Abstract
Purpose
The function of FAM177A1 and its relationship to human disease is largely unknown. Recent studies have demonstrated FAM177A1 to be a critical immune-associated gene. One previous case study has linked FAM177A1 to a neurodevelopmental disorder in four siblings.
Methods
We identified five individuals from three unrelated families with biallelic variants in FAM177A1. The physiological function of FAM177A1 was studied in a zebrafish model organism and human cell lines with loss-of-function variants similar to the affected cohort.
Results
These individuals share a characteristic phenotype defined by macrocephaly, global developmental delay, intellectual disability, seizures, behavioral abnormalities, hypotonia, and gait disturbance. We show that FAM177A1 localizes to the Golgi complex in mammalian and zebrafish cells. Intersection of the RNA-seq and metabolomic datasets from FAM177A1-deficient human fibroblasts and whole zebrafish larvae demonstrated dysregulation of pathways associated with apoptosis, inflammation, and negative regulation of cell proliferation.
Conclusion
Our data sheds light on the emerging function of FAM177A1 and defines FAM177A1-related neurodevelopmental disorder as a new clinical entity.
Keywords: FAM177A1, macrocephaly, neurodevelopment, intellectual disability, developmental delay, inflammation, zebrafish
Introduction:
The FAM177A1 (Family with Sequence Similarity 177 Member A1; MIM: 619181) gene is evolutionarily conserved across species and expressed across diverse tissues of the human body including brain, cerebellum, lung, kidney, thyroid, and colon. Little is known about the function of the protein and its effects in the human body. Association studies have linked a microsatellite in intron 1 of FAM177A1 with susceptibility to autoimmune conditions, including Graves’ disease and juvenile idiopathic arthritis, and race-related increase of FAM177A1 expression has been linked to increased risk for recurrence in breast cancer1–3.
Emerging evidence supports a role of FAM177A1 in the immune system as a mediator of the Toll-like receptor 4 signaling pathway (TLR4) and NF-κB inflammatory cascade. The TLR4 signaling pathway is a canonical pathway critical for the innate immune response. In a study of TLR4 signaling in mouse bone marrow derived macrophages, microRNA-7 (miR-7) negatively regulated Fam177a (mouse ortholog) which resulted in decreased TLR4 signaling. Similarly, downregulation of Fam177a itself in murine macrophage cells led to downregulation of the downstream mediators of TLR4 signaling including Nf-κB, IL-1β, IL-6, TNF-α and IL-124. These findings were corroborated by recent experimental evidence in human cell lines showing that FAM177A1 functions as a negative regulator of the IL-1β and NF-κB inflammatory cascade5.
Studies of FAM177A1 in model organisms are limited. The mouse and zebrafish genome each have two orthologous genes to human FAM177A1. Mouse gene expression databases demonstrate expression of the orthologous genes, Fam177a and Fam177a2, is primarily in the neurologic system6. One prior study has demonstrated a role of FAM177A1 in neurodevelopment. Ko et al. (2009) found that in mouse embryonic carcinoma cells, Fam177a expression is tightly regulated by miR124a and is upregulated during cellular proliferation but suppressed during neuronal differentiation7.
Alazami et al. previously reported four siblings with neurodevelopmental features who harbored homozygous frameshift variants in FAM177A1. These siblings presented with macrocephaly, global developmental delay, characteristic facial features, and mild obesity. While segregation with disease in this family was suggestive of causation, further investigation to establish the gene-disease association was not pursued8.
Here, we describe five individuals from three unrelated families with biallelic, predicted loss-of-function variants in FAM177A1 and a shared neurodevelopmental phenotype. This cohort demonstrates key similarities with the previously described siblings by Alazami et al. (2015) and further expands our understanding of the clinical features associated with biallelic loss-of-function of FAM177A18. We demonstrate that FAM177A1 localizes to the Golgi complex in mammalian cell lines and zebrafish embryos. We also present evidence from molecular and phenotypic characterization of cell models and mutant zebrafish, contributing to the overall understanding of FAM177A1 gene function and disease pathogenesis. This work strengthens the FAM177A1 disease association and defines FAM177A1-related neurodevelopmental disorder as a new clinical entity.
Methods:
Participants:
Approval for human subjects research was obtained from the Stanford University Institutional Review Board (IRB; protocols 47026 and 66629) and National Human Genome Research Institute IRB (protocol 15HG0130). Written consent was obtained from parents/legal guardians in accordance with the standards of the participating institutional review boards (IRB) on human research at each respective institution. Clinical and genetic information was collected via participant medical records and as part of participation in research. Individuals A1 and A2 (Family A) were identified through the Undiagnosed Diseases Network study9,10. Families B and C were identified through a FAM177A1 patient advocacy group.
Genetic Sequencing:
FAM177A1 encodes multiple transcripts (GenBank: NM_001079519.1, NM_001289022.3, NM_173607.5) and produces two protein isoforms which differ in length of the N-terminus. The canonical transcript NM_173607 corresponds to the longest coding transcript (237 amino acids) and demonstrates higher expression across tissues (GTEx database https://gtexportal.org/home/gene/FAM177A1). Alazami et al. (2015) reported pathogenic variants in an alternative transcript, NM_001079519, which were converted to NM_173607.5 in Tables 1 and S1 for consistency8. Variants are reported in the GRCh37 genome build.
Table 1.
Summary of clinical phenotypes of FAM177A1-associated neurodevelopmental disorder. All protein changes are presented as predicted protein changes.
| Family A | Family B | Family C | Alazami et al. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | C1 | C2 | P1 | P2 | P3 | P4 | |
| Genomic DNA (GRCh37; NC_000014.8) | g.35513596_35521681del / g.35546985_35556768del | – | g.35522586G>T / g.35522527_35522528del | g.35546382dup | |||||
| Coding (NM_173607.5) | – | c.(339+1_340−1)_(406+1_407−1)del | c.268G>T / c.209_210del |
c.366dup | |||||
| Predicted protein change (NP_775878.2) | – | – | p.(I70Rfs*15)/ p.(E90*) | p.(W123Mfs*16) | |||||
| Zygosity | Compound heterozygous | Homozygous | Compound heterozygous | Homozygous | |||||
| Age at last evaluation / Sex | 15 yr F | 10 yr M | 16 yr F | 6 yr F | 4 yr F | 10 yr F | 8 yr M | 7 yr F | 4 yr F |
| Macrocephaly | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes |
| Global developmental delay | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Physical features | Syndactyly (2nd, 3rd toe); Clinodactyly (5th toe) | None | Mild coxa valgus deformity of the pelvis | Frontal bossing, Flat feet | Flat feet | Dolichocephaly small midface | Dolichocephaly, frontal bossing, round face, small midface, and deep-set eyes | Dolichocephaly, deep eyes and small mid face | None |
| Behavioral abnormality | AGR, AUT, EL, HYP, IR, ID | AGR, AUT, HYP, IR, ID | AGR, AUT, EL, HYP, IR, ID | AUT, ID, HYP | AUT, ID | HYP, ID | ID | ID | ID |
| Seizures | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | N/A |
| Abnormal MRI brain | Yes | Yes | Yes | No | Yes | N/A | Yes | N/A | N/A |
| Abnormal muscle tone | HYPO, SPAS | HYPO, SPAS | HYPO | HYPO | HYPO | N/A | N/A | N/A | N/A |
| Hyperreflexia | Yes | Yes | No | No | No | N/A | N/A | N/A | N/A |
| Gait disturbance | Yes | Yes | Yes | Yes | Yes | N/A | Yes | N/A | N/A |
| Recurrent infections | No | No | No | No | No | No | Respiratory | N/A | No |
(Abbreviations: N/A = not assessed, Age = age at last evaluation, Behavioral abnormality: AGR = Aggressive behavior; AUT = Autistic behavior; EL = Emotional lability; HYP = Hyperactivity; IR = Irritability. ID= Intellectual disability. Abnormal muscle tone: HYPO = Hypotonia; SPAS = Spasticity.
