.001 | 1.1 | 1.0–1.2 | 0.003 |
| Kome | 1.4 | 1.3–1.5 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Kweru | 1.1 | 1.0–1.2 | 0.003 | 1.1 | 1.0–1.2 | 0.002 |
| Murutilima | 1.4 | 1.2–1.5 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Musozi | 1.4 | 1.3–1.5 | 0.001 | 1.3 | 1.2–1.4 | 0.001 |
| Nebuye | 1.2 | 1.1–1.3 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Nyamanga | 1.3 | 1.2–1.4 | 0.001 | 1.1 | 1.0–1.2 | 0.02 |
| Nyang’ombe | 1.3 | 1.2–1.4 | 0.001 | 1.2 | 1.1–1.3 | 0.001 |
| Liver image pattern | ||||||
| A and B | 1 | 1 | ||||
| C | 1.5 | 1.4–1.6 | 0.001 | 1.5 | 1.4–1.5 | 0.001 |
| D | 1.4 | 1.4–1.5 | 0.001 | 1.4 | 1.4–1.5 | 0.001 |
| E | 1.7 | 1.6–1.8 | 0.001 | 1.6 | 1.5–1.7 | 0.001 |
| F | 1.7 | 1.6–1.9 | 0.001 | 1.6 | 1.5– 1.8 | 0.001 |
Prevalence of both splenomegaly, hepatomegaly, and portal vein dilatation
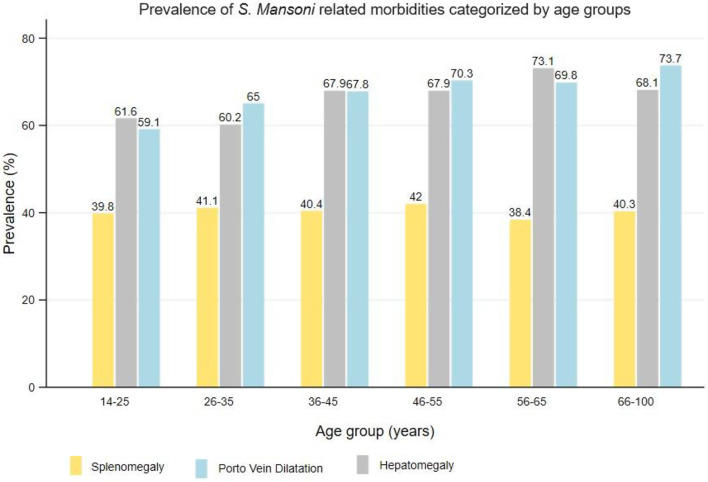
A proportion of participants who had hepatomegaly (66.2%) and splenomegaly (40.5%) had an enlargement of both organs (hepatosplenomegaly). A total of 46.6% of those who had enlargement of either the liver or spleen had hepatosplenomegaly. In relation to the portal vein dilation, a total of 50.9% of those who had splenomegaly, had also a portal vein dilatation. Conversely, 71.9% of those who had hepatomegaly had also a portal vein dilatation. Lastly, 56% of the participants who had hepatosplenomegaly, had portal vein dilatation. Figures 6 and 7 summarizes the prevalence of hepatosplenic morbidities categorised by age and sex among the adult participants on Ukerewe island.
Fig. 6.
Prevalence of S. mansoni related ultrasound detectable morbidities (splenomegaly, hepatomegaly, and portal vein dilatation) categorised by age groups
Fig. 7.
Prevalence of S. mansoni infections and its related ultrasound detectable morbidities (splenomegaly, hepatomegaly, periportal fibrosis and portal vein dilatation) categorised by sex
Other ultrasound detectable morbidities related to S. mansoni infection
Other detectable morbidities included gall bladder wall thickening (≥ 4 mm) with a prevalence of 40.4% (95%CI:38.5–42.3%). The prevalence was higher in males than in females (χ2(1) = 98.8089, P < 0.001), as well as in the older age groups (≥ 26 years, ≥ 40%). Ascites was found in 1.7% and collateral veins in 18.3% of the study participants.
