Abstract
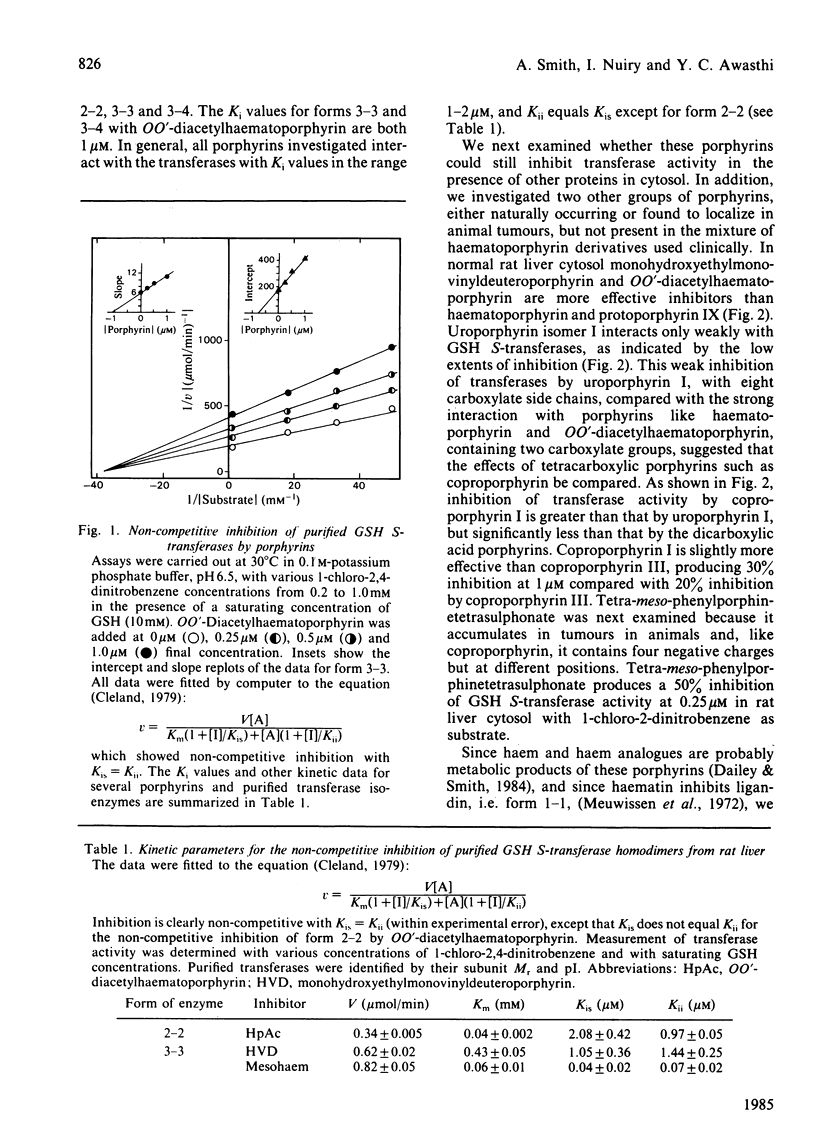
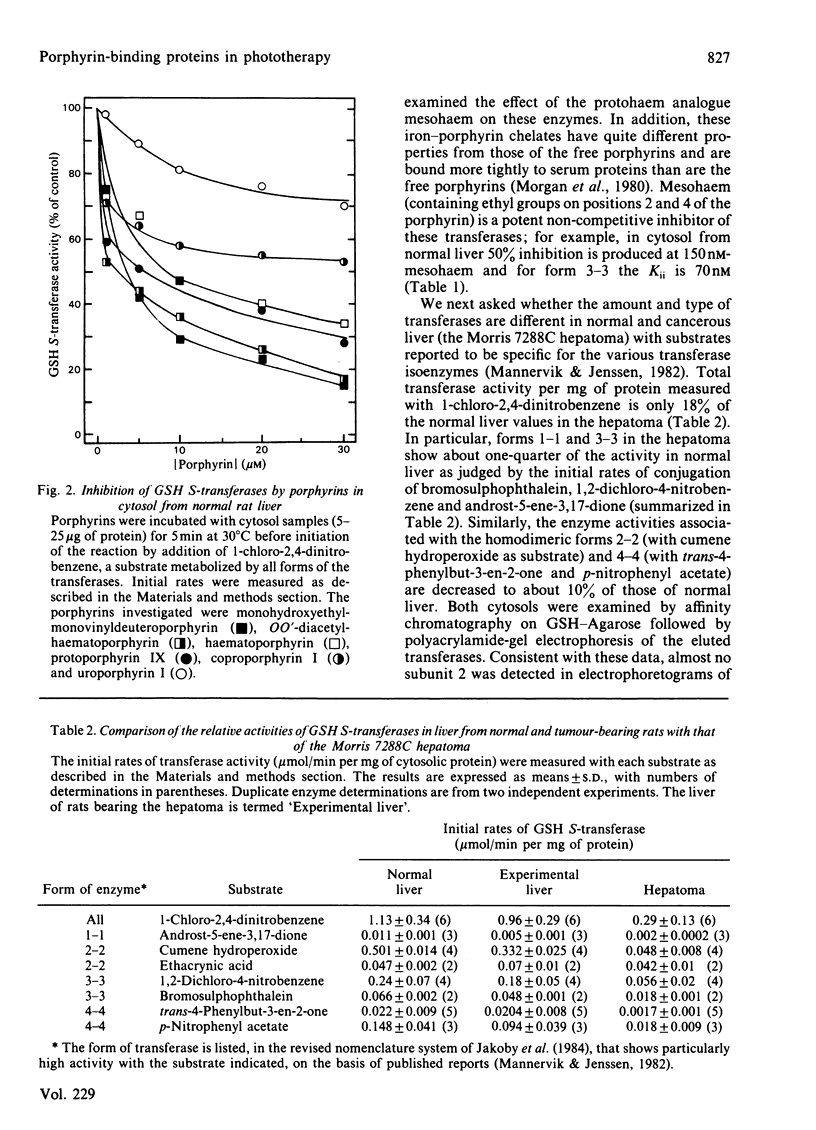
Several naturally occurring porphyrins and porphyrins used in photodynamic therapy inhibit glutathione S-transferase isoenzymes either purified from rat liver or lung or in cytosol from normal and from cancerous (Morris 7288C hepatoma) liver. Although differences occur in the type and amount of transferases in normal and cancerous liver and in the liver of rats bearing an extrahepatic tumour, these enzymes are potential binding sites for porphyrins. Porphyrin structure is an important factor in determining the affinity of binding, as shown by the relative inhibitory effectiveness. Of the dicarboxylic porphyrins in the mixture used clinically, OO'-diacetylhaematoporphyrin and monohydroxyethylmonovinyldeuteroporphyrin are more effective inhibitors than haematoporphyrin and protoporphyrin IX. Of the naturally occurring porphyrins the order of effectiveness is protoporphyrin IX (dicarboxylic) greater than coproporphyrin (tetracarboxylic) greater than uroporphyrin (octacarboxylic) and type I greater than type III isomers of both uroporphyrin and coproporphyrin, and the synthetic tetra-meso-phenylporphinetetrasulphonate is a better inhibitor (apparent Ki = 250 nM) than coproporphyrin, which contains a comparable number of negative charges. In addition, iron-porphyrin chelates are more effective inhibitors of the transferases, with 25-fold decrease in Ki value, than the free porphyrins. These results indicate that one means whereby porphyrins accumulate in tissues is the occupation of intracellular binding sites, such as the transferases. Since porphyrins inhibit the activity of these important detoxifying enzymes, there will be metabolic consequences to the cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M. Ligandin: review and update of a multifunctional protein. Med Biol. 1979 Oct;57(5):328–334. [PubMed] [Google Scholar]
- Arias I. M., Ohmi N., Bhargava M. Ligandin subunits and hepatocellular carcinoma in man and rat. Trans Assoc Am Physicians. 1979;92:113–120. [PubMed] [Google Scholar]
- Awasthi Y. C., Dao D. D., Saneto R. P. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J. 1980 Oct 1;191(1):1–10. doi: 10.1042/bj1910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Talalay P., Keen J. H., Jakoby W. B. Relationship between the soluble glutathione-dependent delta 5-3-ketosteroid isomerase and the glutathione S-transferases of the liver. Proc Natl Acad Sci U S A. 1977 Jan;74(1):158–162. doi: 10.1073/pnas.74.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum M. C., Bonnett R., Scourides P. A. In vivo biological activity of the components of haematoporphyrin derivative. Br J Cancer. 1982 Apr;45(4):571–581. doi: 10.1038/bjc.1982.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnett R., Berenbaum M. C. HPD - a study of its components and their properties. Adv Exp Med Biol. 1983;160:241–250. doi: 10.1007/978-1-4684-4406-3_21. [DOI] [PubMed] [Google Scholar]
