Abstract
Background
Immunosuppressive therapy is the standard management of adults with aplastic anaemia. Antithymocyte globulin is used as first-line treatment of patients not eligible for bone marrow transplantation. This being a rare disease, available evidence in India is scarce. This study aimed to present experience in treating adult aplastic anaemia patients by immunosuppressive therapy using antithymocyte globulin-equine (Thymogam) in two tertiary care centres of northeast India.
Methods
This case series was conducted at the Health city hospital, Guwahati, and Excel Care Hospital, Guwahati from 2018 to 2020. Eighteen adult aplastic anaemia patients who were treated by immunosuppressive therapy with antithymocyte globulin-equine (Thymogam) and followed up for two years were included. Treatment response and relapse are described.
Results
All the 18 patients, (14 severe, four very severe) were uniformly treated with immunosuppressive therapy (Thymogam 40 mg/kg/d for four days with oral Cyclosporine from Day-1). Cyclosporin A was used as a concomitant drug in 94.44 % of the patients. At two years of follow up, 66.7 % showed a response and the mortality rate was 11.1 %.
Conclusion
The results of this case series substantiate the effectiveness of immunosuppressive therapy with a low-cost preparation of horse antithymocyte globulin (Thymogam) along with cyclosporin A in the management of aplastic anaemia patients not suitable for bone marrow transplantation.
Keywords: Aplastic anaemia, Immunosuppressive therapy, Antilymphocyte serum, Antithymocyte globulin, Cyclosporin a, Treatment outcome
Introduction
Aplastic anaemia (AA) is an immune-mediated disorder with features of pancytopenia along with hypocellular bone marrow.1 The bone marrow hypocellularity is caused by destruction of haemopoietic cells of the marrow by deregulated T-cells.2 Allogenic bone marrow transplantation reboots the aberrant immune system. Hence, it is considered the treatment of choice for young patients with AA.3 However, obtaining a matched marrow for transplantation is a difficult task and only a small proportion of the patients have a matched donor.4 This lack of availability of HLA-matched donors and morbidities associated with alternative donor transplants have led to the development of another treatment modality; immunosuppressive therapy (IST).5 Improved survival was observed by the usage of a combination of IST with antithymocyte globulin (ATG), which acts by causing lyses of the lymphocytes, and cyclosporin A (CsA), which acts by blocking the T-cell function.6
From the early years of the 1990s, ATG along with CsA has been recognized as the standard immunosuppressive therapy for AA patients. The overall response rate produced by this combination was 60–70 % and the one-year survival rate was 60 %.6,7 This regimen acts through the ability of ATG to identify lymphocyte surface antigens and subsequently causing lysis of lymphocytes. This leads to immunosuppression, which is complemented by the action of CsA that blocks T cell function. In the past, studies performed to prove the efficacy of this regimen had promising outcomes with the use of horse ATG (hATG) in comparison with rabbit ATG (rATG).8
Considering the rarity of AA, there is only limited evidence available on the efficacy of regimens and the treatment outcome, especially from developing countries. Moreover, all the major studies were undertaken with the innovator product (ATGAM @ Pfizer Ltd). Hence, this study was planned to generate evidence on the role of a hATG preparation using Thymogam (manufactured by Bharat Serums and Vaccines Ltd.), an indigenous product manufactured in India for the treatment of AA.
Objectives: This case series was carried out to study the treatment outcomes of AA patients of immunosuppressive therapy using a hATG preparation produced from Thymogam.
Materials and methods
This case series describes 18 adult AA patients treated with hATG (Thymogam) across two corporate hospitals in Guwahati from 2018 to 2020. Informed written consent was obtained from study participants and data sharing was achieved with confidentiality. The clinical course, response of the patients to treatment and survival over a median follow-up period of two years were recorded.
