Abstract
CKBM is a natural product that exhibits a novel anti-tumor activity through the induction of cell cycle arrest and apoptosis. We have investigated its effects on cell cycle regulation using a gastric cancer cell line, AGS. The effects of CKBM on cell proliferation, cell cycle regulation and apoptosis were analyzed using BrdU (5-bromo-2'-deoxyuridine) cell proliferation assay and flow cytometric analysis, respectively. Specific cellular protein expressions were measured using Western blot analysis. Flow cytometric analysis indicated that CKBM induced G2/M cell cycle arrest and apoptosis, whereas differential protein expressions of p21, p53 and 14-3-3σ (stratifin) using Western blot analysis were enhanced. The differential expressions of p21, p53 and 14-3-3σ in AGS cancer cells after CKBM treatment may play critical roles in the G2/M cell cycle arrest that blocks cell proliferation and induces apoptosis.
Keywords: 14-3-3σ (stratifin), G2/M arrest, cell proliferation, checkpoint protein
1. Introduction
Pharmaceutical research of traditional Chinese medicines on cancer prevention and treatment has attracted much attention. There are a number of herbs that have shown to have the abilities to induce cell cycle arrest and to play an important role in cancer prevention. Genistein, daidzein and isoflavonoids in soybean are thought to play an important role in breast cancer prevention 1. In addition, genistein is found to induce G2/M cell cycle arrest in MCF-7 cells 2. Whereas anti-carcinogenic compounds like lunasin and lectins in soybean were shown to induce apoptosis in malignant cells 3. Schisandra chinensis is shown to reduce prostate cancer cell growth and induce apoptosis by inhibiting androgen receptor expression 4. Panax ginseng and Ziziphus jujube are found to induce cytokine release in macrophages 5. Moreover, soluble β-glucans from Saccharomyces cerevisiae could activate macrophage to secrete TNF-α 6, which is found to have a pro-apoptotic effect on human cancer cells 7.
CKBM is a product that contains the water extracts of wu wei zi (Schisandra chinensis), ginseng (Panax ginseng), hawthorn (Fructus Crataegi), jujube (Ziziphus jujube), soybean (Glycine Max) and Baker's yeast (Saccharomyces cerevisiae) which is processed by a proprietary technology developed by CK Life Sciences Int'l Inc., Hong Kong, China (CKLS). CKBM had previously been found to significantly enhance the secretion of IL-6, IL-8 and TNF-α in human peripheral blood mononuclear cells 8. In addition, it can increase the activities of natural killer cells and the phagocytic index of macrophage 9. The anti-tumor effect of CKBM was demonstrated in nude mice xenograft model with gastric cancer cells 10, in which CKBM showed a dose-dependent inhibition of tumor growth with significant tumor volume reduction in the treatment group. In the present study, tumor suppressive and pro-apoptotic effect of CKBM is further investigated in AGS, a gastric cancer cell line. CKBM is found to inhibit cell proliferation through the induction of apoptosis and G2/M cell cycle arrest. To further elucidate the mechanism of CKBM on apoptosis and G2/M cell cycle arrest, we studied the cellular protein expression profile of human gastric cancer cell line (AGS) before and after CKBM treatment using Western blot analysis.
2. Materials and Methods
Cell culture
Human gastric cancer cell line (AGS) was obtained from American Type Culture Collection (Rockville, MD, USA). Cells were cultured in F12K medium (Invitrogen Life Technologies, Inc., CA, USA) supplemented with 10 % v/v fetal bovine serum (FBS) and 1 % Penicillin/Streptomycin (Invitrogen Life Technologies, Inc., CA, USA) at 5 % CO2, 37 ℃.
Cell proliferation assay (BrdU)
Cells were seeded in a 96-well flat bottom plate at a density of 1 x 104 cells/well. After 24 hr, 20 μl of CKBM (Batch no. 0212191) at a final concentration of 2, 4, 6, 8, 10, 12, 14, 16 or 18 % v/v was added and incubated for either 24 or 48 hr at 5 % CO2, 37 ℃. Cell proliferation was assayed using BrdU (5-bromo-2'-deoxyuridine) kit purchased from Amersham (Uppsala, Sweden) and it was performed according to the kit manual.