Genome sequencing (Family A), exome sequencing (Family B), and panel testing (Family C) were performed using typical clinical protocols. Genome sequencing for individuals A1 and A2 was initially non-diagnostic. Research reanalysis including structural variant calling was subsequently performed and identified biallelic deletions in FAM177A111. These deletions were orthogonally validated at a CLIA certified laboratory via targeted qPCR.
Long-read genome sequencing was also performed for Family A (individuals A1, A2). High molecular weight DNA was extracted from cultured fibroblasts using a Puregene kit (Qiagen, Germany). DNA was sheared using a G-tube (Covaris LLC, Massachusetts). Sequencing libraries were prepared using Nanopore LSK-109 and sequenced on the PromethION48 (Oxford Nanopore Technologies, United Kingdom) to a minimum of 60Gb with a read length N50 of 15 kb. Sequencing data were base called using Guppy (High Accuracy, version 4.6)12, and aligned to HG38 using Minimap213. Structural variants were called and genotyped using Sniffles214.
Generation of human induced pluripotent stem cells (iPSCs):
Fibroblasts isolated from individuals A1 and A2 were cultured until the cells reached appropriate confluency for reprogramming. Next, the following reprogramming factors Oct4, Sox2, Klf4, and c-Myc (OSKM) were introduced into the fibroblasts through Sendai viral transduction according to methods previously described15. The fibroblasts transformed over time into iPSCs by forming characteristic stem cell colonies. These colonies were picked and expanded, and their pluripotency confirmed through various assays.
Immunoblotting iPSCs:
Cells grown on 6 well plates were washed 3 times with PBS. After wash, cells were lysed with ice cold RIPA buffer (Sigma-Aldrich, R0278) supplemented with Protease Inhibitor Cocktail (Roche) and PhosStop phosphatase inhibitor (Roche). Scraped cells were transferred to an Eppendorf tube, rotated for 20 min in 4 °C and centrifuged at 14000 RPM for 5 minutes at 4 °C. 4x Laemmli buffer was added to supernatant to make a final concentration of 2ug/ml protein. Samples were incubated at 95 °C for 5 minutes and were loaded into Mini PROTEAN TGX 4–12% tris-glycine gels (Bio-Rad). After transfer of the gel into membrane, membrane was blocked with 5% milk in TBST and incubated overnight with anti-rabbit anti-FAM177A1 (thermo scientific, A303–366A-M, 1;1000) and mouse anti-GAPDH (40–1246; Proteus Biosciences Inc., 1:10000). Next day, the membrane was washed with TBST and incubated with Licor IRDye 800CW Donkey anti-Rabbit IgG (1:10000) and 680LT Donkey anti-Mouse IgG (1:10000) secondary antibodies for 1hr at room temperature. Membrane was imaged using Biorad Chemidoc.
Human cell culture and transfection:
Cells were cultured at 37°C and 5% carbon dioxide in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine (Gibco, Thermo Fisher Scientific). COS7 cells for imaging experiments were seeded on glass-bottomed dishes (MatTek) at a concentration of 50 to 75 × 103 cells per dish, transiently transfected using FuGene HD (Promega), and imaged 24 hours later.
Live cell imaging of COS7 cells:
Prior to imaging, the growth medium was removed and replaced with a prewarmed Invitrogen Live Cell Imaging Solution (Life Technologies, Thermo Fisher Scientific). Imaging was carried out at 37 °C and 5% carbon dioxide. Spinning-disk confocal microscopy was performed using a Dragonfly Confocal Microscope system (Andor Technology) equipped with a PlanApo objective (63×, 1.4 numerical aperture, oil) and a Zyla sCMOS camera (Oxford Instruments). Images were analyzed in Fiji (ImageJ Schindelin, 2012).
Plasmid construction:
Human FAM177A1 cDNA, provided by Shinya Yamamoto at Baylor College of Medicine, was transferred into pCS2 vector by PCR using the following primers, 5’-GGCGCGCCAGGCACCATGGACCAGGAGCCA-3’ and 5’- CTCGAGGGTTCTATGGTGGGACAGAGACT-3’ (the underline indicates FAM177A1 cDNA sequences). To create FAM177A1-mNeonGreen (mNG) fusion protein construct, mNG DNA fragment was synthesized (Integrated DNA Technologies, IDT) and inserted into the stop codon of FAM177A1 15. Zebrafish fam177a1a cDNA was cloned by RT-PCR using 5’- GGCGCGCCTGAAGAAGACATGGCTGAACTGTCA-3’ and 5’- CTCGAGGATTTTAGGAGGGAATTGGTGCA-3’ (the underline indicates fam177a1a cDNA sequences). The cDNA was subcloned into a pCS2 vector for in vitro transcription. A C-terminal mNG tagged zebrafish fam177a1a construct was also generated by inserting the mNG fragment into the pCS2-fam177a1a plasmid into the stop codon of fam177a1a to study its subcellular localization.
Zebrafish husbandry:
All zebrafish studies were approved by the Institutional Animal Care and Use Committees at Washington University in St. Louis. Zebrafish at all developmental stages were maintained at 28.5 °C unless otherwise specified using the standard operating procedures and guidelines established by the Washington University Zebrafish Facility, described in detail at https://zebrafishfacility.wustl.edu/facility-documents/. The following zebrafish lines were used in this study: AB*, and newly generated lines fam177a1astl638, fam177a1astl700, fam177a1bstl746, and fam177a1bstl747, which are described below.
In vitro mRNA synthesis and microinjection in zebrafish:
For in vitro transcription, the FAM177A1 and fam177a1a and derivatives, membrane localized, memGFP, H2B-GFP (received from John Wallingford, University of Texas in Austin) and GM130-tdTomato (received from C-P Heisenberg, Institute of Science and Technology, Austria) plasmids were used for this study16. All RNAs were synthesized using SP6 mMessage mMachine Kit (Invitrogen).
To study subcellular localization of various FAM177A1-mNG and fam177a1a-mNG fusion proteins, we used 5 pg of synthetic RNAs encoding them. The above RNAs as well as synthetic RNAs encoding memGFP(10 pg), H2B-GFP (30 pg) and GM130-tdTomato (50 pg) were injected into the cytosol of early one-celled zygotes.
Generation of fam177a1a and fam177a1b RNA-less mutant alleles in zebrafish:
Zebrafish fam177a1a and fam177a1b RNA-less alleles were generated using the CRISPR-Cas9 genome editing technology17. Briefly, CHOPCHOP (https://chopchop.cbu.uib.no) was used to design gRNAs targeting 5’-ACAAACGTGAACGTGTCTTT-3’ (gRNA1) in the exon 1 and 5’- GTCGTATATACATAGCTGAC-3’ (gRNA2) in exon 5 of the fam177a1a gene18. For fam177a1b RNA-less variants, we designed gRNAs targeting 5’- GCGTCAGTATAACTGTCCTA −3’ (gRNA3) in the exon 1 and 5’- TTGATGAATACAGCAGGTCA-3’ (gRNA4) in the exon 4 of the fam177a1b gene. gRNAs were prepared for microinjection by mixing chemically synthesized target-specific crRNAs and a common tracrRNA (IDT). A pair of gRNAs (gRNA1+gRNA2 or gRNA3+gRNA4, 30 pg each) and 1.5 ng of HiFiCas9 protein (IDT) were co-injected into one-cell stage zebrafish embryos.
