Abstract
Pancreatic cancer is a highly aggressive cancer with unfavorable prognosis despite the therapeutic interventions. Intraperitoneal chemotherapy has recently shown potential outcomes in the presence of peritoneal metastases. However, a consensus is still lacking on different methods for intraperitoneal chemotherapy in pancreatic cancer. A variety of drugs and doses via three types of intraperitoneal chemotherapy have been studied. The prognosis and treatment strategies for pancreatic ductal adenocarcinoma (PDAC) will be significantly influenced by peritoneal dissemination and resectability of the macroscopic disease. Normothermic intraperitoneal chemotherapy (NIPEC) has been used for the treatment of peritoneal metastases of pancreatic carcinomas. Intraperitoneal chemotherapy is often combined with systemic therapies or surgical procedures which may lead to favorable combination therapies such as cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a relatively new approach that provides a homogenous and deep penetration of the chemotherapy into the peritoneum by producing aerosols. The present study aims to review the literature for recent evidence on intraperitoneal chemotherapy in pancreatic cancer.
Keywords: Pancreatic cancer, Peritoneal metastasis, Intraperitoneal chemotherapy (IP chemotherapy), Cytoreductive surgery (CRS), Hyperthermic intraperitoneal chemotherapy (HIPEC), Pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Background
Pancreatic cancer ranks as the third leading cause of cancer-related mortality in the USA [1]. Almost half of the diagnosed pancreatic ductal adenocarcinomas (PDACs) develop metastasis prior to diagnosis, leading to a median survival time of less than six months [2]. after the liver, peritoneum is the second most frequent site of metastasis for pancreatic cancer [3, 4]. Peritoneal metastases occur before being detectable on imaging. Therefore, in some centers, diagnostic laparoscopy is employed for the detection of hidden intraabdominal metastases at the initial diagnosis of selected patients with pancreatic cancer [5]. Staging laparoscopy is considered positive in the presence of gross metastases or positive peritoneal lavage and cytology.
A multivariate analysis of 1,004 patients who underwent diagnostic laparoscopy revealed that young age, increased serum carbohydrate antigen (CA) 19 − 9, and several tumor-related factors are associated with positive peritoneal involvement. Therefore, diagnostic laparoscopy could be considered in the presence of these elements and is best to precede neoadjuvant chemotherapy since systemic therapy might reduce the sensitivity of laparoscopic assessment [6]. Approximately 20% of the patients may benefit from cytology for the detection of peritoneal metastases in the absence of any macroscopic disease [5]. Peritoneal dissemination in pancreatic cancer is multifactorial (e.g. through venous or lymphatic invasion), and there has been limited research on random sampling of the peritoneum to detect occult disease in patients with otherwise normal-appearing peritoneum. Consequently, such cases may not be detected at the time of diagnosis.
In the existence of peritoneal metastases, PDAC patients would have a median overall survival (OS) of 7.6 months despite systemic chemotherapy [7]. Poor blood supply of the peritoneum and elevated interstitial fluid pressure of the metastatic tumor prevent systemic therapy from adequately entering and influencing peritoneal carcinomatosis [8]. Moreover, in the presence of peritoneal involvement, receiving and continuation of systemic chemotherapy is restricted due to cancer-related signs and symptoms including malnutrition and ascites [9, 10]. Thus, intraperitoneal therapies have emerged for managing pancreatic cancer with peritoneal metastases [5, 11, 12].
Liquid intraperitoneal chemotherapy (L-IPC) for peritoneal metastases is limited due to variable distribution of the solution into the peritoneum and inadequate drug penetration into the tumor tissue [13]. Such limitations have been mostly conquered by pressurized intraperitoneal aerosol chemotherapy (PIPAC), which nebulizes the solution into the abdominal cavity [14]. Conversion surgery which is defined as adding surgical resection to chemo- or chemoradiotherapy in patients with primary unresectable PDAC, has been commonly used for locally advanced tumors [15].
In the presence of peritoneal metastases, intraperitoneal chemotherapy significantly increases the rate of conversion surgery in comparison to systemic chemotherapy; hence provides promising clinical improvements [16], while decreasing systemic exposure [17]. Hyperthermic intraperitoneal chemotherapy (HIPEC), which is often accompanied by surgical resection, has shown favorable oncologic outcomes in cases with PDAC and peritoneal dissemination [5]. Yet, the optimal intraperitoneal chemotherapy regimen and method of delivery are undefined for pancreatic cancer [3, 18]. Here, we reviewed the current literature on intraperitoneal chemotherapy for the management of pancreatic cancer.
Liquid intraperitoneal chemotherapy (L-IPC)
Normothermic intraperitoneal chemotherapy (NIPEC)
This type of intraperitoneal chemotherapy has been referred to as NIPEC by Frassini and colleagues [18]. Liquid-based intraperitoneal chemotherapy is a proven method, particularly in the setting of multimodal treatment regimens for managing peritoneal metastases [19]. Recently, Öman et al. administered intraperitoneal 5-Fluorouracil (5-FU) for resectable PDAC tumors the day before pancreatic resection and observed that 5-FU and its active metabolite were absorbed by pancreatic tissue, lymph nodes, and hepatic tissue [20]. Several studies investigated the outcomes of NIPEC in pancreatic cancer (Table 1.). Cytology of peritoneal washing is currently the gold standard method for the assessment of response to intraperitoneal chemotherapy in the presence of peritoneal dissemination [16].
Table 1.
NIPEC for pancreatic cancer
| Author, Year | Study design | Country | N | Age (median) |
Tumor status | Chemotherapy agent, dosage | Median OS (months) |
Note |
|---|---|---|---|---|---|---|---|---|
| Meguro, 2023 [22] | Case report | Japan | 1 | 57 | Unresectable peritoneal and liver metastases | I.p. PTX (30 mg/m2) + i.v. nab-PTX (125 mg/m2) + i.v. GEM (1000 mg/m2) | 4.7 after start of therapy | Both patients underwent CART before combined chemotherapy |
| 1 | 58 | Unresectable peritoneal and liver metastases | I.p. PTX + i.v. nab-PTX + i.v. GEM | 9 after start of therapy | ||||
|
Yamamoto, 2022 [16] |
Retrospective cohort |
Japan | 101 | 69 | Unresectable with PM |
I.p. PTX + oral S-1 + i.v. PTX Or I.p. PTX + oral S-1 + i.v. GEM Or I.p. PTX + i.v. nab-PTX + i.v. GEM |
17.9 |