Discussion
The result of the current study confirm that Ukerewe District remains endemic for S. mansoni infection and that its prevalence varies depending on the age, gender, and geographical locations of the study villages. Ultrasound detectable S. mansoni related hepatosplenic morbidities characterised by hepatomegaly of the left liver lobe, splenomegaly, hepatosplenomegaly, ascites, collateral veins, gall bladder wall thickening and portal vein dilatation were common in the adult population involved in the current study. Hepatitis B, C and HIV have been found to occur in the same population which resulting into a proportion of adult individuals being co-infected with S. mansoni and these viral diseases simultaneously. We identified several of demographic, clinical, and epidemiological factors that partially explain the occurrence of S. mansoni related hepatosplenic morbidities in the current study population.
The results of the current study confirm the results of the previous studies on Ukerewe island, which suggested that the area is endemic only for S. mansoni infection [6, 10] and the affected groups have high infection intensity. Previous studies in pre- and school-aged children [6, 7, 10] and adults [4, 9] found a high prevalence and intensity of infections on Ukerewe island, suggesting that these areas are hotspots for S. mansoni infections for a long time. The current study found a lower prevalence of S. mansoni infection based on the KK-technique than 78% [4] and 86.3% [9] reported in previous studies in adults on the same island. The recorded lower prevalence observed in the current study may be explained by the changes in the epidemiology of S. mansoni infection in northwestern Tanzania, where accumulative decline in the prevalence of the disease was observed due to repeated school-based mass drug administration in this area [2, 3, 17]. Despite this positive observation, there are still areas of high S. mansoni prevalence and infection intensity in communities living along the Lake Victoria shoreline, particularly on Ukerewe island [3]. This shows that an intervention that focuses only on a part of the population, mainly school-age children, and excludes the other population groups, namely adults and preschool-age children, from control efforts will not lead to disease control. An integrative MDA will be used in the current study population, which include all community members. While the Tanzanian government offers annual MDA to the SAC through the primary school platform, the current project activities are focusing on adult community members. They will receive annual MDA within their villages and the plan is to provide five rounds of treatment during the five-year project period. Previous studies have shown that a combination of school-and-community based MDA had a major impact on the disease prevalence and infection intensity [28, 29].
In the current study population, a significant difference in sensitivity between the KK technique and the POC-CCA test in detecting S. mansoni cases was observed in the subpopulation of the participants screened with both tests. The POC-CCA test detected more cases of the disease in the adult population compared to the KK technique in the adult population. Studies along the Lake Victoria have shown similar results in the adult population [7, 21, 30, 31] and in pre-school children and school aged children [7, 30]. The POC-CCA test remain highly sensitive than the KK technique and is recommended for the rapid assessment of schistosomiasis in endemic areas [32–35]. However, the test has several limitations including reduced specificity, inability to quantify eggs and is still an expensive test [32]. As intensity of infection continue to be an indicator for measuring the elimination of schistosomiasis as a public health challenge [19] and the decline in intensities of infection due to repeated rounds of MDA impacting the performance of the KK technique [36]. It will be important to continue combining the multiple diagnostic as countries approach the elimination phase.
In the current study, some of the participants had hepatitis B, C, and HIV infections that resulted in co-infection with S. mansoni parasite. Due to their different pathogenic mechanisms, these co-infections can lead to an exacerbation of hepatosplenic pathologies, which in turn can result in liver fibrosis [37]. We have found similar results in our previous studies in the adult population in villages around the Lake Victoria shoreline [38]. However, the co-infections of these viral infections with S. mansoni parasite occurs at lower rate than in other endemic areas of Africa [39], particularly in Egypt, where the prevalence of HCV is high [39, 40]. It is worth noting that in the present study, the prevalence of the hepatitis viruses HCV and hepatitis B is low, therefore, the prevalence of co-infection patterns is also low. Regarding HIV infections, the fishing villages on the southern shoreline of Lake Victoria have lower prevalence of HIV than comparable villages in western Kenya and Uganda [41]. A number of behavioural, cultural, and socio-economic conditions may partially explain the observed differences. Overall, our results demonstrate the need of integrating health services rather than implement parallel programs when targeting communities living in tropical environments where multiple diseases transmission occurs. This can reduce the costs in the program, as in the present survey, which included the district’s HIV program and offered HIV screening services.