To test whether an expected large deletion in the target locus was generated, we isolated genomic DNA from the F0 injected embryos at 1 day post fertilization (dpf) and performed genotyping using the following primers, 5’- ACGAGTTTTGTAAATTTGTCAGCGTGTT-3’ and 5’- GCGAATTGTTGTAGTGACTAAGAGAGGT-3’ for fam177a1a deletion and 5’- GTGCTTTTACCCTAGAGACGTACAGTGATA-3’ and 5’- TCGCACTATGGAGAAAACAACAAAACA-3’ for fam177a1b deletion. The resulting PCR products were analyzed by an agarose-gel electrophoresis. The sperm genomic DNA from the resulting adult F0 founders was used to screen for heterozygotes of the deletions to establish two deletion alleles for each gene, fam177a1astl638, fam177a1astl700, fam177a1bstl746, and fam177a1bstl747. To identify homozygotes for each allele, the combination of above primers as well as the following primers, 5’- ATGTTGAACTTGGAGATCTGGG-3’ and 5’- ACTCACCGGATCCACTGTACTT-3’ to detect wild-type fam177a1a and 5’- TGTGTGGAGCTTGGTGATCTG-3’ and 5’-TGTCCTCCTCCTCATCTGTACT-3’ for wild-type fam177a1b sequences, were used.
Analysis of FAM177A1-mNG and fam177a1a-mNG fusion proteins in zebrafish embryos:
Embryos were co-injected with synthetic RNAs encoding FAM177A1-mNG and fam177a1a-mNG fusion proteins, memGFP, and GM130-tdTomato at one-cell stage. The injected Embryos at 4 hours post fertilization (hpf) were mounted in 0.6% low-melting temperature agarose (Lonza) on the glass bottom culture dishes (MatTek). After the agarose gel was hardened, the Petri dish containing embryos was filled with 0.3X Danieau’s Solution. Images were collected with a spinning disk confocal microscope (Quorum) using an inverted Olympus IX-81 microscope and an Olympus 40X (N.A. 0.75) air objective, a Hamamatsu EMCCD camera (C9100–13), and Metamorph acquisition software (Molecular Devices).
Immunohistochemistry:
Zebrafish embryos were co-injected with synthetic mRNAs encoding memGFP and H2B-GFP at one-cell stage. At 1-somite stage, embryos were fixed in 4% paraformaldehyde at 4°C for 12 hours. After washing out paraformaldehyde, we dechorionated and performed staining with mouse anti-GM130 antibody (1:100, BD Biosciences) and goat anti-mouse IgG antibody conjugated with Alexa Fluor 568 (1:200, ThermoFisher Scientific).
RNA-sequencing (RNA-seq) analysis:
Zebrafish:
WT (wild-type), fam177a1astl700/stl700 and fam177a1astl700/stl700 ;fam177a1bstl746/stl746 8 dpf zebrafish larvae were used for analyses. For bulk RNA-seq analyses, we isolated total RNAs using RNeasy micro kit (Qiagen) from 10 larvae per each genotype with DNase digestion to eliminate possible DNA contamination. The RNA samples were subjected to QC measurements utilizing Agilent Bioanalyzer. Libraries were processed for mRNA-Seq with Ribo-depletion and sequenced on an Illumina NovoSeq (GTAC). The RNA-seq data were processed by Cutadapt (v2.7; --quality-cutoff=15,10 --minimum-length=36), FastQC (v0.11.4), and STAR (v2.5.2b; -- quantMode TranscriptomeSAM --outWigType bedGraph --outWigNorm RPM) to do the trimming, QC report and zebrafish genome mapping (zv11). The QC report of RNA-seq can be found in Supplemental table S2. Then, gene expressions of each sample were calculated by featureCounts (-p -T 4 -Q 10) based on UCSC RefSeq gene annotation of zebrafish. See supplemental methods for differential expression analysis of RNA-seq data.
Human:
Untargeted RNA sequencing was performed utilizing blood and fibroblasts of individuals A1 and A2. Methods for RNA extraction and sequencing have been described19. FASTQs were demultiplexed using bcl2fastq2 conversion software (v2.20, Illumina, 2017), and trimmed with Cutadapt20. Reads were aligned with STAR to the GRCh38 primary assembly with the GENCODEv35 annotation21. Optical duplicates were filtered with Picard (Broad Institute), multi-mapped reads were removed, and quantification was done with RSEM22.
Exon-level quantification was generated using featureCounts23. Outlier-based analyses were then performed per sample using tissue-matched samples from healthy patients enrolled in the Undiagnosed Diseases Network study (66 fibroblast and 283 whole blood) to determine z-scores for expression and splicing. For expression, we used a custom script. Briefly, expression outlier methods were adapted from Frésard et al19. A TPM threshold was set for expression, counts were log transformed and normalized, and genes with no variance were removed. Known technical covariates were regressed, and residuals were normalized to generate final z-scores. Splicing z-scores were determined using LeafCutterMD annotated with RegTools24,25.
Quantification and statistical analysis:
Statistical analysis in Figure 5 was performed by one-way ANOVA tests with GraphPad Prism version 9. Significance was defined as *0.01<p<0.05, **0.001p<0.01, ***0.001<p<0.0001 and ****p<0.0001.
Figure 5. Morphometric analysis of zebrafish fam177a1a, fam177a1b and fam177a1a/b geneless mutants.
(A) Measurement of body length from swim bladder to the end of the tail in WT, fam177a1a, fam177a1b and fam177a1a/b mutants at 25 hpf. (B-E) Lateral views at 25 hpf. (B) WT. (C) fam177a1a single mutant. (D) fam177a1b single mutant. (E) fam177a1a/b double mutant. Scale bar, 200 mm.
Results:
Clinical characteristics:
We present a cohort of five individuals from three unrelated families with biallelic variants in FAM177A1 (Figure 1A, Table 1). The cohort includes four females and one male spanning ages 4–16 years. Disease onset occurred in infancy or early childhood in all individuals. Clinical findings and genotypes of this cohort are summarized in Table 1 and further detailed in the supplemental data (Table S1, supplemental case reports).
Figure 1. Genetic findings of individuals harboring homozygous variants in FAM177A1.
(A) Pedigrees representing affected individuals (shaded black) in the three families reported in this cohort, as well as variants in FAM177A1 and predicted protein changes in individuals tested. (B) Gene schematic of the location of variants and deletions in the mRNA and predicted protein changes.
Development and behavior:
Global developmental delay, autism, and behavioral abnormalities are present in all individuals in this cohort. This includes delayed developmental milestones such as sitting independently and walking, as well as delayed speech and language development. Three of five individuals are able to speak in small, simple sentences but were unable to read, write, or count by their early teenage years. All individuals were formally evaluated and met diagnostic criteria for autism. Behavioral abnormalities are variable across individuals but include hyperactivity, anxiety, irritability, and/or aggressive tendencies. Individual B1 also has hyperphagia.
Neurological manifestations:
Seizures, hypotonia, and gait disturbance are features shared across all individuals in this cohort. Siblings from Family C presented with seizures as early as one and two years of age, whereas individuals from Families A and B presented with seizures between childhood and early adolescence. Seizure types and frequency are variable. Hypotonia manifested in the neonatal period in three individuals (A1, A2, C2). Gait disturbance is variable, ranging from intermittent limping to a grossly ataxic gait. Additionally, three individuals have a history of spasticity, contractures, and hyperreflexia (A1, A2, B1) and tremor was observed in two (A1, B1). Ocular findings are present in three individuals: congenital cataracts (A2), amblyopia (B1), and esotropia (C2).
