Abstract
Background:
Triple-negative breast cancer (TNBC) is more aggressive as compared to other subtypes of breast cancer with characteristic metastatic patterns and a poor prognosis. The standard of care for early-stage TNBC is historically anthracycline and taxane-based chemotherapy (ATAX). Despite the effectiveness of this regimen, anthracyclines carry a small but important risk of cardiotoxicity, which is specifically a concern in the older population. This study evaluates major adverse cardiovascular events (MACE) in older women with TNBC treated with ATAX compared to taxane-based chemotherapy (TAX).
Methods:
Using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, we identified women aged 66 and older with TNBC diagnosed between 2010 and 2015 (N = 2215). We compared patient and clinical characteristics according to adjuvant chemotherapy regimen (chemotherapy versus no chemotherapy and ATAX versus TAX). Logistic regression was performed to estimate the odds ratios (OR) and 95% confidence intervals (CIs), Kaplan-Meier survival curves were generated to estimate three-year overall survival (OS) and cancer specific survival (CSS). Cox proportional hazards models were used to analyze OS and CSS while controlling for patient and tumor characteristics. MACE was defined as acute myocardial infarction, heart failure, potentially fatal arrhythmia, and cerebral vascular incidence. Few patients experienced a cardiac death and therefore this was excluded in the analysis.
Results:
Of the 2215 patients in our cohort, most patients (n = 1334; 60.26%) received TAX compared to ATAX (n = 881; 39.78%). Patients who received ATAX were not statistically significantly more likely than those who received TAX to experience acute myocardial infarction, cerebral vascular accident (CVA), or potentially fatal arrhythmia when controlling for traditional risk factors. Among patients who experienced MACE, there was no difference in OS or CSS in patients who received TAX vs ATAX. Patients who received ATAX were less likely to develop heart failure than those who received TAX (OR 0.63, 95% CI [0.45–0.88], p < 0.01). Patients who developed MACE and who were ≥ 76 years old had worse OS compared to those who experienced MACE and were age 66–75 years old (HR 1.67, 95% CI [1.07–2.62], p = 0.02).
Conclusion:
Among older women with TNBC, receipt of adjuvant chemotherapy with ATAX was not associated with increased risk of major adverse cardiac events. For those who experienced a cardiac event, there was no difference in survival amongst those who received TAX vs ATAX. Other factors including additional chemotherapy toxicities should be investigated as a potential etiology for the inferior OS previously observed with ATAX vs TAX in older women with node negative or 1–3 positive lymph nodes.
Keywords: Breast cancer, Older adults, Chemotherapy, Triple-negative breast cancer, Anthracyclines, Cardiotoxicity
1. Introduction
Triple-negative breast cancer (TNBC) is an aggressive breast cancer (BC) subtype characterized by a lack of estrogen and progesterone receptor expression and negative or low human epidermal growth factor 2 (HER2) expression [1]. TNBC is associated with a higher risk of metastatic recurrence, visceral and brain metastasis, and overall a poor prognosis compared to other BC subtypes [2]. TNBC accounts for 15–20% of BCs and chemotherapy is the mainstay of adjuvant treatment to prevent recurrence.
Age is one of the most important risk factors for BC, and although TNBC is associated with a younger median age of diagnosis compared with other subtypes, approximately 35% of patients diagnosed with TNBC are older than age 65 [3–5]. With an aging population, the number of older women with BC is projected to increase over the coming decades, affecting 21.5% of women age 65 and older by 2050. [6] Understanding the optimal treatment for older patients with early-stage TNBC is an area of unmet clinical need, especially in light of small numbers of patients older than age 65 years enrolled in clinical trials.
Chemotherapy is the mainstay in the neoadjuvant, adjuvant, and metastatic treatment of TNBC. The standard of care for early-stage TNBC is anthracycline and taxane-based chemotherapy (ATAX). As demonstrated in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis, the addition of a taxane to an anthracycline improved the 8-year BC mortality rate compared to the anthracyclines only control group [7]. When compared head-to-head, TC (docetaxel, cyclophosphamide) was superior to AC (doxorubicin, cyclophosphamide) with a benefit in disease-free and overall survival (OS) [8]. The anthracyclines in early breast cancer (ABC) trials and a recent EBCTCG meta-analysis demonstrated improved invasive disease-free survival for ATAX compared to TAX chemotherapy, with more benefit in patients with TNBC and those with node positive disease in the ABC trials [9,10]. In the phase III KEYNOTE-522 trial, a carboplatin and anthracycline-containing neoadjuvant regimen with concurrent pembrolizumab demonstrated an improvement in pathological complete response (pCR) and event free survival in patients with untreated stage II or III TNBC, establishing it as the current standard of care [11]. This is important considering that pembrolizumab also possesses the potential for immune-related cardiotoxicity.
In the context of metastatic TNBC, treatment decisions are influenced by treatment history, the expression of programmed cell death ligand 1 (PD-L1), and presence of germline BRCA mutation but chemotherapy continues to be a vital component of therapy. Sacituzumab govitecan, an antibody-drug conjugate, is effective in patients with metastatic TNBC who have undergone a minimum of two prior therapies, at least one of which was for metastatic disease [12]. Another antibody drug conjugate, trastuzumab deruxtecan, has been shown to significantly extend progression-free survival and OS when compared to physician’s choice of chemotherapy in patients with previously treated HER2-low metastatic breast cancer [13].
Despite their effectiveness, anthracyclines carry a small but important risk of cardiotoxicity through multiple mechanisms such as myocardial cellular disruption and free radical formation. Cardiotoxicity may present clinically with symptoms related to arrhythmia or reduced systolic function, or be detected in asymptomatic patients during surveillance cardiac testing. Cardiotoxicity is classified into Type I, characterized by direct, dose-related, and irreversible damage, and Type II, seen as reversible and dose dependent. However, these categories have some overlap, as patients treated solely with Type II-associated agents may have persistent LV dysfunction [14]. The risk factors for development of cardiotoxicity as outlined by the cardio-oncology guidelines from the American Society of Clinical Oncology (ASCO) in 2017 and the European Society of Cardiology (ESC) in 2022 include individuals treated with high-dose anthracycline or high-dose radiotherapy involving the heart in the treatment field. This also includes patients receiving treatment with anthracyclines or trastuzumab with multiple CV risk factors including older age, compromised cardiac function, a history of myocardial infarction, or a sequence of anthracycline followed by trastuzumab [15,16]. Baseline cardiovascular toxicity risk stratification can be calculated using the Heart Failure Association-International Cardio-Oncology Society baseline (HFA-ICOS) risk assessment tool [16]. Regular monitoring of cardiac function is advised, especially in patients who receive high-dose anthracyclines (eg, doxorubicin ≥ 250 mg/m2, epirubicin ≥ 600 mg/m2). Consideration should be given to cardioprotective measures, such as dexrazoxane, in high-risk patients or those receiving high cumulative doses. Treatment includes discontinuation of anthracycline chemotherapy and guideline-based heart failure therapy in patients who develop symptoms or asymptomatic patients with moderate/severe cardiac dysfunction.
If cardiotoxicity occurs, it can compromise the survival and quality of life of survivors [17–19]. A recent study among adult BC patients undergoing treatment with an anthracycline determined a high incidence rate of cardiotoxicity at 9.68% [20]. Cardiotoxicity is specifically a concern in the older population as age 65 and older is considered an independent risk factor along with having pre-existing cardiovascular (CV) conditions like hypertension and diabetes [21,22]. Therefore, older BC patients are at a higher risk of treatment-related cardiotoxicity [23, 24].
In a prior published study, our team completed retrospective analyses of adjuvant chemotherapy in older women (≥ 65 years old) with both node-negative and node-positive TNBC. We found that ATAX was associated with inferior 3-year overall survival (OS) and cancer-specific survival (CSS) compared to TAX in the node-negative and 1–3 positive lymph node groups and that there was a trend toward superior 3-year OS and CSS compared to TAX in the node-positive group with 4 or more lymph nodes involved [25,26]. We expand upon this work to evaluate the incidence of major adverse cardiovascular events (MACE) in both cohorts of older women with node-negative and node-positive TNBC. We hypothesized that cardiac event rates in the ATAX group would be higher thus providing a potential explanation for inferior survival in older women treated with ATAX.
2. Methods
Using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, we identified 178,228 patients diagnosed with primary BC from 2010 to 2015. We then identified women with TNBC who were treated with ATAX or TAX diagnosed at ages 66 and older meeting eligibility criteria for inclusion in this study (N = 2215). Individuals with missing data of interest were excluded from the analysis. Fig. 1 shows how the sample was derived. Data regarding year of diagnosis, age, race, marital/partnered status, reporting registry region, urban/rural residency, tumor stage, number of positive nodes, census poverty level, facilities visited in the first six months, tumor laterality, treatment, and presence of cardiovascular risk factors including hyperlipidemia, hypertension, diabetes, obesity, tobacco use, family history of heart disease, polysubstance/alcohol use, receipt of trastuzumab, personal history of myocardial infarction, personal history of heart failure, heart failure after diagnosis, cerebral vascular accident (CVA) after diagnosis, potentially fatal arrhythmias after diagnosis, and cardiac death were extracted. An important risk factor, radiotherapy where the heart is in the treatment field, was unavailable for analysis. To address this missing information, data on tumor laterality, left-sided tumors being a known risk factor for heart radiotherapy, was extracted. Potentially fatal arrhythmia was defined as ventricular fibrillation/flutter, ventricular tachycardia, and cardiac arrest. Cardiac death was defined as death from acute coronary syndrome, heart failure, cerebrovascular accident, or fatal arrhythmias including cardiac arrest, ventricular tachycardia, and ventricular fibrillation. We controlled for the covariates of traditional cardiovascular risk factors. A small number of patients had a personal history of acute myocardial infarction, personal history of heart failure, hypertension, polysubstance use, and cardiac death. Therefore, these factors were excluded from the analysis as they were not good predictors to include in the model. Cardiovascular risk factors were identified based on their corresponding ICD codes, while cardiac events were defined using the criteria outlined above and were also identified by their respective ICD codes (Appendix).