It has been repeatedly reported that communities living on the shoreline of Lake Victoria and on its islands where S. mansoni is highly endemic have the highest burden of hepatosplenic disease. The pathology results mainly from Th-2 immune responses due to S. mansoni eggs shed at various sites of the body [11]. The results of the current study and previous studies [9, 18] on Ukerewe island have found a high prevalence of hepatosplenic disease in the adult population. Previous studies reported a prevalence of 35% [18] and 80% of hepatomegaly and splenomegaly [9]. Similarly, a high prevalence of PPF was observed among adult at Kome (42%) and Msozi (42%) villages of Ukerewe island [18]. In the current study, similar trends were noted in the high prevalence of hepatosplenic diseases characterized by splenomegaly (40.5%), PPF (48.1%), left hepatic lobe hepatomegaly (66.2%) and portal vein dilatation (67.7%). We observed that the prevalence of these chronic S. mansoni chronic morbidities increases with age and that they occur frequently after the age of 40 years. Overall, these results suggest that S. mansoni infection reaches its chronic morbidities with increasing age in this population. Our previous study of pre-school children from the same area shows that, in this population, S. mansoni infection begins at two years of age [7] and we have recorded visible signs of hepatosplenic diseases by ultrasound in children aged 5 to 6 years [21]. In the hospital setting in northwestern Tanzania, our studies have found a high prevalence of hepatosplenic disorders, characterized by upper gastrointestinal bleeding. In adults with oesophageal varices, 50% of cases are associated with S. mansoni and almost 25% of these patients die within six months [14]. These results suggest that the adult population suffers from S. mansoni infection for a long time without treatment and eventually develops life threatening morbidities. Overall, our results highlight the need to involve the entire community in schistosomiasis control programs along the Lake Victoria basin. To reduce and control the transmission of schistosomiasis and its associated hepatosplenic morbidities, it is strongly recommended that annual or biannual community-based MDA be offered to the exposed population for an extended period of time to reduce the transmission potential of the parasite and control the hepatosplenic morbidities. Treatment of hepatosplenic disease in adult patients (on a case-by-case basis) in healthcare facilities should be part of this effort. Activities of the current project include improving the detection and treatment of severe hepatosplenic diseases in primary healthcare facilities and transferring patients to the next level of healthcare when necessary to reduce severe morbidity and mortality. In the district hospital, the project is building the capacity of healthcare workers to deal with these serious cases through endoscopic procedures established within this framework. Currently, the country is working to achieve the 2030 vision of controlling and eliminating schistosomiasis as a public health challenge. In the elimination phase, the remaining work is mainly to treat the chronic hepatosplenic disease caused by S. mansoni, which may not resolve or may take time to resolve. Therefore, capacity building of the primary healthcare system is strongly recommended, especially in endemic areas.
In S. mansoni endemic areas, various demographic, clinical and epidemiological factors have been described to explain the continuity of transmission of S. mansoni infections and the development of associated morbidities. In the current study setting, S. mansoni infection was observed to be associated with the youngest age groups, male gender, blood in stool, no use of praziquantel and place of residence. These demographic factors have been described previously to be associated with S. mansoni infection along the Lake Victoria areas [21, 42]. The current study participants reported enterocolitis symptoms and signs such as abdominal pain, diarrhoea, hematemesis, and bloody stools. This was similar to a previous study in the same setting [9]. We found that reported bloody stools continue to be independently associated with S. mansoni infection. From a pathogenic perspective, S. mansoni eggs that pass through the intestinal wall and are accompanied by inflammatory reactions can cause blood to seep into the intestinal lumen and be detectable in excreted stool. Due to rupture of oesophageal varices, digested blood maybe found in stool [43]. In tropical environmental, blood in stool can also be caused by other endemic intestinal infections such as intestinal amoebiasis [44] and other bacterial and viral infections that can cause dysentery. Due to their geographical location on the Lake Victoria shorelines, the population living in these villages frequently contacts lake water during fishing, recreational activities, farming, carrying household chores and farming along the shoreline. Almost, all study villages remain at risk of S. mansoni infection. Epidemiological studies have shown that, S. mansoni infection is associated with village of residence, age, occupation, specifically fishing, being male, and hepatosplenic morbidities [21, 42].
