Abstract
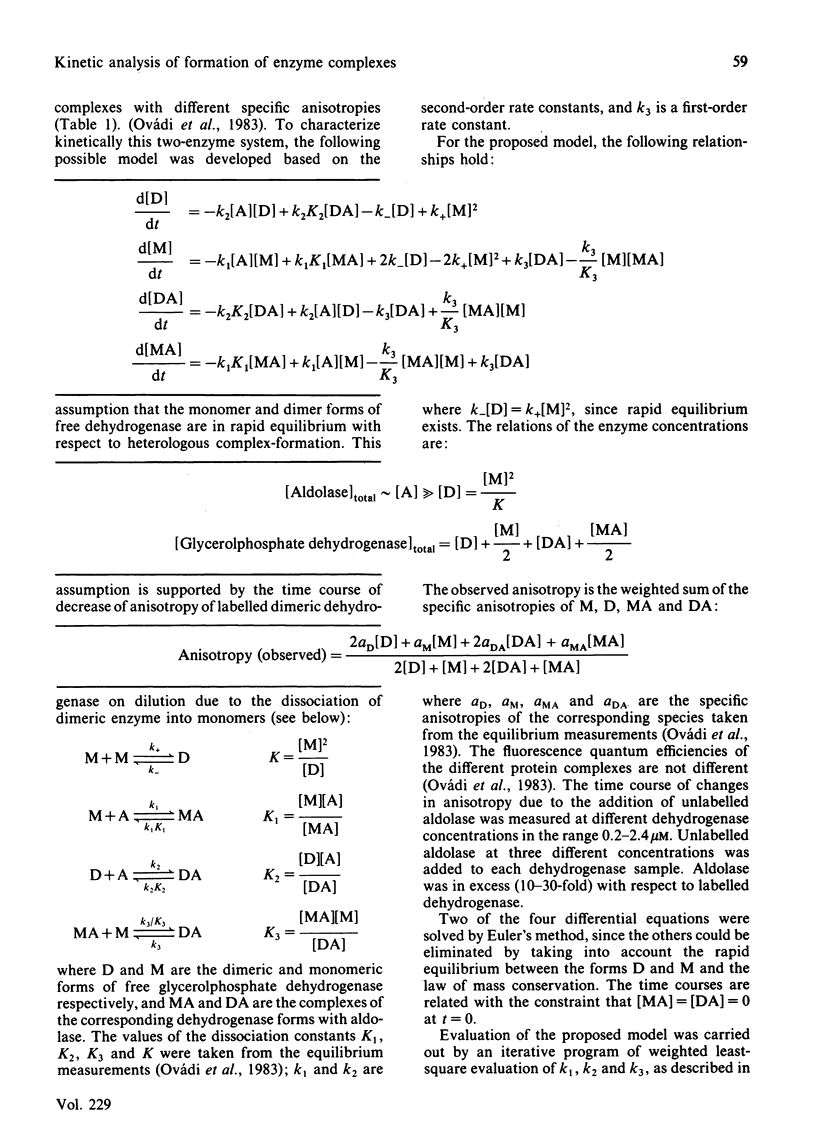
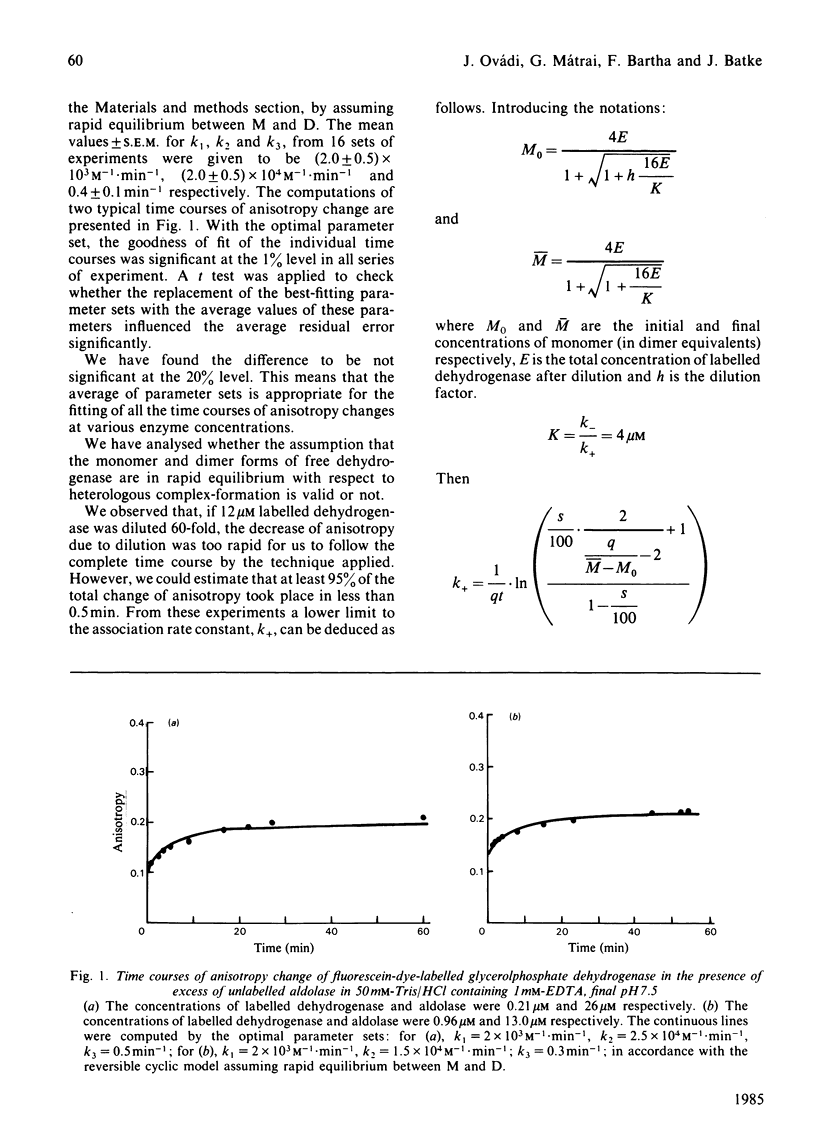
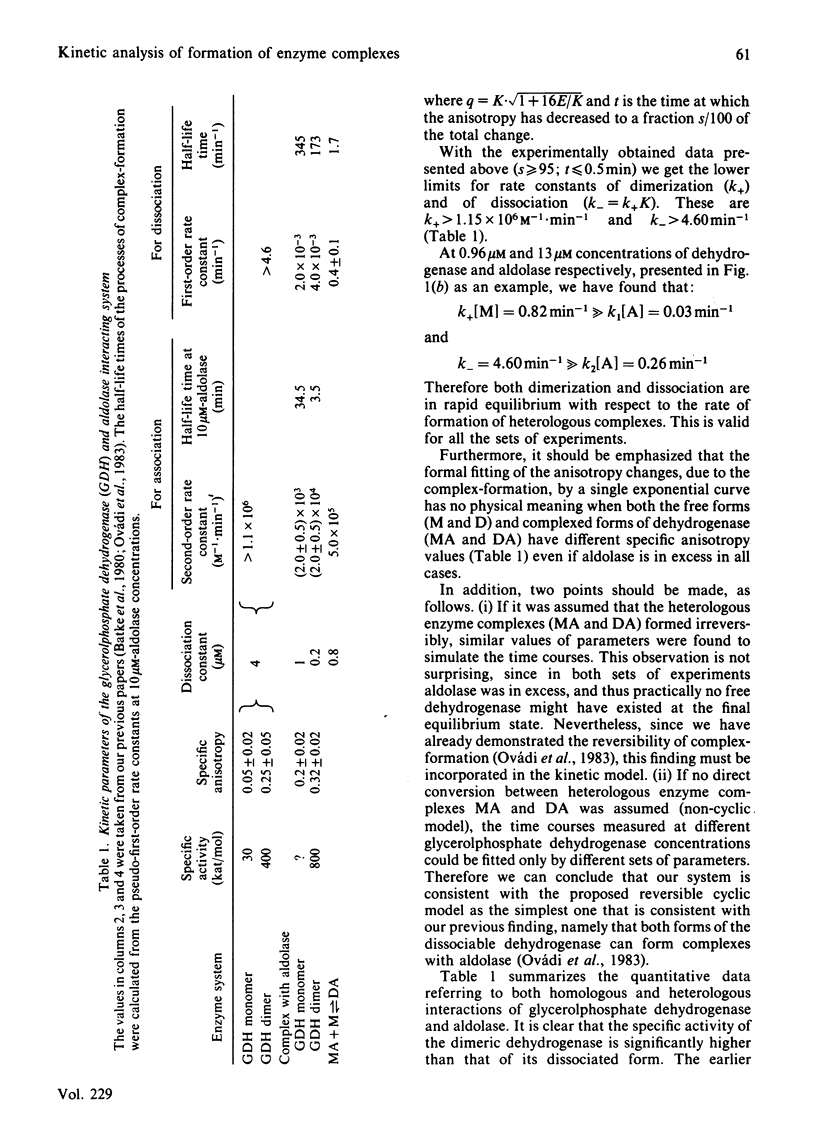
Quantitative analysis of the time courses of fluorescence anisotropy changes due to the binding of fructose-1,6-bisphosphate aldolase to the dissociable cytoplasmic glycerol-3-phosphate dehydrogenase covalently labelled with fluorescent dye was carried out. The behaviour of the aldolase-dehydrogenase system seems to be consistent with a cyclic reversible model characterized by the formation and dissociation of complexes of both the monomeric and the dimeric forms of dehydrogenase with aldolase, and rapid equilibrium between the free monomeric and dimeric forms of dehydrogenase. The half-life time of the formation of dimeric dehydrogenase-aldolase complex at the concentration of the enzymes expected to exist in the cell (i.e. in the micromolar range) is some minutes, and the time needed for equilibration between the aldolase-bound dimeric and monomeric forms of dehydrogenase is a few minutes as well. Consequently, one may expect that both the formation and the dissociation of this heterologous enzyme complex have physiological