Fig. 1.
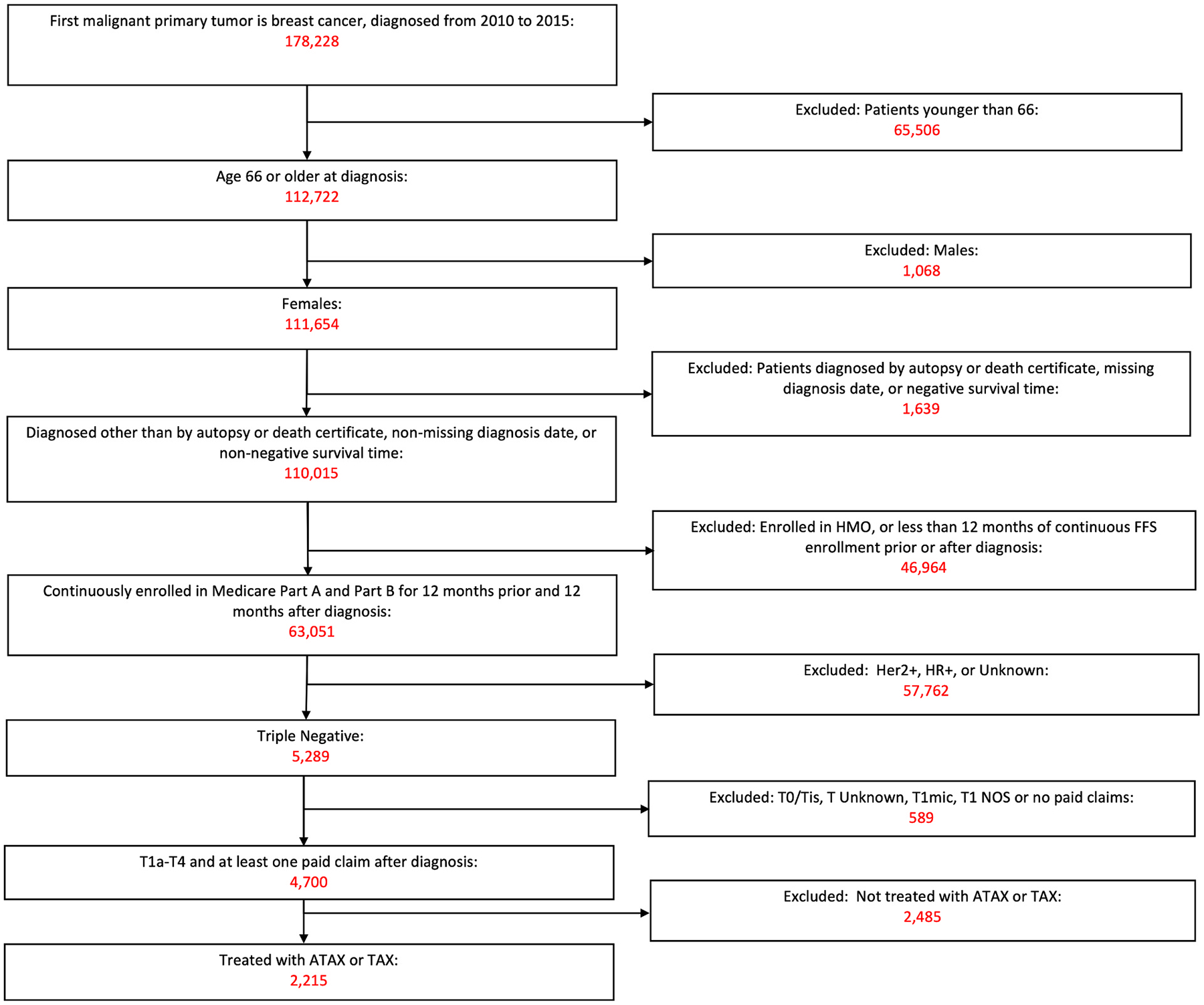
Sample selection. Newly diagnosed TNBC in women aged 66 or older between 2010 and 2015 in SEER-Medicare.
Patients with T1a and T1b disease and T3 and T4a-d disease were grouped together in T1a/b and T3/4 groups to maximize sample size. To control for lymph node status, patients were grouped into 1–3 positive lymph nodes (N1), ≥ 4 positive lymph nodes (N2), negative nodes (N0), or unknown lymph node status (NX).
Treatment categories are based on the initial treatment administered after BC diagnosis which was patients’ first primary tumor. There were no patients with recorded anthracycline use prior to their cancer diagnosis. The administration of neoadjuvant or adjuvant chemotherapy was determined using CPT and HCPCS procedure codes for commonly used chemotherapy drugs. Co-morbid conditions were defined by ICD9 and ICD10 codes (Appendix). Patients were identified as receiving ATAX if they received doxorubicin or epirubicin plus paclitaxel, docetaxel or nab-paclitaxel and as receiving TAX if they received docetaxel, paclitaxel or nab-paclitaxel without doxorubicin or epirubicin. Receipt of other drugs in combination with these agents was not included in this analysis.
We summarized demographic characteristics across the treatment groups. Chi-square analysis was used to determine statistical significance of differences in descriptive characteristics between treatment groups. OS was defined as death due to any cause from the month of cancer diagnosis to death. CSS was defined as death from cancer determined by the time from the month of diagnosis to death. Kaplan-Meier 3-year all-cause and CSS curves were generated for treatment groups and those patients with or without a cardiac outcome. OS and CSS between chemotherapy regimens was estimated using adjusted Cox proportional hazards models. Violations of the proportional hazard assumption were addressed using time-dependent variables. Logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CIs) for the association between covariates and cardiac outcomes of interest. All analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC) and were evaluated with a p value < 0.05.
3. Results
3.1. Patient characteristics
Of the 4700 patients identified with TNBC aged 66 and older, 2615 (55.64%) patients received adjuvant chemotherapy. Among those who received adjuvant chemotherapy, 2215 patients received either ATAX or TAX. Baseline patient characteristics are summarized in Table 1. More patients were aged 66–75 years old (77.8%). Most patients were non-Hispanic white (76.1%), followed by Black (13.5%), or other (10.4%). Patients were nearly even distributed by marital status with 46.5% non-married and 53.5% married or partnered. Many patients were diagnosed in the West region (41.7%) followed by the South (25.9%), Northeast (19.5%) and Midwest (13.0%). Most patients were diagnosed in an urban commuting area (88.3%), had T2 tumors (42.6%) and were node negative (61.8%). Of those with nodal involvement, there were more patients with N1 v. N2 disease (18.9% vs 11.0%). Most patients were free of comorbidities with a Charlston Comorbidity Index (CCI) score of 0 (60.6%), followed by a CCI score of 1 (22.3%) and CCI score of 2 or more (17.1%). Patients more commonly visited teaching hospitals (55.5%) than National Cancer Institute (NCI) centers (15.6%) or other facilities (28.9%). The most prevalent cardiovascular risk factors were hyperlipidemia (82.8%) and hypertension (84.5%).
Table 1.
Descriptive characteristics and statistics, women diagnosed with TNBC by chemotherapy administration, SEER-Medicare 2010–2015.