For hepatosplenic morbidities (left liver lobe hepatomegaly, PPF and PVD), sex, older age groups, non-use of praziquantel drugs, reported ever having vomited blood, abdominal distension, liver image pattern (C-F) and village of residences were common explanatory factors that remained independently associated with these morbidities. The association of these morbidities with older age groups clearly shows that the current study population suffering from S. mansoni infection for a long time without treatment. The development of S. mansoni related morbidities is a function of intensity of infection, duration of infection exposure, and a genetical component of the host [11, 42]. These morbidities develop slowly, and the severe manifestations and life-threatening symptoms and signs occur in older age groups [43]. Some of these factors, such as the intensity of infection and duration of exposure can be modified through various interventions. For example, the delivery of PZQ drug through community MDA can be expanded to reach the entire population. This will help to reduce the transmission of S. mansoni infections and lead to regression of these hepatosplenic morbidities [28, 45]. In addition, providing water, sanitation and hygiene facilities to endemic communities can reduce human faecal pollution and human-water contact, thereby helping to facilitates effort to control the disease and push the country to the elimination phase. Public health education that focuses on understanding disease transmission routes and compliance with prevention and control measures can increase target audience awareness, which in turn can lead to improved compliance with control measures such as MDA. These efforts should be accompanied by the search for alternative sources of income for these populations whose livelihood are highly dependent on fishing.
We acknowledge that the current study was not conducted without any limitations. The use of duplicate KK thick smears for a single stool sample to screen for S. mansoni infection may have resulted in an underestimate of the true prevalence of the disease due to daily fluctuation in egg output of infected individuals [46]. To reduce the impact of this limitation, a point-of-care CCA test was used, which has higher sensitivity than the KK technique, but this test also has its own limitations. The cross-sectional nature of the study design, with limited ability to demonstrate the association of various explanatory factors with the outcome, may have contribute to the lack of association between some of the explanatory variables and the outcome of interest. Nevertheless, the current epidemiological study was one of the largest ultrasound surveys in an endemic foci of S. mansoni in northwestern Tanzania and has clearly demonstrated that the disease is still endemic and associated with life-threatening morbidities and therefore requires integrative measures. The current project activities close this gap.
Conclusion
The current study setting is endemic for S. mansoni infection and the population has a high prevalence of hepatosplenic morbidities associated with the disease, characterized by hepatomegaly of the liver lobe, splenomegaly, ascites, thickness of the gall bladder wall thickness, periportal fibrosis and portal vein dilatation. Several demographic, clinical and epidemiological factors remain independently associated with S. mansoni infection and associated morbidities. These findings call for integrative intervention measures, starting with whole- community MDA, that covers all out-of-school going community members. The current project conducts annual MDA for high-risk communities living in 20 villages. However, all the villages on Ukerewe Island require community MDA interventions, which should be combined with other intervention measures such as WASH, public health education and capacity building of the health facilities to detect and treat advanced complications of hepatosplenic schistosomiasis.
Acknowledgements
We would like to acknowledge the participants, community leaders and community healthcare workers of the 20 villages involved in the study for their cooperation and support during the fieldwork. We are also grateful to the district authorities for supporting this study and ensuring that community members have access to the study. We acknowledge the technical team from the National Institute for Medical Research, Mwanza centre and the Bugando Medical Centre, Mwanza for supporting the fieldwork in the 20 villages.
Author contributions
HDM, SK, CK, AM and AF fund acquisition and study designCEC, TB, ESM, GMK, MM and SM fieldwork coordination, data collection and data management HDM and TL data analysisHDM and CEC wrote the first draft of the manuscript SK, CK, AF and AM reviewed the manuscriptHDM, CEC, SK and CK were responsible for the overall project administration.
Funding
This project is funded by the Else Kröner-Fresenius-Stiftung (EKFS) in Germany (Grant number: 2018_HA10SP), through the Else Kröner Center for advanced medical and medical humanitarian studies.
Data availability
The datasets used for analysing the presented data are available from the corresponding authors upon reasonable request. This is because the ethical approval process made no specific mention of making the data publicly available.
Declarations
Ethics approval and consent to participate
The ethical approval to implement the project activities including the current study was obtained from the National Ethical Review Committee board (NIMR/HQ/R.8a/Vol.IX/3590 and NIMR/HQ/R.8 C/Vol.I/1973) and further implementation permission were received from the Prime Minister office for local governments, Mwanza region administrative office and the Ukerewe district council, where the study is being implemented. Written Informed Consent to participate in the current study was obtained from all study participants. The informed consent form was written in Kiswahili language and each participant received a copy of written informed consent. For illiterate participants, the consent form was signed using thumbprint after they had received a clear oral description of the study objectives and the treatment options. The consent form described the objective, durations, risks, and benefits of participating in the study. Participation in the study was voluntary and participants had the right to continue or to withdrawal their consent from the study at any time.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Humphrey D. Mazigo, Email: humphreymazigo@gmail.com
Crecencia Edward Chiombola, Email: Crecencia.edward@yahoo.com.