Treatment responders (26%) underwent conversion surgery. The median OS of 27.4 months in the conversion surgery group |
|
Yamada, 2021 [24] |
Clinical trial | Japan | 79 | 69 | Unresectable with PM |
I.p. PTX (20 mg/m2) + i.v. PTX (50 mg/m2) + oral S-1 (80 mg/m2) Or I.p. PTX + i.v. GEM + i.v. nab-PTX |
32.5 |
16 (20%) patients underwent conversion surgery PFS = 9.2 months |
| Takahara, 2021 [12] | Phase I clinical trial | Japan | 12 | 56 | Unresectable with PM |
I.p. PTX (30 mg/m2) + i.v. GEM (1000 mg/m2) + i.v. nab-PTX (125 mg/m2) |
Median PFS 5.4 |
8 (66%) patients became cytology-negative after peritoneal lavage |
| Öman, 2021 [20] | Case series | Sweden | 22 | 65 |
Radiologically Resectable without PM |
I.p. 5-FU 1250 mg/m2 | ~ 11 | Followed by pancreatic surgery |
| Yamada, 2020 [21] | Phase I/II clinical trial | Japan | 46 | - | Unresectable with PM | I.p. PTX (20 mg/m2) + i.v. GEM (800 mg/m2) + i.v. nab-PTX (75 mg/m2) | 14.5 | 8 (17%) patients underwent conversion surgery |
|
Satoi, 2017 [11] |
Phase II clinical trial | Japan | 33 | 69 | Unresectable with PM | I.p. PTX (20 mg/m2) + oral S-1 (80 mg/m2) + i.v. PTX (50 mg/m2) | 16.3 | 8 (24%) patients underwent conversion surgery |
|
Satoi, 2017 [26] |
Retrospective cohort | Japan | 49 | 69 | Unresectable with PM | I.p. PTX (20 mg/m2) + oral S-1 (80 mg/m2) + i.v. PTX (50 mg/m2) | 20 | - |
| Takahara, 2016 [23] | Phase II clinical trial | Japan | 35 | 66 | Malignant ascites caused by PM | I.p. PTX (20 mg/m2) + i.v. PTX (50 mg/m2) + oral S-1 (80 mg/m2) | 4.8 | 8 (22%) patients achieved negative peritoneal cytology |
N, Number of patients; PM, Peritoneal metastases; OS, Overall survival; I.p., Intraperitoneal; I.v., Intravenous; GEM, Gemcitabine; PTX, Paclitaxel; Nab-PTX, Nanoparticle albumin-bound-paclitaxel; PFS, Progression free survival; CART, Concentrated ascites reinfusion therapy; 5-FU, 5-Fluorouracil
Combination of intraperitoneal (i.p.) with intravenous (i.v.) regimen
A phase I trial on 12 patients with pancreatic cancer and peritoneal metastases did not reach the maximum tolerated dose but recommended the dose of 30 mg/m2 for i.p. paclitaxel. Combination of i.p. paclitaxel with systemic gemcitabine and nanoparticle albumin-bound (nab)-paclitaxel showed a response rate of 25% [12]. A phase I clinical trial with the previously mentioned drugs determined the recommended dose of 20 mg/m2 for i.p. paclitaxel, which revealed a response rate of 21/43 patients in phase II of the study. Additionally, the study reported that 8/46 (17%) patients became eligible for conversion surgery with a median OS of 12.4 months after the operation [21]. A report of two cases with pancreatic metastases to both liver and peritoneum, presenting with massive ascites, indicated that combination of systemic and intraperitoneal chemotherapy following the concentrated ascites reinfusion therapy (CART) might be a promising palliative management for such patients [22].
Combination of i.p., i.v., and oral regimens
Intraperitoneal paclitaxel of 20 mg/m2 has also been used in combination with oral S-1 (a fluoropyrimidine-derived medication) and i.v. paclitaxel in a phase II study. Following the treatment, patients with peritoneum-isolated metastasis had a response rate of 36% [11]. Intraperitoneal plus i.v. paclitaxel and S-1 have been employed in a group of patients with gemcitabine resistance with malignant ascites and remote metastasis. It has indicated a median progression-free survival (PFS) of 2.8 months and a response rate of 8% [23]. Yamada et al. conducted a trial with a combined treatment protocol which allowed for 20% of the patients to undergo conversion surgery following the effective treatment. After the conversion surgery, the median PFS was reported to be 9.2 months [24]. Yamamoto et al. designed a phase III trial to compare the combination of systemic and intraperitoneal therapy with systemic chemotherapy alone in PDAC with peritoneal metastasis, which is still ongoing [25].
Comparing i.p. and systemic therapy
A retrospective study, comparing conventional systemic therapy and intraperitoneal paclitaxel, showed improved survival in patients with PDAC and peritoneal metastases who underwent intraperitoneal therapy (17.9 months vs. 10.2 months). Responders to treatment who underwent conversion surgery had a median OS of 27.4 months in the i.p. group versus 11.3 months in the systemic therapy group. However, in multivariate analysis, intraperitoneal paclitaxel did not show any positive effect on survival. Whereas, conversion surgery was significantly more applied in the group with intraperitoneal versus systemic chemotherapy group (23% vs. 4%) [16]. Satoi et al. carried out a retrospective cohort study, with intraperitoneal paclitaxel in a combination regimen that has shown decreased ascites development (25% vs. 62%), increased chance of conversion surgery (30% vs. 7%), and improved OS (20 months vs. 10 months) compared with systemic therapy alone in patients with PDAC and peritoneal dissemination [26].
Adverse events
Adverse events reported in phase II studies were mostly hematologic toxicities such as neutropenia, leukopenia, and anemia. Non-hematologic events included appetite loss, nausea, vomiting, and diarrhea. Peritoneal access-related complications include infection and device dislocation [11, 21, 23]. Yamada et al. [21] reported that 76% (35/46) of the cases developed grade 3–4 hematologic toxicity which is particularly high but comparable to standard systemic chemotherapy [27]. A summary of the 2021 Japanese guideline for clinical practice in PDAC with peritoneal metastases stated a weak recommendation regarding NIPEC for patients who do not have massive ascites [10].
Hyperthermic intraperitoneal chemotherapy (HIPEC)
It has been shown that hyperthermia (40–43℃) enhances the cytotoxicity of the chemotherapy agents [28]. There is evidence suggesting that an increase of HIPEC pressure to 20–34 mmHg would not be associated with postoperative complications or prolonged hospital stay, but elevated core body temperature would be [29]. In the published literature, HIPEC has been implemented through a closed or open procedure that lasts 30 to 90 min [5, 30, 31]. A new HIPEC technology (PRS Combat) employs an additional catheter to recirculate the drug and CO2, enhancing intraperitoneal chemotherapy distribution. It also uses a gas exchanger that controls intra-abdominal pressure following the circulation of CO2 in a closed HIPEC procedure [32] (Fig. 1). Cytoreductive surgery (CRS), which is performed to clear all the macroscopic disease, is often combined with HIPEC to eliminate micrometastases [33]. It is suggested that different dimensions of quality of life such as cognitive, social, emotional, physical, and functional health recover or surpass the baseline by the first year after CRS/HIPEC [34].
Fig. 1.