Analysis of cell cycle progression
Cells were seeded in a 25 cm2 flask at a density of 1 x 106 cells/ flask . After 24 hr, at a final concentration of 0, 5 or 10 % v/v of CKBM was added to the respective flask and incubated for 24, 48 or 72 hr. Cells were trypsinized, harvested, and fixed in 1 ml 80 % cold ethanol in test tubes and incubated at 4 ℃ for 15 min. After incubation, cells were centrifuged at 1,500 rpm for 5 min and the cell pellets were resuspended in 500 μl propidium iodine (10 μg/ml) containing 300μg/ml RNase (Sigma, MO, USA). Then cells were incubated on ice for 30 min and filtered with 53 μm nylon mesh. Cell cycle distribution was calculated from 10,000 cells with ModFit LTTM software (Becton Dickinson, CA, USA) using FACScaliber (Becton Dickinson, CA, USA).
Apoptotic analysis with Annexin V Staining
AGS cells were seeded in a 6-well plate at a density of 1.2 x 106 cells/well. After 2, 4 or 6 hr of CKBM treatment at a concentration of 5, 10 or 15 % v/v, cells were trypsinized. Then cells were washed twice with cold PBS and 1 x 106 cells were resuspended in 500 ml 1 X binding buffer. A hundred microliter of cell suspension was transferred to a 5 ml culture tube and incubated with 10 μl of Annexin V antibodies and 10 μl of propidium iodine (10 μg/ml) containing 300 μg/ml RNase (Sigma, MO, USA). Gently vortex the cells and incubate for 15 min at RT in the dark. Four hundred microliter of 1X binding buffer was added to each tube and the cells were analyzed with flow cytometry within 1 hr.
Western blot analysis
Cells were seeded in a 150 mm Petri dish at a density of 1 x 105 cells/ml . After 24 hr, a final concentration of 0, 0.25, 0.5, 1, 2.5, 5 or 10 % v/v of CKBM was added and incubated for 48 hr. The cells were then trypsinized and washed twice with PBS and lysed with lysis buffer (50 mM Tris-HCl, pH 7.5, 250 mM NaCl, 5 mM EDTA, pH 8.0, 0.2 % Triton X-100 and add 10 μl protease inhibitor cocktail per 1 ml cell lysate at a cell density of 108 cells/ml) at 4 ºC. Lysates in sample buffer (2 % SDS, 10 % glycerol, 80 mM Tris-HCl (pH 6.8), 720 mM 2-mercaptoethanol and 0.001 % bromophenol blue) were denatured at 95 ºC for 5 min. Total cellular proteins (20-100 μg) were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and the proteins were transferred to Hybond-P PVDF membrane (Amersham, Buckinghamshire, England). The membranes were blocked with 5 % non-fat dried milk in 1 X PBS/1 % Tween 20 at 4 ºC overnight and incubated with primary antibodies (anti-p21, anti-14-3-3σ and anti-p53 at 1:5000 in TBST; anti-actin at 1:10000 in TBST) (Santa Cruz Biotechnology Inc., CA, USA) at room temperature for 2 hours followed by incubation of horseradish peroxidase-conjugated rabbit anti-mouse IgG (1:25000 in TBST) (Santa Cruz Biotechnology Inc., CA, USA) at room temperature for 2 hours . Reactive protein bands were visualized using ECL Plus Western blotting detection reagents (Amersham, Uppsala, Sweden) and the bands intensities were scanned and quantified using a densitometer.