Thymogam, an indigenous Indian preparation of hATG, was administered to patients.9 Following dosage calculation based on patient's weight, about 40 mg/kg/day of Thymogam was administered via a central line over 4–6 h per day for four days. Methylprednisolone and paracetamol were used as premedication. Patient details were collected using a predesigned case record form. The collected data include demographic details, duration of treatment, concomitant medications and the treatment outcome at the end of the follow-up period. CsA was given at a dose of 4–6 mg/kg/day starting on day-1 of hATG administration to maintain trough levels of 200–400 ng/mL for 6–9 months. Second-line drugs were used in case of relapse or no response to the first-line medication. The severity of AA was assessed as per Camitta,10 and Bacigalupo.11 The response was categorized as complete response (CR) or partial response (PR) as recommended by the 2009 version of the British Committee for Standards in Haematology guidelines.1
Statistical analysis
Descriptive statistics are used to present the data. Data are expressed as mean, 95 % confidence interval (CI; lower and upper bounds), median, minimum and maximum, and percentage, where appropriate. Data are also represented using appropriate diagrams (bar chart, pie chart). coGuide software was used for data analysis.
Results
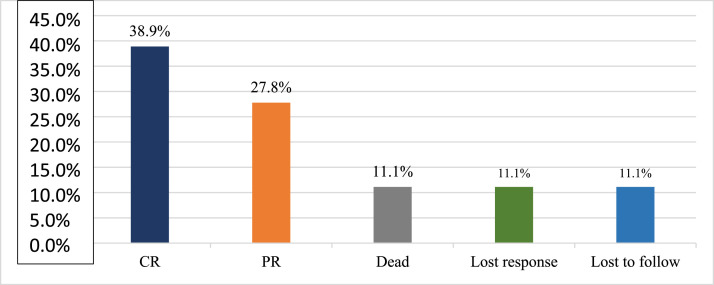
A total of 18 subjects were included in the final analysis. Table 1 depicts the demographic, treatment and outcome characteristics of all 18 AA patients included in the study. The mean age was 43.94 ± 13.48 years ranging from 19 to 63 years. Out of the 18 participants, 11 (61.11 %) were female. The majority (77.78 %) of the patients had severe disease, while 22.22 % had very severe disease. The mean dose of Thymogam used was 38.78 ± 4.18 mg ranging from 32 to 46 mg. amongst the concomitant drugs used, 17 (94.44 %) participants were given CsA and two (11.11 %) patients each were given romiplostin and eltrombopag. Six (33.33 %) patients were on danazol. At the end of the two-year follow-up period, seven (38.89 %) had a complete response and five (27.78 %) had a partial response. Lost response and loss to follow-up were observed in two (11.11 %) participants each. The overall response rate amongst patients was 66.7 % and the death rate was 11.1 % (Table 2).
Table 1.
Demographic, treatment, and outcome characteristics of aplastic anaemia patients (n = 18).
| Patient n° | Sex | Age | Total units of Thymogam used | Cyclosporin A | Romiplostim | Eltrombopag | Duration from the start of therapy to first response in days | Danazol | Romiplostim A | Erythropoietin | Mycophenolate mofetil | Best response | Current status (after 2-year follow up) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 40 | 41 | Y | N | N | 44 | N | N | N | N | Complete response | Complete response |
| 2 | Female | 54 | 34 | Y | Y | N | 111 | N | N | N | N | Complete response | Complete response |
| 3 | Male | 29 | 40 | Y | N | N | 158 | N | N | N | N | Partial response | Partial response |
| 4 | Male | 36 | 38 | Y | N | N | 81 | N | N | N | N | Complete response | Complete response |
| 5 | Female | 45 | 42 | Y | N | N | 61 | Y | N | N | N | Partial response | Lost response |
| 6 | Male | 54 | 41 | Y | N | N | 365 | Y | Y | Y | N | Complete response after 2nd line | Lost response |
| 7 | Female | 40 | 40 | Y | N | N | 120 | N | N | N | N | Complete response | Complete response |
| 8 | Female | 25 | 32 | Y | N | Y | 150 | N | N | N | N | Partial response | Partial response |
| 9 | Female | 50 | 35 | Y | N | N | Never achieved | Y | N | N | Y | No Response | Lost to follow |
| 10 | Female | 55 | 32 | Y | N | N | 153 | Y | N | N | N | Partial response | Partial response |
| 11 | Female | 63 | 33 | N | Y | N | Never achieved | N | N | N | Y | No Response | Dead |
| 12 | Female | 52 | 40 | Y | N | Y | 395 | Y | N | N | Y | Complete response | Complete response |