Stella Mugassa, Email: stlmugassa@gmail.com.
Magreth Magambo, Email: magambomeg@yahoo.com.
Godfrey M. Kaatano, Email: gmkaatano@yahoo.com
Titus Leeyio, Email: titusleeyio@gmail.com.
Erick Simon Mwangoka, Email: eringoks@gmail.com.
Tumaini Baumba, Email: tumaibaumba@yahoo.com.
Saskia Kreibich, Email: Saskia.Kreibich@dahw.de.
Christa Kasang, Email: Christa.Kasang@dahw.de.
Antje Fuss, Email: antje.fuss@medmissio.de.
Andreas Mueller, Email: andreas.mueller@kwm-klinikum.de.
References
- 1.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuente LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128(2):423–40. [DOI] [PubMed] [Google Scholar]
- 2.Mazigo HD, Nuwaha F, Kinung’hi SM, Morona D, Pinot de Moira A, Wilson S, Heukelbach J, Dunne DW. Epidemiology and control of human schistosomiasis in Tanzania. Parasit Vectors. 2012;5:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazigo HD, Zinga MM, Kepha S, Yard E, McRee-Mckee K, Kabona G, Ngoma DD, Nshala A. Precision and geographical prevalence mapping of schistosomiasis and soil-transmitted helminthiasis among school-aged children in selected districts of north-western Tanzania. Parasit Vectors. 2022;15(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malenganisho WLM, Magnussen P, Friis H, Siza J, Kaatano G, Temu M, Vennervald B. Schistosoma mansoni morbidity among adults in two villages along Lake Victoria shores in Mwanza District, Tanzania. Trans Royal Soc Trop Med Hygiene 2008;102(6):532–541 [DOI] [PubMed]
- 5.Mazigo HD, Dunne DW, Morona D, Lutufyo TE, Kinung’hi SM, Kaatano G, Nuwaha F. Periportal fibrosis, liver and spleen sizes among S. Mansoni mono or co-infected individuals with human immunodeficiency virus-1 in fishing villages along Lake Victoria shores, North-Western, Tanzania. Parasit Vectors. 2015;8:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugono M, Konje E, Kuhn S, Mpogoro FJ, Morona D, Mazigo HD. Intestinal schistosomiasis and geohelminths of Ukara Island, North-Western Tanzania: prevalence, intensity of infection and associated risk factors among school children. Parasit Vectors. 2014;7:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruganuza DM, Mazigo HD, Waihenya R, Morona D, Mkoji GM. Schistosoma mansoni among pre-school children in Musozi village, Ukerewe Island, North-Western-Tanzania: prevalence and associated risk factors. Parasit Vectors. 2015;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parisi S, Mazigo HD, Kreibich S, Puchner K, Kasang C, Mueller A. Factors associated with relevant knowledge of intestinal schistosomiasis and intention to participate in treatment campaigns: a cross sectional survey among school children at Ijinga Island on Lake Victoria, North-Western Tanzania. BMC Public Health. 2019;19(1):1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kardorff R, Gabone RM, Mugashe C, Obiga D, Ramarokoto CE, Mahlert C, Spannbrucker N, Lang A, Gunzler V, Gryseels B, et al. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Intern Health. 1997;2(3):230–9. [DOI] [PubMed] [Google Scholar]
- 10.El Scheich T, Hofer L, Kaatano G, Foya J, Odhiambo D, Igogote J, Lwambo N, Ekamp H, Karst K, Haussinger D, et al. Hepatosplenic morbidity due to Schistosoma mansoni in schoolchildren on Ukerewe Island, Tanzania. Parasitol Res. 2012;110(6):2515–20. [DOI] [PubMed] [Google Scholar]
- 11.Dunne DW, Pearce EJ. Immunology of hepatosplenic schistosomiasis mansoni: a human perspective. Micro Infect. 1999;1(7):553–60. [DOI] [PubMed] [Google Scholar]
- 12.Vennervald BJ, Dunne DW. Morbidity in schistosomiasis: an update. Curr Opin Infect Dis. 2004;17(5):439–47. [DOI] [PubMed] [Google Scholar]