| Characteristic | Overall n (%) |
TAX only n (%) |
ATAX n (%) |
p-value | |||
|---|---|---|---|---|---|---|---|
| All Patients | 2215 | 1334 | 881 | . | |||
| Year of Diagnosis | |||||||
| 2010 | 354 | (16.0) | 217 | (16.3) | 137 | (15.6) | 0.948 |
| 2011 | 367 | (16.6) | 222 | (16.6) | 145 | (16.5) | . |
| 2012 | 344 | (15.5) | 209 | (15.7) | 135 | (15.3) | . |
| 2013 | 347 | (15.7) | 206 | (15.4) | 141 | (16.0) | . |
| 2014 | 388 | (17.5) | 238 | (17.8) | 150 | (17.0) | . |
| 2015 | 415 | (18.7) | 242 | (18.1) | 173 | (19.6) | . |
| Age Category | |||||||
| 66–75 | 1724 | (77.8) | 943 | (70.7) | 781 | (88.6) | < .001 |
| 76 and older | 491 | (22.2) | 391 | (29.3) | 100 | (11.4) | . |
| Race/Ethnicity Category | |||||||
| White NH | 1685 | (76.1) | 1016 | (76.2) | 669 | (75.9) | 0.665 |
| Black NH | 300 | (13.5) | 175 | (13.1) | 125 | (14.2) | . |
| Other | 230 | (10.4) | 143 | (10.7) | 87 | (9.9) | . |
| Marital Status Category | |||||||
| Non-Married | 1030 | (46.5) | 659 | (49.4) | 371 | (42.1) | < .001 |
| Married or Partnered | 1185 | (53.5) | 675 | (50.6) | 510 | (57.9) | . |
| Registry Region at Diagnosis | |||||||
| Northeast | 431 | (19.5) | 238 | (17.8) | 193 | (21.9) | < .001 |
| Midwest | 287 | (13.0) | 152 | (11.4) | 135 | (15.3) | . |
| South | 574 | (25.9) | 340 | (25.5) | 234 | (26.6) | . |
| West | 923 | (41.7) | 604 | (45.3) | 319 | (36.2) | . |
| Patient Urban/Rural Recode Category | |||||||
| Urban Commuting Area | 1955 | (88.3) | 1178 | (88.3) | 777 | (88.2) | 0.937 |
| Non-Urban Commuting Area | 260 | (11.7) | 156 | (11.7) | 104 | (11.8) | . |
| DAJCC 7th Ed T Category | |||||||
| T1a/T1b | 247 | (11.2) | 189 | (14.2) | 58 | (6.6) | < .001 |
| T1c | 723 | (32.6) | 497 | (37.3) | 226 | (25.7) | . |
| T2 | 943 | (42.6) | 513 | (38.5) | 430 | (48.8) | . |
| T3/T4 | 302 | (13.6) | 135 | (10.1) | 167 | (19.0) | . |
| Number of positive nodes | |||||||
| No nodes positive | 1369 | (61.8) | 959 | (71.9) | 410 | (46.5) | < .001 |
| 1–3 nodes positive | 419 | (18.9) | 201 | (15.1) | 218 | (24.7) | . |
| 4 + nodes positive | 243 | (11.0) | 97 | (7.3) | 146 | (16.6) | . |
| Unknown | 184 | (11.1) | 77 | (5.8) | 107 | (12.1) | . |
| Charlson Comorbidity Index Category | |||||||
| 0 | 1343 | (60.6) | 756 | (56.7) | 587 | (66.6) | < .001 |
| 1 | 493 | (22.3) | 310 | (23.2) | 183 | (20.8) | . |
| 2 or more | 379 | (17.1) | 268 | (20.1) | 111 | (12.6) | . |
| Census Poverty Level | |||||||
| 0% to <20% poverty | 1758 | (79.4) | 1063 | (79.7) | 695 | (78.9) | 0.650 |
| 20% or higher poverty | 457 | (20.6) | 271 | (20.3) | 186 | (21.1) | . |
| Facilities Visited in First 6 months | |||||||
| NCI Center | 345 | (15.6) | 200 | (15.0) | 145 | (16.5) | 0.546 |
| Teaching Hospital | 1230 | (55.5) | 740 | (55.5) | 490 | (55.6) | . |
| Other | 640 | (28.9) | 394 | (29.5) | 246 | (27.9) | . |
| Tumor Laterality | |||||||
| Right | 1098 | (49.6) | 671 | (50.3) | 427 | (48.4) | 0.399 |
| Left | 1117 | (50.4) | 663 | (49.7) | 454 | (51.5) | |
| HLD | |||||||
| No | 382 | (17.2) | 218 | (16.3) | 164 | (18.6) | 0.166 |
| Yes | 1833 | (82.8) | 1116 | (83.7) | 717 | (81.4) | . |
| HTN | |||||||
| No | 344 | (15.5) | 186 | (13.9) | 158 | (17.9) | 0.011 |
| Yes | 1871 | (84.5) | 1148 | (86.1) | 723 | (82.1) | . |
| Diabetes | |||||||
| No | 1357 | (61.3) | 786 | (58.9) | 571 | (64.8) | 0.005 |
| Yes | 858 | (38.7) | 548 | (41.1) | 310 | (35.2) | . |
| Obesity | |||||||
| No | 1637 | (73.9) | 981 | (73.5) | 656 | (74.5) | 0.628 |
| Yes | 578 | (26.1) | 353 | (26.5) | 225 | (25.5) | . |
| Tobacco use | |||||||
| No | 1954 | (88.2) | 1179 | (88.4) | 775 | (88.0) | 0.768 |
| Yes | 261 | (11.8) | 155 | (11.6) | 106 | (12.0) | . |
| Family History of Heart Disease | |||||||
| No | 1956 | (88.3) | 1185 | (88.8) | 771 | (87.5) | 0.345 |
| Yes | 259 | (11.7) | 149 | (11.2) | 110 | (12.5) | . |
| Polysubstance use | |||||||
| No | 2171 | (98.0) | 1305 | (97.8) | 866 | (98.3) | 0.437 |
| Yes | 44 | (2.0) | 29 | (2.2) | 15 | (1.7) | . |
| Alcohol use | |||||||
| No | 1954 | (88.2) | 1177 | (88.2) | 777 | (88.2) | 0.980 |
| Yes | 261 | (11.8) | 157 | (11.8) | 104 | (11.8) | . |
| Receipt of Trastuzumab | |||||||
| No | 2151 | (97.1) | 1287 | (96.5) | 864 | (98.1) | 0.028 |
| Yes | 64 | (2.9) | 47 | (3.5) | 17 | (1.9) | . |
| Personal history of HF | |||||||
| No | 2121 | (95.8) | 1262 | (94.6) | 859 | (97.5) | < .001 |
| Yes | 94 | (4.2) | 72 | (5.4) | 22 | (2.5) | . |
| Acute MI after dx | |||||||
| No | 2130 | (96.2) | 1284 | (96.3) | 846 | (96.0) | 0.788 |
| Yes | 85 | (3.8) | 50 | (3.7) | 35 | (4.0) | . |
| HF admit after dx | |||||||
| No | 1987 | (89.7) | 1177 | (88.2) | 810 | (91.9) | 0.005 |
| Yes | 228 | (10.3) | 157 | (11.8) | 71 | (8.1) | . |
| CVA after dx | |||||||
| No | 2138 | (96.5) | 1285 | (96.3) | 853 | (96.8) | 0.534 |
| Yes | 77 | (3.5) | 49 | (3.7) | 28 | (3.2) | . |
| Dysrhythmia after dx | |||||||
| No | 2181 | (98.5) | 1311 | (98.3) | 870 | (98.8) | 0.373 |
| Yes | 34 | (1.5) | 23 | (1.7) | 11 | (1.2) | . |
3.2. Patterns of chemotherapy administration
Younger patients aged 66–75 received ATAX at a significantly higher rate (781/1724, 45.3%) compared to patients ages 76 and older (100/491, 20.37%), p < 0.01). Married or partnered patients received ATAX at a higher frequency (510/1185, 43.04%) compared to non-married or partnered patients (371/1030, 36.02%, p < 0.01). Patients free of comorbidities with a CCI of 0 received ATAX at the highest frequency (587/1343, 43.71%), followed by a CCI score 1(183/493, 37.12%), and a CCI score of 2 or more (111/379, 29.30%, p < 0.01). Patients with hypertension had a significantly lower frequency of receiving ATAX (723/1871, 38.64%) than those patients without hypertension (158/344, 45.93%, p = 0.01). The same was true for patients with diabetes (310/858, 36.13%) than those without diabetes (571/1357, 42.08%, p < 0.01). Among patients who received trastuzumab, fewer received ATAX (17/64, 26.57%) compared to those who did not receive trastuzumab (864/2151, 40.17%, p = 0.03). Patients who had a personal history of heart failure (22/94, 23.40%) received ATAX at a lower frequency compared to patients who did not have a personal history of heart failure (859/2121, 40.50%, p < 0.01). Finally, those with patients who subsequently developed heart failure after diagnosis received ATAX at a lower frequency (71/228, 24.65%) than those patients who did not develop heart failure (810/1987, 40.76%, p < 0.01).
3.3. Factors associated with Major Adverse Cardiovascular Event (MACE) outcomes
3.3.1. Acute myocardial infarction
Of those patients who received adjuvant chemotherapy with either ATAX or TAX (N = 2215), 85 (3.84%) developed an acute myocardial infarction after diagnosis (Table 2). Patients with diabetes were more likely to experience an acute myocardial infarction than those without diabetes (OR 2.10, 95% CI [1.29–3.43], p < 0.01). This was also true for those patients with an unknown number of nodes compared to patients with 1–3 positive lymph nodes (OR 2.71, 95% CI [1.15–6.39], p = 0.02). Those patients who received ATAX were not more likely to have an acute myocardial infarction than those who received TAX (OR 1.03, 95% CI [0.62–1.70], p = 0.91).
Table 2.
