Abstract
A prospective cohort study was conducted to assess the prognostic significance of heart rate variability (HRV) measured by the HRV Camera application in predicting major adverse cardiovascular events (MACE) and all-cause mortality within 3 months after percutaneous coronary intervention (PCI). Of 101 patients, 25 developed MACE and 6 died. Low HRV (SDNN and rMSSD) is associated with increased MACE (p < 0.001 and p = 0.014, respectively) and all-cause mortality rates (p = 0.025 and p = 0.032, respectively). Our study concludes that HRV measured by smartphone applications has significant potential as predictive indicators of MACE and all-cause mortality after PCI, particularly SDNN.
Keywords: Heart rate variability, Acute myocardial infarction, MACE
Graphical abstract
1. Introduction
Heart rate variability (HRV) has become the term most commonly mentioned to measure the variation in the time interval between two heartbeats. Regulated by neurohumoral factors, HRV can reflect changes in the balance of the autonomic nervous system. Many studies have shown the significance of heart rate variability for early prognosis and long-term prognosis in patients after acute myocardial infarction (AMI).1 HRV can be measured using a variety of methods (time-domain measures, frequency-domain measures and nonlinearity-based measures.1,2 Analysis of HRV signals obtained from 3-lead ECG and earlobe photoplethysmography (PPG) recordings show nearly identical results. Therefore, the pulse sensor serves as a reliable method of capturing signals from which HRV metrics can be derived.3 Our study utilizes SDNN and rMSSD time-domain indices measured by the Camera HRV application, which provides a readily available and convenient solution compared to other existing methods and techniques.4,5 The purpose of this study is to assess the prognostic role of HRV in patients with AMI undergoing PCI through PPG methods on smartphones.
2. Methods
This study was designed as a prospective cohort analysis in patients with a confirmed diagnosis of AMI who were treated with PCI in a central hospital in Vietnam from September 2022 to March 2023. The selection criteria comprised patients diagnosed with AMI according to the Fourth Universal Definition of Myocardial Infarction,6 while patients with arrhythmia and conduction disorders, those treated with positive inotropic or chronotropic drugs, and patients who underwent late or delayed interventions were excluded.
All enrolled patients had time-domain HRV indexes measured (in milliseconds), including SDNN (Standard deviation of all normal R–R intervals) and rMSSD (Square root of the average number of squared differences between consecutive normal R–R intervals) within 1 min using the HRV Camera application upon admission.
Afterward, all patients underwent PCI following the 2018 ESC/EACTS Guidelines on myocardial revascularization.7 Upon discharge, all patients received optimized treatment according to the 2018 ESC/EACTS Guidelines on myocardial revascularization.7 After 3 months of PCI, these patients were evaluated for MACE, including all-cause death, recurrent myocardial infarction, stroke, and hospitalization due to heart failure.
SPSS 20 was used for statistical analyses.
3. Results
A total of 101 patients were enrolled, of whom 25 patients (24.8 %) experienced a 3-month MACE. There were 6 cases of all-cause mortality, 3 cases of recurrent myocardial infarction, 2 cases of stroke, and 14 hospitalizations due to heart failure.
Table 1 shows the demographic and HRV characteristics of the patients.
Table 1.
Demographic and HRV characteristics of the patients.