3. Results
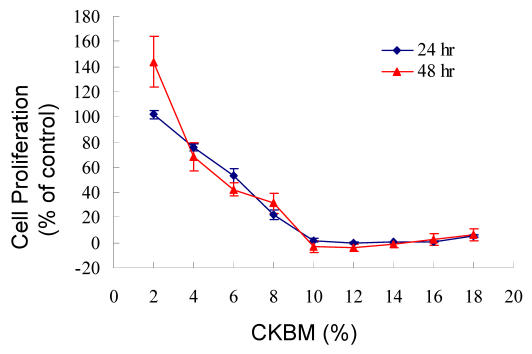
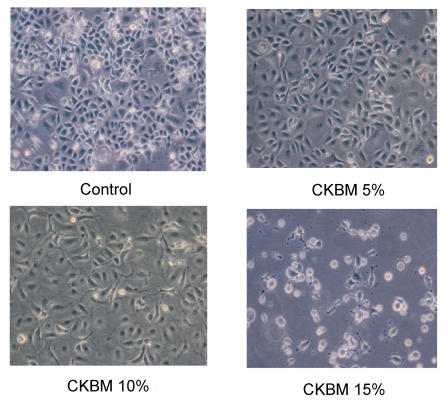
We examined the effect of CKBM on the proliferation of AGS using BrdU cell proliferation assay. The dosage of CKBM ranged from 2 to 18 %. CKBM inhibited cell proliferation in a dose-dependent manner (Fig. 1), the higher the CKBM dosage, the higher the degree of inhibition. The IC50 value for AGS was found to be approximately 6 % and proliferation was suppressed at 10 % or higher (Fig. 1). AGS cells underwent morphological changes from a polygonal appearance to an elongated shape after CKBM treatment (5 and 10 %) (Fig. 2).
Figure 1.
The effect of CKBM on cell proliferation. Data points represent the mean of 4 replicates. Percentage of proliferation was calculated by (Abs cells – Abs background) treated/(Abs cells – Abs background) control X 100
Figure 2.
Morphological changes of AGS cancer cells treated for 48 hr with the indicated CKBM concentration.
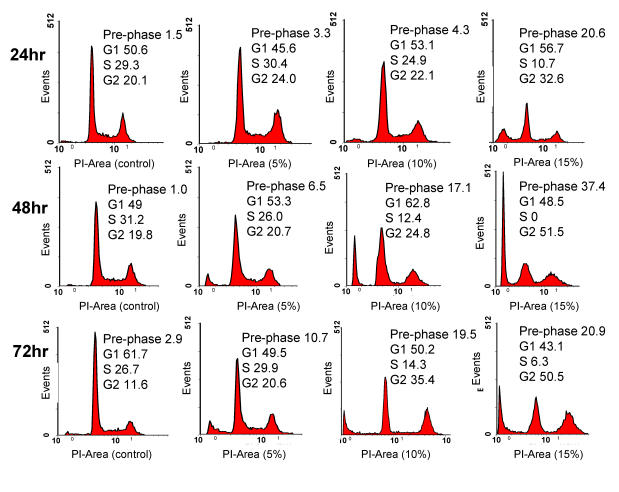
The effect of CKBM on cell cycle was evaluated using flow cytometric analysis. We observed a dose-dependent but time-independent effect of CKBM on the cell cycle. After 72 hr of 5 % CKBM treatment, cells in the G2/M population increased from 11.6 % to 20.6 % compared to control whereas cells with 15 % CKBM treatment, the G2/M population increased to 50.5 % The increase of cell population at the G2/M phase was accompanied by a decrease of cell population in the G1 phase of the cell cycle (Fig. 3). The effect of CKBM on AGS cells appears to be dose-dependent, the higher the dosage the greater the G2/M population increases. This dose-dependent effect was also demonstrated at the three dosages that we had examined however the length of CKBM treatment did not further contribute to the G2/M population increase (Fig. 3).
Figure 3.
Analysis of cell cycle progression. AGS cancer cells were untreated or treated with CKBM. Cells were harvested after 24, 48 or 72 hr then they were fixed, stained and analyzed for DNA content. The distribution and percentage of cells in pre-phase, G1, S and G2/M phase of the cell cycle are indicated.