Logistic regression analysis estimating OR across variables to predict myocardial infarction, SEER-Medicare 2010–2015.
| Predictors of acute MI | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p-value |
| Year of Diagnosis | |||
| 2010 (ref) | . | . | . |
| 2011 | 1.50 | (0.76–2.98) | 0.244 |
| 2012 | 0.82 | (0.37–1.81) | 0.627 |
| 2013 | 1.02 | (0.48–2.14) | 0.965 |
| 2014 | 0.58 | (0.25–1.31) | 0.188 |
| 2015 | 0.65 | (0.28–1.48) | 0.300 |
| Age at Diagnosis | |||
| 66–75 (ref) | . | . | . |
| 76 and older | 1.36 | (0.79–2.34) | 0.263 |
| Race/Ethnicity | |||
| White NH (ref) | . | . | . |
| Black NH | 1.32 | (0.71–2.45) | 0.387 |
| Other | 0.80 | (0.34–1.89) | 0.617 |
| Marital Status | |||
| Married or Partnered (ref) | . | . | . |
| Non-Married | 1.28 | (0.80–2.04) | 0.309 |
| Region | |||
| West (ref) | . | . | . |
| Midwest | 1.16 | (0.57–2.37) | 0.689 |
| Northeast | 1.17 | (0.63–2.16) | 0.620 |
| South vs West | 0.62 | (0.32–1.21) | 0.160 |
| Urban/Rural Category | |||
| Urban Area (ref) | . | . | . |
| Rural Area | 0.68 | (0.30–1.56) | 0.362 |
| DAJCC7 T Category | |||
| T1a/T1b (ref) | . | . | . |
| T1c | 0.60 | (0.26–1.38) | 0.228 |
| T2 | 0.95 | (0.44–2.08) | 0.901 |
| T3/T4 | 1.43 | (0.59–3.48) | 0.428 |
| Number of positive nodes | |||
| 1–3 nodes positive (ref) | . | . | . |
| 4 + nodes positive | 1.87 | (0.80–4.35) | 0.150 |
| No nodes positive | 1.53 | (0.77–3.06) | 0.229 |
| Unknown | 2.71 | (1.15–6.39) | 0.023 |
| Census-Level Poverty | |||
| 0–20% poverty (ref) | . | . | . |
| 20% or higher poverty | 1.22 | (0.69–2.16) | 0.487 |
| Facility Type | |||
| Other (ref) | . | . | . |
| NCI Center | 0.88 | (0.42–1.84) | 0.741 |
| Teaching Hospital | 0.90 | (0.52–1.54) | 0.692 |
| Tumor Laterality | |||
| Right (ref) | |||
| Left | 0.91 | (0.58–1.42) | 0.685 |
| Chemo | |||
| Taxane only (ref) | . | . | . |
| Taxane + Anthracycline | 1.03 | (0.62–1.70) | 0.909 |
| HLD | |||
| No (ref) | . | . | . |
| Yes | 1.80 | (0.82–3.95) | 0.143 |
| Diabetes | |||
| No (ref) | . | . | . |
| Yes | 2.10 | (1.29–3.43) | 0.003 |
| Obesity | |||
| No (ref) | . | . | . |
| Yes | 1.34 | (0.83–2.19) | 0.236 |
| Tobacco Use | |||
| No (ref) | . | . | . |
| Yes | 1.56 | (0.45–5.37) | 0.480 |
| Family history of heart disease | |||
| No (ref) | . | . | . |
| Yes | 1.22 | (0.65–2.28) | 0.537 |
| Alcohol Use | |||
| No (ref) | . | . | . |
| Yes | 1.60 | (0.46–5.55) | 0.456 |
| Receipt of Trastuzumab | |||
| No (ref) | . | . | . |
| Yes | 0.67 | (0.15–2.93) | 0.597 |
3.3.2. Heart failure
Of those patients who received adjuvant chemotherapy with either ATAX or TAX, there were 228 patients (10.29%) who developed heart failure after diagnosis (Table 3). Patients ages 76 and older were more likely to develop heart failure than those patients aged 66–75 (OR 1.60, 95% CI [1.14–2.24], p < 0.01). Non-married patients were more likely to develop heart failure than married or partnered patients (OR 1.39, 95% CI [1.03–1.88], p = 0.03). Patients with an unknown number of positive nodes were more likely to develop heart failure than those with 1–3 positive lymph nodes (OR 1.80, 95% CI [1.04–3.14], p = 0.04). Patients in a non-urban or rural commuting area were more likely to develop heart failure compared to those patients in an urban commuting area (OR 1.73, 95% CI [1.15–2.61], p < 0.01). Patients who resided in an area with 20% or higher poverty level were more likely to develop heart failure than those who resided in an area with < 20% poverty (OR 1.59, 95% CI [1.13–2.26], p < 0.01). Patients with cardiovascular risk factors such as hyperlipidemia (OR 1.71, 95% CI [1.05–2.79], p = 0.03), diabetes (OR 2.39, 95% CI [1.75–3.26], p < 0.01), and obesity (OR 1.59, 95% CI [1.17–2.18], p < 0.01) were more likely to develop heart failure. Interestingly, those who received ATAX had a lower risk at subsequently developing of heart failure than those who received TAX (OR 0.63, 95% CI [0.45–0.88], p < 0.01).
Table 3.
Logistic regression analysis estimating OR across variables to predict heart failure, SEER-Medicare 2010–2015.
| Predictors of heart failure | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p-value |
| Year of Diagnosis | |||
| 2010 (ref) | . | . | . |
| 2011 | 1.14 | (0.68–1.90) | 0.630 |
| 2012 | 1.33 | (0.80–2.21) | 0.272 |
| 2013 | 1.34 | (0.80–2.23) | 0.264 |
| 2014 | 0.94 | (0.56–1.59) | 0.824 |
| 2015 | 1.27 | (0.77–2.10) | 0.355 |
| Age at Diagnosis | |||
| 66–75 (ref) | . | . | . |
| 76 and older | 1.60 | (1.14–2.24) | 0.007 |
| Race/Ethnicity | |||
| White NH (ref) | . | . | . |
| Black NH | 0.73 | (0.47–1.14) | 0.162 |
| Other | 1.15 | (0.70–1.88) | 0.581 |
| Marital Status | |||
| Married or Partnered (ref) | . | . | . |
| Non-Married | 1.39 | (1.03–1.88) | 0.032 |
| Region | |||
| West (ref) | . | . | . |
| Midwest | 1.26 | (0.79–2.01) | 0.339 |
| Northeast | 1.12 | (0.73–1.71) | 0.611 |
| South vs West | 0.90 | (0.60–1.36) | 0.615 |
| Urban/Rural Category | |||
| Urban Area (ref) | . | . | . |
| Rural Area | 1.73 | (1.15–2.61) | 0.008 |
| DAJCC7 T Category | |||
| T1a/T1b (ref) | . | . | . |
| T1c | 1.22 | (0.66–2.23) | 0.530 |
| T2 | 1.69 | (0.94–3.05) | 0.082 |
| T3/T4 | 2.14 | (1.10–4.14) | 0.025 |
| Number of positive nodes | |||
| 1–3 nodes positive (ref) | . | . | . |
| 4 + nodes positive | 1.37 | (0.82–2.30) | 0.226 |
| No nodes positive | 0.97 | (0.65–1.45) | 0.883 |
| Unknown | 1.80 | (1.04–3.14) | 0.037 |
| Census-Level Poverty | |||
| 0–20% poverty (ref) | . | . | . |
| 20% or higher poverty | 1.59 | (1.13–2.26) | 0.009 |
| Facility Type | |||
| Other (ref) | . | . | . |
| NCI Center | 0.95 | (0.58–1.57) | 0.841 |
| Teaching Hospital | 1.29 | (0.91–1.84) | 0.153 |
| Tumor Laterality | |||
| Right (ref) | |||
| Left | 1.02 | (0.76–1.36) | 0.922 |
| Chemo | |||
| Taxane only (ref) | . | . | . |
| Taxane + Anthracycline | 0.63 | (0.45–0.88) | 0.006 |
| HLD | |||
| No (ref) | . | . | . |
| Yes | 1.71 | (1.05–2.79) | 0.032 |
| Diabetes | |||
| No (ref) | . | . | . |
| Yes | 2.39 | (1.75–3.26) | < 0.001 |
| Obesity | |||
| No (ref) | . | . | . |
| Yes | 1.59 | (1.17–2.18) | 0.004 |
| Tobacco Use | |||
| No (ref) | . | . | . |
| Yes | 1.54 | (0.69–3.43) | 0.288 |
| Family history of heart disease | |||
| No (ref) | . | . | . |
| Yes | 1.36 | (0.91–2.05) | 0.134 |
| Alcohol Use | |||
| No (ref) | . | . | . |
| Yes | 1.17 | (0.52–2.65) | 0.702 |
| Receipt of Trastuzumab | |||
| No (ref) | . | . | . |
| Yes | 0.70 | (0.28–1.71) | 0.431 |
3.3.3. Potentially fatal arrhythmia
Of those patients who received adjuvant chemotherapy with either ATAX or TAX, 34 (1.53%) developed potentially fatal arrhythmia (e.g. VT/VF, cardiac arrest) after diagnosis (Table 4). Patients with a family history of heart disease were more likely to develop potentially fatal arrhythmia (OR 2.47, 95% CI [1.08–5.67], p = 0.03). Those patients who received ATAX were not more likely to develop potentially fatal arrhythmia than those who received TAX (OR 0.52, 95% CI [0.23–1.17], p = 0.12).
Table 4.