relevance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBERSON W. R., BAUER A. C., PHILPOTT D. E., ROISEN F. PROTEINS AND ENZYME ACTIVITIES OF PRESS JUICES, OBTAINED BY ULTRACENTRIFUGATION OF WHITE, RED AND HEART MUSCLES OF THE RABBIT. J Cell Physiol. 1964 Feb;63:7–24. doi: 10.1002/jcp.1030630103. [DOI] [PubMed] [Google Scholar]
- Batke J., Asbóth G., Lakatos S., Schmitt B., Cohen R. Substrate-induced dissociation of glycerol-3-phosphate dehydrogenase and its complex formation with fructose-bisphosphate aldolase. Eur J Biochem. 1980 Jun;107(2):389–394. doi: 10.1111/j.1432-1033.1980.tb06041.x. [DOI] [PubMed] [Google Scholar]
- Frieden C. Slow transitions and hysteretic behavior in enzymes. Annu Rev Biochem. 1979;48:471–489. doi: 10.1146/annurev.bi.48.070179.002351. [DOI] [PubMed] [Google Scholar]
- Grazi E., Trombetta G. The aldolase-substrate intermediates and their interaction with glyceraldehyde-3-phosphate dehydrogenase in a reconstructed glycolytic system. Eur J Biochem. 1980 Jun;107(2):369–373. doi: 10.1111/j.1432-1033.1980.tb06038.x. [DOI] [PubMed] [Google Scholar]