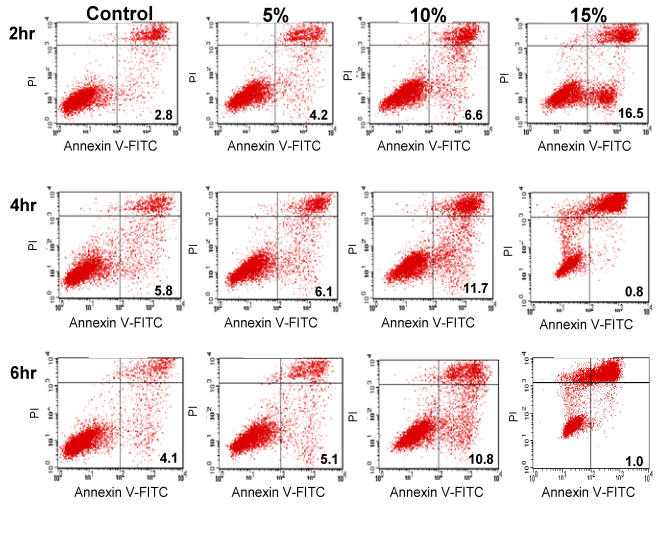
Apoptotic effect of CKBM was evaluated with Annexin V staining and a dose-dependent relationship was found (Fig. 4). After 2 hr of 0, 5, 10 or 15 % CKBM incubation, the apoptotic population increased from 2.8 % in the control group compared to 16.5 % in the 15 % treatment group. The dose-dependent effect was also observed in the 4 and 6 hr CKBM treatment groups, however, the apoptotic population decreased with 15 % treatment because the cells in the late stage of apoptosis were indistinguishable from the necrotic population. The increase in the apoptotic population is in concomitant with the increase in the pre-phase population that was observed in the cell cycle analysis (Fig. 3).
Figure 4.
AGS cells stained with anti-Annexin V. The percentage of apoptotic cells is indicated.
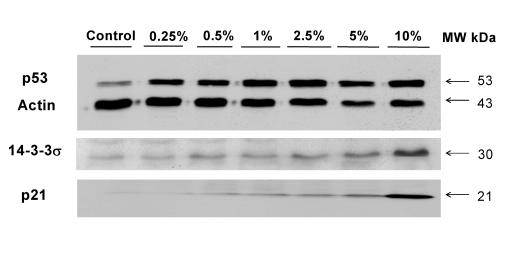
In CKBM treated AGS cancer cells, p21, p53 and 14-3-3σ all showed an increase in protein expressions in a dose-dependent manner and the changes were more significant for p21 and 14-3-3s (Fig. 5). Protein concentration in each lane was normalized with actin before the comparisons amongst the proteins were made. The protein expression of p53, p21 and 14-3-3σ were enhanced with 5, 14 and 3 fold increase respectively at 10 % CKBM treatment (Table 1). The increase in the protein expressions of these proteins correlated well with the BrdU cell proliferation assay, in which we found the cell proliferation of AGS cancer cells decreased.
Figure 5.
Western blot analysis on the effect of CKBM on the protein expression of p21, p53 and 14-3-3σ. Actin was used as the internal control.
Table 1.
The Ratio of p53, p21 and 14-3-3s protein expression between the control and the CKBM-treated AGS cells after actin normalization.
| Protein \ [CKBM]% | 0.25 | 0.5 | 1 | 2.5 | 5 | 10 |
|---|---|---|---|---|---|---|
| p53 | 2.7 | 2.9 | 4.0 | 4.7 | 5.0 | 5.0 |
| p21 | 0.8 | 2.5 | 3.0 | 3.4 | 5.0 | 14.3 |
| 14-3-3 | 1.0 | 1.5 | 1.2 | 2.0 | 2.4 | 3.3 |
4. Disscussion
Cell cycle checkpoints are important control mechanisms that ensure the proper execution of cell cycle events. One of the checkpoints, the G2/M checkpoint blocks the entry into mitosis when DNA is damaged 11. p53 can regulate the G2/M transition either through the induction of p21 and 14-3-3σ, a protein that normally sequesters cyclin B1-Cdc2 complexes in the cytoplasm 12, 13 or through the induction of apoptosis 14, 15. The p53 protein can also activate the transcription of a variety of apoptosis-associate d genes, and to program cell death in response to genotoxic stresses 16, 17. 14-3-3σ (stratifin), p21, and Gadd45 are three major transcriptional targets of p53 that can simultaneously inhibit Cdc2, which is essential for the entry into mitosis 11, 18.