Neurological imaging:
White matter abnormalities of the brain are present in all four individuals for whom brain magnetic resonance imaging (MRI) information was available. This included nonspecific T2/FLAIR lesions, either hypo- or hyperintense punctate lesions of the white matter tracts. Delayed myelination was appreciated in one individual (A1), and simplified gyral pattern was appreciated in another (C2). For one individual, brain MRI was reportedly normal (C1).
Physical features:
Macrocephaly is present in four out of five individuals (A1, A2, B1, C1), documented at >99th percentile by 12 months of age in individuals A1, A2, and B1 and at 99th percentile by 5 years of age in individual C1. Other features are variable, including a single transverse palmar crease (A1, A2), clinodactyly of the 5th toe (A1, A2), 2,3 toe syndactyly (A1, A2), flat feet (C1, C2), deep set eyes (A1), and mild coxa valgus deformity of the pelvis (B1).
Immunologic:
Siblings from Family A developed findings of (A1) or suspicious for (A2) enthesitis of the bilateral femur greater trochanters, and individual A2 also has a diagnosis of juvenile rheumatoid arthritis thought to be the underlying etiology of his enthesitis.
Genomic findings:
A total of five unique variants in FAM177A1 (1 nonsense, 1 frameshift, and 3 exon-level deletions) were identified in this cohort (Figure 1B). In Family A, compound heterozygous deletions were identified including a maternally inherited 8 kilobase deletion encompassing the 5’ untranslated region (UTR) and exon 1, and a paternally inherited 9.7 kilobase deletion encompassing exons 4–5, the 3’ UTR of FAM177A1, and the 3’ end of the downstream gene PPP2R3C. Individual B1 harbors a homozygous deletion of exon 3, exact breakpoints of which were not determined by exome sequencing and likely lie in the intronic regions flanking the exon. Therefore, the annotation c.(339+1_340−1)_(406+1_407−1)del is used to represent the entire deletion of exon 3. All three deletions (the 8kb and 9.7kb deletions in Family A, and the exon 3 deletion in Family B) are absent from the gnomAD database (SV v2.1). In Family C, compound heterozygous variants were identified including a nonsense variant p.(E90*) and a frameshift deletion p.(I70Rfs*15), both located in exon 2. The p.(E90*) variant is present in gnomAD v2.1.1 in 12 alleles overall (0.0048% allele frequency), all of which are in the Latino/Admixed American subpopulation with no homozygotes. The p.(I70Rfs*15) variant is also present in gnomAD v2.1.1 in two alleles overall (0.0008% allele frequency), one allele each detected in the European (non-Finnish) and other subpopulations with no homozygotes. All variants lead to predicted protein changes which most likely result in loss of function due to early protein truncation and absent protein expression due to nonsense-mediated mRNA decay, and absent mRNA expression. Disease features appear to follow an autosomal recessive mode of inheritance as all affected individuals have biallelic FAM177A1 variants and heterozygous parents are clinically unaffected.
Long-read genome sequencing analysis validated the genomic deletions identified in the short-read genome sequencing data from individuals A1 and A2 (Figure S1). Additionally, precise deletion breakpoints were resolved through long-read sequencing analysis: chr14:35513596–35521681 (maternal deletion) and chr14:35546985–35556768 (paternal deletion). According to the RepeatMasker program, there are short interspersed nuclear elements (SINEs) flanking the 5’ and 3’ breakpoints of each of the two deletions, which may have contributed to their origin26.
Effects of DNA deletions on human RNA transcript:
RNA sequencing from blood and fibroblasts from individuals A1 and A2 show findings consistent with the compound heterozygous deletions identified by genome sequencing. Review of RNA sequencing data demonstrates a reduced number of reads covering exons 1–3 when compared to controls, and an almost complete absence of reads covering exons 4–5 (Figure 2A–C). In both siblings, exon-level quantification analysis also showed predominantly an absence of reads covering the exon 4–5 splice junction, consistent with the observed lack of expression of these two exons (Figure 2A, 2B). Neither sibling was an expression outlier for the FAM177A1 gene in the outlier analysis. In individual A1, expression z-scores were 0.2 (fibroblast) and −1.9 (blood); in A2, expression z-scores were −0.9 (fibroblast) and 0.3 (blood). Expression outlier status is calculated across the whole gene rather than by exon; since individuals A1 and A2 had reads covering exons 1–3 of the gene, we believe this underlies the finding that FAM177A1 was not an expression outlier in the analysis.
Figure 2. Deletions in FAM177A1 gene result in altered mRNA expression and loss of protein expression in individuals A1 and A2.
(A) RNA sequencing reads from Family A showing near complete absence of reads covering exons 4, 5, and the 3’UTR (blue arrows and bars). (B) Sashimi plot of RNA-sequencing data from Family A showing splicing from exon 3 into the downstream gene, PPP2R3C (blue arrows). (C) Exon-level quantifications showing the number of RNA-sequencing reads covering each exon of FAM177A1 in Individuals A1 (blue) and A2 (green); background distributions represent exon-level quantifications across an internal control cohort (D) Western blot analysis of FAM177A1 and GAPDH levels in iPSCs derived from fibroblasts of healthy donor, Individual A1, and Individual A2.
Splicing outlier analysis from both individuals A1 and A2 was significant for the FAM177A1 gene. In individual A1, splicing z-scores were 5.7 (fibroblast) and 2.6 (blood); in A2, splicing z-scores were 2.1 (fibroblast) and 2.7 (blood). Interestingly, Sashimi plots show splicing from FAM177A1 exon 3 into multiple junctions in the downstream gene, PPP2R3C (Figure 2A, B). The potential consequence(s) of the observed fusion transcripts is not clear; however, they are inconsistent across individuals A1 and A2 and across sample type and are unlikely to lead to a functional protein.
We further studied the effect of FAM177A1 deletions in family A by western blot analysis of protein expression. For this assay, induced pluripotent stem cells (iPSCs) were generated from fibroblast cells obtained from individual A1 and A2. Consistent with splicing analysis, results show striking lack of FAM177A1 protein expression compared to health donor iPSCs (Figure 2D).
FAM177A1 is localized to Golgi complex in zebrafish embryos and mammalian cells:
We generated a zebrafish model to better understand the function of homologous FAM177A1 genes. The zebrafish genome encodes two FAM177A1 homologs, fam177a1a and zgc:153383/fam177a1b that could be functionally redundant. To investigate the function of human FAM177A1 and zebrafish fam177a1a, we first explored their intracellular distribution by an overexpression assay. We generated constructs encoding the zebrafish fam177a1a and its C-terminal fusion with mNeonGreen and synthetized capped sense RNAs. Injection of synthetic RNAs encoding FAM177A1 or fam177a1a-mNeongreen into one-celled zebrafish embryos caused gastrulation defects, embryonic body malformation and tissue degeneration in dose-dependent fashion (data not shown). Confocal microscope imaging of zebrafish embryos injected with lower doses of fam177a1a-mNeongreen RNA that did not impair development showed a spotty pattern within the cytoplasm, reminiscent of the Golgi complex-associated proteins in early zebrafish embryos. To verify whether fam177a1a-mNeonGreen is localized to the Golgi complex, we co-expressed in embryos fam177a1a-mNeonGreen and GM130-tdTomato, a Golgi marker, and observed a striking co-localization of fam177a1a-mNeonGreen protein with GM130-tdTomato (Figure 3). A similar concentration in the Golgi complex, as assessed by colocalization with GM130, was observed for human FAM177A1-mNeonGreen in zebrafish (Figure 3 G–L).
Figure 3. Subcellular localization of zebrafish fam177a1a-mNeonGreen and human FAM177A1-mNeonGreen in zebrafish embryos and mammalian COS7 cell line.