- 13.King CH, Magak P, Salam EA, Ouma JH, Kariuki HC, Blanton RE. Measuring morbidity in schistosomiasis mansoni: relationship between image pattern, portal vein diameter and portal branch thickness in large-scale surveys using new WHO coding guidelines for ultrasound in schistosomiasis. Trop Med Internl Health. 2003;8(2):109–17. [DOI] [PubMed] [Google Scholar]
- 14.Chofle AA, Jaka H, Koy M, Smart LR, Kabangila R, Ewings FM, Mazigo HD, Johnson WD Jr., Fitzgerald DW, Peck RN, et al. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infect Dis. 2014;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO guideline on control and elimination of human schistosomiasis. Geneva, Switzerland: World Health Organization; 2021. [PubMed] [Google Scholar]
- 16.Kokaliaris C, Garba A, Matuska M, Bronzan RN, Colley DG, Dorkenoo AM, Ekpo UF, Fleming FM, French MD, Kabore A, et al. Effect of preventive chemotherapy with praziquantel on schistosomiasis among school-aged children in sub-saharan Africa: a spatiotemporal modelling study. Lancet Infect Dis. 2022;22(1):136–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazigo HD, Mwingira UJ, Zinga MM, Uisso C, Kazyoba PE, Kinung’hi SM, Mutapi F. Urogenital schistosomiasis among pre-school and school aged children in four districts of north western Tanzania after 15 years of mass drug administration: geographical prevalence, risk factors and performance of haematuria reagent strips. PLoS Negl Trop Dis. 2022;16(10):e0010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malenganisho WL, Magnussen P, Friis H, Siza J, Kaatano G, Temu M, Vennervald BJ. Schistosoma mansoni morbidity among adults in two villages along Lake Victoria shores in Mwanza District, Tanzania. Trans R Soc Trop Med Hyg. 2008;102(6):532–41. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Ending the neglect to attain the sustainable development goals A road map for neglected tropical diseases 2021–2030 2020.
- 20.Tanzania National Bureau of Statistics. Tanzania Populations census 2012 Tanzania Government 2022.
- 21.Mueller A, Fuss A, Ziegler U, Kaatano GM, Mazigo HD. Intestinal schistosomiasis of Ijinga Island, north-western Tanzania: prevalence, intensity of infection, hepatosplenic morbidities and their associated factors. BMC Infect Dis. 2019;19(1):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charan J, Tamoghna B. How to calculate sample size for different study designs in Medical Research? Indian J Psychol Med. 2013;35(2):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kardorff R, Gabone RM, Mugashe C, Obiga D, Ramarokoto CE, Mahlert C, Spannbrucker N, Lang A, Gunzler V, Gryseels B. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Intern Health 1997;2(3):230–239 [DOI] [PubMed]
- 24.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis Mansoni. Rev Inst Med Trop Sao Paulo 1972;14(6):392–400 [PubMed]
- 25.Villar L, Cruz H, Barbosa J, Bezerra C, Portilho M, Scalioni Lde P. Update on hepatitis B and C virus diagnosis. World J Virol 2015;4(4):323 – 42 2015. [DOI] [PMC free article] [PubMed]
- 26.Lyamuya EF, Aboud S, Urassa WK, Sufi J, Mbwana J, Ndugulile F, Massambu C. Evaluation of simple rapid HIV assays and development of national rapid HIV test algorithms in Dar Es Salaam, Tanzania. BMC Infect Dis. 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter J, Hatz C, Campagne G, Bergquist N, Jenkins JM. Ultrasound in schistosomiasis: a practical guide to the standard use of ultrasonography for assessment of schistosomiasis-related morbidity: Second international workshop, October 22–26 1996, Niamey, Niger. Tropical Diseases Research, World Health Organization, Geneva 2000.