- Bonnett R., Charalambides A. A., Jones K., Magnus I. A., Ridge R. J. The direct determination of porphyrin carboxylic acids. High-pressure liquid chromatography with solvent systems containing phase-transfer agents. Biochem J. 1978 Aug 1;173(2):693–696. doi: 10.1042/bj1730693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Carrano C. J., Tsutsui M., McConnell S. Localization of meso-tetra(p-sulfophenyl)porphine in murine sarcoma virus-induced tumor-bearing mice. Cancer Treat Rep. 1977 Oct;61(7):1297–1300. [PubMed] [Google Scholar]
- Carrano C. J., Tsutsui M., McConnell S. Tumor localizing agents: the transport of meso-tetra(p-sulfophenyl) porphine by Vero and HEp-2 cells in vitro. Chem Biol Interact. 1978 Jun;21(2-3):233–248. doi: 10.1016/0009-2797(78)90022-4. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Smith A. Differential interaction of porphyrins used in photoradiation therapy with ferrochelatase. Biochem J. 1984 Oct 15;223(2):441–445. doi: 10.1042/bj2230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. M., Smith A., Muller-Eberhard U., Morgan W. T. Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1504–1511. doi: 10.1016/0006-291x(79)91235-x. [DOI] [PubMed] [Google Scholar]
- El-Far M. A., Pimstone N. R. Tumour localization of uroporphyrin isomers I and III and their correlation to albumin and serum protein binding. Cell Biochem Funct. 1983 Oct;1(3):156–160. doi: 10.1002/cbf.290010307. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. Evidence that the coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem J. 1978 May 15;172(2):345–347. doi: 10.1042/bj1720345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg T., Bentley P., Stasiecki P., Glatt H. R., Raphael D., Oesch F. The identification, solubilization, and characterization of microsome-associated glutathione S-transferases. J Biol Chem. 1979 Dec 10;254(23):12028–12033. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Jagt D. L., Wilson S. P., Dean V. L., Simons P. C. Bilirubin binding to rat liver ligandins (glutathione S-transferases A and B). Relationship between bilirubin binding and transferase activity. J Biol Chem. 1982 Feb 25;257(4):1997–2001. [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Jakoby W. B. Glutathione transferases. Catalysis of nucleophilic reactions of glutathione. J Biol Chem. 1978 Aug 25;253(16):5654–5657. [PubMed] [Google Scholar]