Developing Notochord and enveloping layer (EVL) in the embryos. (A-F) Embryos co-injected with fam177a1a-mNeonGreen and GM130-tdTomato RNAs at one-cell stage and imaged at 12 hpf. (G-L) Embryos co-injected with FAM177A1-mNeonGreen and GM130-tdTomato RNAs at embryo one-cell stage and imaged at 12 hpf. (A, D) Zebrafish fam177a1a-mNeonGreen. (B, E, H, K) GM130-tdTomato. (C, F, I, L) Merged images. (G, J) Human FAM177A1-mNeongGreen. Scale bars, 20 mm. (M, N, O) Human FAM177A1 is predominantly in the Golgi complex in mammalian cells. COS7 cell lines expressing FAM177A1-GFP. Note the co-localization with a marker of the trans-Golgi complex, GalT (Galactosyltransferase, shown in magenta). Image is z-stacked image showing the max projection of all signals. Dotted white lines indicate cell margins, and nucleus (n) respectively. Scale bar = 10 mm.
To determine the localization of FAM177A1 in mammalian cells, we overexpressed a C terminal-tagged human FAM177A1-GFP in COS7 cells. The results revealed that, as observed in zebrafish embryos, FAM177A1 also localizes to the Golgi complex in these cells (Figure 3M, N, O). Together these studies indicate that FAM177A1 is predominantly localized in Golgi complex.
Generation of zebrafish fam177a1a, fam177a1b geneless mutants:
Given that the human variants likely represent null alleles, we generated in zebrafish a fam177a1a and fam177a1b allelic series. fam177a1a, stl638 (Δ6165) and fam177a1a, stl700 (Δ6165, +25) “geneless” alleles removing large portions of the fam177a1a coding region which model FAM177A1 deletions observed in some participants (Figure 4A). We also generated two such “geneless” alleles of fam177a1b, stl746 (Δ19008, +4) and stl747 (Δ19010, +3) (Figure 4B) in the fam177a1astl700/stl700 background. RNA quantification of fam177a1a and fam177a1b/b gene expression was performed in zebrafish larvae with lacking either fam177a1a or fam177a1a and fam177a1b (fam177a1a/b double mutant) at 8 dpf (Figure 4C). Interestingly, expression of fam177a1b was strongly reduced in fam177a1a single mutants.
Figure 4. Generation of zebrafish fam177a1a, fam177a1b and fam177a1a/b geneless mutants.
(A) Genetic locus of fam177a1a and the sequences of geneless alleles. (B) Genetic locus of fam177a1b and the sequences of the geneless alleles. (C) Gene expression of fam177a1a and fam177a1a/b compared to WT as measured by quantitative RNA sequencing in the fam177a1a and fam177a1b genetic background (KO- knockout −/−, CPM- copies per million).
fam177a1a/b double mutant zebrafish exhibit transient developmental growth delay:
We observed that fam177a1a stl638/stl638 and fam177a1astl700/stl700 single and fam177a1astl700/stl700; fam177a1bstl747/stl747 (thereafter fam177a1a/b) double mutant embryos generated by heterozygous females (zygotic mutants) and those generated by homozygous females (maternal zygotic, MZ, mutants lacking both maternal and zygotic gene function) underwent embryogenesis normally and grew into viable and fertile adults. We also observed such viable phenotypes for fam177a1bstl746/stl746 and fam177a1astl700/stl700; fam177a1bstl746/stl746 mutants. However, morphometric analyses at 25 hours post fertilization (hpf) revealed that MZfam177a1a and MZfam177a1b single mutants exhibited 8% and 12% shorter body length compared to WT embryos, respectively (Figure 5 A, B–E). Whereas fam177a1a/b double mutants exhibited an additive phenotype, with their body length being 19% shorter compared to WT (Figure 5E). The phenotype of reduced body length gradually diminished over time. Whereas fam177a1a/b double mutants body length was 19% shorter than wild-type embryos at 1 day post fertilization (dpf), by 5 dpf, the difference in body length between wild-type and fam177a1a/b double mutants decreased to as little as 4%. This observation led us to classify this phenotype as a transient abnormality. In addition, the milder phenotype of fam177a1a (Figure 5C) and fam177a1b single mutants (Figure 5D) compared to fam177a1a/b double mutant (Figure 5E) supports the notion that fam177a1a and fam177a1b genes are functionally redundant.
fam177a1a/b deficiency does not impair gross Golgi complex morphology:
Since FAM177A1 is localized in the Golgi complex, we hypothesized that the Golgi complex could form aberrantly in zebrafish fam177a1a/b double mutants. To test this, we injected GM130-tdTomato RNA along with memGFP and H2B-GFP RNAs into WT and fam177a1a/b double mutant embryos at one-cell stage and analyzed the intracellular localization of GM130-tdTomato in embryos during gastrulation stages using confocal microscopy (Figure 6). The intracellular expression pattern of GM130-tdTomato in the superficial enveloping layer (EVL) cells of the fam177a1a/b double mutants and WT embryos during gastrulation was comparable.
Figure 6. Intracellular distribution of the Golgi complex in developing notochord during late gastrulation.

Dorsal view, anterior to the left. Brackets indicate notochord area. (A-F) Embryos injected with H2B-GFP, memGFP and GM130-tdTomato RNAs at one-cell stage and imaged at 11 hpf. (G-L) Embryos injected with H2B-GFP and memGFP RNAs at one-cell stage, fixed at 11 hpf, and stained with ɑ-GM130 antibody. (A-C, G-I) WT embryos. (D-F, J-L) fam177a1a/b double mutant embryos. (A, D, G, J) Expression of H2B-GFP in the cell nuclei and memGFP in cell membranes. (B, E) Expression of GM130-tdTomato in the Golgi vesicles. (H, K) Detection of the endogenous GM130 protein by immunohistochemistry (C, F, I, L) Merged images. Scale bar, 20 ums.
Moreover, during early segmentation GM130-tdTomato expressing vesicles became properly polarized to the midline of the notochord tissue as observed in WT embryos. We further confirmed normal expression of GM130 in the notochord of fam177a1a/b double mutant embryos using immunohistochemistry (Figure 6G–L), corroborating that there was no gross change in the Golgi body morphology in the mutants.
Analysis of gene expression patterns in fam177a1a/b double mutant zebrafish
Although the morphological phenotypes of fam177a1a single mutant and double mutant, fam177a1a/b, were mild and the mutants became fertilized adults, we hypothesized that the gene expression would be altered. Bulk RNA-sequencing (RNA-seq) analysis showed that the transcriptomes of fam177a1a single mutant and fam177a1a/b double mutants were significantly different from those of WT controls (Figure 7). Bioinformatic analysis showed negligible levels of fam177a1a and fam177a1b gene transcripts in fam177a1a/b double mutants compared to WT, consistent with the stl700 and stl746 deletion alleles removing coding sequences of these two genes. From 885 genes significantly upregulated in the single mutants and 633 in the double mutants, the majority, 481 genes, were commonly upregulated. Similarly, from 565 genes downregulated in the single mutants and 343 in the double mutants, 256 were shared (Figure S2).
Figure 7. Bulk RNA-sequencing analysis of WT, fam177a1a and fam177a1a/b replicates in zebrafish 8dpf.
(A) Heatmap of correlation of three independent RNA samples. The color represents the strength and direction of correlation. Three replicates of each genotype. (B) Principal component analysis (PCA) using RNA-sequencing data. Percent variability within each principal component is listed on each axis.