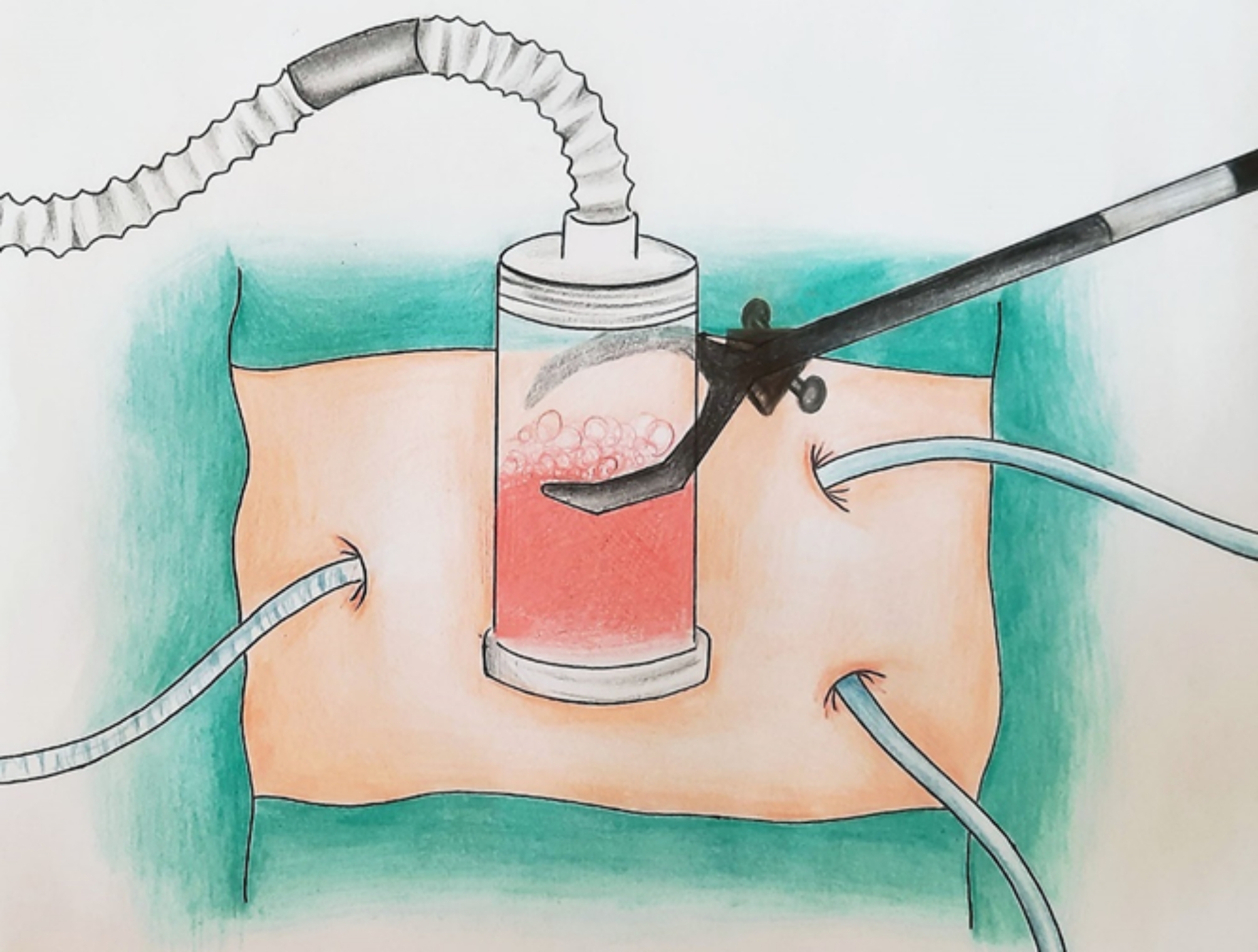
HIPEC using a CO2 recirculation system. HIPEC, Hyperthermic intraperitoneal chemotherapy
Frassini and colleagues have found that completeness of surgical cytoreduction is associated with a survival advantage in patients with pancreatic adenocarcinoma. In fact, the study emphasizes that in cases with borderline resectable and locally advanced pancreatic cancer, in which CRS and surgical resection are possible after neoadjuvant chemotherapy, HIPEC could improve survival without adding to the morbidity [18]. Table 2. summarizes the studies using HIPEC for pancreatic cancer.
Table 2.
HIPEC in pancreatic cancer
| Author, Year | Study design | Country | N | Mets | Mean Initial PCI |
Age | Method | Time (min) | Tem (℃) |
I.p. chemotherapy agent, dosage | Note | PFS (mo) |
OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Padilla-Valverde, 2024 [32] |
Phase II/III randomized clinical trial | Spain | 21 | No | - | Median 68 | Close | 30 | 41–42 |
GEM (120 mg/m2) |
CRS/HIPEC followed by adjuvant therapy | Median 14 | Median 17.1 |
| Zimmermann, 2024 [49] | Case report | Germany | 1 | No | - | ~ 60 | Close | 60 | 42 | GEM (1600 mg) | Left-sided pancreatic resection followed by HIPEC and six cycles of adjuvant systemic chemotherapy | 65 | 65 |
|
Elhariri, 2023 [50] |
Case report | USA | 1 | PM | 9 | 74 | - | 90 | 41–42 |
MMC (30 mg/m2) + CIS (171 mg/m2) |
Chemotherapy and pancreatoduodenectomy followed by CRS/HIPEC then adjuvant therapy | 36 | 72 |
|
Sugarbaker, 2023 [31] |
Case report | USA | 1 | No | - | 55 | Open | 60 | 42 |
GEM (1000 mg/m2) |
Additional 6 cycles of NIPEC | 60 | 120 |
|
Gudmundsdottir, 2023 [5] |
Cohort | USA | 23 | PM | < 7 | Median 57 | Close | 60–90 | 42.5 |
MMC (30 mg) + CIS (200 mg) Or PTX (175 mg/m2) + CIS (100 mg/m2) |
≥ 6 mo of effective chemotherapy before CRS/HIPEC | 17 | 41 |
|
Grotz, 2023 [3] |
Prospective pilot study | USA | 18 | PM | 2 | Median 57 | Close | 60 | 42.5 |
MMC (30 mg) + CIS (200 mg) |
≥ 6 mo of effective chemotherapy before neoadjuvant HIPEC and CRS/HIPEC or IRE/IORT and HIPEC | 20 | 26 |
|
Nogueiro, 2022 [30] |
Case report | Portugal | 1 | PM | 5 | 43 | - | 30 | 43 | Oxa (360 mg/m2) + irinotecan (360 mg/m2) |
Complete CRS/HIPEC Pancreatic solid pseudopapillary neoplasm |
24 | 24 |
|
Sugarbaker, 2021 [45] |
Phase I/II of a pilot protocol | USA | 12 | No | - | Median 56 | Open | 60 |
Median 41.8 |
GEM (1000 mg/m2) | CRS/HIPEC followed by 6 mo of long-term NIPEC | - | 29 (in 8 patients) |
|
Yurttas, 2021 [44] |
Phase I/II pilot trial | Germany | 16 | No | - | Median 62.5 | Close | 60 | 42 | GEM (1000 mg/m2) | - | - | 16.1 after surgery |
|
Azzam, 2020 [41] |
Case series | Saudi Arabia | 5 | No | 4.2 | Median 51 | Open | 60 | 40–42 | GEM (1000 mg/m2) |
CRS followed by IORT and then HIPEC. No death until a median follow-up of 8 months |
- | - |
|
Tentes, 2018 [35] |
Case series | Greece | 6 | PM | 12 | Mean 51.8 | - | - | - |
GEM (1000 mg/m2) Or CIS (50 mg/m2) + MMC (15 mg/m2) |
CRS/HIPEC followed by adjuvant chemotherapy with gemcitabine | - | > 12 (in 4 patients) |
|
Tentes, 2016 [48] |
Case series | Greece | 33 | No | - | Mean 67.8 | Open | 60 | 42.5–43 | GEM (1000mg/m2) | Complete surgical resection followed by HIPEC and for stage-3 patients additional systemic chemotherapy | 9 | 13 |
N, Number of patients who underwent HIPEC; Tem, Temperature; PM, Peritoneal metastases; Mets, Metastases; Mo, Months; OS, Overall survival from diagnosis; PFS, Progression-free survival; IRE, Irreversible electroporation; IORT, Intraoperative radiation therapy; GEM, Gemcitabine; MMC, Mitomycin C; CIS, Cisplatin; PTX, Paclitaxel; Oxa, Oxaliplatin
CRS/HIPEC in pancreatic cancer with peritoneal metastases
The application of CRS and HIPEC in pancreatic cancer with peritoneal metastases was first reported in 2018 by Tentes et al. who indicated that selected patients with tumors located in the tail of the pancreas may benefit from this approach [35]. Peritoneal carcinomatosis index (PCI) is a parameter that characterizes the extent of peritoneal carcinomatosis preoperatively [36]. Patients with a low volume of peritoneal metastasis (PCI < 7) who received induction systemic therapy and underwent CRS plus HIPEC had a three-year OS of 59% compared to systemic therapy alone with the three-year OS of 8% in a cohort study of 61 individuals [5]. However, a systematic review demonstrated that CRS and adjuvant HIPEC are possibly unsafe for patients with pancreatic cancer and peritoneal metastasis due to overall 34% morbidity and 8.5% mortality [37]. A recent systematic review revealed no significant survival benefit in resection of isolated liver metastases of PDAC compared with standard chemotherapy [38]. However, a case report offered the possible benefit of CRS/HIPEC for the synchronous liver and peritoneal metastases of pancreatic cancer [39].