Logistic regression analysis estimating OR across variables to predict potentially fatal arrhythmia, SEER-Medicare 2010–2015.
| Predictors of potentially fatal arrhythmia | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p-value |
| Year of Diagnosis | |||
| 2010 (ref) | . | . | . |
| 2011 | 0.72 | (0.23–2.23) | 0.570 |
| 2012 | 0.96 | (0.32–2.88) | 0.943 |
| 2013 | 0.69 | (0.21–2.25) | 0.540 |
| 2014 | 0.47 | (0.13–1.66) | 0.241 |
| 2015 | 0.59 | (0.18–1.93) | 0.380 |
| Age at Diagnosis | |||
| 66–75 (ref) | . | . | . |
| 76 and older | 0.83 | (0.34–2.00) | 0.671 |
| Race/Ethnicity | |||
| White NH (ref) | . | . | . |
| Black NH | 0.75 | (0.26–2.16) | 0.591 |
| Other | 0.62 | (0.14–2.84) | 0.542 |
| Marital Status | |||
| Married or Partnered (ref) | . | . | . |
| Non-Married | 1.05 | (0.51–2.16) | 0.903 |
| Region | |||
| West (ref) | . | . | . |
| Midwest | 0.83 | (0.24–2.87) | 0.769 |
| Northeast | 1.80 | (0.69–4.66) | 0.228 |
| South vs West | 0.95 | (0.35–2.54) | 0.915 |
| Urban/Rural Category | |||
| Urban Area (ref) | . | . | . |
| Rural Area | 1.02 | (0.33–3.15) | 0.973 |
| DAJCC7 T Category | |||
| T1a/T1b (ref) | . | . | . |
| T1c | 0.86 | (0.22–3.45) | 0.833 |
| T2 | 1.54 | (0.41–5.75) | 0.519 |
| T3/T4 | 3.19 | (0.76–13.38) | 0.114 |
| Number of positive nodes | |||
| 1–3 nodes positive (ref) | . | . | . |
| 4 + nodes positive | 2.36 | (0.64–8.77) | 0.198 |
| No nodes positive | 1.65 | (0.54–5.05) | 0.383 |
| Unknown | 2.83 | (0.70–11.44) | 0.144 |
| Census-Level Poverty | |||
| 0–20% poverty (ref) | . | . | . |
| 20% or higher poverty | 1.98 | (0.85–4.62) | 0.113 |
| Facility Type | |||
| Other (ref) | . | . | . |
| NCI Center | 1.42 | (0.42–4.78) | 0.569 |
| Teaching Hospital | 1.52 | (0.61–3.80) | 0.371 |
| Tumor Laterality | |||
| Right (ref) | |||
| Left | 1.52 | (0.75–3.09) | 0.248 |
| Chemo | |||
| Taxane only (ref) | . | . | . |
| Taxane + Anthracycline | 0.52 | (0.23–1.17) | 0.116 |
| HLD | |||
| No (ref) | . | . | . |
| Yes | 1.24 | (0.45–3.45) | 0.679 |
| Diabetes | |||
| No (ref) | . | . | . |
| Yes | 0.70 | (0.32–1.50) | 0.355 |
| Obesity | |||
| No (ref) | . | . | . |
| Yes | 1.82 | (0.86–3.86) | 0.117 |
| Tobacco Use | |||
| No (ref) | . | . | . |
| Yes | 2.40 | (0.39–14.67) | 0.345 |
| Family history of heart disease | |||
| No (ref) | . | . | . |
| Yes | 2.47 | (1.08–5.67) | 0.032 |
| Alcohol Use | |||
| No (ref) | . | . | . |
| Yes | 0.42 | (0.06–3.03) | 0.392 |
| Receipt of Trastuzumab | |||
| No (ref) | . | . | . |
| Yes | 0.77 | (0.10–6.03) | 0.392 |
3.3.4. Cerebral vascular accident
Of those patients who received adjuvant chemotherapy with either ATAX or TAX, there were 77 patients (3.48%) who developed CVA after diagnosis (Table 5). Patients with no positive nodes were more likely to develop CVA than those patients with 1–3 positive nodes (OR 0.50, 95% CI [0.27–0.92], p = 0.03). Patients with left-sided tumors, or those at higher risk of receiving heart RT, were found to be more susceptible to developing CVA than those patients with right-sided tumors (OR 1.69, 95% CI [1.04–2.74], p = 0.03). Patients with diabetes were also statistically more likely to develop a CVA (OR 1.71, 95% CI [1.03–2.83], p = 0.04). Patients who received adjuvant chemotherapy with ATAX were not more statistically significantly more likely to develop a CVA than those who received TAX (OR 0.71, 95% CI [0.42–1.20], p = 0.20).
Table 5.
Logistic regression analysis estimating OR across variables to predict CVA, SEER-Medicare 2010–2015.
| Predictors of CVA | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p-value |
| Year of Diagnosis | |||
| 2010 (ref) | . | . | . |
| 2011 | 1.07 | (0.47–2.44) | 0.866 |
| 2012 | 1.01 | (0.43–2.37) | 0.986 |
| 2013 | 1.58 | (0.72–3.46) | 0.257 |
| 2014 | 1.00 | (0.43–2.30) | 0.996 |
| 2015 | 1.19 | (0.52–2.71) | 0.680 |
| Age at Diagnosis | |||
| 66–75 (ref) | . | . | . |
| 76 and older | 1.14 | (0.65–1.99) | 0.641 |
| Race/Ethnicity | |||
| White NH (ref) | . | . | . |
| Black NH | 1.36 | (0.70–2.62) | 0.365 |
| Other | < 0.001 | (<0.001–>999.999) | 0.968 |
| Marital Status | |||
| Married or Partnered (ref) | . | . | . |
| Non-Married | 1.07 | (0.66–1.75) | 0.783 |
| Region | |||
| West (ref) | . | . | . |
| Midwest | 1.23 | (0.56–2.69) | 0.614 |
| Northeast | 1.76 | (0.93–3.32) | 0.083 |
| South vs West | 0.91 | (0.48–1.75) | 0.779 |
| Urban/Rural Category | |||
| Urban Area (ref) | . | . | . |
| Rural Area | 1.17 | (0.56-–.43) | 0.675 |
| DAJCC7 T Category | |||
| T1a/T1b (ref) | . | . | . |
| T1c | 0.73 | (0.29–1.81) | 0.498 |
| T2 | 1.16 | (0.50–2.73) | 0.726 |
| T3/T4 | 1.08 | (0.40–2.93) | 0.881 |
| Number of positive nodes | |||
| 1–3 nodes positive (ref) | . | . | . |
| 4 + nodes positive | 1.52 | (0.75–3.09) | 0.246 |
| No nodes positive | 0.50 | (0.27–0.92) | 0.026 |
| Unknown | 0.74 | (0.29–1.87) | 0.521 |
| Census-Level Poverty | |||
| 0–20% poverty (ref) | . | . | . |
| 20% or higher poverty | 0.82 | (0.43–1.57) | 0.551 |
| Facility Type | |||
| Other (ref) | . | . | . |
| NCI Center | 0.64 | (0.28–1.44) | 0.277 |
| Teaching Hospital | 0.79 | (0.46–1.38) | 0.413 |
| Tumor Laterality | |||
| Right (ref) | |||
| Left | 1.69 | (1.04–2.74) | 0.033 |
| Chemo | |||
| Taxane only (ref) | . | . | . |
| Taxane + Anthracycline | 0.71 | (0.42–1.20) | 0.201 |
| HLD | |||
| No (ref) | . | . | . |
| Yes | 1.63 | (0.75–3.55) | 0.219 |
| Diabetes | |||
| No (ref) | . | . | . |
| Yes | 1.71 | (1.03–2.83) | 0.038 |
| Obesity | |||
| No (ref) | . | . | . |
| Yes | 0.98 | (0.57–1.66) | 0.925 |
| Tobacco Use | |||
| No (ref) | . | . | . |
| Yes | 0.87 | (0.25–3.08) | 0.833 |
| Family history of heart disease | |||
| No (ref) | . | . | . |
| Yes | 1.30 | (0.69–2.48) | 0.417 |
| Alcohol Use | |||
| No (ref) | . | . | . |
| Yes | 2.27 | (0.66–7.85) | 0.194 |
| Receipt of Trastuzumab | |||
| No (ref) | . | . | . |
| Yes | 1.64 | (0.48–5.60) | 0.430 |
3.3.5. Analysis of chemotherapy type and survival
Overall, patients who received ATAX had worse CSS and OS than those who received TAX (see Fig. 2A and B). Those who experienced a cardiac outcome had worse CSS and OS than those who did not (see Fig. 2B and C). Patients who received TAX and had no cardiac event had the best survival, followed by those who received ATAX and had no cardiac event, those who received TAX and developed a cardiac event, and finally those who received ATAX and developed a cardiac event (see Fig. 2E and F).
Fig. 2.