RNA network analysis in FAM177A1-deficient human and fam177a1a/b double mutant zebrafish cells:
To gain further insights into the functions of FAM177A1 we performed bulk RNA-seq and metabolomic analyses of WT, fam177a1a single and fam177a1a/b double mutant larvae at 8 dpf (Figure 7; Figure S2). Metabolomic analysis can be found in supplemental information. Untargeted analysis of metabolites was performed with the goal to identify upregulated or downregulated pathways indicative of gene function. Broadly, FAM177A1-deficient human tissues and fam177a1/b double mutant zebrafish showed abnormal phospholipid profile relative to WT.
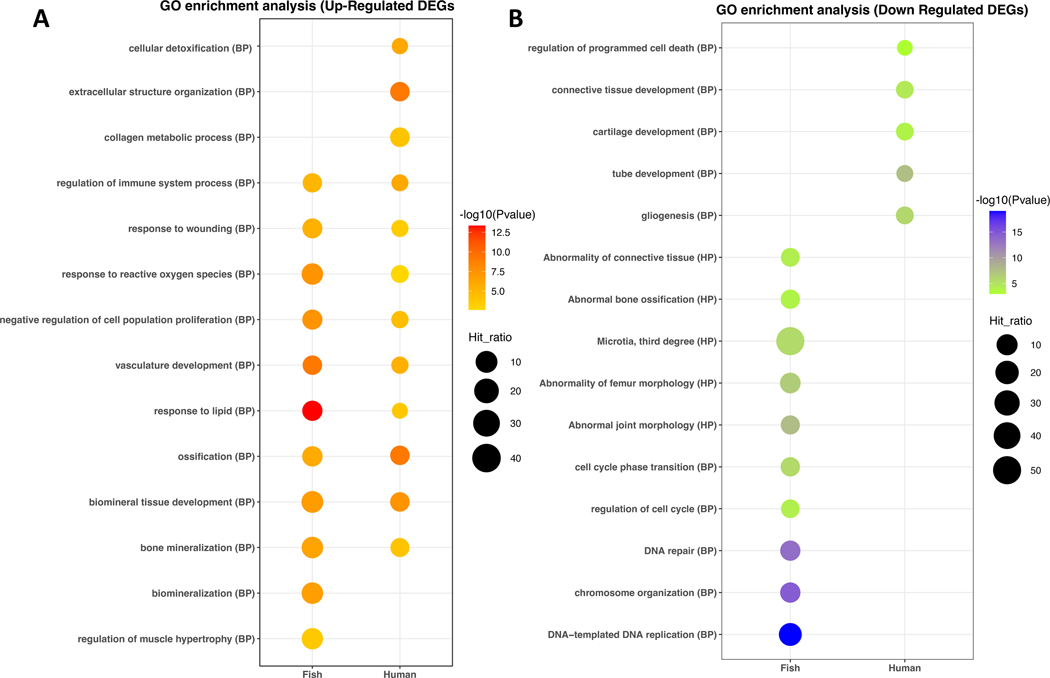
We also compared transcriptomes of fibroblasts from the FAM177A1-deficient participants (individuals A1 and A2) and control fibroblasts (Figure 8). For human fibroblasts, heatmap and PCA showed the transcriptomes of the two FAM177A1-deficient samples from Family A correlated with one another but were distinct from three control samples (Figure 8A, 8B). Subsequently, we carried out an intersection of the human and zebrafish RNA-seq datasets to identify nine sets of commonly upregulated genes by GO enrichment analysis (Figure 9A, 9B). This analysis pointed to biological processes including regulation of the immune system, response to wounding, response to reactive oxygen species, negative regulation of cell proliferation, response to lipid, and biomineral tissue development. Downregulated DEGs were inconsistent between human and zebrafish FAM177A1 deficient transcriptome datasets (Figure 9B).
Figure 8. RNA-seq analysis FAM177A-deficient and WT fibroblasts.
(A) Heatmap of correlation within the human RNA samples from the affected participants’ fibroblasts and control fibroblasts. (B) Principal component analysis using human RNA-seq data.
Figure 9. Gene Ontology (GO) enrichment analysis of differentially expressed genes among human and zebrafish RNA-sequencing.
GO terms pertaining to biological processes (BP) and human phenotype ontology (HP) are listed on the y-axis. The color of the bubbles indicates significance of the enrichment (log10(Pvalue). The hit ratio (hit_ratio) refers to the size of the bubble and indicates the relative number of genes associated with the term. (A) Up-regulated genes. (B) Down-regulated genes.
Discussion:
Here, we describe five individuals from three families with a FAM177A1-related neurodevelopmental disorder characterized by global developmental delay, seizures, behavioral abnormalities, macrocephaly, hypotonia, and gait disturbance. The siblings previously described by Alazami et al. (2015) share similar clinical features with this cohort8. Our cohort broadens and defines the clinical picture for FAM177A1-related neurodevelopmental disorder. As more individuals with this FAM177A1-related neurodevelopmental disorder are identified, a more complete understanding of the phenotypic spectrum and associated clinical characteristics will emerge.
Affected individuals harbor biallelic variants that are predicted to result in loss of protein function. There are 28 distinct predicted loss-of-function variants present in the gnomAD database (v2.1.1 and v3.1.2), and seven structural variants (SVs v2.1). None of these are present in the homozygous state, which is consistent with an autosomal recessive mode of inheritance and that observed in this cohort. While missense variants in FAM177A1 have not been identified in association with disease to date, it is possible that missense variation with deleterious effect on protein function could result in the FAM177A1-related disorder as well. It is notable that Family A described here carries multi-exon level deletions in FAM177A1 which were identified via structural variant analysis from short- and long-read genome sequencing. Single and multi-exon deletion detection via next-generation sequencing is a known challenge as such deletions may not be reliably detected. This emphasizes the importance of including appropriate deletion-duplication analysis when interrogating the FAM177A1 gene for disease-causing variation.
In previous studies, FAM177A1 was found to be highly localized to the cytoplasm and nucleus of mouse macrophages and undifferentiated embryonic carcinoma cells4,7. In our basic cellular research, we show a striking co-localization of zebrafish and human FAM177A1-fusion proteins with the Golgi markers, such as GM130, in zebrafish embryos and mammalian cells. Moreover, FAM177A1 appears to be a Golgi-associated protein throughout dynamic cellular processes, including polarization and repolarization. These observations motivate future studies of Golgi organization and function on downstream lipid processing and protein organization.
In our novel animal model of homologous FAM177A1-deficiency, two zebrafish homologs of FAM177A1, fam177a1a and fam177a1b, were knocked out to analyze the phenotypes of the resulting single and compound mutants. Interestingly, fam177a1b transcripts are significantly downregulated in fam177a1a single mutants, suggesting a potential co-regulation that should be investigated in the future. Whereas fam177a1a single, fam177a1b single, and fam177a1a/b double zebrafish mutants develop into morphologically normal and fertile adults, they all exhibit transient body shortening during embryonic and larval stages.
Our finding that both zebrafish and human FAM177A1-GFP fusion protein localize to the Golgi complex in zebrafish embryo and human cells leads us to a plausible hypothesis that fam177a1a and/or fam177a1b play a role in Golgi dynamics and function. We hypothesize that the absence of both genes could impair functionality of the Golgi complex, subsequently causing a delay in embryonic development and a temporary reduction in body length during the early stages. The temporary reduction in body length during the early stages could be due to compensation by other genes in zebrafish embryos, which can substitute for the function of fam177a1a and/or fam177a1b at later stages. Furthermore, the preserved architecture of the Golgi in fam177a1a/b double mutant samples suggests that the gene is not involved in Golgi structural integrity. Future studies on Golgi dynamics in the setting of FAM177A1-deficiency are ongoing.