Currently, CRS/HIPEC is not regarded as the standard of care for PDAC with peritoneal metastases due to the lack of sufficient evidence in the literature [3]. Treatment with CRS/HIPEC has also been successful in patients with pancreatic malignancies other than PDAC such as a Pancreatic solid pseudopapillary neoplasm with peritoneal dissemination [30]. Moreover, a case of Pseudomyxoma peritonei (PMP) originating from a perforated intraductal papillary mucinous neoplasm of the pancreas has been treated with CRS/HIPEC, which was considered safe and feasible [40].
Combination of intraoperative radiotherapy (IORT) with CRS/HIPEC
Grotz et al. conducted a prospective pilot study of 18 patients with only peritoneal metastasis of pancreatic cancer who have had at least six months of multiagent systemic chemotherapy. Pursuing systemic therapy, patients underwent neoadjuvant laparoscopic HIPEC and then a group with resectable tumors underwent CRS and HIPEC. Due to the lack of safety evidence on pancreatoduodenal resection in cases with locally advanced or borderline resectable primary tumors, a combination of irreversible electroporation (IRE) or intraoperative radiation therapy (IORT) with HIPEC was employed. After a median follow-up of 16 months, the median OS was reported to be 26 months [3]. Azzam and Amin retrospectively studied patients with resectable pancreatic cancer who underwent CRS followed by IORT and then HIPEC. They reported that this combination of procedures increases the advantages of each one alone, without adding to the perioperative complications [41].
Morbidity and mortality regarding CRS/HIPEC
A multicenter collaborative study of 2,364 patients with peritoneal malignancies of different origins who underwent CRS plus HIPEC demonstrated a postoperative morbidity of 56% and 30-day mortality of 3% [42]. These results were consistent with a study on pancreatic cancer patients with peritoneal invasion who received CRS/HIPEC and had a 30-day mortality of 4.3% and grade 3–4 complications of 43% [5]. Grotz et al. reported a 30-day mortality of 5.5% and a major complication rate of 44% in PDAC patients with limited peritoneal dissemination who underwent laparoscopic HIPEC induction followed by CRS/HIPEC and indicated that these are safe approaches with minimal complications [3].
A study from the United States HIPEC Collaborative included patients with peritoneal metastases of various sources with a median PCI of 13 who underwent CRS with/without HIPEC. The investigation demonstrated that over 17 years Clavien III/IV adverse events were similarly high (55% vs. 57%) while the 90-day mortality has decreased significantly (5% vs. 3%) as time passed [42]. These results were confirmed by a study evaluating distal pancreatic resection and HIPEC [43] and a phase I/II pilot trial of CRS/HIPEC [44]. Application of CRS/HIPEC followed by NIPEC has also shown favorable outcomes since just 1/12 patients developed class III morbidity [45]. Yurttas et al. in a pilot phase I/II trial of patients who underwent CRS and HIPEC reported that 3/13 patients had pancreatic fistula as a key adverse effect. This study supported that HIPEC with PDAC surgery has a mortality rate of less than 10% [44].
The application of HIPEC for curative purposes in PDAC has been recently reported in a phase II/III randomized trial of 42 patients. This study demonstrated that the group who underwent CRS/HIPEC had similar perioperative Clavien-Dindo complications, duration of hospital stay, and cost compared with the CRS group [32]. A retrospective cohort study comparing CRS alone and CRS/HIPEC demonstrated that adding HIPEC to CRS did not raise the likelihood of pooled major adverse events or deaths from major complications at 30 days postoperatively [46]. Downs-Canner et al. have found that the incidence of postoperative pancreatic fistula is the same comparing CRS/HIPEC with distal pancreatectomy alone, although the severity of the fistula is increased when CRS is accompanied by HIPEC [47].
Prophylactic role of CRS/HIPEC in resectable pancreatic cancer
Recently, a systematic review and meta-analysis by Frassini et al. demonstrated that prophylactic treatment with HIPEC in borderline resectable and/or locally advanced pancreatic cancer leads to a three-year survival rate of 25.5%. However, this rate drops to 6.2% in the presence of peritoneal metastases. Therefore, they considered HIPEC as a promising method for prophylactic and curative intents [18]. A 55-year-old man with pancreatic cancer without evidence of any metastases who underwent pancreatic resection plus intraoperative HIPEC followed by six cycles of NIPEC postoperatively survived 10 years after diagnosis of pancreatic cancer [31]. A series of 33 patients with resectable pancreatic carcinoma indicated the potential influence of complete cytoreduction plus adjuvant HIPEC on locoregional recurrence [48]. A case with locally advanced PDAC was treated with oncological resection, HIPEC, and six cycles of adjuvant systemic chemotherapy and did not show any signs of recurrence in CT scan or serum CA 19 − 9 levels after five years of follow-up [49]. In a phase II/III randomized clinical trial, Padilla-Valverde et al. demonstrated that CRS/HIPEC for resectable PDAC is associated with lower locoregional recurrence but comparable OS, disease-free survival (DFS), and distant recurrence compared to resection alone after a median follow up of 18 months [32]. Nevertheless, the available evidence is insufficient to definitely suggest the use of HIPEC for prophylactic treatment of resectable PDAC, and further controlled studies are required to conclude [10, 37].
Pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Intraperitoneal chemotherapy has gained attention for 30 years and PIPAC has become of interest for Peritoneal carcinomatosis over the past decade [8, 17]. The application of PIPAC for palliative therapy was initiated in 2011 for patients with peritoneal dissemination of various malignancies [14, 51]. A systematic review confirmed the safety, tolerability, and effectiveness of PIPAC for the treatment of peritoneal dissemination in a variety of malignancies [52]. A retrospective study of 118 patients with primary or metastatic peritoneal malignancies who underwent high-pressure/high-dose PIPAC at least once demonstrated that perioperative complications are equivalent to standard pressure/dose PIPAC, but therapeutic effects have yet to be evaluated [19].
Characteristics of the PIPAC procedure
Performing a standardized PIPAC procedure and using safety checklists, the procedure would have a minimal learning curve [53]. PIPAC provides the opportunity to increase the drug concentration of the aerosols by only decreasing the volume of the carrier solution without adding to the administered dose [19]. A minimum flow rate of 25 mL/min is required for stable aerosol formation during PIPAC, while volumetric rise only contributes to faster aerosol formation [54].
Newly developed PIPAC devices with diverse manufacturing properties and costs have been launched. Differences in droplet sizes, diffusion angles, and the pressure required for droplet formation may have variable impacts on patient safety and therapeutic effectiveness [55]. It is advised that new PIPAC nozzles with properties different from the original technology should undergo preclinical testing with regard to spatial distribution of chemotherapy drugs, tissue permeation, and concentration before being used clinically [56]. Despite the common belief that PIPAC causes widespread peritoneal drug delivery, Göhler et al. claimed that with standard microinjection pump operation in PIPAC, homogenous drug distribution into the abdominal cavity is not achieved. Therefore, it may lead to inadequately treated tumoral tissue and therapy failure [54].
Using a conventional PIPAC nebulizer, a 12 mmHg of CO2 pneumoperitoneum at 37℃ is applied and lasts for 30 min [14]. Following the achievement of a desirable pneumoperitoneum, a high-pressure injector is attached to a nebulizer and placed into the abdominal cavity via a trocar [10]. Administration of PIPAC can be repeated two to five times with four to six weeks intervals [10, 17].