| 13 | Female | 24 | 37 | Y | N | N | Never achieved | N | N | N | N | Never achieved | Dead |
| 14 | Female | 19 | 42 | Y | N | N | 30 | N | N | N | N | Complete response | Complete response |
| 15 | Male | 60 | 45 | Y | N | N | 214 | N | N | N | N | Partial response | Partial response |
| 16 | Male | 43 | 46 | Y | N | N | 92 | N | N | N | N | Complete response | Partial response |
| 17 | Female | 62 | 41 | Y | N | N | 267 | N | N | Y | Y | Complete response after 2nd line | Complete response |
| 18 | Male | 40 | 39 | Y | N | N | Never achieved | Y | N | N | Y | No Response | Lost to follow |
Y: Yes; N: No.
Table 2.
Summary of baseline parameters (n = 18).
| Parameter | Summary |
|---|---|
| Age (in years) mean ± SD | 43.94 ± 13.48 (Range: 19 to 63) |
| Gender | |
| Male | 7 (38.89 %) |
| Female | 11 (61.11 %) |
| Mean units of Thymogam used | 38.78 ± 4.18 (Range: 32 to 46) |
| Concomitant Drugs Used | |
| Cyclosporin A (CsA) | 17 (94.44 %) |
| Romiplostin | 2 (11.11 %) |
| Eltrombopag | 2 (11.11 %) |
| Second line | |
| Danazol | 6 (33.33 %) |
| Mycophenolate mofetil | 5 (27.78 %) |
| Erythropoietin | 2 (11.11 %) |
| Romiplostim | 1 (5.56 %) |
| Best Response | |
| Complete Response | 7 (38.89 %) |
| Partial response | 5 (27.78 %) |
| No Response | 4 (22.22 %) |
| Complete Response after 2nd line | 2 (11.11 %) |
| Overall response rate | 12 (66.7 %) |
| Overall death rate | 2 (11.1 %) |
Figure 1 depicts the severity of AA amongst the study participants and Figure 2 depicts the status at the end of the two-year follow-up period.
Figure 1.
Pie chart of the severity of aplastic anaemia of the study population (n = 18).
Figure 2.
Bar chart of current status (after 2-year follow-up period) (n = 18).
Discussion
This case series reports responses to hATG (Thymogam) in the management of AA cases. This study shows that the two-year survival rate was 66 % of the patients on immunosuppressive therapy using a hATG (Thymogam) preparation.
Previous available studies have documented response rates ranging from 50 to 70 % for ATG-based immunosuppressive therapy for the management of AA in periods ranging from three months to one year.12 Studies from India have reported response rates of 33.3–87.9 % in adults.13,14 In this current study, the overall response rate at the end of a two-year follow-up period was 66.7 %.
In the current study, CsA was used as a concomitant drug in 94.44 % of the patients. Globally, the hATG and CsA combination is considered the standard immunosuppressive regimen for severe AA. The recent addition of the thrombopoietin receptor agonist, eltrombopag, is an effective and safe first-line regimen. For younger adults without a matched sibling donor and older adults, IST, including ATG and CsA, is considered to be the first-line option. In the current study, as a second-line drug treatment, 33.3 % of the patients received danazol. Danazol acts by increasing T-regulatory cells in acquired idiopathic AA. In a study by Zhang W et al., it was found that low-dose CsA combined with danazol has a better therapeutic effect in the treatment of chronic AA by significantly improving the hemograms and myelograms of the patients.15
The cost of hATG is expensive and its availability is sparse. In India, there are only two preparations of hATG available - ATGAM (Pfizer) and Thymogam. In a developing country like India, with people belonging to varied socioeconomic statuses, cost limits the use of ATG in a large number of eligible patients.13 Given this situation, an indigenous Indian preparation of hATG (Thymogam), with a comparable efficacy and safety profile as reported globally and its availability at our centre, is an advantage for low socioeconomic patients. The treatment response rate observed in this study is comparable to global studies and substantiates the role of IST with the indigenous preparation of hATG in AA along with CsA.