Further insights into FAM177A1 function came from bulk RNA-seq and metabolomic analyses of zebrafish larvae and that from human fibroblasts. Bioinformatic analyses of these datasets revealed hundreds of genes commonly dysregulated in zebrafish fam177a1a single and fam177a1a/b double mutants compared to wild-type. GO enrichment analysis of zebrafish and human (Family A fibroblast) datasets identified commonly upregulated biological processes and pathways, including regulation of the immune system, negative regulation of cell proliferation, response to lipid, and biomineral tissue development. Network analysis of untargeted metabolomics data combined with RNA-seq data from fam177a1a single and fam177a1a/b double mutant zebrafish larvae tissues demonstrated dysregulation of amino acid and lipid pathways (see metabolomics data in supplemental information). These studies, combined with the wide and robust expression patterns of FAM177A1, suggest that FAM177A1 affects diverse physiologic processes in the human body.
Interestingly, the limited research available on this gene has demonstrated FAM177A1 to be an immune-associated gene. Several genome-wide association studies implicated FAM177A1 with diseases ranging from primary biliary cholangitis to juvenile idiopathic arthritis1,2,28. The individuals with FAM177A1-related neurodevelopmental disorder in this cohort do not have a strong immune/inflammatory component, except for the history of including arthritis and enthesitis of the greater trochanters in Family A. In the current study, we found DEGs commonly upregulated in FAM177A1-deficient samples involving processes such as cytokine signaling pathways of IL-2, IL-18, 1L-6 and TNF. Consistent with this evidence, Liao et al, published confirmatory functional data of FAM177A1 as a negative regulator of the IL-1β immunogenic cascade in mammalian cells5. Further evidence for immunologic involvement of this disorder may emerge as a key finding as more individuals are diagnosed and evaluated.
An important limitation to the RNA-seq and metabolome differential expression analyses are that no shared dysregulated genes were observed in FAM177A1-deficient fibroblasts and zebrafish mutants, except for FAM177A1 and its zebrafish homologs, which were all strongly downregulated. The differences in specific dysregulated genes could be because the transcriptomic analyses were performed only on human fibroblast but on whole zebrafish larvae. Whereas FAM177A1 is broadly expressed in human tissues and is expressed ubiquitously throughout zebrafish development and adult tissues, requirements for its function can vary between tissues and cell types. Identifying cell types most sensitive to FAM177A1 loss of function will be important to understanding FAM177A1-dependent disease. There are similar limitations to the untargeted metabolomic analyses, which were also performed on fibroblasts and plasma from FAM177A1-deficient and unaffected control individuals and whole 8 dpf fam177a1a single, fam177a1a/b double mutant, and WT larvae. While significant conclusions cannot be drawn from these analyses at this time, we hope they may serve as a starting point from which to build in the future.
In conclusion, we describe an emerging neurodevelopmental disease in five individuals caused by loss-of-function variants in FAM177A1. We illustrate a comprehensive clinical phenotype of the individuals in this cohort and provide further evidence of FAM177A1 as a neurologic disease gene. In our basic research, we show that FAM177A1 is a Golgi-associated protein and provide a dataset investigating RNA and metabolomic anomalies in the setting of FAM177A1 deficiency. In terms of clinical validity, our work supports moderate to strong evidence of this disease-gene association29. Thus, future work elucidating gene function and disease pathogenesis will be critical for identifying avenues for therapeutic targets which may prevent or slow disease progression.
Supplementary Material
Acknowledgements
The authors thank the participants and their families. Thanks to Shinya Yamamoto for providing research samples.
Funding statement
Research reported in this manuscript was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under award numbers U01HG010218. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 03/16/22 (https://gtexportal.org/home/gene/FAM177A1)
Consortia
Members of the Undiagnosed Diseases Network include: Maria T. Acosta, Margaret Adam, David R. Adams, Raquel L. Alvarez, Justin Alvey, Laura Amendola, Ashley Andrews, Euan A. Ashley, Carlos A. Bacino, Guney Bademci, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Michael Bamshad, Deborah Barbouth, Pinar Bayrak-Toydemir, Anita Beck, Alan H. Beggs, Edward Behrens, Gill Bejerano, Hugo J. Bellen, Jimmy Bennett, Beverly Berg-Rood, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, Stephanie Bivona, Elizabeth Blue, John Bohnsack, Devon Bonner, Lorenzo Botto, Brenna Boyd, Lauren C. Briere, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, Peter Byers, William E. Byrd, John Carey, Olveen Carrasquillo, Thomas Cassini, Ta Chen Peter Chang, Sirisak Chanprasert, Hsiao-Tuan Chao, Ivan Chinn, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, Matthew Coggins, F. Sessions Cole, Heather A. Colley, Heidi Cope, Brian Corner, Rosario Corona, William J. Craigen, Andrew B. Crouse, Michael Cunningham, Precilla D’Souza, Hongzheng Dai, Surendra Dasari, Joie Davis, Jyoti G. Dayal, Esteban C. Dell’Angelica, Patricia Dickson, Katrina Dipple, Daniel Doherty, Naghmeh Dorrani, Argenia L. Doss, Emilie D. Douine, Dawn Earl, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Kimberly Ezell, Marni Falk, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, William A. Gahl, Ian Glass, Bernadette Gochuico, Page C. Goddard, Rena A. Godfrey, Katie Golden-Grant, Alana Grajewski, Don Hadley, Sihoun Hahn, Meghan C. Halley, Rizwan Hamid, Kelly Hassey, Nichole Hayes, Frances High, Anne Hing, Fuki M. Hisama, Ingrid A. Holm, Jason Hom, Martha Horike-Pyne, Alden Huang, Sarah Hutchison, Wendy Introne, Rosario Isasi, Kosuke Izumi, Fariha Jamal, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Orpa Jean-Marie, Vaidehi Jobanputra, Lefkothea Karaviti, Shamika Ketkar, Dana Kiley, Gonench Kilich, Shilpa N. Kobren, Isaac S. Kohane, Jennefer N. Kohler, Susan Korrick, Mary Kozuira, Deborah Krakow, Donna M. Krasnewich, Elijah Kravets, Seema R. Lalani, Byron Lam, Christina Lam, Brendan C. Lanpher, Ian R. Lanza, Kimberly LeBlanc, Brendan H. Lee, Roy Levitt, Richard A. Lewis, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, AudreyStephannie Maghiro, Rachel Mahoney, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Kenneth Maravilla, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Jacob McCauley, Allyn McConkie-Rosell, Alexa T. McCray, Elisabeth McGee, Heather Mefford, J. Lawrence Merritt, Matthew Might, Ghayda Mirzaa, Eva Morava, Paolo Moretti, John Mulvihill, Mariko Nakano-Okuno, Stanley F. Nelson, Serena Neumann, John H. Newman, Sarah K. Nicholas, Deborah Nickerson, Shirley Nieves-Rodriguez, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina G.S. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, Lorraine Potocki, Barbara N. Pusey Swerdzewski, Aaron Quinlan, Deepak A. Rao, Anna Raper, Wendy Raskind, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Natalie Rosenwasser, Francis Rossignol, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Mario Saporta, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, C. Ron Scott, Elaine Seto, Vandana Shashi, Jimann Shin, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Edward C. Smith, Kevin S. Smith, Lilianna Solnica-Krezel, Ben Solomon, Rebecca C. Spillmann, Joan M. Stoler, Kathleen Sullivan, Jennifer A. Sullivan, Angela Sun, Shirley Sutton, David A. Sweetser, Virginia Sybert, Holly K. Tabor, Queenie K.-G. Tan, Amelia L. M. Tan, Arjun Tarakad, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Rachel A. Ungar, Tiina K. Urv, Adeline Vanderver, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Melissa Walker, Stephanie Wallace, Nicole M. Walley, Jennifer Wambach, Jijun Wan, Michael F. Wangler, Patricia A. Ward, Daniel Wegner, Monika Weisz Hubshman, Mark Wener, Tara Wenger, Monte Westerfield, Matthew T. Wheeler, Jordan Whitlock, Lynne A. Wolfe, Kim Worley, Changrui Xiao, Shinya Yamamoto, John Yang, Zhe Zhang, Stephan Zuchner.