Various PIPAC regimens and outcomes
On a recent consensus for PIPAC in peritoneal carcinomatosis both the drug regimens cisplatin/doxorubicin and oxaliplatin have been validated by the experts [57]. Lately, in another consensus on PIPAC protocol for peritoneal disease, doxorubicin (2.1 mg/m2) and cisplatin (10.5 mg/m2) combination has been suggested by 90% of the experts, 72% approved oxaliplatin (90–120 mg/m2), and 77% supported combination of 5-FU with PIPAC oxaliplatin [51]. A phase I clinical trial reported that patients tolerated the high dose (120 mg/m2) of oxaliplatin administered via PIPAC for different gastrointestinal cancers with peritoneal metastases [58]. However, adding systemic chemotherapy to PIPAC cycles would lead to a maximum safe dose of 90 mg/m2 for oxaliplatin [33, 59].
In a phase II controlled trial, cisplatin and doxorubicin were administered for pancreatic cancer with peritoneal metastases through PIPAC which resulted in a median OS of 15.6 months [8]. Di Giorgio et al. demonstrated the safety, feasibility, and antitumor activity of PIPAC with oxaliplatin or cisplatin-doxorubicin in a retrospective study on patients affected by peritoneal metastases of pancreatic and biliary origins. They also found a pathological regression in 50% of the patients [60]. Ceelen et al. conducted a phase I clinical trial to study PIPAC with nab-paclitaxel in patients with unresectable peritoneal metastases from various cancers. They reported a maximum tolerated and recommended dose of 140 mg/m2 for future phase II study, a median OS of 10 months, and a one-year survival rate of 50% [61]. Table 3. provides recent evidence on PIPAC for pancreatic cancer.
Table 3.
PIPAC for pancreatic cancer
| Author, Year | Study design | Country | N | Age (Median) |
Tumor status | Technique | Time (min) |
Chemotherapy agent, dosage | MOS (months) |
Note |
|---|---|---|---|---|---|---|---|---|---|---|
|
Graversen, 2023 [8] |
Phase II trial | Denmark | 21 | 63 | Unresectable with PM | Close | 30–35 |
CIS-Dox (7.5 mg/m2- 1.5 mg/m2) |
8.2 | MOS since PM diagnosis = 15.6 months |
|
Nielsen, 2021 [63] |
Case series | Denmark | 16 |
Mean 59 |
Unresectable | Close | 30 |
CIS-Dox (7.5 mg/m2- 1.5 mg/m2) |
9.9 | - |
|
Di Giorgio, 2020 [60] |
Case series | Italy and France | 14 | 64 | Unresectable with PM | Close | 30 |
CIS-Dox (7.5 mg/m2- 1.5 mg/m2) Or Oxa (92 mg/m2) |
9.7 |
MOS since PM diagnosis = 16.2 months Pathological regression rate = 50% |
|
Graversen, 2017 [67] |
Case series | Denmark | 5 | 62 | Unresectable with PM | Close | 30–35 |
CIS-Dox (7.5 mg/m2- 1.5 mg/m2) |
6 | MOS since PM diagnosis = 14 months |
|
Khosrawipour, 2017 [68] |
Case series | Germany | 20 | Mean 64.9 | Unresectable with PM | Close | 30 |
CIS-Dox (7.5 mg/m2- 1.5 mg/m2) |
9.1 | Pathological regression rate = 35% |
N, Number of patients; MOS, Median overall survival from first PIPAC; PM, Peritoneal metastases; CIS, Cisplatin; Dox, Doxorubicin; Oxa, Oxaliplatin
Evaluation of PIPAC response
At present, response to PIPAC in patients with peritoneal metastasis is mostly evaluated based on histopathological methods such as peritoneal regression grading score (PRGS); whereas, a variety of invasive and non-invasive modalities have been reported such as serum biomarkers, radiology, PCI, and cytology of peritoneal lavage fluid or ascites [62]. In 2021, next generation sequencing (NGS) was first used to evaluate the frequency of KRAS mutations in peritoneal quadrant biopsies and peritoneal fluids following the PIPAC for pancreatic carcinoma with Peritoneal involvement. NGS may be utilized particularly when there is access only to post-PIPAC peritoneal biopsies or fluids [63].
A systematic review and meta-analysis on peritoneal malignancies of different origins showed a one-year survival rate of 37% in patients with pancreatic cancer who were treated with PIPAC. Furthermore, the pathological response appeared to be the most reliable outcome for evaluating the anticancer activity of PIPAC with a permissible heterogeneity (I2 28.41%, p = 0.09). However, the correlation of pathological, radiological, and macroscopic response with patient survival has yet to be investigated [64]. Graversen et al. discovered that PRGS < 2 at the third PIPAC was the only independent prognostic factor in a multivariate analysis of age, sex, and bidirectional treatment [8]. A retrospective study by Kryh-Jensen et al. revealed that combination of PIPAC and systemic chemotherapy allows for 63% of the patients with pancreatic peritoneal carcinomatosis to reach long-term survival, which was defined as the minimum survival of 15 months [65].
PIPAC-related complications
Di Giorgio et al. systematically reviewed 10 years of PIPAC and revealed that severe complications (grade 3–4) had been reported in 4% of the procedures and death occurred in 1.3% of the patients mostly due to disease progression [64]. Generally, adverse events associated with PIPAC are considered acceptable [8]. A retrospective international cohort study has shown the safety of combining PIPAC with additional surgical procedures since it does not affect surgical complications or deaths but increases hospital stay, operation length, and minor medical complications [66]. However, based on a recent consensus on PIPAC, a combination of PIPAC with other surgical procedures was controversial among the expert panel [57]. Through PIPAC, Platin-based drugs are highly absorbed into the systemic circulation which may cause neurotoxicity in multiple PIPAC cycles or with prior platin-based chemotherapy [33, 59].
Comparison of different methods of intraperitoneal chemotherapy
At the end of the PIPAC, chemotherapy droplets are left in place; however, considering HIPEC the chemotherapy solution is pulled out. The brief exposure of chemotherapy in HIPEC results in lower absorption as well as decreased systemic toxicity. Moreover, unless HIPEC, PIPAC is performed by a minimally invasive method that can be repeated [33].
A systematic review of preclinical studies on peritoneal dissemination of different origins revealed that PIPAC is safe and provides better drug distribution and concentration into the peritoneum in comparison with traditional intraperitoneal chemotherapy by lavage [69]. Regardless of the type, intraoperative chemotherapy is associated with potential complications. For instance, a meta-analysis of gastric cancer patients has shown that intraperitoneal chemotherapy is associated with intra-abdominal abscess formation, fever, and bone marrow suppression [70]. Frassini et al. systematically reviewed the complications following HIPEC, PIPAC, and NIPEC; based on the Clavien-Dindo classification, the occurrence of grade III and IV side effects was found to be 5.5%, 5.1%, and 6.2%, respectively [18].
Future of intraperitoneal chemotherapy for pancreatic cancer
An ongoing phase II-III clinical trial (NCT03251365) studying HIPEC with gemcitabine following the CRS for pancreatic cancer is estimated to be completed in December 2024 [18]. A phase III RCT (UMIN000027229/jRCTs051180199) is in progress which will address the controversies regarding the safety and therapeutic effects of intraperitoneal chemotherapy in PDAC with peritoneal dissemination [25]. Clinical studies have shown the advantages of intraperitoneal immunotherapy for patients with malignant ascites and peritoneal metastasis from various cancer types such as PDAC [71–73]. Recently, 3DNA nanocarriers have been used for more effective and selective intraperitoneal drug delivery in a mice model of PDAC [74].