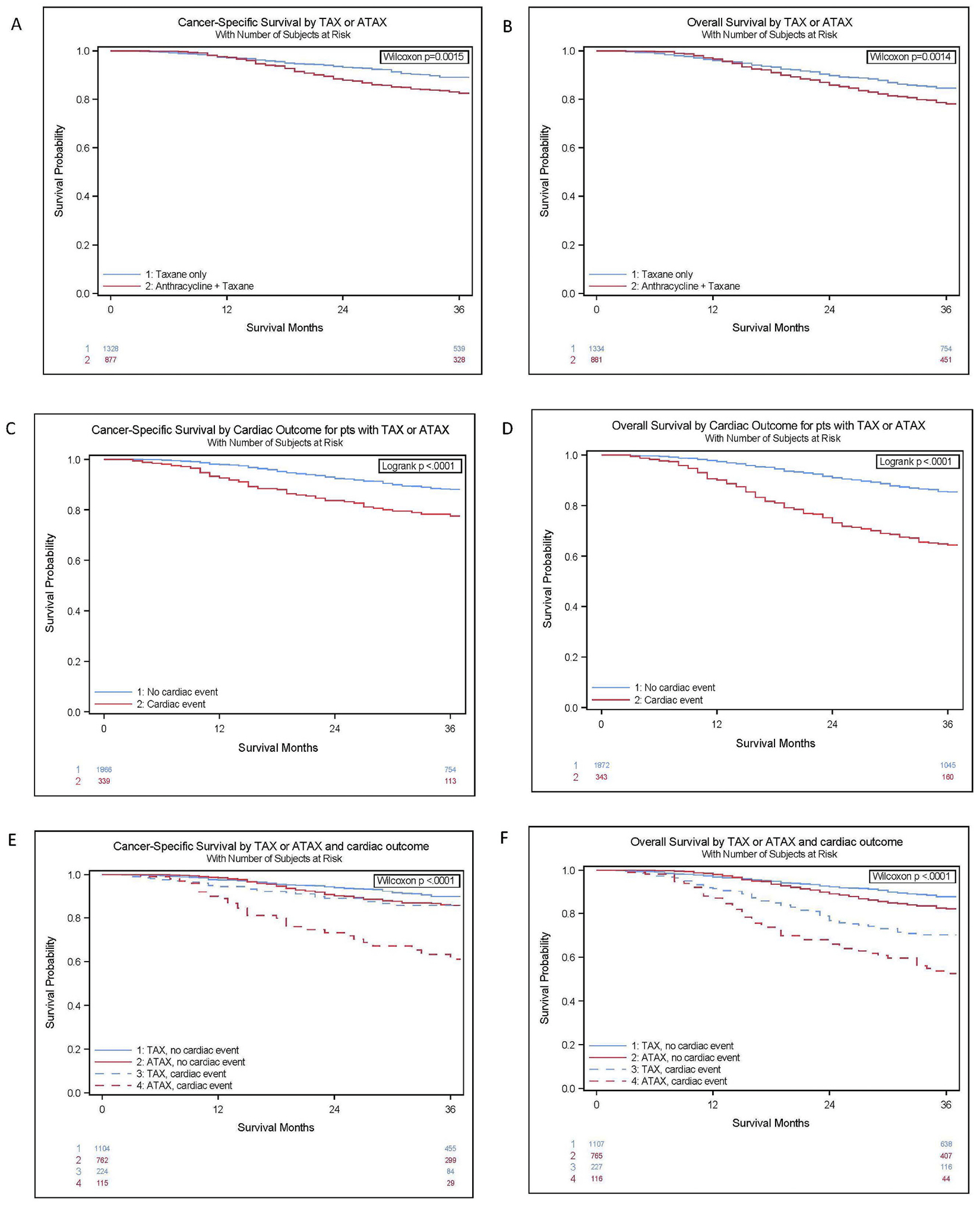
Kaplan Meier Survival Curves.
3.3.6. Analysis of CSS and OS controlling for covariates
We evaluated factors that might influence the occurrence of MACE (Figs. 3 and 4). Patients who were 76 and older had worse OS compared to those ages 66–75 (HR 1.67, 95% CI [1.07–2.62], p = 0.02), and a trend toward worse CSS (HR 1.98, 95% CI [0.99–3.96], p = 0.05), Patients diagnosed in the Midwest had a worse CSS than those in the West (HR 3.29, 95% CI [1.09–9.95], p = 0.03), although there was no difference in OS by geography. Patients who experienced a cardiac event in a non-urban or rural commuting area had worse CSS and OS than those who experienced a cardiac outcome in an urban commuting area (HR 2.99, 95% CI [1.32 – 6.78] p < 0.01 and HR 1.84, 95% CI [1.10–3.05], p = 0.02). Patients with no positive nodes had improved CSS and OS compared to those patients with 1–3 positive nodes (HR 0.29, 95% CI [0.12–0.75], p = 0.01 and HR 0.50, 95% CI [0.29 – 0.89] p = 0.02). Among patients with a cardiac outcome, patients who visited an NCI center had improved CSS and a trend toward improved OS compared to those who visited other facilities (HR 0.20, 95% CI [0.04 – 0.91] p = 0.04 and HR 0.40, 95% CI [0.16–0.99, p = 0.05).
Fig. 3.
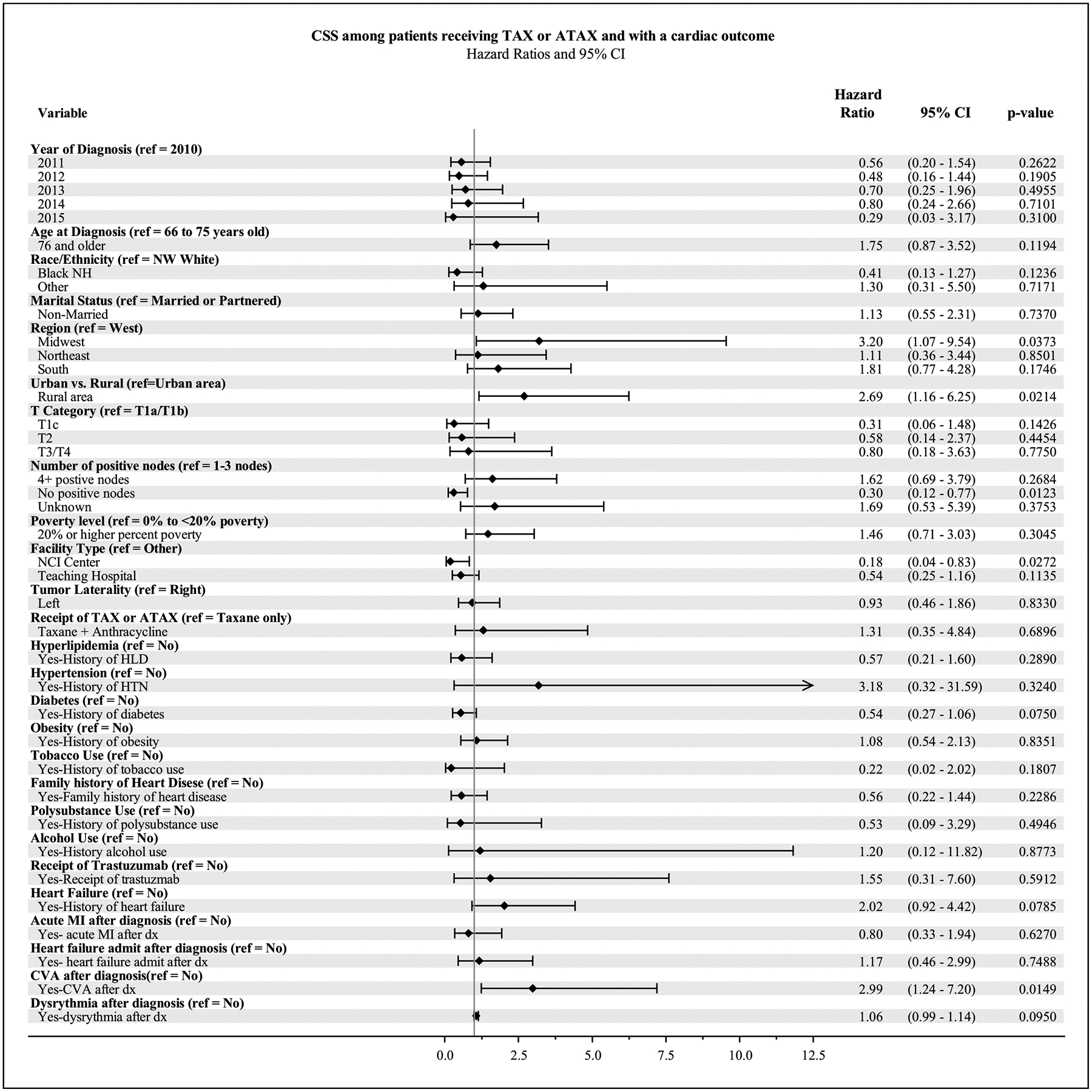
Forest Plot for multivariate analysis of Cancer-Specific Survival (CSS) among patients receiving TAX or ATAX who experienced a cardiac outcome.
Fig. 4.