In this study, we demonstrated that CKBM is able to inhibit cell proliferation in human gastric cancer cell line, AGS. The data from a subcutaneous implantation model of gastric cancer tissue was also agreeable to our observation in which anti-tumor effect was demonstrated showing a significant decrease in tumor volume 10. The mechanism for the inhibition was further investigated with cell cycle analysis, apoptotic study and Western blot analysis. The results showed that CKBM is able to induce a G2/M cell cycle arrest and a dose-dependent apoptotic effect in AGS cells. Using Western blot analysis, it was revealed that the expressions of p21, p53 and 14-3-3σ proteins in AGS are enhanced after CKBM treatment. It is well known that these three proteins play an important role in cell cycle regulation. p21 is an inhibitor of cyclin/cyclin-dependent kinase complexes, and it interacts with other regulators of signal transduction 19. Its induction is mediated by both p53 and p53-independent mechanisms and is essential for the onset of both G1 and G2 cell cycle arrest in damage response and cell senescence 19. The 14-3-3 proteins belong to a family of highly conserved proteins (alpha, beta, delta, sigma, tau, zeta etc.) with molecular weight of 25- to 30-kDa. They are expressed in all eukaryotic cells and modulate a wide variety of cellular processes 20. They play a critical role in the regulation of signal transduction pathways associated with the control of cell proliferation, differentiation, and survival 21. This family of proteins is known to interact directly or indirectly with signaling proteins such as the insulin-like growth factor I receptor, Raf, MEK kinases, PI3-kinase. However, the exact mechanism by which they activate or inhibit these elements remains unclear 21. It had been shown that 14-3-3σ is a p53-dependent inhibitor of G2/M progression, and that its over-expression can cause G2/M cell cycle arrest 11. Furthermore, it is able to up-regulate Cdc2 phosphorylation via Wee1 and down-regulate Cdc25c thus controlling the entry of cells into mitosis by maintaining the G2/M checkpoint 22. Other researchers had also found that over-expression of 14-3-3σ in breast cancer cell lines was able to inhibit cell proliferation and prevent anchorage-independent growth 23. These results have le d them to speculate that 14-3-3σ might be a potential candidate as tumor suppressor 23. In addition, previously study showed that proteolysis of 14-3-3σ by Efp promotes breast tumor growth and suggested that 14-3-3σ protein might play an important role in regulating tumor growth 24.
5. Conclusion
In this study, we demonstrated that CKBM inhibits cell proliferation through the induction of G2/M cell cycle arrest and dose-dependent apoptotic effect. From the results, we speculate that the induction of G2/M arrest and apoptosis may be partly due to the increase of the p21, p53 and 14-3-3σ protein expressions evidenced by the Western blot analysis. Whether other proteins are also involved in the induction of G2/M cell cycle arrest and inhibition in cell proliferation still need further studies. In summary, we have used flow cytometry and Western blot analysis to examine the effect of CKBM on AGS cell cycle regulation. The results indicate that the increase in p21, p53, and 14-3-3s after treatment of CKBM may contribute to the G2/M cell cycle arrest and the induction of apoptosis in human AGS cancer cell line.
Biographies
Sharon Chui-Wah Luk (Ph.D.) is Project Manager of CK Life Sciences Int'l Inc. She graduated from University of Alberta with a bachelor degree before completing Ph.D. study in the Department of Biochemistry, The Chinese University of Hong Kong. Her research interest is in cancer therapy.
Stephanie Wing-Fai Siu (M. Phil.) is Science Officer of CK Life Sciences Int'l Inc. She graduated from University of Hong Kong in the Department of Physiology. Her current research is in anti-cancer research.
Chun-Kit Lai (B.Sc.) is Science Assistant of CK Life Sciences Int'l Inc. He graduated from The Chinese University of Hong Kong in the Department of Biology. His current research is in proteomics.
Ying-Jye Wu (Ph.D.) has over 20 years of experience with US biomedical industry and is knowledgeable in the development of FDA-approved cancer, AIDS and hepatitis B products including proteomics-based diagnostic products for early cancer detection.