Conclusions
Regarding pancreatic cancer, recent studies have examined the effectiveness and optimal therapeutic regimens of intraperitoneal anticancer treatments. Despite low safety concerns based on a small phase II/III randomized clinical trial, current evidence on the efficacy of HIPEC for pancreatic cancer is limited since most studies are case reports and case series with a low sample size. Therefore, intraperitoneal chemotherapy in the management of PDAC has unclear long-term outcomes [18]. Based on the recent phase II/III randomized clinical trial, CRS/HIPEC shows similar survival and complication rates in comparison with complete resection alone. However, there is still a lack of evidence on the benefits of these approaches for the overall survival of patients with pancreatic cancer. Well-designed Phase I and II studies should be conducted before a Phase III study to determine the safety and effectiveness of different intraperitoneal chemotherapies in pancreatic cancer to have more realistic perspective on the future of these approaches for pancreatic carcinomas.
Acknowledgements
None.
Abbreviations
- CRS/HIPEC
Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
- NIPEC
Normothermic Intraperitoneal Chemotherapy
- PIPAC
Pressurized Intraperitoneal Aerosol Chemotherapy
- PDAC
Pancreatic Ductal Adenocarcinoma
- OS
Overall Survival
- L-IPC
Liquid Intraperitoneal Chemotherapy
- 5-FU
5-Fluorouracil
- I.p.
Intraperitoneal
- I.v.
Intravenous
- CA 19 − 9
Carbohydrate Antigen 19 − 9
- PFS
Progression-Free Survival
- PM
Peritoneal Metastasis
- GEM
Gemcitabine
- PTX
Paclitaxel
- Nab-PTX
Nanoparticle Albumin-Bound-Paclitaxel
- CART
Concentrated Ascites Reinfusion Therapy
- PCI
Peritoneal Carcinomatosis Index
- PMP
Pseudomyxoma Peritonei
- DFS
Disease-Free Survival
- IRE
Irreversible Electroporation
- IORT
Intraoperative Radiation Therapy
- MMC
Mitomycin C
- CIS
Cisplatin
- Oxa
Oxaliplatin
- NGS
Next Generation Sequencing
- Dox
Doxorubicin
Author contributions
S.S. and D.S. had the idea for the article. M.F. and D.S. performed the literature search. S.S., D.S., and M.F. analyzed the data. D.S. drafted the manuscript. S.S. and M.F. critically revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
No funding received.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda S, Nagai M, Terai T, Kohara Y, Sho M. Essential updates 2021/2022: Surgical outcomes of oligometastasis in pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2023;7(3):358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grotz TE, Yonkus JA, Thiels CA, Warner SG, McWilliams RR, Mahipal A, Bekaii-Saab TS, Cleary SP, Kendrick ML, Truty MJ. Cytoreduction with Hyperthermic Intraperitoneal Chemoperfusion for Pancreatic Cancer with low-volume peritoneal metastasis: results from a prospective pilot study. Ann Surg Oncol. 2023;30(1):395–403. [DOI] [PubMed] [Google Scholar]
- 4.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133(3):413–22. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsdottir H, Yonkus JA, Thiels CA, Warner SG, Cleary SP, Kendrick ML, Truty MJ, Grotz TE. Oncologic outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for highly selected patients with metastatic pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2023. [DOI] [PubMed]
- 6.Gudmundsdottir H, Yonkus JA, Alva-Ruiz R, Kendrick ML, Smoot RL, Warner SG, Starlinger P, Thiels CA, Nagorney DM, Cleary SP. Yield of staging Laparoscopy for Pancreatic Cancer in the modern era: analysis of more than 1,000 consecutive patients. J Am Coll Surg. 2023;237(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, Scheithauer W, Ramanathan RK, Goldstein D, Penenberg DN, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20(2):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graversen M, Detlefsen S, Ainsworth AP, Fristrup CW, Knudsen AO, Pfeiffer P, Tarpgaard LS, Mortensen MB. Treatment of peritoneal metastasis with pressurized Intraperitoneal Aerosol Chemotherapy: results from the prospective PIPAC-OPC2 study. Ann Surg Oncol. 2023;30(5):2634–44. [DOI] [PubMed] [Google Scholar]
- 9.Satoi S, Yanagimoto H, Yamamoto T, Toyokawa H, Hirooka S, Yamaki S, Opendro SS, Inoue K, Michiura T, Ryota H. A clinical role of staging laparoscopy in patients with radiographically defined locally advanced pancreatic ductal adenocarcinoma. World J Surg Oncol. 2015;14(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoi S, Takahara N, Fujii T, Isayama H, Yamada S, Tsuji Y, Miyato H, Yamaguchi H, Yamamoto T, Hashimoto D. Synopsis of a clinical practice guideline for pancreatic ductal adenocarcinoma with peritoneal dissemination in Japan; Japan Peritoneal Malignancy Study Group. J Hepato-Biliary‐Pancreatic Sci. 2022;29(6):600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoi S, Fujii T, Yanagimoto H, Motoi F, Kurata M, Takahara N, Yamada S, Yamamoto T, Mizuma M, Honda G. Multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients with peritoneal metastasis. Ann Surg. 2017;265(2):397–401. [DOI] [PubMed] [Google Scholar]
- 12.Takahara N, Nakai Y, Ishigami H, Saito K, Sato T, Hakuta R, Ishigaki K, Saito T, Hamada T, Mizuno S. A phase I study of intraperitoneal paclitaxel combined with gemcitabine plus nab-paclitaxel for pancreatic cancer with peritoneal metastasis. Investig New Drugs. 2021;39:175–81. [DOI] [PubMed] [Google Scholar]
- 13.Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst. 1997;89(7):480–7. [DOI] [PubMed] [Google Scholar]
- 14.Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc. 2012;26:1849–55. [DOI] [PubMed] [Google Scholar]
- 15.Satoi S, Yamamoto T, Yamaki S, Sakaguchi T, Sekimoto M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Annals Gastroenterological Surg. 2020;4(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto T, Satoi S, Yamaki S, Hashimoto D, Ishida M, Ikeura T, Hirooka S, Matsui Y, Boku S, Nakayama S et al. Intraperitoneal Paclitaxel Treatment for Patients with pancreatic ductal adenocarcinoma with peritoneal dissemination provides a Survival Benefit. Cancers (Basel). 2022;14(5). [DOI] [PMC free article] [PubMed]
- 17.Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, Zieren J, Schwab M, Reymond MA. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frassini S, Calabretto F, Granieri S, Fugazzola P, Viganò J, Fazzini N, Ansaloni L, Cobianchi L. Intraperitoneal chemotherapy in the management of pancreatic adenocarcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48(9):1911–21. [DOI] [PubMed] [Google Scholar]
- 19.Ramos Arias G, Sindayigaya R, Ouaissi M, Buggisch JR, Schmeding M, Giger-Pabst U, Zieren J. Safety and feasibility of High-Pressure/High-Dose pressurized Intraperitoneal Aerosol Chemotherapy (HP/HD-PIPAC) for primary and metastatic peritoneal surface malignancies. Ann Surg Oncol. 2023;30(4):2497–505. [DOI] [PubMed] [Google Scholar]
- 20.Öman M, Wettergren Y, Odin E, Westermark S, Naredi P, Hemmingsson O, Taflin H. Pharmacokinetics of preoperative intraperitoneal 5-FU in patients with pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol. 2021;88(4):619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada S, Fujii T, Yamamoto T, Takami H, Yoshioka I, Yamaki S, Sonohara F, Shibuya K, Motoi F, Hirano S. Phase I/II study of adding intraperitoneal paclitaxel in patients with pancreatic cancer and peritoneal metastasis. J Br Surg. 2020;107(13):1811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meguro Y, Yamaguchi H, Sasanuma H, Shimodaira K, Aoki Y, Chinen T, Morishima K, Miyato H, Miki A, Endo K et al. Combined intraperitoneal paclitaxel and systemic chemotherapy for patients with massive malignant ascites secondary to pancreatic Cancer: a report of two patients. Intern Med. 2023. [DOI] [PMC free article] [PubMed]