The key limitation of this study is that it is a simple case series with a limited number of patients, hence the potential role of chance, bias and confounding factors could not be assessed. In addition, it was not possible to assess the factors that can influence the overall response rate to the treatment.
Conclusion
In conclusion, the usage of an indigenous preparation, Thymogam, in AA has shown a response rate of 66.7 %. This strengthens existing evidence regarding the effectiveness of immunosuppressive therapy with an indigenous ATG preparation (Thymogam) along with CsA for AA. There is a need for further research with a larger sample size and more robust study designs to document the comparative efficacy and safety.
Authorship contributions
Conceptualization, data curation, formal analysis, project administration, methodology, supervision, writing, reveiw and editing: Asif Iqbal; Data curation, resources, software: Abhijit Phukan; Data curation, visualization, investigation: Chandana Sharma.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Acknowledgements
We acknowledge the technical support in data entry, analysis and manuscript editing by Evidencian Research Associates.
Source of funding
The project was self-funded. No external agency funded the project.
References
- 1.Marsh J.C., Ball S.E., Cavenagh J., Darbyshire P., Dokal I., Gordon-Smith E., et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 2.Young N.S. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013:76–81. doi: 10.1182/asheducation-2013.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locasciulli A., Oneto R., Bacigalupo A., Socié G., Korthof E., Bekassy A., et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11–18. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M.M. Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol. 2000;37:30–42. doi: 10.1016/s0037-1963(00)90028-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld S., Follmann D., Nunez O., Young N.S. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. J Am Med Assoc. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld S.J., Kimball J., Vining D., Young N.S. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- 7.Frickhofen N., Kaltwasser J.P., Schrezenmeier H., Raghavachar A., Vogt H.G., Herrmann F., et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297–1304. doi: 10.1056/NEJM199105093241901. [DOI] [PubMed] [Google Scholar]
- 8.Jeong D.C., Chung N.G., Cho B., Zou Y., Ruan M., Takahashi Y., et al. Long-term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99:664–671. doi: 10.3324/haematol.2013.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharath serum and vaccines ltd. Thymogam. thymogam insert. 2021. Available from: https://www.bharatserums.com/assets/pdf/products/Thymogam.pdf
- 10.Camitta B.M .TE., Nathan D.G., Santos G., Gordon-Smith E.C., Gale R.P.RJ., Storb R. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 11.Bacigalupo A., Hows J., Gluckman E., Nissen C., Marsh J., Van Lint M., et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA Working Party. Br J Haematol. 1988;70:177–182. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 12.Scheinberg P., Wu C.O., Nunez O., Scheinberg P., Boss C., Sloand E., et al. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus:a prospective randomized study. Haematologica. 2009;94:348–354. doi: 10.3324/haematol.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V., Kumar A., Tilak V., Saini I., Bhatia B. Immunosuppressive therapy in aplastic anemia. Indian J Pediatr. 2012;79:1587–1591. doi: 10.1007/s12098-012-0691-2. [DOI] [PubMed] [Google Scholar]
- 14.Shah S., Jain P., Shah K., Patel K., Parikh S., Patel A., et al. Immunosuppressive therapy for aplastic anemia: a single-center experience from western India. Ann Hematol. 2019;98:41–46. doi: 10.1007/s00277-018-3487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Shi C., Wang C. Effect of low-dose cyclosporine combined with danazol on chronic aplastic anemia. Chinese J Biochem Pharm. 2017;37:243–245. [Google Scholar]