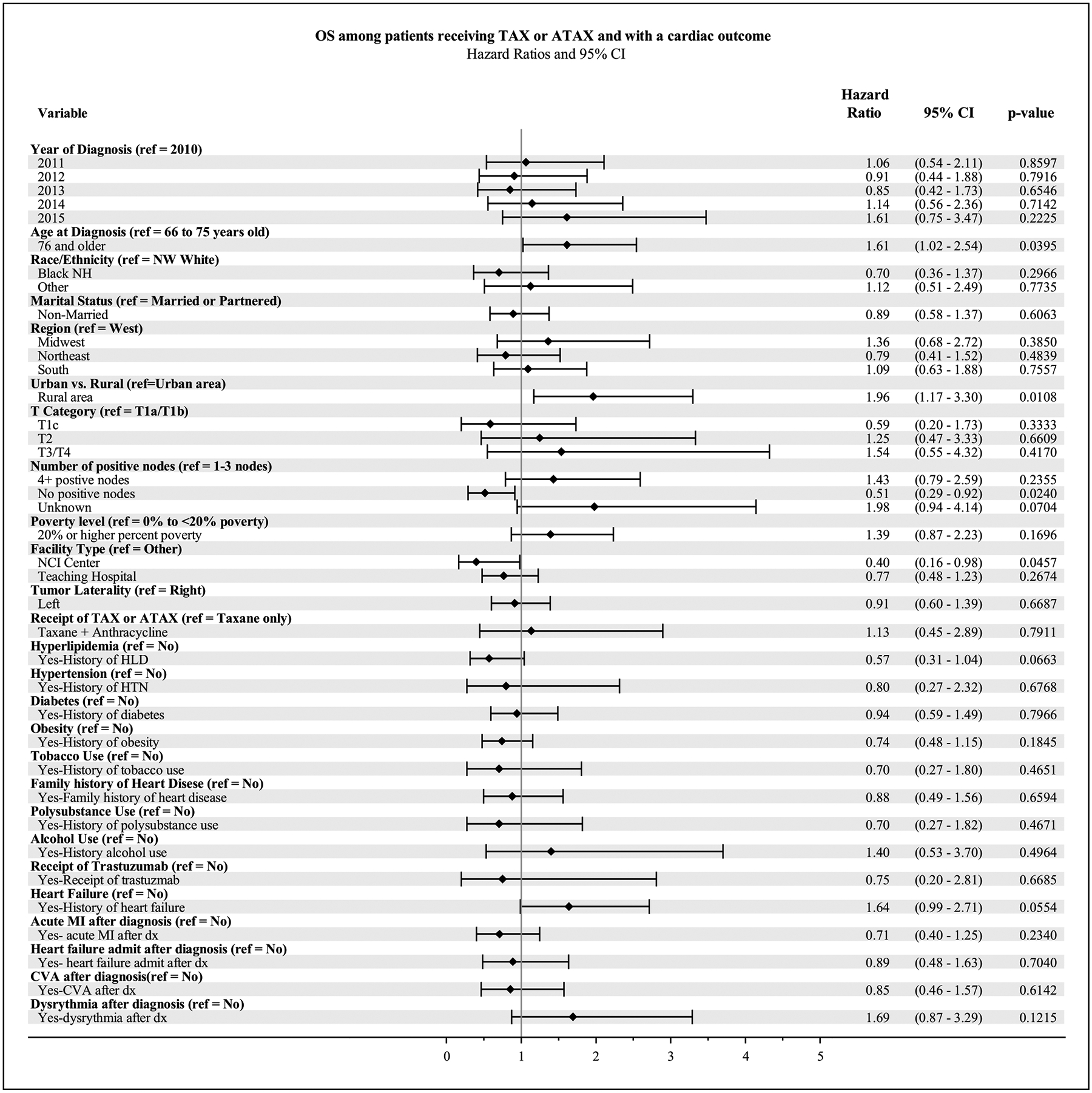
Forest Plot for multivariate analysis of Overall Survival (OS) among patients receiving TAX or ATAX who experienced a cardiac outcome.
Patients with a potentially fatal arrhythmia after diagnosis had worse CSS (HR 2.88, 95% CI [1.20–6.92], p = 0.02), although no difference in OS. Patients with diabetes had a trend toward improved CSS (HR 0.53, 95% CI [0.27–1.06], p = 0.07), although no difference in OS.
Among patients who experienced a cardiac outcome, there was no statistically significant difference in OS or CSS in patients who received TAX vs ATAX.
4. Discussion
Using the SEER-Medicare database, we conducted a retrospective evaluation of major adverse cardiovascular events and survival for older women diagnosed with TNBC treated with ATAX or TAX chemotherapy. Our study builds upon a previous investigation by Doyle et al. that examined the cardiac effects of chemotherapy using the SEER-Medicare database in BC patients diagnosed between 1992 and 1999 and found that chemotherapy with anthracyclines was associated with a substantially increased risk of cardiomyopathy [23]. The novelty of our current study lies in examining this specific population of older patients with TNBC diagnosed between 2010 and 2015 and comparing the adjuvant chemotherapy of ATAX vs TAX.
Several landmark studies including Lefrak et al. (1973), Von Hoff et al. (1979), Swain et al. (2003) have demonstrated the cardiotoxic effects of anthracyclines, revealing a dose-dependent increase in cardiac dysfunction, heart failure, and other cardiac abnormalities in patients treated with these drugs [18,27,28]. In contrast, we found that patients who received ATAX were not more susceptible to major adverse cardiovascular events such as acute myocardial infarction, heart failure, CVA, or potentially fatal arrhythmia when controlling for traditional cardiovascular risk factors. These findings underscore the significance of pre-existing cardiovascular risk factors as the dominant driver of MACE following adjuvant chemotherapy. However, few patients experienced cardiac outcomes in our study, which could affect the precision of our estimates. Among patients who underwent adjuvant chemotherapy with either ATAX or TAX, few experienced an acute myocardial infarction (3.84%), developed heart failure (10.29%), experienced potentially fatal arrhythmia (1.53%), or had a CVA (3.48%). Improved identification of patients at increased cardiac risk, preventative strategies with cardiologists and cardio-oncologists, and enhanced surveillance and monitoring during treatment may all have contributed to these low cardiac event rates.
Treatment with trastuzumab, a monoclonal antibody targeting HER2, is associated with an increased risk of anthracycline-induced cardiotoxicity. Numerous studies assessed the cardiac safety of combining anthracyclines with trastuzumab and consistently reported a higher incidence of cardiac dysfunction compared to those treated with anthracyclines alone [29,30]. Our study specifically focused on patients with TNBC, which lacks estrogen, progesterone, and HER2 receptors. Therefore, the use of trastuzumab was not indicated in our treatment approach. Only a small number of patients in our cohort (N = 64, 0.03%), received trastuzumab, and the reasons for this utilization in this cohort remain unclear. It is possible this factor may have contributed to the lower occurrence of cardiac events in our study compared to studies that encompass various BC types including a high proportion of patients receiving anthracyclines and trastuzumab.
Previous research suggests that anthracycline-induced cardiotoxicity may be more pronounced in older patients. Timóteo et al. (2019) found a significant worsening in diastolic dysfunction during the first year of BC treatment, with age as the only independent predictor. [31] Numerous other studies have also independently reported an increased risk of developing cardiac toxicity among older individuals, often defined as those above 65 years of age [23,32,33].
Although our study did not find a significant association between older age and cardiac toxicity, we observed a difference in survival rates between age groups. Among patients who experienced a cardiac outcome, those aged 76 and older exhibited worse OS and a trend toward worse CSS than those aged 66–75. No difference in OS or CSS was observed among patients who experienced a cardiac outcome who received TAX vs ATAX. Therefore, our results suggest that age may serve as a predictor for worse survival once a cardiac event occurs. This finding is logical, considering that CV events are inherent to human aging and continue to be a leading cause of death among women in the United States.
Our prior findings indicate that in older patients with no or limited nodal involvement, the addition of an anthracycline to a taxane-containing regimen does not confer any discernible benefit in OS or CSS [25]. Conversely, for older patients with 4 or more positive lymph nodes, there appears to be an advantage in using an anthracycline and taxane-containing regimen [26]. When we analyzed data by age groups, we observed that patients aged 76 and older with 4 or more positive lymph nodes exhibited a statistically significant improvement in CSS when treated with ATAX compared to TAX.
Our current study suggests that the risk of cardiotoxicity is low when treating with ATAX in older women aged 76 and above with significant nodal involvement with the current preventative measures in place. In fact, our study found that patients who received ATAX were less likely to develop heart failure even after adjusting for all other covariates. This result is likely impacted by the standard practice of assessing cardiac function prior to anthracycline administration and only treating patients without significant cardiac comorbidities. Although the incidence of heart failure was low in women aged 76 and above treated with ATAX, patients in this age group in our study who experienced a cardiac outcome had worse survival outcomes. That is, in older women who have received ATAX, if they develop a cardiac outcome, their prognosis tends to be worse.
Overall, ATAX would typically be administered to older individuals who are in better physical condition and have higher clinical risk of breast cancer recurrence. Conversely, patients who are older, more frail or have a lower risk BC would typically receive TAX. When considering the interplay of competing risks, those who received ATAX likely have a lower baseline CV risk and are routinely monitored and offered prompt cardioprotective measures. In contrast, patients receiving TAX may start with a higher baseline risk for CV events but are not monitored closely for cardiotoxicity following therapy.
Our study found that patients in a non-urban or rural commuting area or in an area with 20% or higher poverty level were more likely to develop heart failure. Additionally, patients experiencing cardiac events in non-urban areas had worse CSS and OS outcomes than their urban counterparts. It is known that low socioeconomic status impacts both quality and access to healthcare and is associated with adverse cancer and CV outcomes [34,35]. Patients from non-urban or rural commuting areas and poorer neighborhoods were likely influenced by social and structural determinants of health, such as health insurance status, geographical distance from specialized care, and transportation barriers. These factors likely resulted in reduced screening and surveillance, providing a plausible explanation for the observed poorer outcomes.
Non-married patients were more likely to develop heart failure than married or partnered patients. Past research has demonstrated that marriage serves as a protective factor against mortality and increased healthcare utilization. Moreover, transitions from a married to unmarried status have been linked to an escalation in adverse health behaviors [36–38]. Hence, non-married patients in our cohort may have had reduced screening and surveillance for cardiotoxicity due to lower healthcare system utilization. They may have exhibited more adverse health behaviors typically associated with heart failure than married patients, leading to this observed result.