| Characteristics | MACE Group (n = 25) | Non-MACE Group (n = 76) | p | Mortality (n = 6) | Non-mortality (n = 95) | p |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 73.08 ± 10.83 | 70.13 ± 9.64 | 0.201 | 74.33 ± 7.20 | 70.64 ± 10.11 | 0.382 |
| Gender | ||||||
| Male | 17 | 52 | 0.969 | 3 | 66 | 0.320 |
| Female | 8 | 24 | 3 | 29 | ||
| Heart rate, b.p.m (mean ± SD) | 81.44 ± 13.60 | 81.16 ± 18.86 | 0.945 | 75.17 ± 13.44 | 81.61 ± 17.86 | 0.388 |
| LVEF | ||||||
| <50 % | 16 | 35 | 0.119 | 3 | 48 | 0.98 |
| ≥50 % | 9 | 41 | 3 | 47 | ||
| Killip Classification | ||||||
| Class I | 17 | 65 | 0.052 | 5 | 77 | 0.89 |
| Class II - IV | 8 | 11 | 1 | 18 | ||
| Multivessel coronary artery disease | ||||||
| No | 5 | 36 | 0.016 | 0 | 41 | 0.037 |
| Yes | 20 | 40 | 6 | 54 | ||
| Heart rate variabity | ||||||
| SDNN, ms (mean ± SD) | 14.93 ± 6.71 | 22.60 ± 10.44 | <0.001 | 11.67 ± 4.28 | 21.19 ± 10.17 | 0.025 |
| rMSSD, ms (mean ± SD) | 18.57 ± 11.08 | 25.44 ± 12.38 | 0.014 | 13.20 ± 3.63 | 24.33 ± 12.45 | 0.032 |
Abbreviations: SD - Standard deviation, MACE - Major adverse cardiovascular events, LVEF – Left ventricular ejection fraction, SDNN - Standard deviation of all normal R–R intervals), rMSSD - Square root of the average number of squared differences between consecutive normal R–R intervals.
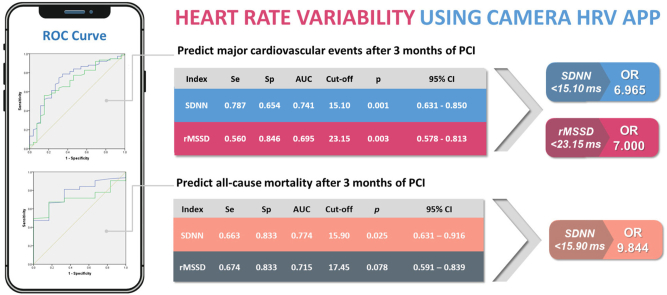
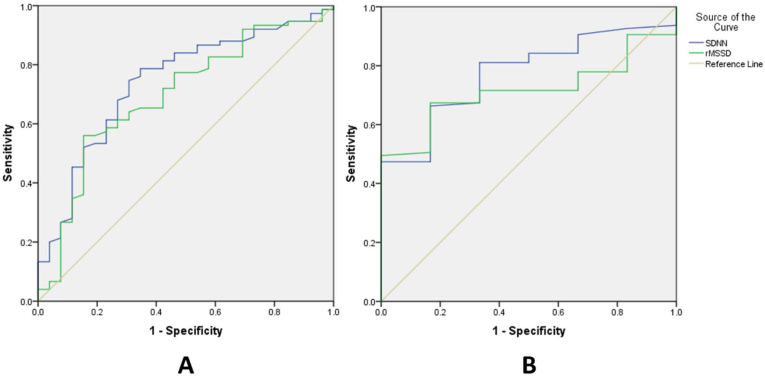
Fig. 1 shows the ROC curves that demonstrate the role of HRV in the prediction of MACE and all-cause mortality.
Fig. 1.
A - ROC Curve of SDNN and rMSSD for predicting MACE. B - ROC Curve of SDNN and rMSSD for predicting all-cause mortality.
Abbreviations: ROC - Receiver Operating Characteristic, SDNN - Standard deviation of all normal R–R intervals), rMSSD - Square root of the average number of squared differences between consecutive normal R–R intervals).
The AUC of SDNN and rMSSD in predicting 3-month MACE was 0.741 (95 % confidence interval [CI]: 0.631–0.850, p < 0.001), 0.695 (95 % CI: 0.578–0.827, p = 0,003), respectively. SDNN <15.10 ms showed a sensitivity of 78.7 % and a specificity of 65.4 %. The odds ratio (OR) was 6.965 (95 % CI: 2.617–18.536, p < 0.001) (Supplementary Table S1). For rMSSD, with a cut-off value of 23.15 ms, the sensitivity is 56.0 % and the specificity is 84.6 %. The OR was 7.000 (95 % CI: 2.197–22.303, p < 0,001) (Supplementary Table S1).