Shiu-Fun Pang (Ph.D.) is Vice President and Chief Technology Officer of CK Life Sciences Int'l Inc. Dr. Pang was the Head of Department of Physiology at University of Hong Kong prior joining the company. He had been the founding Editor and Editor-in-Chief of Biological Signals and Biological Signals and Receptors, Adjunct Professor of University of Toronto and is Honorary or Visiting Professor of over ten universities.
References
- 1.Wang HZ, Zhang Y, Xie LP, Yu XY, Zhang RQ. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells. J Nutr Biochem. 2002;13:421–426. doi: 10.1016/s0955-2863(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim Biophys Acta. 2003;1638:187–196. doi: 10.1016/s0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 3.de Mejia EG, Bradford T, Hasler C. The anticarcinogenic potential of soybean lectin and lunasin. Nutr Rev. 2003;61:239–246. doi: 10.1301/nr.2003.jul.239-246. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh TC, Lu X, Guo J, Xiong W, Kunicki J, Darzynkiewicz Z, Wu JM. Effects of herbal preparation Equiguard on hormone-responsive and hormone-refractory prostate carcinoma cells: mechanistic studies. Int J Oncol. 2002;20:681–689. [PubMed] [Google Scholar]
- 5.Shin YJ, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 6.Lee DY, Ji IH, Chang HI, Kim CW. High-level TNF-α secretion and macrophage activity with soluble β-glucans from Saccharomyces cerevisiae. BioSci Biotechnol Biochem. 2002;66:233–238. doi: 10.1271/bbb.66.233. [DOI] [PubMed] [Google Scholar]
- 7.Gillio Tos A, Cignetti A, Rovera G, Foa R. Retroviral vector-mediated transfer of the tumour necrosis factor alpha gene into human cancer cells restores an apoptotic cell death program and induces a bystander-killing effect. Blood. 1996;87:2486–2495. [PubMed] [Google Scholar]
- 8.Chan BP, Yuen WF, Lee WH, Wong SN, Chung TY, Wu YJ, Pang SF. Immunomodulating effects of CKBM-T01 on the cytokine production in peripheral blood mononuclear cells (PBMCs) from healthy volunteers. Immunopharmacol Immunotoxicol. 2004;26:177–192. doi: 10.1081/iph-120037713. [DOI] [PubMed] [Google Scholar]
- 9.Pang JJ, Shan HM, Xu RK, Luk CW, Pang SF, Xu JN. Chang Ke Zhi Ji Zeng Qiang Shi Yan Dong Wu De Mian Yi Gong Leng. World Clinical Drugs. 2004;25(7):444–448. [Google Scholar]
- 10.Shin VY, So WHL, Liu ESL, Wu YJ, Pang SF, Cho CH. Anti-tumorigenic and Pro-apoptotic effects of CKBM-T01 on gastric cancer growth in nude mice. Int J Med Sci. 2004;1(3):137–145. doi: 10.7150/ijms.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor WR, Stark G.R. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 12.Hermeking H, Lengauer C, Polyak K, He T-C, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 13.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 14.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 15.Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci. 1992;89:4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 1994;9:1767–1773. [PubMed] [Google Scholar]
- 17.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 18.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 19.Roninson I. Oncogenic functions of tumour suppressor p21Waf1/Cip1/Sdi1: association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Letters. 2002;179:1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- 20.Adriaenssens E, Lemoine J, EI Yazidi-Belkoura I, Hondermarck H. Growth signaling in breast cancer cells: outcomes and promises of proteomics. Biochemical Pharmacology. 2002;64:797–803. doi: 10.1016/s0006-2952(02)01141-3. [DOI] [PubMed] [Google Scholar]
- 21.Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 22.Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3 sigma is required to prevent mitotic catastrophe after DNA damage. Nature (Lond) 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 23.Laronga C, Yang HY, Neal C, Lee MH. Association of the cyclin-dependent kinases and 14-3-3 sigma negatively regulates cell cycle progression. JBC. 2000;275:23106–23112. doi: 10.1074/jbc.M905616199. [DOI] [PubMed] [Google Scholar]
- 24.Urano T, Saito T, Tsukui T, Fujita M, Hosoi T, Muramatsu M, Ouchi Y, Inoue S. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature. 2002;417:871–875. doi: 10.1038/nature00826. [DOI] [PubMed] [Google Scholar]