- 28.King CH, Kittur N, Binder S, Campbell CH, N’Goran EK, Meite A, Utzinger J, Olsen A, Magnussen P, Kinung’hi S, et al. Impact of different Mass Drug Administration strategies for gaining and sustaining control of Schistosoma mansoni and Schistosoma haematobium infection in Africa. Am J Trop Med Hyg. 2020;103(1Suppl):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronzan RN, Dorkenoo AM, Agbo YM, Halatoko W, Layibo Y, Adjeloh P, Teko M, Sossou E, Yakpa K, Tchalim M, et al. Impact of community-based integrated mass drug administration on schistosomiasis and soil-transmitted helminth prevalence in Togo. PLoS Negl Trop Dis. 2018;12(8):e0006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazigo HD, Fuss A, Mueller A. High Egg reduction rate but poor clearance of Circulating Cathodic Antigen three weeks after Praziquantel treatment among school children on Ijinga Island, north-western Tanzania. Acta Trop 2021;218:105871. [DOI] [PubMed]
- 31.Mazigo HD, Kepha S, Kinung’hi SM. Sensitivity and specificity of point-of-care circulating Cathodic antigen test before and after praziquantel treatment in diagnosing Schistosoma mansoni infection in adult population co-infected with human immunodeficiency virus-1, North-Western Tanzania. Arch Public Health. 2018;76:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamberton PH, Kabatereine NB, Oguttu DW, Fenwick A, Webster JP. Sensitivity and specificity of multiple Kato-Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl Trop Dis. 2014;8(9):e3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mwinzi PN, Kittur N, Ochola E, Cooper PJ, Campbell CH Jr., King CH, Colley DG. Additional evaluation of the point-of-contact circulating Cathodic Antigen Assay for Schistosoma mansoni infection. Front Public Health. 2015;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kittur N, Castleman JD, Campbell CH Jr., King CH, Colley DG. Comparison of Schistosoma mansoni prevalence and Intensity of Infection, as determined by the Circulating Cathodic Antigen Urine Assay or by the Kato-Katz Fecal Assay: a systematic review. Am J Trop Med Hyg. 2016;94(3):605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straily A, Kavere EA, Wanja D, Wiegand RE, Montgomery SP, Mwaki A, Eleveld A, Secor WE, Odiere MR. Evaluation of the Point-of-Care Circulating Cathodic Antigen Assay for Monitoring Mass Drug Administration in a Schistosoma mansoni Control Program in Western Kenya. Am J Trop Med Hyg. 2021;8(106):303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bärenbold O, Raso G, Coulibaly JT, N’Goran EK, Utzinger J. (2017) VP: Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl Trop Dis 2017, 11(10):e0005953. [DOI] [PMC free article] [PubMed]
- 37.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatitis. 2009;49(4):1335–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazigo HD, Kepha S, Kaatano GM, Kinung’hi SM. Co-infection of Schistosoma mansoni/hepatitis C virus and their associated factors among adult individuals living in fishing villages, north-western Tanzania. BMC Infect Dis. 2017;17(1):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angelico M, Renganathan E, Gandin C, Fathy M, Profili MC, Refai W, De Santis A, Nagi A, Amin G, Capocaccia L, et al. Chronic liver disease in the Alexandria governorate, Egypt: contribution of schistosomiasis and Hepatitis virus infections. J Hepatol. 1997;26(2):236–43. [DOI] [PubMed] [Google Scholar]
- 40.el-Esnawy NA, Al-Herrawy AZ. Seroprevalence of certain hepatitis viruses among Egyptian workers infected with schistosomiasis. J Egypt Public Health Assoc. 2000;75(3–4):357–66. [PubMed] [Google Scholar]
- 41.Mazigo HD, Dunne DW, Wilson S, Kinung Hi SM, de Moira A, Jones FM, Morona D, Nuwaha F. Co-infection with Schistosoma mansoni and human immunodeficiency Virus-1 (HIV-1) among residents of fishing villages of north-western Tanzania. Parasit Vectors. 2014;7(1):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berhe N, Myrvang B, Gundersen SG. Intensity of Schistosoma mansoni, Hepatitis B, age, and sex predict levels of hepatic periportal thickening/fibrosis (PPT/F): a large-scale community-based study in Ethiopia. Am J Trop Med Hyg. 2007;77(6):1079–86. [PubMed] [Google Scholar]
- 43.Jordan P, Webbe G, Sturrock R. Human schistosomiasis. Wallingford, England, CAB 1993 1993.
- 44.Dans LF, Martínez EG. Amoebic dysentery. BMJ Clin Evid 2007, 1(0918). [PMC free article] [PubMed]
- 45.Berhe N, Myrvang B, Gundersen SG. Reversibility of schistosomal periportal thickening/fibrosis after praziquantel therapy: a twenty-six month follow-up study in Ethiopia. Am J Trop Med Hyg. 2008;78(2):228–34. [PubMed] [Google Scholar]
- 46.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, Moore R, Habte E, Redda A, Gebre-Michael T. Variations in helminth faecal egg counts in Kato–Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. 2004;92(3):205–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for analysing the presented data are available from the corresponding authors upon reasonable request. This is because the ethical approval process made no specific mention of making the data publicly available.