Footnotes
Ethics Declaration
The research ethics committee at Stanford University approved this study through their institutional review board which upholds tenets such as those described in the Declaration of Helsinki (IRB; protocols 47026 and 66629).A single IRB was used across institutions with each family signing written and informed consent. Clinical data was collected by the PI of the IRB and de-identified for use in the study. Family A was initially studying under the approval of the NIH Undiagnosed Diseases IRB protocol (15HG0130). IRB materials and written consents are available for review upon request.
WEB resources
https://zebrafishfacility.wustl.edu/facility-documents/
https://gtexportal.org/home/gene/FAM177A1
https://support.illumina.com/downloads/bcl2fastq-conversion-software-v2-20.html
http://star.mit.edu/index.html
Additional information
Supplemental text and data are available.
Conflicts of Interest
The authors declare no conflict of interest
Data availability statement
Further clinical and genetic data is available upon request to the corresponding author.
References
- 1.Sjakste T, Trapina I, Rumba-Rozenfelde I, Lunin R, Sugoka O, Sjakste N. Identification of a novel candidate locus for juvenile idiopathic arthritis at 14q13.2 in the Latvian population by association analysis with microsatellite markers. DNA Cell Biol. 2010;29(9):543–551. doi: 10.1089/DNA.2009.0970 [DOI] [PubMed] [Google Scholar]
- 2.Sjakste T, Eglite J, Sochnevs A, et al. Microsatellite genotyping of chromosome 14q13.2–14q13 in the vicinity of proteasomal gene PSMA6 and association with Graves’ disease in the Latvian population. Immunogenetics. 2004;56(4):238–243. doi: 10.1007/S00251-004-0687-9 [DOI] [PubMed] [Google Scholar]
- 3.Parada H, Sun X, Fleming JM, et al. Race-associated biological differences among luminal A and basal-like breast cancers in the Carolina Breast Cancer Study. Breast Cancer Res 2017 191. 2017;19(1):1–9. doi: 10.1186/S13058-017-0914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Guo M, Yue D, et al. MicroRNA-7 negatively regulates Toll-like receptor 4 signaling pathway through FAM177A. Immunology. 2021;162(1):44–57. doi: 10.1111/imm.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao BW, Zhang HY, Du WT, Ran Y, Wang YY, Xu ZS. FAM177A1 Inhibits IL-1β–Induced Signaling by Impairing TRAF6–Ubc13 Association. J Immunol. 2021;207(12):3090–3097. doi: 10.4049/jimmunol.2100561 [DOI] [PubMed] [Google Scholar]
- 6.Magdaleno S, Jensen P, Brumwell CL, et al. BGEM: An In Situ Hybridization Database of Gene Expression in the Embryonic and Adult Mouse Nervous System. PLoS Biol. 2006;4(4):497–500. doi: 10.1371/JOURNAL.PBIO.0040086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko HY, Lee DS, Kim S. Noninvasive imaging of microRNA124a-mediated repression of the chromosome 14 ORF 24 gene during neurogenesis. FEBS J. 2009;276(17):4854–4865. doi: 10.1111/j.1742-4658.2009.07185.x [DOI] [PubMed] [Google Scholar]
- 8.Alazami AM, Patel N, Shamseldin HE, et al. Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep. Published online 2015. doi: 10.1016/j.celrep.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 9.Splinter K, Adams DR, Bacino CA, et al. Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease. N Engl J Med. 2018;379(22):2131–2139. doi: 10.1056/nejmoa1714458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahl WA, Wise AL, Ashley EA. The Undiagnosed Diseases Network of the National Institutes of Health: A National Extension. JAMA. 2015;314(17):1797–1798. doi: 10.1001/JAMA.2015.12249 [DOI] [PubMed] [Google Scholar]
- 11.Holt JM, Birch CL, Brown DM, et al. Identification of pathogenic structural variants in rare disease patients through genome sequencing. bioRxiv. Published online 2019. doi: 10.1101/627661 [DOI] [Google Scholar]
- 12.Ueno Y, Arita M, Kumagai T, Asai K. Processing sequence annotation data using the Lua programming language. Genome Inform. 2003;14:154–163. [PubMed] [Google Scholar]
- 13.Li H Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–3100. doi: 10.1093/BIOINFORMATICS/BTY191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolka M, Paulin LF, Grochowski CM, et al. Comprehensive Structural Variant Detection: From Mosaic to Population-Level. bioRxiv. Published online April 5, 2022:2022.04.04.487055. doi: 10.1101/2022.04.04.487055 [DOI] [Google Scholar]
- 15.Miller JD, Schlaeger TM. Generation of Induced Pluripotent Stem Cell Lines from Human Fibroblasts via Retroviral Gene Transfer. Methods Mol Biol. 2011;767:55–65. doi: 10.1007/978-1-61779-201-4_5/COVER [DOI] [PubMed] [Google Scholar]
- 16.Moriyoshi K, Richards LJ, Akazawa C, Dennis MORD, Nakanishi S. Labeling Neural Cells Neurotechnique Using Adenoviral Gene Transfer of Membrane-Targeted GFP. Vol 16.; 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hwang WY, Fu Y, Reyon D, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31(3):227–229. doi: 10.1038/nbt.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labun K, Montague TG, Krause M, Torres Cleuren YN, Tjeldnes H, Valen E. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019;47(W1):W171–W174. doi: 10.1093/NAR/GKZ365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frésard L, Smail C, Ferraro NM, et al. Identification of rare-disease genes using blood transcriptome sequencing and large control cohorts. Nat Med. 2019;25(6):911–919. doi: 10.1038/s41591-019-0457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12. [Google Scholar]
- 21.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15. doi: 10.1093/BIOINFORMATICS/BTS635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Dewey CN. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12. doi: 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/BIOINFORMATICS/BTT656 [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson G, Li YI, Basu S, Cousin MA, Oliver GR, Klee EW. LeafCutterMD: an algorithm for outlier splicing detection in rare diseases. Bioinformatics. 2020;36(17):4609–4615. doi: 10.1093/BIOINFORMATICS/BTAA259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotto KC, Feng YY, Ramu A, et al. Integrated analysis of genomic and transcriptomic data for the discovery of splice-associated variants in cancer. Nat Commun. 2023;14(1):1589. doi: 10.1038/S41467-023-37266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RepeatMasker. Smit AFA, Hubley R & Green P. RepeatMasker Open-4.0. Published 2013. Accessed November 2, 2023. http://www.repeatmasker.org
- 27.Sepich DS, Solnica-Krezel L. Intracellular Golgi Complex organization reveals tissue specific polarity during zebrafish embryogenesis. Dev Dyn. 2016;245(6):678–691. doi: 10.1002/dvdy.24409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Chen L, Liu Y. A large-scale plasma proteome Mendelian randomization study identifies novel causal plasma proteins related to primary biliary cholangitis. Front Immunol. 2023;14. doi: 10.3389/fimmu.2023.1052616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strande NT, Riggs ER, Buchanan AH, et al. Evaluating the Clinical Validity of Gene-Disease Associations: An Evidence-Based Framework Developed by the Clinical Genome Resource. Am J Hum Genet. 2017;100(6):895–906. doi: 10.1016/J.AJHG.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further clinical and genetic data is available upon request to the corresponding author.