- 23.Takahara N, Isayama H, Nakai Y, Ishigami H, Satoi S, Mizuno S, Kogure H, Matsubara S, Yamamoto N, Yamaguchi H. Intravenous and intraperitoneal paclitaxel with S-1 for treatment of refractory pancreatic cancer with malignant ascites. Investig New Drugs. 2016;34:636–42. [DOI] [PubMed] [Google Scholar]
- 24.Yamada S, Fujii T, Yamamoto T, Takami H, Yoshioka I, Yamaki S, Sonohara F, Shibuya K, Motoi F, Hirano S. Conversion surgery in patients with pancreatic cancer and peritoneal metastasis. J Gastrointest Oncol. 2021;12(Suppl 1):S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto T, Fujii T, Hirano S, Motoi F, Honda G, Uemura K, Kitayama J, Unno M, Kodera Y, Yamaue H, et al. Randomized phase III trial of intravenous and intraperitoneal paclitaxel with S-1 versus gemcitabine plus nab-paclitaxel for pancreatic ductal adenocarcinoma with peritoneal metastasis (SP study). Trials. 2022;23(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoi S, Yanagimoto H, Yamamoto T, Hirooka S, Yamaki S, Kosaka H, Inoue K, Hashimoto Y, Matsui Y, Kon M. Survival benefit of intravenous and intraperitoneal paclitaxel with S-1 in pancreatic ductal adenocarcinoma patients with peritoneal metastasis: a retrospective study in a single institution. J Hepato‐Biliary‐Pancreatic Sci. 2017;24(5):289–96. [DOI] [PubMed] [Google Scholar]
- 27.Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T, Omuro Y, Nakajima TE, Furuse J. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595–603. [DOI] [PubMed] [Google Scholar]
- 28.Urano M. Invited review: for the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperth. 1999;15(2):79–107. [DOI] [PubMed] [Google Scholar]
- 29.Goldenshluger M, Zippel D, Ben-Yaacov A, Dux J, Yalon T, Zendel A, Rayman S, Mor E, Berkenstadt H, Fogel-Grinvald H. Core body temperature but not intraabdominal pressure predicts postoperative complications following closed-system hyperthermic intraperitoneal chemotherapy (HIPEC) administration. Ann Surg Oncol. 2018;25:660–6. [DOI] [PubMed] [Google Scholar]
- 30.Nogueiro J, Gomes F, Pacheco J, Santos-Sousa H, Meireles S, Bessa Melo R, Aral M, Barbosa E. Late recurrence of pancreatic solid Pseudopapillary Neoplasm with peritoneal carcinomatosis treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a Case Report. Cureus. 2022;14(11):e31189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugarbaker PH, Steves MA. Ten-year survival of pancreas cancer with liver metastases treated by intraoperative and long-term intraperitoneal gemcitabine. A case report. Int J Surg Case Rep. 2023;107:108313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padilla-Valverde D, Bodoque-Villar R, García-Santos E, Sanchez S, Manzanares-Campillo C, Rodriguez M, González L, Ambrós A, Cano JM, Padilla-Marcote M et al. Safety and Effectiveness of Perioperative Hyperthermic Intraperitoneal Chemotherapy with Gemcitabine in patients with resected pancreatic ductal adenocarcinoma: clinical trial EudraCT 2016-004298-41. Cancers (Basel). 2024;16(9). [DOI] [PMC free article] [PubMed]
- 33.de Jong LA, van Erp NP, Bijelic L. Pressurized intraperitoneal aerosol chemotherapy: the road from promise to proof. Clin Cancer Res. 2021;27(7):1830–2. [DOI] [PubMed] [Google Scholar]
- 34.Leimkühler M, Hentzen JE, Hemmer PH, Been LB, van Ginkel RJ, Kruijff S, van Leeuwen BL, de Bock GH. Systematic review of factors affecting quality of life after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2020;27:3973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tentes A-A, Pallas N, Karamveri C, Kyziridis D, Hristakis C. Cytoreduction and HIPEC for peritoneal carcinomatosis of pancreatic cancer. J buon. 2018;23(2):482–7. [PubMed] [Google Scholar]
- 36.Sugarbaker PH, Chang D, Koslowe P. Prognostic features for peritoneal carcinomatosis in colorectal and appendiceal cancer patients when treated by cytoreductive surgery and intraperitoneal chemotherapy. In: Peritoneal Carcinomatosis: Drugs and Diseases edn. Edited by Sugarbaker PH. Boston, MA: Springer US; 1996: 89–104. [DOI] [PubMed]
- 37.Larentzakis A, Anagnostou E, Georgiou K, Vrakopoulou G-Z, Zografos CG, Zografos GC, Toutouzas KG. Place of hyperthermic intraperitoneal chemotherapy in the armament against pancreatic adenocarcinoma: a survival, mortality and morbidity systematic review. Oncol Lett. 2021;21(4):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halle-Smith JM, Powell-Brett S, Roberts K, Chatzizacharias NA. Resection of isolated liver oligometastatic disease in pancreatic ductal adenocarcinoma: is there a survival benefit? A systematic review. World J Gastrointest Surg. 2023;15(7):1512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tentes AA, Kyziridis D, Kalakonas A, Iliadis A, Fotiadou A. Pancreatic cancer with synchronous peritoneal and hepatic metastases: a case report. Int J Surg Case Rep. 2024;118:109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshizaki Y, Gohda Y, Inagaki F, Kataoka A, Takemura N, Miyazaki H, Igari T, Kiyomatsu T, Yano H, Kokudo N. A case of pseudomyxoma peritonei arising from a perforated intraductal papillary mucinous neoplasm that underwent cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Clin J Gastroenterol. 2024;17(1):188–97. [DOI] [PubMed] [Google Scholar]
- 41.Azzam AZ, Amin TM. Combined intraoperative Radiotherapy (IORT) and hyperthermic intraperitoneal chemotherapy (HIPEC) with cytoreduction surgery (CRS) as a Novel Approach in the management of Resectable Pancreatic Cancer. Gulf J Oncol. 2020;1(33):19–26. [PubMed] [Google Scholar]
- 42.Beal EW, Ahmed A, Grotz T, Leiting J, Fournier KF, Lee AJ, Dineen S, Dessureault S, Baumgartner JM, Veerapong J. Trends in the indications for and short-term outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Am J Surg. 2020;219(3):478–83. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz L, Votanopoulos K, Morris D, Yonemura Y, Deraco M, Piso P, Moran B, Levine EA, Tuech J-J. Is the combination of distal pancreatectomy and cytoreductive surgery with HIPEC reasonable? Ann Surg. 2016;263(2):369–75. [DOI] [PubMed] [Google Scholar]
- 44.Yurttas C, Horvath P, Fischer I, Meisner C, Nadalin S, Koenigsrainer I, Koenigsrainer A, Beckert S, Löffler MW. A prospective, phase I/II, open-label pilot trial to assess the safety of hyperthermic intraperitoneal chemotherapy after oncological resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2021;28(13):9086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugarbaker PH, Stuart OA. Intraperitoneal gemcitabine chemotherapy is safe for patients with resected pancreatic cancer: final clinical and pharmacologic data from a phase II protocol and recommended future directions. J Gastrointest Oncol. 2021;12(Suppl 1):S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macfie RC, Cha DE, Gleeson E, Yu A, Cohen N, Sarpel U, Golas B, Hiotis S, Labow D. Hyperthermic intraperitoneal chemotherapy does not increase risk of major complication or failure to rescue in cytoreductive surgery. J Surg Oncol. 2022;126(4):781–6. [DOI] [PubMed] [Google Scholar]