- Kessel D. Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy. Photochem Photobiol. 1984 Jun;39(6):851–859. doi: 10.1111/j.1751-1097.1984.tb08871.x. [DOI] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Ketterer B., Beale D., Meyer D. The structure and multiple functions of glutathione transferases. Biochem Soc Trans. 1982 Apr;10(2):82–84. doi: 10.1042/bst0100082. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Beale D., Taylor J. B., Meyer D. J. The genetic relationships and inducibility of soluble glutathione transferases of the rat liver. Biochem Soc Trans. 1983 Aug;11(4):466–467. doi: 10.1042/bst0110466. [DOI] [PubMed] [Google Scholar]
- Kraus P. Resolution, purification and some properties of three glutathione transferases from rat liver mitochondria. Hoppe Seylers Z Physiol Chem. 1980 Jan;361(1):9–15. doi: 10.1515/bchm2.1980.361.1.9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Morgan W. T., Smith A., Koskelo P. The interaction of human serum albumin and hemopexin with porphyrins. Biochim Biophys Acta. 1980 Jul 24;624(1):271–285. doi: 10.1016/0005-2795(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Romslo I., Husby P. Iron, porphyrin and heme transport in mitochondria. Int J Biochem. 1980;12(5-6):709–712. doi: 10.1016/0020-711x(80)90148-2. [DOI] [PubMed] [Google Scholar]
- Schacter B. A., Kurz P. Alterations in hepatic heme and cytochrome P-450 metabolism in Murphy-Sturm lymphosarcoma-bearing rats. Implications for drug metabolism. Biochem Pharmacol. 1984 Mar 1;33(5):815–820. doi: 10.1016/0006-2952(84)90467-2. [DOI] [PubMed] [Google Scholar]
- Scully N. C., Mantle T. J. Tissue distribution and subunit structures of the multiple forms of glutathione S-transferase in the rat. Biochem J. 1981 Jan 1;193(1):367–370. doi: 10.1042/bj1930367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Neuschatz T. Haematoporphyrin and OO'-diacetylhaematoporphyrin binding by serum and cellular proteins. Implications for the clearance of these photochemotherapeutic agents by cells. Biochem J. 1983 Aug 15;214(2):503–509. doi: 10.1042/bj2140503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Koskelo P. The binding of porphyrins by ligandin. Biochem J. 1978 Mar 1;169(3):509–516. doi: 10.1042/bj1690509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Winkelman J., Slater G., Grossman J. The concentration in tumor and other tissues of parenterally administered tritium- and 14-C-labeled tetraphenylporphinesulfonate. Cancer Res. 1967 Nov;27(11):2060–2064. [PubMed] [Google Scholar]
- Woods J. S. Alterations in hepatic heme biosynthetic capability and mixed function oxidase activity during ethionine exposure in rats. Mol Pharmacol. 1978 Sep;14(5):900–910. [PubMed] [Google Scholar]