SDNN possesses predictive capability for all-cause mortality 3 months after PCI, with a threshold value of 15.9 ms. The AUC of SDNN was 0.774 (95 % CI: 0.631–0.916, p = 0.025). The sensitivity and specificity values were 66.3 % and 83.3 %. The OR is 9.844 (95 % CI = 1.103–87.849, p = 0.001) (Supplementary Table S2). In contrast, rMSSD's prognostic capacity for all-cause mortality after 3 months of intervention did not reach statistical significance, with p = 0.078.
4. Discussion
In our study, a total of 25 patients (24.8 %) experienced 3-month MACE. This finding is also consistent with some other research.8,9 This high proportion can be explained by the fact that the study was conducted in a central hospital that received severely ill patients with multiple comorbidities.
The results show that both SDNN and rMSSD measured by the PPG method have separate values to predict patients who develop MACE 3 months after PCI due to AMI. The prognostic value of SDNN for MACE in patients with acute myocardial infarction appears to be higher than the values for rMSSD (AUC 0.741 versus AUC 0.695). The study conducted by Pop-Busui R et al (2022) also concurs that SDNN's ability to classify cardiovascular autonomic neuropathy surpasses that of rMSSD.10
The findings of our study also indicate that SDNN and rMSSD measured by this method were statistically significantly lower in the all-cause mortality group. ROC curve analysis revealed that SDNN at admission exhibited a good predictive value for all-cause mortality in patients with AMI. Its specificity is high, albeit with a modest sensitivity. On the contrary, when analyzing the ROC curve, the rMSSD index did not demonstrate any predictive capacity for all-cause mortality in patients with acute myocardial infarction. Research results by James Nolan et al (1998) also found that the SDNN index was significantly related to all-cause mortality, rMSSD measurements were similar in survivors and deceased patients in univariate analysis.11
5. Conclusions
Time-domain HRV parameters (SDNN, rMSSD) HRV parameters calculated from smartphone apps using the PPG method are a useful predictor of cardiovascular events and all-cause mortality in AMI patients undergoing PCI. This method is low cost, easy to use, and can serve as a remote monitoring method to aid clinicians in evaluating the patient's condition after AMI.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2024.07.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kleiger R.E., Miller J.P., Bigger J.T., Jr., Moss A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 2.Johnston B.W., Barrett-Jolley R., Krige A., Welters I.D. Heart rate variability: measurement and emerging use in critical care medicine. J Intensive Care Soc. 2020;21:148–157. doi: 10.1177/1751143719853744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvaraj N., Jaryal A., Santhosh J., Deepak K.K., Anand S. Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. J Med Eng Technol. 2008;32:479–484. doi: 10.1080/03091900701781317. [DOI] [PubMed] [Google Scholar]
- 4.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 5.Almar shad M.A., Islam M.S., Al-Ahmadi S., BaHammam A.S. Diagnostic features and potential applications of PPG signal in healthcare: a systematic review. Healthcare (Basel) 2022;10 doi: 10.3390/healthcare10030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thygesen K., Alpert J.S., Jaffe A.S., et al. Task force for the universal definition of myocardial infarction. Circulation. 2018;138:618–651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 7.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 8.Anh Tuan N., Thi Thu Hoai N., Nguyen Son P. Predictive value of myocardial strain for major adverse cardiac events and mortality in patients with acute ST elevation myocardial infarction after primary percutaneous coronary intervention. Journal of 108 - Clinical Medicine and Phamarcy. 2022;17:39–48. [Google Scholar]
- 9.Cai A., Dillon C., Hillegass W.B., et al. Risk of major adverse cardiovascular events and major hemorrhage among white and black patients undergoing percutaneous coronary intervention. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pop-Busui R., Backlund J.C., Bebu I., et al. Utility of using electrocardiogram measures of heart rate variability as a measure of cardiovascular autonomic neuropathy in type 1 diabetes patients. J Diabetes Investig. 2022;13:125–133. doi: 10.1111/jdi.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan J., Batin P.D., Andrews R., et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.