- 47.Downs-Canner S, Ding Y, Magge DR, Jones H, Ramalingam L, Zureikat A, Holtzman M, Ahrendt S, Pingpank J, Zeh HJ. A comparative analysis of postoperative pancreatic fistulas after surgery with and without hyperthermic intraperitoneal chemoperfusion. Ann Surg Oncol. 2015;22:1651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tentes AA, Stamou K, Pallas N, Karamveri C, Kyziridis D, Hristakis C. The effect of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) as an adjuvant in patients with resectable pancreatic cancer. Int J Hyperth. 2016;32(8):895–9. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann V, Frauenfeld L, Löffler MW, Mihaljevic AL, Yurttas C. Long-term recurrence-free survival following pancreatic surgery with HIPEC treatment for locally advanced pancreatic adenocarcinoma. BMJ Case Rep. 2024;17(2). [DOI] [PMC free article] [PubMed]
- 50.Elhariri A, Starr JS, Bagaria S, Tran N, Babiker H. A Unicorn Disease: the large Duct variant of Invasive Ductal Adenocarcinoma of the pancreas. Cureus. 2023;15(7):e41430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sgarbura O, Eveno C, Alyami M, Bakrin N, Guiral DC, Ceelen W, Delgadillo X, Dellinger T, Di Giorgio A, Kefleyesus A, et al. Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum. 2022;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alyami M, Hübner M, Grass F, Bakrin N, Villeneuve L, Laplace N, Passot G, Glehen O, Kepenekian V. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20(7):e368–77. [DOI] [PubMed] [Google Scholar]
- 53.Hübner M, Grass F, Teixeira-Farinha H, Pache B, Mathevet P, Demartines N. Pressurized intraperitoneal aerosol chemotherapy–practical aspects. Eur J Surg Oncol (EJSO). 2017;43(6):1102–9. [DOI] [PubMed] [Google Scholar]
- 54.Göhler D, Khosrawipour V, Khosrawipour T, Diaz-Carballo D, Falkenstein TA, Zieren J, Stintz M, Giger-Pabst U. Technical description of the microinjection pump (MIP®) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg Endosc. 2017;31:1778–84. [DOI] [PubMed] [Google Scholar]
- 55.Pocard M, So JBY, Huchon C, Robella M, Chavatte-Palmer P, Eveno C, Glehen O, Peng Yong W. PIPAC nebulizer: how to test the new devices in the market, expert recommendations. J Visc Surg. 2023;160(1):52–4. [DOI] [PubMed] [Google Scholar]
- 56.Göhler D, Oelschlägel K, Ouaissi M, Giger-Pabst U. Performance of different nebulizers in clinical use for pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). PLoS ONE. 2024;19(5):e0300241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hübner M, Alyami M, Villeneuve L, Cortés-Guiral D, Nowacki M, So J, Sgarbura O. Consensus guidelines for pressurized intraperitoneal aerosol chemotherapy: technical aspects and treatment protocols. Eur J Surg Oncol. 2022;48(4):789–94. [DOI] [PubMed] [Google Scholar]
- 58.Kim G, Tan HL, Sundar R, Lieske B, Chee CE, Ho J, Shabbir A, Babak MV, Ang WH, Goh BC. PIPAC-OX: a phase I study of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy in patients with peritoneal metastases. Clin Cancer Res. 2021;27(7):1875–81. [DOI] [PubMed] [Google Scholar]
- 59.Dumont F, Passot C, Raoul J-L, Kepenekian V, Lelièvre B, Boisdron-Celle M, Hiret S, Senellart H, Pein F, Blanc-Lapierre A. A phase I dose-escalation study of oxaliplatin delivered via a laparoscopic approach using pressurised intraperitoneal aerosol chemotherapy for advanced peritoneal metastases of gastrointestinal tract cancers. Eur J Cancer. 2020;140:37–44. [DOI] [PubMed] [Google Scholar]
- 60.Di Giorgio A, Sgarbura O, Rotolo S, Schena CA, Bagalà C, Inzani F, Russo A, Chiantera V, Pacelli F. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Therapeutic Adv Med Oncol. 2020;12:1758835920940887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ceelen W, Sandra L, de Sande LV, Graversen M, Mortensen MB, Vermeulen A, Gasthuys E, Reynders D, Cosyns S, Hoorens A, et al. Phase I study of intraperitoneal aerosolized nanoparticle albumin based paclitaxel (NAB-PTX) for unresectable peritoneal metastases. EBioMedicine. 2022;82:104151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roensholdt S, Detlefsen S, Mortensen MB, Graversen M. Response evaluation in patients with Peritoneal Metastasis Treated with pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). J Clin Med. 2023;12(4). [DOI] [PMC free article] [PubMed]
- 63.Nielsen M, Graversen M, Ellebæk SB, Kristensen TK, Fristrup C, Pfeiffer P, Mortensen MB, Detlefsen S. Next-generation sequencing and histological response assessment in peritoneal metastasis from pancreatic cancer treated with PIPAC. J Clin Pathol. 2021;74(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Giorgio A, Macrì A, Ferracci F, Robella M, Visaloco M, De Manzoni G, Sammartino P, Sommariva A, Biacchi D, Roviello F et al. 10 years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): a systematic review and Meta-analysis. Cancers (Basel). 2023;15(4). [DOI] [PMC free article] [PubMed]
- 65.Kryh-Jensen CG, Fristrup CW, Ainsworth AP, Detlefsen S, Mortensen MB, Pfeiffer P, Tarpgaard LS, Graversen M. What is long-term survival in patients with peritoneal metastasis from gastric, pancreatic, or colorectal cancer? A study of patients treated with systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum. 2023;8(4):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robella M, Hubner M, Sgarbura O, Reymond M, Khomiakov V, di Giorgio A, Bhatt A, Bakrin N, Willaert W, Alyami M, et al. Feasibility and safety of PIPAC combined with additional surgical procedures: PLUS study. Eur J Surg Oncol. 2022;48(10):2212–7. [DOI] [PubMed] [Google Scholar]
- 67.Graversen M, Detlefsen S, Bjerregaard JK, Pfeiffer P, Mortensen MB. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Clin Exp Metastasis. 2017;34:309–14. [DOI] [PubMed] [Google Scholar]
- 68.Khosrawipour T, Khosrawipour V, Giger-Pabst U. Pressurized intra peritoneal aerosol chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS ONE. 2017;12(10):e0186709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. J Br Surg. 2017;104(6):669–78. [DOI] [PubMed] [Google Scholar]
- 70.Huang J-Y, Xu Y-Y, Sun Z, Zhu Z, Song Y-X, Guo P-T, You Y, Xu H-M. Comparison different methods of intraoperative and intraperitoneal chemotherapy for patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2012;13(9):4379–85. [DOI] [PubMed] [Google Scholar]
- 71.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127(9):2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ai Y-Q, Cai K, Hu J-H, Jiang L-W, Gao Y-R, Zhao H, Jia S-C. The clinical effects of dendritic cell vaccines combined with cytokine-induced killer cells intraperitoneal injected on patients with malignant ascites. Int J Clin Exp Med. 2014;7(11):4272. [PMC free article] [PubMed] [Google Scholar]
- 73.Ströhlein MA, Lordick F, Rüttinger D, Grützner K-U, Schemanski OC, Jäger M, Lindhofer H, Hennig M, Jauch K-W, Peschel C. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Oncol Res Treat. 2011;34(3):101–8. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy GA, Jain A, Di Niro R, Schultz CW, Jiang W, Yeo CJ, Bowers J, Finan J, Rhodes K, Casta L, et al. A novel 3DNA® nanocarrier effectively delivers payloads to pancreatic tumors. Transl Oncol. 2023;32:101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