- Ovádi J., Mohamed Osman I. R., Batke J. Interaction of the dissociable glycerol-3-phosphate dehydrogenase and fructose-1,6-bisphosphate aldolase. Quantitative analysis by an extrinsic fluorescence probe. Eur J Biochem. 1983 Jun 15;133(2):433–437. doi: 10.1111/j.1432-1033.1983.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Ovádi J., Salerno C., Keleti T., Fasella P. Physico-chemical evidence for the interaction between aldolase and glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1978 Oct 16;90(3):499–503. doi: 10.1111/j.1432-1033.1978.tb12629.x. [DOI] [PubMed] [Google Scholar]
- Salerno C., Ovádi J. Interaction between D-fructose-1,6-bisphosphate aldolase and triosephosphate isomerase. Mutual protection against perchloric acid denaturation. FEBS Lett. 1982 Feb 22;138(2):270–272. doi: 10.1016/0014-5793(82)80458-4. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Enzyme concentrations in tissues. Science. 1967 Nov 17;158(3803):936–937. doi: 10.1126/science.158.3803.936. [DOI] [PubMed] [Google Scholar]
- TELEGDI M. ISOLATION OF CRYSTALLINE ALPHA-GLYCEROPHOSPHATE DEHYDROGENASE (L-GLYCEROL-3-PHOSPHATE: NAD OXIDOREDUCTASE 1.1.1.8) FROM RABBIT MUSCLE. Acta Physiol Acad Sci Hung. 1964;25:177–180. [PubMed] [Google Scholar]