Recent studies offer alternative treatment options for women with TNBC and significant cardiovascular risk factors. The KEYNOTE-522 trial demonstrated an improvement in pCR rate and event-free survival with the addition of pembrolizumab to an anthracycline, taxane, and platinum-based neoadjuvant chemotherapy regimen in patients with T2 + or node positive TNBC [11]. However, it is important to note that more than > 88% of patients were younger than 65 years old. The NeoCART trial showed that compared with ATAX, docetaxel carboplatin resulted in a higher pCR rate in patients with T2 + or node-positive TNBC [39]. However, it is worth mentioning that previous geriatric clinical trials, such as the ADVANCE trial, did not achieve targeted feasibility thresholds with the platinum and taxane-based regimen. [40] Furthermore, the CREATE-X trial examined the use of capecitabine in TNBC patients who had residual disease after neoadjuvant chemotherapy containing anthracyclines, taxanes, or both. The capecitabine group demonstrated effective prolongation of disease-free survival [41]. Again, the median age of enrollment in the CREATE-X trial was 48 years old, highlighting the need for more studies focused on an older population. In summary, while these alternative options show promise for women with TNBC and significant cardiovascular risk factors, further research is necessary to better define the efficacy and safety of these regimens in older patient populations.
Our study has a number of limitations which should be acknowledged. The use of a retrospective dataset may increase bias in our results. We relied on claims that may not have captured all relevant variables or potential confounders. Our study did not consider specific chemotherapy toxicities or changes in quality of life, which could have impacted treatment decisions and outcomes. Additionally, the follow up time in our study was 3 years which limits our ability to identify late-onset cardiovascular events. Unobservable differences in health status could have influenced treatment decisions, potentially resulting in selection bias where women in poorer health were not given ATAX. We accounted for cardiovascular risk factors; however, the SEER registries do not collect information about risk factors. Medicare data include diagnoses codes related to some risk factors, but their sensitivity is limited which may have affected our results. Our dataset also did not include administration of radiotherapy involving the heart within the treatment filed which could represent a significant risk factor for cardiotoxicity that was not accounted for in our analysis. It is important to note that our sample size of patients with cardiac outcomes was small, which hinders our ability to draw definitive conclusions. Further studies with larger sample sizes are warranted to validate and expand upon our findings. Lastly, our population may not accurately represent current patients receiving the standard of care, as they did not receive adjuvant capecitabine or immunotherapy, both of which are known to have potential cardiotoxic effects.
5. Conclusion
This study represents the largest cohort study to date evaluating major adverse cardiovascular events in older patients with TNBC. Among older women with TNBC, adjuvant chemotherapy with ATAX did not increase the risk of cardiac outcomes, including acute myocardial infarction, potentially fatal arrhythmia, heart failure, or CVA. Survival did not differ between those who received ATAX vs TAX among patients experiencing a cardiac outcome. However, stratifying patients with cardiac events by age revealed that those aged 76 and older may have worse survival compared to those ages 66–76. Thus, age may serve as a predictor for poorer survival once a cardiac event occurs. Clinical trials evaluating non-anthracycline containing regimens, like docetaxel carboplatin in combination with pembrolizumab, should be designed to include older patients as an alternative for women with cardiovascular risk factors.
Supplementary Material
Funding
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. This study was funded by the Women’s Cancer Developmental Therapeutics (WCDT) Program at the University of Colorado Cancer Center. Cathy Bradley and Elizabeth Molina are supported by the Cancer Center Support Grant P30CA046934.
Funding
Women’s Cancer Developmental Therapeutics Program, University of Colorado Cancer Center.
Footnotes
Ethics Approval and Consent to Participate
This study was conducted following local institutional review board approval and a limited data set was obtained via the National Cancer Institute’s Surveillance, Epidemiology and End Results program’s policies.
CRediT authorship contribution statement
Savannah Roy, Stephanie Lakritz, Anna R. Schreiber, Cathy J. Bradley, Lavanya Kondapalli, Jennifer R. Diamond contributed to study design and concept. Savannah Roy, Stephanie Lakritz, Anna R. Schreiber, Elizabeth Molina Kuna, Cathy J. Bradley, Lavanya Kondapalli, Jennifer R. Diamond contributed to analysis and interpretation of data. Savannah Roy, Jennifer R. Diamond wrote and edited the original draft. All authors contributed to the critical revision of the manuscript.
All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejca.2023.113426.
Availability of Data and Materials
The datasets used to conduct this study are available upon approval of a research protocol from the National Cancer Institute. Instructions for obtaining these data are available at https://healthcaredelivery.cancer.gov/seermedicare/obtain/.
References
- [1].Geyer FC, Pareja F, Weigelt B, et al. The spectrum of triple-negative breast disease: high- and low-grade lesions. Am J Pathol 2017;187(10):2139–51. 10.1016/j.ajpath.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13(15 Pt 1):4429–34. 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- [3].McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. Effects of age on the detection and management of breast cancer. Cancers (Basel) 2015;7(2):908–29. 10.3390/cancers7020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106 (5). 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17–48. 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- [6].Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The Population 65 Years and Older in the United States: 2016. US Census Bureau: American Community Survey Reports, ACS-38,; 2018. [Google Scholar]
- [7].Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379(9814): 432–44. 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 2006;24(34):5381–7. 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- [9].bc.overview@ctsu.ox.ac.uk EBCTCGEEa, (EBCTCG) EBCTCG. Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: a patient-level meta-analysis of 100 000 women from 86 randomised trials. Lancet 2023;401(10384):1277–92. 10.1016/S0140-6736(23)00285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06–090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017;35(23):2647–55. 10.1200/JCO.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schmid P, Cortes J, Dent R. Pembrolizumab in early triple-negative breast cancer. Reply. N Engl J Med 2022;386(18):1771–2. 10.1056/NEJMc2203316. [DOI] [PubMed] [Google Scholar]
- [12].Bardia A, Hurvitz SA, Rugo HS. Sacituzumab govitecan in metastatic breast cancer. Reply. N Engl J Med 2021;385(3):e12. 10.1056/NEJMc2108478. [DOI] [PubMed] [Google Scholar]
- [13].Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387(1):9–20. 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 2010;28 (25):3910–6. 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- [15].Armenian SH, Lacchetti C, Barac A, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35(8):893–911. 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- [16].Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J Cardiovasc Imaging 2022;23(10):e333–465. 10.1093/ehjci/jeac106. [DOI] [PubMed] [Google Scholar]
- [17].Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998; 339(13):900–5. 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- [18].Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32(2):302–14. . [DOI] [PubMed] [Google Scholar]
- [19].Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharm 1999;57(7):727–41. 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- [20].Gerodias FR, Tan MK, De Guzman A, et al. Anthracycline-induced cardiotoxicity in breast cancer patients: a five-year retrospective study in 10 centers. Cardiol Res 2022;13(6):380–92. 10.14740/cr1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bash LD, Weitzman D, Blaustein RO, Sharon O, Shalev V, Chodick G. Comprehensive healthcare resource use among newly diagnosed congestive heart failure. Isr J Health Policy Res 2017;6:26. 10.1186/s13584-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14(8):803–69. 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- [23].Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol 2005;23(34):8597–605. 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- [24].Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol 1997;15(4):1544–52. 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- [25].Schreiber AR, Kagihara J, Eguchi M, et al. Evaluating anthracycline + taxane versus taxane-based chemotherapy in older women with node-negative triple-negative breast cancer: a SEER-Medicare study. Breast Cancer Res Treat 2022;191 (2):389–99. 10.1007/s10549-021-06424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roy S, Lakritz S, Schreiber AR, et al. Clinical outcomes of adjuvant taxane plus anthracycline versus taxane-based chemotherapy regimens in older adults with node-positive, triple-negative breast cancer: A SEER-Medicare study. Eur J Cancer 28 2023;185:69–82. 10.1016/j.ejca.2023.02.014. [DOI] [PubMed] [Google Scholar]
- [27].Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91(5):710–7. 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- [28].Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97(11):2869–79. 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- [29].Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365(14):1273–83. 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353(16):1673–84. 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- [31].Timóteo AT, Moura Branco L, Filipe F, et al. Cardiotoxicity in breast cancer treatment: What about left ventricular diastolic function and left atrial function? Echocardiography 2019;36(10):1806–13. 10.1111/echo.14487. [DOI] [PubMed] [Google Scholar]
- [32].Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012;104(17):1293–305. 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25(25):3808–15. 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- [34].Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 2018;137(20):2166–78. 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54(2):78–93. 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- [36].Umberson D. Gender, marital status and the social control of health behavior. Soc Sci Med 1992;34(8):907–17. 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- [37].Mineau GP, Smith KR, Bean LL. Historical trends of survival among widows and widowers. Soc Sci Med 2002;54(2):245–54. 10.1016/s0277-9536(01)00024-7. [DOI] [PubMed] [Google Scholar]
- [38].Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med 2003;57(11):2137–47. 10.1016/s0277-9536(02)00546-4. [DOI] [PubMed] [Google Scholar]
- [39].Zhang L, Wu ZY, Li J, et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): Results from a multicenter, randomized controlled, open-label phase II trial. Int J Cancer 2022;150(4):654–62. 10.1002/ijc.33830. [DOI] [PubMed] [Google Scholar]
- [40].Freedman RA, Li T, Sedrak MS, et al. ADVANCE’ (a pilot trial) ADjuVANt chemotherapy in the elderly: Developing and evaluating lower-toxicity chemotherapy options for older patients with breast cancer. J Geriatr Oncol 2022. 10.1016/j.jgo.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376(22):2147–59. 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used to conduct this study are available upon approval of a research protocol from the National Cancer Institute. Instructions for obtaining these data are available at https://healthcaredelivery.cancer.gov/seermedicare/obtain/.


